Abstract
Cancer vaccines that have utilized various immunization strategies to induce antitumor immunity have largely failed in clinical settings. We have recently developed a cancer vaccine using a cytomegalovirus (CMV) based vector that expressed a modified melanoma antigen that elicited a robust antitumor CD8+ T cell response and tumor rejection.
Recent advances in our understanding of T cell biology coupled with the demonstration that tumor infiltrating T cells are associated with tumor rejection have given rise to a new age of cancer immunotherapy where targeting the immune system has become a powerful weapon in fighting cancer. Use of immune checkpoint blockade such as anti-CTLA4 and anti-PD-1 antibodies to rejuvenate the T cell response against tumors has provided new hope and enthusiasm in the fight against cancer.Citation1 An additional avenue of intense investigation for cancer immunotherapy is the use of cancer vaccines to stimulate antitumor immunity. Several vaccine strategies have been designed including peptide vaccines, dendritic cell based vaccines and viral vector based vaccines. However, despite years of effort, and substantial investments, the clinical efficacy of cancer vaccines against melanoma and other tumors have largely yielded disappointing results.Citation2,3 The inadequate performance of current cancer vaccines begs for the development of alternative strategies.
Generating an effective cancer vaccine has proven to be challenging, because most tumor antigens used thus far have been derived from self-antigens. Due to tolerance mechanisms, T cells that are reactive to self-antigens activate poorly, fail to expand adequately and do not differentiate into memory cells. An effective cancer vaccine must be able to (1) elicit a strong T cell immune response that (2) is capable of infiltrating the tumor tissue, and (3) tumor specific T cells must be able to overcome the immune suppressive environment at the tumor site to deliver effector functions. Finally, (4) effector T cells must develop into a robust memory population to provide long term protection. The choice of tumor antigen, antigen delivery vehicle and the type of adjuvant all need to be carefully considered when designing cancer vaccines. Keeping these criteria in mind we recently reported the development of a unique CMV based vaccine against melanoma that was effective at tumor rejection.Citation4 We chose CMV as our tumor antigen delivery vehicle for specific reasons. CMV belongs to the herpesviridae family and establishes a life-long, asymptomatic infection in immunocompetent hosts. CMV infection induces a strong inflammatory innate immune response that drives robust CD8+ T cell expansion. Moreover, in mice and in humans several CMV specific CD8+ T cell populations continue to slowly expand for the life of the host, long after the establishment of latency, a process called T cell inflation.Citation5 A large percentage of these ‘inflationary’ CD8+ T cells are functional as exhibited by their ability to secrete multiple cytokines, and these inflationary CD8+ T cell populations are widely distributed in lymphoid and non-lymphoid organs such as lung, liver and the brain. Thus, we reasoned that the aforementioned unique properties of CMV infection would make it an excellent vaccine candidate against melanoma and potentially other cancer types. Hence, we engineered murine CMV to express a melanoma antigen gp100 (MCMV-gp100) and tested the ability of the vaccine to break immune tolerance and induce a gp100-specific CD8+ T cell response. However, immunization with MCMV-gp100 failed to elicit a gp100-specific CD8+ T cell response, which prompted us to test an alternative strategy. Our alternative approach was based on reports that showed that highly mutagenic cancers such as melanoma resulted in the emergence of a pool of modified tumor epitopes (termed as neoantigens) that exhibited enhanced immunogenicity.Citation6 Therefore, we attempted to improve the effectiveness of the vaccine by generating a CMV vector that expressed a modified tumor antigen. A previous study had demonstrated that changing gp10025–33 epitope from EGSRNQDWL to KGPRNQDWL greatly increased the binding affinity to MHCCitation7 making it a potential ‘proof of concept’ neoantigen. Hence, we engineered MCMV to express gp100 with the altered epitope (MCMV-gp100KGP) and tested its ability to induce gp100-reactive CD8+ T cells (). Our data showed that MCMV-gp100KGP effectively overcame immune tolerance and elicited a robust, polyfunctional gp100-reactive CD8+ T cell response. Remarkably, these gp100-reactive CD8+ T cells inflated over time, and remained polyfunctional even up to 10 mo after immunization. We further demonstrated that MCMV-gp100KGP was highly effective at inducing tumor rejection in an antigen dependent manner. Both prophylactic and therapeutic vaccinations with MCMV-gp100KGP strongly protected mice from highly metastatic lung B16-F10 melanoma.
Figure 1. CMV based cancer vaccines induce robust antitumor CD8+ T cell reponses. (A) CD8+ T cell response to different vaccination strategies. Peptide-based vaccination protocols or vaccines using acute-viral vectors elicit classical CD8+ T cell responses consisting of a primary expansion of effector cells followed by a dramatic contraction and maintenance of a low frequency of memory cells. In contrast, CMV-based vaccines produce an ‘inflationary’ response in which CD8+ T cells continue to accumulate over the lifetime of the host. (B) Proposed strategy for utilizing CMV-based vectors to target neoantigens. Potential immunogenic neoantigens can be engineered into the CMV genome to generate recombinant CMV vectors expressing multiple neoantigens. Vaccination with these recombinant vectors may produce ‘inflationary’ CD8+ T cell responses to tumor-specific antigens.
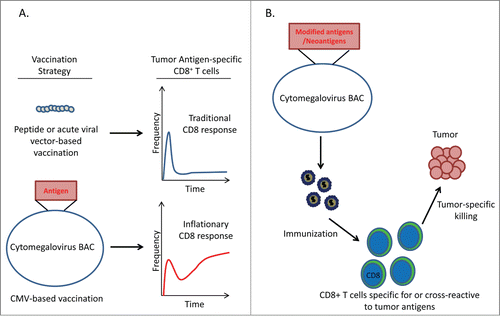
The advantages of using CMV based vaccinesCitation8 against cancers provide a new avenue for exploration and may represent a credible opportunity for future clinical trials. CMV has a large stable double-stranded DNA genome that can be manipulated to express several exogenous genes and thus can be used to express multiple T cell tumor antigens. As noted above, with the advent of cheaper RNA sequencing technology it has been demonstrated that several neoantigens are created by random mutations in tumor cellsCitation6. Several clinical trials are underway or being planned where individual cancer patients are being immunized with multiple ‘personal’ neoantigensCitation6 with the hope of inducing a robust antitumoral CD8+ T cell immune response. Such an approach coupled with the use of algorithm-based neoepitope immunogenicity predictionCitation9 may be used in the future to generate attenuated recombinant CMV vectors that express multiple cancer neoantigens for generating tumor specific CD8+ T cells responsive to the altered epitopes, thereby minimizing the risk of autoimmunity.
CMV is a ubiquitous infection with more than 90% of the population in developing countries and 40–60% in developed nations being seropositive. Remarkably, we and others have shown that in both mice and humans a robust superinfection occurs even when the host harbors latent CMV,Citation4,10 which makes it feasible to widely use CMV as a vaccine vector. Although CMV causes asymptomatic infection in immunocompetent individuals, it still posts significant risk in pregnant women and immunocompromised individuals and so future studies will be needed to generate attenuated CMV vectors that can be safely used.
In conclusion, we developed an effective cancer vaccine using a CMV-based vector that expressed a modified tumor antigen. Moving forward, we are currently testing several combination strategies including further stimulating CD8+ T cell responses with IL-2, use of immune checkpoint inhibitors, and adoptive T cell therapy, with the hope of further improving the effectiveness of CMV-based vaccines.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013; 342:1432-3; PMID:24357284; http://dx.doi.org/10.1126/science.342.6165.1432
- Ott PA, Fritsch EF, Wu CJ, Dranoff G. Vaccines and melanoma. Hematol Oncol Clin North Am 2014; 28:559-69; PMID:24880947; http://dx.doi:org/10.1016/j.hoc.2014.02.008
- Butterfield LH. Cancer vaccines. BMJ 2015; 350:h988; PMID:25904595; http://dx.doi.org/10.1136/bmj.h988
- Qiu Z, Huang H, Grenier JM, Perez OA, Smilowitz HM, Adler B, Khanna KM. Cytomegalovirus-based vaccine expressing a modified tumor antigen induces potent tumor-specific CD8+ T-cell response and protects mice from melanoma. Cancer Immunol Res 2015; 3:536-46; PMID:25633711; http://dx.doi.org/10.1158/2326-6066.CIR-14-0044
- Snyder CM, Cho KS, Bonnett EL, van DS, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity 2008; 29:650-9; PMID:18957267; http://dx.doi.org/10.1016/j.immuni.2008.07.017
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348:69-74; PMID: 25838375; http://dx.doi.org/10.1126/science.aaa4971
- Van Stipdonk MJ, Badia-Martinez D, Sluijter M, Offringa R, van HT, Achour A. Design of agonistic altered peptides for the robust induction of CTL directed towards H-2Db in complex with the melanoma-associated epitope gp100. Cancer Res 2009; 69:7784-92; PMID:19789338; http://dx.doi.org/10.1158/0008-5472.CAN-09-1724
- Xu G, Smith T, Grey F, Hill AB. Cytomegalovirus-based cancer vaccines expressing TRP2 induce rejection of melanoma in mice. Biochem Biophys Res Commun 2013; 437:287-91; PMID:23811402; http://dx.doi.org/10.1016/j.bbrc.2013.06.068
- Duan F, Duitama J, Al SS, Ayres CM, Corcelli SA, Pawashe AP, Blanchard T, McMahon D, Sidney J, Sette A et al. Genomic and bioinformatic profiling of mutational neoepitopes reveals new rules to predict anticancer immunogenicity. J Exp Med 2014; 211:2231-48; PMID:25245761; http://dx.doi.org/10.1084/jem.20141308
- Hansen SG, Powers CJ, Richards R, Ventura AB, Ford JC, Siess D, Axthelm MK, Nelson JA, Jarvis MA, Picker LJ et al. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science 2010; 328: 102-6; PMID:20360110; http://dx.doi.org/10.1126/science.1185350
