Abstract
Ibrutinib (PCI-32765) is an irreversible dual Btk/Itk inhibitor shown to be effective in treating several B cell malignancies. However, limited studies have been conducted to study the effect of this drug on myeloid cell function. Hence, we studied the effect of ibrutinib treatment on TLR-4 mediated activation of bone marrow derived dendritic cell culture (DCs). Upon ibrutinib treatment, LPS-treated DCs displayed lower synthesis of TNF-α and nitric oxide (NO) and higher induction of IL-6, TGF-β, IL-10 and IL-18. While ibrutinib dampened MHC-II and CD86 expression on DCs, CD80 expression was upregulated. Further, ibrutinib-treated DCs promoted T cell proliferation and enhanced IL-17 production upon co-culture with nylon wool enriched T cells. Taken together, our results indicate that ibrutinib modulates TLR-4 mediated DC activation to promote an IL-17 response. We describe a novel mode of action for ibrutinib on DCs which should be explored to treat other forms of cancer besides B cell malignancies.
Abbreviations
| CD | = | Cluster of differentiation |
| DC | = | Dendritic cell |
| IFN | = | Interferon |
| IL | = | Interleukin |
| LPS | = | Lipopolysaccharide |
| MHC | = | Major Histocompatibility Complex |
| NO | = | Nitric oxide |
| TGF | = | Transforming Growth Factor |
| TLR | = | Toll-like receptor |
| TNF | = | Tumor Necrosis Factor |
Introduction
Ibrutinib is a potent irreversible inhibitor of Tec kinases, BtkCitation1 and Itk.Citation2 Extensive studies have demonstrated that ibrutinib effectively treats a variety of hematological malignancies.Citation3-7 Additionally, its efficacy against immune complex disease models where myeloid cells such as neutrophils, monocytes, macrophages and DCs mediate chronic inflammation suggests that ibrutinib may also target myeloid cells.Citation8 Btk is expressed in different cell types within the myeloid lineage such as DCs and macrophages.Citation9,10 Further, Btk enhances innate immune responses, participates in TLR-4 mediated myeloid cell activationCitation9,11-14 and also enables DCs to regulate T cell proliferation and differentiation.Citation9 Although ibrutinib has been shown to have varied effects on B cells, T cells and NK cells,Citation1,5-8 its effect on myeloid cells is still incompletely understood. Since DCs play a critical role in initiating and directing protective T cell responses during cancer,Citation15,16 we wanted to study how ibrutinib affects the activation of DCs and their ability to prime T cell responses. Therefore, we determined the impact of ibrutinib treatment on DC culture and subsequent T cell activation using an in vitro model of murine bone marrow derived DCs.
Results and Discussion
Ibrutinib treatment alters cytokine and nitric oxide responses in LPS-treated DCs
Ligands to Toll-like receptors (TLRs) are potent activators of DCs and are being evaluated as adjuvants for DC based cancer therapies.Citation15 Further, it is known that Btk participates in TLR signaling in myeloid cells including DCs.Citation9-14 Hence, we studied how ibrutinib affects immune responses in TLR-activated DCs using lipopolysaccharide (LPS), a TLR-4 ligand, as an immunogen for our studies. We examined whether ibrutinib modulates cytokine and NO production in DCs upon LPS stimulation. We studied these responses at various time points after LPS stimulation and at different concentrations of ibrutinib. LPS/ibrutinib-treated DCs dampened TNF-α production compared to LPS/control-treated DCs (), while IL-12 production was comparable between both groups (). There was reduced NO production in LPS/ibrutinib-treated DCs at a later time point compared to LPS/control-treated DCs (). Additionally upon ibrutinib treatment there was higher induction of IL-18, an increase in IL-6 and TGF-β at earlier time points of LPS stimulation, and an increase in IL-10 at a later time point compared to controls (). The differences for IL-6, IL-10, IL-18 and NO were observed to be greatest mostly at the higher concentration of ibrutinib ( and ). Taken together, our results indicate that ibrutinib decreases TNF-α and NO production, increases the expression of IL-6, IL-10, IL-18 and TGF-β and does not alter IL-12 production upon LPS stimulation ( and ). Our observations for reduced TNF-α and NO is consistent with previous reports of deficient TNF-α and NO in LPS stimulated myeloid cells from Btk−/− mice and XID mice which have a mutation in the PH domain of Btk that interferes with normal Btk signaling.Citation11-14 Enhanced IL-6 production has also been reported in LPS stimulated Btk−/− macrophages.Citation11 Further, a recent study comparing LPS-mediated cytokine production in WT and Btk−/− DCs supports some of our observations. The authors reported a decrease in TNF-α production in Btk−/− mice and increase in IL-10 production by Btk−/− DCs.Citation17 However, there were contrasting differences in cytokines such as IL-6, IL-12 and IL-18 compared to the cytokine responses observed upon Btk inhibition with ibrutinib in our system. The authors observed lower IL-12 and IL-18 production by Btk−/− DCs while there were no differences in IL-6 production. We noted that the authors employed a different method of DC generation compared to our studies. The authors generated DCs by culturing bone marrow cells in the presence of FMS-like tyrosine kinase 3 ligand (Flt3L) while we generated DCs in the presence of granulocyte macrophage colony stimulating factor (GMCSF) for our studies. Previous reports have demonstrated that Flt3L and GMCSF promote the development of different subsets of DCs.Citation18,19 Further, Flt3L- and GMCSF-derived DCs also differ in their profiles of cytokine production in response to LPS activation.Citation19 It is possible that Btk differentially modulates TLR-4 signaling in Flt3L- and GMCSF-derived DCs and thereby, mediates different cytokine responses in these DC subsets. Taken together, our results indicate that ibrutinib alters TLR-4 mediated cytokine and NO production in DCs. These changes in cytokine responses upon ibrutinib treatment on DCs could subsequently reprogram T cell responses.
Figure 1. Ibrutinib dampens TNF-α and nitric oxide production in dendritic cells upon LPS stimulation. (A) TNF-α, (B) nitric oxide (NO) and (C) IL-12 production in control- and ibrutinib-treated DCs stimulated with LPS. DCs were treated with control (DMSO) or ibrutinib (1 µM or 10 µM), washed twice and treated with LPS (1 µg/mL). After 4, 12 and 24 h of LPS treatment, cytokine production was determined in the culture supernatants by ELISA. At these time points, NO levels were determined in the culture supernatants by measuring nitrite concentrations using Griess assay. The data are presented as mean + SEM of triplicate sample values from 2 independent experiments. *p < 0.05, **p < 0.001, ***p < 0.0001.
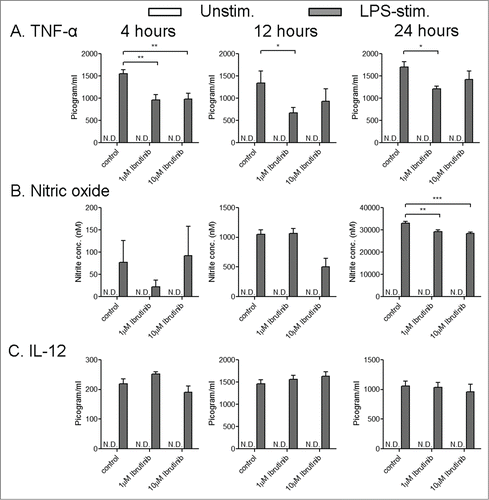
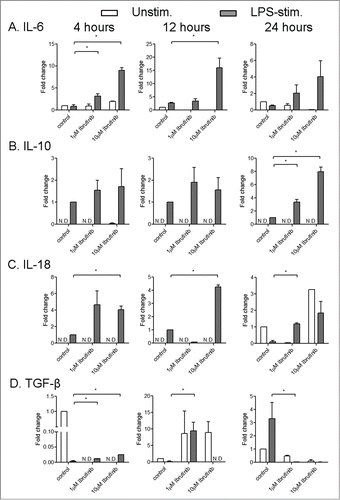
Figure 2. Ibrutinib enhances the induction of IL-6, IL-10, IL-18 and TGF-β in dendritic cells upon LPS stimulation. (A) IL-6, (B) IL-10, (C) IL-18 and (D) TGF-β mRNA induction in control and ibrutinib-treated DCs upon LPS stimulation. DCs were treated with control (DMSO) or ibrutinib (1 µM or 10 µM), washed twice and treated with LPS (1 µg/mL). After 4, 12 and 24 h of LPS treatment, cells were treated with TRIzol Reagent, RNA was isolated from cells and mRNA levels of respective cytokines were determined by real-time qPCR. The data are presented as mean + SEM of duplicates obtained by pooling 3 samples in 2 independent experiments. *p < 0.05.
Treatment with ibrutinib modulates the expression of MHC-II and co-stimulatory molecules on LPS-stimulated DCs
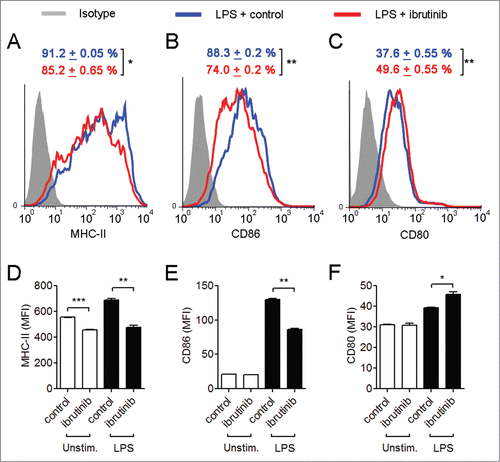
Since LPS treatment upregulates the expression of MHC-II and co-stimulatory molecules in DCs,Citation20 we wanted to determine how ibrutinib affects the expression of these molecules in LPS-stimulated DCs. Ibrutinib treatment reduced the percentage of MHC-II+ and CD86+ cells and increased the percentage of CD80+ cells compared to control treatment in LPS-stimulated DCs (). Further, LPS/ibrutinib-treated DCs displayed lower levels of MHC-II and CD86 expression and higher CD80 expression compared to LPS/control-treated DCs (). However, percentages of CD40+ DCs and expression levels of CD40 remained similar between LPS/ibrutinib- and LPS/control-treated DCs (data not shown). It has been previously shown that IL-6 and TGF-β dampen the surface expression of MHC-II and co-stimulatory molecules.Citation21,22 Hence, it is possible that the increased production of IL-6 and TGF-β in ibrutinib-treated DCs () contributes to the reduction of MHC-II and CD86 expression in these cells.
Figure 3. Ibrutinib differentially regulates the surface expression of MHC-II and co-stimulatory molecules in LPS-treated dendritic cells. Histogram plots show expressions of (A) MHC-II, (B) CD86 and (C) CD80 in LPS/control and LPS/ibrutinib-treated DC cultures. Numbers represent mean percentage of cells + SEM of the respective surface molecule on DCs. The data presented are representative plots of 3 independent experiments. Mean fluorescence intensities (MFIs) of (D) MHC-II, (E) CD86 and (F) CD80 expression in LPS/control and LPS/ibrutinib-treated DC cultures. The data are presented as mean + SEM of representative MFI values of 3 independent experiments. DCs were treated with control (DMSO) or ibrutinib (1 µM), washed twice and treated with LPS (1 µg/mL). After 24 h of LPS treatment, cells were blocked, stained with conjugated antibodies for the respective surface molecules and expressions of the surface molecules were determined by flow cytometry. Analyses were conducted by gating on CD11c+ DCs. *p < 0.05, **p < 0.001, ***p < 0.0001.
Ibrutinib-treated DCs promote an IL-17 response upon culture with T cells
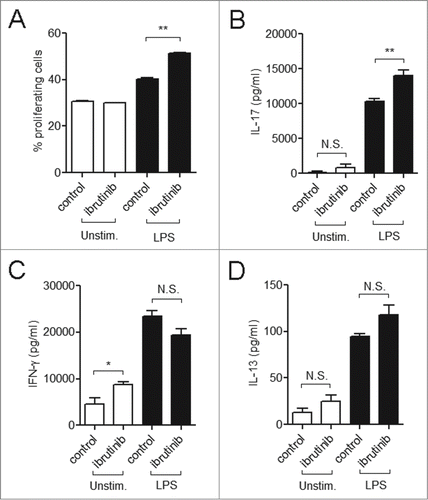
DC-derived cytokines such as IL-6, IL-18 and TGF-β are critical in initiating and directing IL-17 production by T cells.Citation20 Based on the cytokine profile generated upon ibrutinib treatment ( and ), we hypothesized that ibrutinib-treated DCs would promote an IL-17 response from T cells. We investigated this by using an in vitro antigen-specific DC:T cell co-culture model. T cells co-cultured with LPS/ibrutinib-treated DCs displayed higher proliferation rates compared to T cells co-cultured with LPS/control-treated DCs (). We also evaluated the production of T cell cytokines in the co-culture supernatants. LPS/ibrutinib-treated DCs enhanced the production of IL-17, but not IFNγ or IL-13 compared to LPS/control-treated DCs (). We could not detect the presence of IL-4 in either treatment (data not shown).
Figure 4. Ibrutinib treated DCs promote IL-17 response upon culture with T cells. (A) Analysis of T cell proliferation upon co-culture with control-, ibrutinib-, LPS/control- or LPS/ibrutinib-treated DCs. DCs were treated with control (DMSO) or ibrutinib (1 µM), washed twice, pulsed with OVA (10 µg/mL) for 2 h and treated with LPS (1 µg/mL) for 22 h. After OVA/LPS stimulation, DCs were cultured in 1:4 ratio with CFSE-stained T cells enriched from spleens of OT-II mice for 5 d. At day 5, cells from co-culture were blocked, stained with anti-CD4 antibody and T cell proliferation was measured by flow cytometry. Analyses were conducted by gating on CD4+ population. The data are presented as mean + SEM of duplicates and are representative of 2 independent experiments. (B) Production of T cell cytokines IL-17, (C) IFNγ and (D) IL-13 in co-culture experiments performed as mentioned in A. At Day 5 of co-culture, cell culture supernatants were collected and the respective cytokines were measured by ELISA. The data are presented as mean + SEM of duplicates and are representative of 2 independent experiments. *p < 0.05, **p < 0.001.
The balance of cytokines and co-stimulatory molecules expressed by DCs determines the T-helper subset generated during T cell differentiation. The production of both IL-6 and TGF-β by DCs promotes IL-17 production by T cells. Further, IL-17 synthesis by T cells can also be mediated by other cytokines such as IL-18,Citation20 which we observed to be increased in LPS/ibrutinib-treated DCs (). Although CD80-CD86/CD28-CTLA4 mediated co-stimulation has been suggested by some researchers to suppress IL-17 production by T cells,Citation23,24 our studies suggest that differential regulation of CD80 and CD86 expression may complement the cytokine signals driving IL-17 synthesis by T cells. Taken together, our results indicate that ibrutinib modulates DC activation and thereby promotes an IL-17 response by T cells.
The precise role of IL-17 response in cancer immunity is still being evaluated with studies indicating that IL-17 plays both pro-tumor as well as antitumor roles depending on the type of cancer.Citation25 In a murine melanoma model, Th17 immune response was more effective than Th1 immune response in rejecting melanoma tumors.Citation26 In this model, Th17 cells activated tumor specific CD8+ T cells and promoted the recruitment of DCs into tumor sites.Citation27 Alternatively, in healthy individuals and patients with indolent Chronic Lymphocytic Leukemia (CLL), the frequency of IL-17 producing T cells was higher and inversely correlated with immunosuppressive T cells such as regulatory T cells (Tregs) which are found at relatively-higher frequency in progressive CLL patients.Citation28 This suggests that IL-17 may play a protective role in the pathogenesis of CLL. The frequency of IL-17 producing T cells was higher in mice with reduced tumor burden with primary intraocular B-cell lymphomaCitation29 and increased IL-17 in the tumor microenvironment was associated with improved clinical survival in patients with ovarian cancer.Citation30 Such studies provide a rationale for the development of IL-17 based immunotherapies for the treatment of certain types of cancer. TLR-activated DCs are being studied for their potential anticancer effects due to their ability to generate potent T cell immune responses against various hematological malignancies and solid tumors.Citation15,31 We propose that ibrutinib should be further explored for its use in DC-based therapies due to its ability to modulate DC function and enhance DC-mediated T cell responses in a TLR-dependent manner. Our results also reveal a new mode of action for ibrutinib on DCs besides its previously known effects on B cells, T cells and NK cells.Citation1,5-8 Hence, the effects of ibrutinib on DCs and other myeloid cell populations must be further elucidated, especially in diseases where ibrutinib is currently being employed for treatment.
Materials and Methods
Mice strains
Female C57BL/6 (age 8–10 weeks) and OT-II TCR transgenic mice (age 8–10 weeks) were purchased from Harlan and Jackson Laboratories respectively. All animals were housed in a pathogen-free animal facility in The Ohio State University in accordance with National Institutes of Health and institutional guidelines.
Cultivation and stimulation of bone marrow derived dendritic cells
Bone marrow derived DCs from C57BL/6 mice were cultivated as described previously.Citation32,33 Briefly, bone marrow cells were isolated from femurs and tibias of mice, treated with ACK lysis buffer and plated @ 5 million cells/ plate in sterile 100 × 15 mm vented petri dishes along with complete RPMI medium supplemented with 10% fetal bovine serum (Atlanta Biologicals), 1% penicillin (20 Units/mL)/streptomycin (20 µg/mL) (Life Technologies) and 20ng/mL GMCSF (Peprotech) for 6 d At Day 6, cells from DC culture were collected by gently harvesting the floating fraction of cells to obtain >75% purity of CD11c+ DCs in the floating fraction. The remaining cells in the floating fraction did not display lineage specific markers for T cells, B cells, NK cells and macrophages upon flow cytometric analysis. A small percentage of neutrophils were observed (approximately 6–8% of total cell population). However, these cells were short-lived and were observed to die when the DC culture was seeded in 24-well plates (Corning Life Sciences) and rested for 24 h prior to subsequent ibrutinib treatment and activation studies. For LPS activation studies, rested DCs were washed once with 1X PBS (Life Technologies), treated with DMSO (control), 1 µM ibrutinib or 10 µM ibrutinib (Pharmacyclics, Inc..) for 30 min, washed twice with 1X PBS and treated with 1 µg/mL LPS (Sigma Aldrich) at 37°C and 5% CO2. After 4, 12 and 24 h of LPS treatment, RNA was extracted from cells for real-time PCR analysis and cell culture supernatants were harvested to determined cytokine and NO production. Further at 24 h time point, cells were isolated for flow cytometric analysis.
T cell co-culture and proliferation studies
Rested DCs were treated with control or 1µM ibrutinib as mentioned above, pulsed with 10 µg/mL OVA-peptide (323–339) (Anaspec) for 2 h and treated with 1 µg/mL LPS (Sigma Aldrich) for 22 h. After OVA/LPS stimulation, DCs were washed twice with 1X PBS and cultured with CFSE-stained T cells from OT-II mice. Prior to culture with OVA/LPS-stimulated DCs, T cells were enriched from total splenocytes of OT-II mice by nylon wool enrichment as described previously.Citation34 Briefly, single cell suspensions were prepared from spleens from OT-II mice in complete RPMI medium supplemented with 10% fetal bovine serum (Atlanta Biologicals), 1% penicillin (20 Units/mL)/streptomycin (20 µg/mL) (Life Technologies), treated with ACK Lysis buffer and incubated in nylon wool columns for 1 h. After incubation, cells were eluted from the column, washed with 1X PBS, stained with 5 µM CFSE (Life Technologies) and cultured with OVA, OVA/Ibrutinib, OVA/LPS and OVA/LPS/Ibrutinib-treated DCs in 1:4 ratio for 5 d. After 5 d, T cell proliferation was evaluated by flow cytometry and culture supernatants were harvested to determine the concentration of cytokines by ELISA.
Real-time PCR analysis
Total RNA from LPS-stimulated DCs was extracted by adding TRIzol reagent to cells (Life Technologies) and performing chloroform-isopropanol extraction of RNA according to the manufacturer's protocol. RNA was washed with 75% ethanol and resuspended in 1X TE buffer (Life Technologies). RNA concentration was determined by absorbance at 260 nm. One µg RNA was used for first strand cDNA synthesis with SuperScript VILO cDNA synthesis kit (Invitrogen). Primer sequences and cycling conditions were obtained from PRIMER BANK.Citation35 PCR amplification was conducted in an Opticon Real-Time PCR cycler (Biorad) using SYBR Green (BioRad) for detection. Data were normalized to β-actin and represented as fold induction over control-treated cells by ΔΔCT method.
Cytokine ELISA
Cytokine ELISA was performed on culture supernatants of LPS-stimulated DCs and DC-T cell co-cultures as described previously.Citation34 Briefly, purified anti-mouse TNF-α, IL-12, IFNγ, IL-17 (Biolegend) and IL-13 (eBiosciences) monoclonal antibodies were used as capture antibodies for the respective ELISA. Recombinant mouse TNF-α, IL-12, IFNγ, IL-13 (BD Biosciences) and IL-17 (eBiosciences) were used as standards. Detection of cytokines was performed using biotinylated anti-mouse antibodies for TNF-α, IL-12, IFNγ, IL-17 (Biolegend) and IL-13 (eBiosciences), streptavidin conjugated alkaline phosphatase (BD PharMingen) and p-nitrophenyl phosphate (PNPP) tablets (Thermo Fisher Scientific) as substrate. Plates were read using Spectramax M3 microplate reader (Molecular Devices LLC) at an absorbance of 405 nm. Cytokine concentrations were determined by extrapolation from the generated standard curve using Softmax Pro software (Molecular Devices LLC).
Nitric oxide assay
Culture supernatants of LPS-stimulated DCs were tested for the presence of NO as described previously.Citation36 Briefly, nitrite was measured in culture supernatants using Griess Reagent (Sigma Aldrich) with sodium nitrite as the standard. Plates were read using a Spectramax M3 microplate reader (Molecular Devices LLC) at the absorbance of 570 nm. The concentrations of NO were determined by extrapolation of generated standard curves using Softmax Pro software (Molecular Devices LLC).
Flow cytometry
LPS-stimulated DCs were washed with 1X PBS, blocked using normal mouse serum and incubated with conjugated antibodies against various cell surface markers including CD11c, MHC-II, CD80 and CD86 (Biolegend). Cells from co-cultures assays were washed and blocked as mentioned above and incubated with conjugated antibody against CD4 (Biolegend). Samples were acquired on a BD FACS Calibur (BD Biosciences). Data analysis was performed using FlowJo software (Tree Star, Inc..). During analysis, gating was performed based on respective isotype controls for the corresponding conjugated antibody. Analysis of surface marker expression on DCs was performed by gating on CD11c+ cells. Percentage of proliferating cells in T cell co-culture assay was measured on CD4+ T cells using the Proliferation platform in the FlowJo software.
Statistical analysis
All statistical analyses were done using Prism 5 (GraphPad Software). Student's unpaired t test was employed to determine statistical significance of values obtained. The p values less than 0.05 were considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Institutes of Health grants R03AI090231, RC4AI092624, R34AI100789, R21AT004160 and R03CA164399 awarded to A.R.S., National Institute of Dental and Craniofacial Research Training Grant T32DE014320 awarded to S.O. and National Cancer Institute Grants (P01 CA95426 and P50-CA140158) awarded to J.C.B.
References
- Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A 2010; 107:13075-80; PMID:20615965; http://dx.doi.org/10.1073/pnas.1004594107
- Dubovsky JA, Beckwith KA, Natarajan G, Woyach J, Jaglowski S, Zhong Y, Hessler JD, Liu T-M, Chang BY, Larkin KM et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013; 122:2539-49; PMID:23886836; http://dx.doi.org/10.1182/blood-2013-06-507947
- Akinleye A, Chen Y, Mukhi N, Song Y, Liu D. Ibrutinib and novel BTK inhibitors in clinical development. J Hematol Oncol 2013; 6:59; PMID:23958373; http://dx.doi.org/10.1186/1756-8722-6-59
- Maddocks K, Blum KA. Ibrutinib in B-cell Lymphomas. Curr Treat Options Oncol 2014; 15:226-37; PMID:24481980; http://dx.doi.org/10.1007/s11864-014-0274-8
- Burger JA, Buggy JJ. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765). Leuk Lymphoma 2013; 54:2385-91; PMID:23425038; http://dx.doi.org/10.3109/10428194.2013.777837
- Ponader S, Chen S-S, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, Keating MJ, O'Brien S, Chiorazzi N, Burger JA. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 2012; 119:1182-9; PMID:22180443; http://dx.doi.org/10.1182/blood-2011-10-386417
- Kohrt HE, Sagiv-Barfi I, Rafiq S, Herman SE, Butchar JP, Cheney C, Zhang X, Buggy JJ, Muthusamy N, Levy R, Johnson AJBJ. Ibrutinib antagonizes rituximab-dependent NK cell-mediated cytotoxicity. Blood 2014; 123:1957-60; PMID:24652965; http://dx.doi.org/10.1182/blood-2014-01-547869
- Chang BY, Huang MM, Francesco M, Chen J, Sokolove J, Magadala P, Robinson WH, Buggy JJ. The Bruton tyrosine kinase inhibitor PCI-32765 ameliorates autoimmune arthritis by inhibition of multiple effector cells. Arthritis Res Ther 2011; 13:R115; PMID:21752263; http://dx.doi.org/10.1186/ar3400
- Kawakami Y, Inagaki N, Salek-ardakani S, Kitaura J, Tanaka H, Nagao K. Regulation of dendritic cell maturation and function by Bruton's tyrosine kinase via IL-10 and Stat3. Proc Natl Acad Sci U S A 2006; 103:153-8; PMID:16371463; http://dx.doi.org/10.1073/pnas.0509784103
- Brunner C, Müller B, Wirth T. Bruton's Tyrosine Kinase is involved in innate and adaptive immunity. Histol Histopathol 2005; 20:945-55; PMID:15944945
- Schmidt NW, Thieu VT, Mann BA, Ahyi A-NN, Kaplan MH. Bruton's Tyrosine Kinase Is Required for TLR-Induced IL-10 Production. J Immunol 2006; 177:7203-10; PMID:17082638; http://dx.doi.org/10.4049/jimmunol.177.10.7203
- Mukhopadhyay S, Mohanty M, Mangla A, George A, Bal V, Rath S, Ravindran B. Macrophage Effector Functions Controlled by Bruton's Tyrosine Kinase Are More Crucial Than the Cytokine Balance of T Cell Responses for Microfilarial Clearance. J Immunol 2002; 168:2914-21; PMID:11884462; http://dx.doi.org/10.4049/jimmunol.168.6.2914
- Mangla A, Khare A, Vineeth V, Panday NN, Mukhopadhyay A, Ravindran B, Bal V, George A, Rath S. Pleiotropic consequences of Bruton tyrosine kinase deficiency in myeloid lineages lead to poor inflammatory responses. Blood 2004; 104:1191-7; PMID:15117762; http://dx.doi.org/10.1182/blood-2004-01-0207
- Mukhopadhyay S, George A, Bal V. Bruton's tyrosine kinase deficiency in macrophages inhibits nitric oxide generation leading to enhancement of IL-12 induction. J Immunol 1999; 163:1786-92; PMID:10438910
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12:265-77; PMID:22437871; http://dx.doi.org/10.1038/nrc3258
- Fuertes MB, Kacha AK, Kline J, Woo S-R, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J Exp Med 2011; 208:2005-16; PMID:21930765; http://dx.doi.org/10.1084/jem.20101159
- Ní Gabhann J, Spence S, Wynne C, Smith S, Byrne JC, Coffey B, Stacey K, Kissenpfennig A, Johnston J, Jefferies CA. Defects in acute responses to TLR4 in Btk-deficient mice result in impaired dendritic cell-induced IFN-γ production by natural killer cells. Clin Immunol 2012; 142:373-82; PMID:22281426; http://dx.doi.org/10.1016/j.clim.2011.12.009
- Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O'Keeffe M, Shao Q-X, Chen W-F et al. Cutting edge: generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol 2005; 174:6592-7; PMID:15905497; http://dx.doi.org/10.4049/jimmunol.174.11.6592
- Xu Y, Zhan Y, Lew AM, Naik SH, Kershaw MH. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol 2007; 179:7577-84; PMID:18025203; http://dx.doi.org/10.4049/jimmunol.179.11.7577
- Walsh KP, Mills KHG. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol 2013; 34:521-30; PMID:23973621; http://dx.doi.org/10.1016/j.it.2013.07.006
- Park S-J, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S-I, Kamimura D, Ueda N, Iwakura Y, Ishihara K et al. IL-6 Regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol 2004; 173:3844-54; PMID:15356132; http://dx.doi.org/10.4049/jimmunol.173.6.3844
- Fainaru O, Shay T, Hantisteanu S, Goldenberg D, Domany E, Groner Y. TGFbeta-dependent gene expression profile during maturation of dendritic cells. Genes Immun 2007; 8:239-44; PMID:17330136; http://dx.doi.org/10.1038/sj.gene.6364380
- Bouguermouh S, Fortin G, Baba N, Rubio M, Sarfati M. CD28 co-stimulation down regulates Th17 development. PLoS One 2009; 4:e5087; PMID:19333372; http://dx.doi.org/10.1371/journal.pone.0005087
- Ying H, Yang L, Qiao G, Li Z, Zhang L, Yin F, Xie D, Zhang J. Cutting edge: CTLA-4–B7 interaction suppresses Th17 cell differentiation. J Immunol 2010; 185:1375-8; PMID:20601598; http://dx.doi.org/10.4049/jimmunol.0903369
- Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol 2009; 183:4169-75; PMID:19767566; http://dx.doi.org/10.4049/jimmunol.0901017
- Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 2014; 112:362-74; PMID:18354038; http://dx.doi.org/10.1182/blood-2007-11-120998
- Martin-Orozco N, Muranski P, Chung Y, Yang XO, Lu S, Hwu P, Restifo NP, Overwijk WW. Th17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 2009; 31:787-98; PMID:19879162; http://dx.doi.org/10.1016/j.immuni.2009.09.014
- Jadidi-Niaragh F, Ghalamfarsa G, Memarian A, Asgarian-Omran H, Razavi SM, Sarrafnejad A, Shokri F. Downregulation of IL-17-producing T cells is associated with regulatory T cell expansion and disease progression in chronic lymphocytic leukemia. Tumour Biol 2013; 34:929-40; PMID:23269607; http://dx.doi.org/10.1007/s13277-012-0628-4
- Galand C, Donnou S, Crozet L, Brunet S, Touitou V, Ouakrim H, Fridman WH, Sautès-Fridman C, Fisson S. Th17 cells are involved in the local control of tumor progression in primary intraocular lymphoma. PLoS One 2011; 6:e24622; PMID:21949734; http://dx.doi.org/10.1371/journal.pone.0024622
- Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 2009; 114:1141-9; PMID:19470694; http://dx.doi.org/10.1182/blood-2009-03-208249
- Kitawaki T. DC-based immunotherapy for hematological malignancies. Int J Hematol 2014; 99:117-22; PMID:24379028; http://dx.doi.org/10.1007/s12185-013-1496-4
- Dey R, Natarajan G, Bhattacharya P, Cummings H, Dagur PK, Terrazas C, Selvapandiyan A, McCoy JP, Duncan R, Satoskar AR et al. Characterization of cross-protection by genetically modified live-attenuated Leishmania donovani parasites against Leishmania mexicana. J Immunol 2014; 193:3513-27; PMID:25156362; http://dx.doi.org/10.4049/jimmunol.1303145
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 1992; 176:1693-702; PMID:1460426; http://dx.doi.org/10.1084/jem.176.6.1693
- Oghumu S, Varikuti S, Terrazas C, Kotov D, Nasser MW, Powell CA, Ganju RK, Satoskar AR. CXCR3 deficiency enhances tumor progression by promoting macrophage M2 polarization in a murine breast cancer model. Immunology 2014; 143:109-119; PMID:24679047; http://dx.doi.org/10.1111/imm.12293
- Wang X. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res 2003; 31:154e-154; PMID:14654707; http://dx.doi.org/10.1093/nar/gng154
- Sanchez Y, Rosado JDD, Vega L, Elizondo G, Estrada-Muñiz E, Saavedra R, Juárez I, Rodríguez-Sosa M. The unexpected role for the aryl hydrocarbon receptor on susceptibility to experimental toxoplasmosis. J Biomed Biotechnol 2010; 2010:505694; PMID:20111744; http://dx.doi.org/10.1155/2010/505694
