Abstract
Melanoma patients with regional metastatic disease are at high risk for recurrence and metastatic disease, despite radical lymph node dissection (RLND). We investigated the immunologic response and clinical outcome to adjuvant dendritic cell (DC) vaccination in melanoma patients with regional metastatic disease who underwent RLND with curative intent. In this retrospective study, 78 melanoma patients with regional lymph node metastasis who underwent RLND received autologous DCs loaded with gp100 and tyrosinase and were analyzed for functional tumor-specific T cell responses in skin-test infiltrating lymphocytes. The study shows that adjuvant DC vaccination in melanoma patients with regional lymph node metastasis is safe and induced functional tumor-specific T cell responses in 71% of the patients. The presence of functional tumor-specific T cells was correlated with a better 2-year overall survival (OS) rate. OS was significantly higher after adjuvant DC vaccination compared to 209 matched controls who underwent RLND without adjuvant DC vaccination, 63.6 mo vs. 31.0 mo (p = 0.018; hazard ratio 0.59; 95%CI 0.42–0.84). Five-year survival rate increased from 38% to 53% (p < 0.01). In summary, in melanoma patients with regional metastatic disease, who are at high risk for recurrence and metastatic disease after RLND, adjuvant DC vaccination is well tolerated. It induced functional tumor-specific immune responses in the majority of patients and these were related to clinical outcome. OS was significantly higher compared to matched controls. A randomized clinical trial is needed to prospectively validate the efficacy of DC vaccination in the adjuvant setting.
Abbreviations
| DC(s) | = | Dendritic cell(s) |
| DTH | = | Delayed-type hypersensitivity |
| OS | = | Overall survival |
| RFS | = | Recurrence free survival |
| RLND | = | Radical lymph node dissection. |
Introduction
The incidence of cutaneous melanoma continues to rise worldwide.Citation1,2 Adequate surgical resection remains the standard of care for patients with non-systemic disease. However, approximately 15–20% of patients with cutaneous melanoma will develop regional (lymph node) metastasis.Citation3 The likelihood of the primary cutaneous melanoma to metastasize mainly depends on thickness, presence of ulceration and mitotic rate.Citation4,5 If patients have a positive sentinel node or develop palpable lymph node metastasis, RLND is potentially curative, although the survival is poor.Citation6 The 5-year survival rate ranges between 27% and 69%, depending on the size and number of involved nodes and characteristics of the primary melanoma.Citation7 In case of metastatic melanoma the prognosis is poor, despite recent therapeutic developments such as targeted therapies and immune checkpoint inhibitors and their positive impact on OS.Citation8-11 This has initiated numerous trials over the last few decades in search of an effective adjuvant treatment in early stage high-risk melanoma (stage IIB, IIC and III). Adjuvant radiotherapy after RLND can be considered for patients with extranodal extension, incomplete surgery or numerous positive lymph nodes to improve regional control, however, without any recurrence-free survival (RFS) or OS benefit.Citation3,12
Since melanoma is considered one of the most immunogenic types of cancer, different immunomodulatory approaches have been tested in melanoma, however mostly without showing any therapeutic effect.Citation13 IFN-α is the only approved adjuvant therapy based on significant improvement of RFS shown in phase III trials.Citation14 Nonetheless, adjuvant IFN-α is not offered universally because of substantial toxicity and only minimal OS benefit, at best 3% shown in meta-analyses.Citation15 Recently, anti-CTLA-4 antibodies in the adjuvant setting showed improvement on RFS compared to placebo, although with substantial toxicity.Citation16 Data on OS are awaited.
Specific stimulation of the immune system with the use of vaccines aims to induce melanoma-specific responses and avoids the toxicity associated with the enhanced activity across multiple subsets of effector cells, as seen with cytokines and immune checkpoint inhibitors. Thus far trials with various vaccines illustrated the potential of vaccination strategies as they showed the ability of inducing tumor-specific immune responses, however, without consistent improvement in OS.Citation17-19
DCs, the most effective antigen-presenting cells of the immune system, are exploited to induce melanoma-specific cytotoxic T cells in melanoma patients. Immature DCs are very effective in antigen uptake and when stimulated by inflammatory mediators and ‘danger signals’ they mature and migrate from peripheral tissues to lymphoid organs. Here the DCs are able to activate the specific immune system.Citation20-22 Since 1996, there have been several clinical studies investigating tumor antigen-loaded DC-based vaccines, mainly in metastatic melanoma patients.Citation23,24 Over the years, many parameters in DC vaccination have been optimized in clinical studies.Citation25 Furthermore, DC vaccination has only minimal side effects and thus provides a well-tolerable treatment.
In this study, we retrospectively analyzed the safety and survival of melanoma patients with regional metastatic disease who underwent a RLND and received adjuvant DC vaccination.
Results
Characteristics of the study population
Seventy-eight melanoma patients received adjuvant DC vaccination and were matched with 209 control patients who had undergone RLND without adjuvant DC vaccination in the same time period. Baseline characteristics are summarized in . In general, patient populations were comparable in terms of the major factors determining prognoses in regionally spread melanoma such as N substage, thickness and ulceration of the primary tumor, sex and age.Citation4,26 However, matching did not prevent significant differences between the sites of RLND. After RLND, 8 patients (3.8%) in the control population received adjuvant radiotherapy and 15 patients (7.6%) received adjuvant IFN-α (in trials), while none of the patients treated with DC vaccination received additional adjuvant therapy. In patients who developed distant metastasis during follow-up, less control patients received systemic therapy (). Systemic therapy mainly consisted of chemotherapy, whereas a small number of patients received other immunotherapy. The median follow-up in the entire study population was 39 mo (range 1–165 mo). In a few patients follow-up was extremely short, due to early death due to rapid progressive disease.
Table 1. Baseline characteristics of dendritic cell vaccinated patients and controls
Table 2. Systemic therapy in patients with metastatic disease
Dendritic cell vaccination
DC vaccination consisted of a maximum of three cycles of three biweekly vaccinations. Two patients did not complete the first cycle of vaccinations due to rapid progressive disease. Another 13 patients did not receive a second cycle due to development of distant metastasis within 6 mo after RLND. The majority of patients, 54 out of 78, completed all three cycles of vaccinations. The two patients with rapid progressive disease both were screened for metastases before RLND. One patient had a chest X-ray but was later diagnosed with non-pulmonary metastases. The other patient had a CT of the chest and abdomen which showed one dubious lesion in the lungs, but developed multiple lung and liver metastases shortly after.
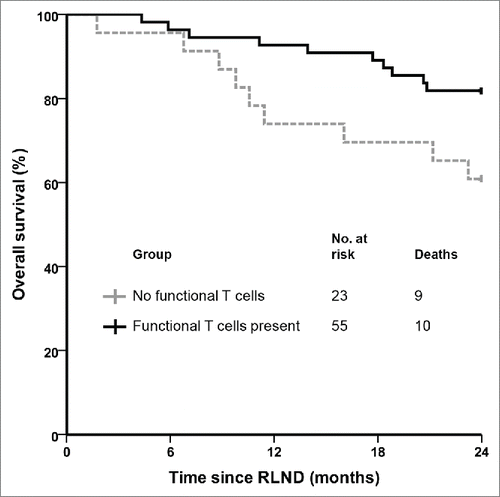
To determine the presence of functional tumor-specific T cells, tetramer staining for tyrosinase and gp100 epitopes were performed after each vaccination cycle on skin-test infiltrating lymphocytes and functionality was tested. Functional tumor-specific T cells were detected in 55 out of 78 vaccinated patients (71%). The 2-year survival rate was significantly higher (82%) in patients with functional tumor-specific T cells compared to DC vaccinated patients without functional tumor-specific T cells (61%; p = 0.04; ).
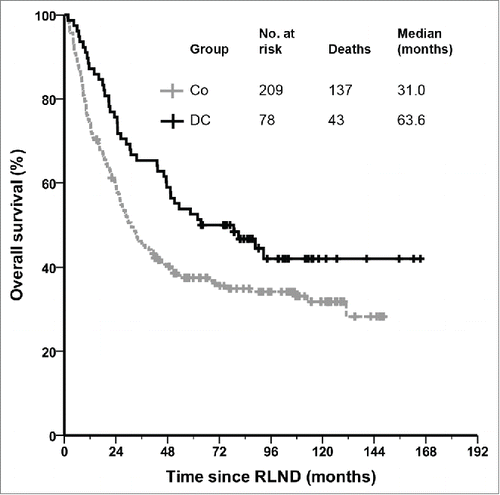
Figure 1. Functional tumor-specific T cells correlate with clinical outcome in stage III melanoma patients. Kaplan–Meier curve of patients with regional metastasized melanoma who received adjuvant dendritic cell vaccination after radical lymph node dissection according to the presence or absence of functional tumor-specific T cells in skin-test infiltrating lymphocytes. RLND, radical lymph node dissection.
Toxicity
DC vaccinations were generally well tolerated. Forty-eight out of 57 patients (84%) receiving only DC vaccinations and 20 out of 21 patients (95%) receiving both DC vaccination and IL-2 suffered at least one mild adverse event (CTC grade 1 or 2; ). The most common side effects that are associated with DC vaccination are transient flu-like symptoms, including fatigue and fever and erythema at the site of injection. No treatment related grade 3 or 4 toxicity was observed. Treatment was discontinued in two patients at their own request due to vaccine-related grade two rash in the second cycle, both patients are still alive (94 and 115 mo after RLND).
Table 3. Dendritic cell vaccination protocols and toxicity
Survival
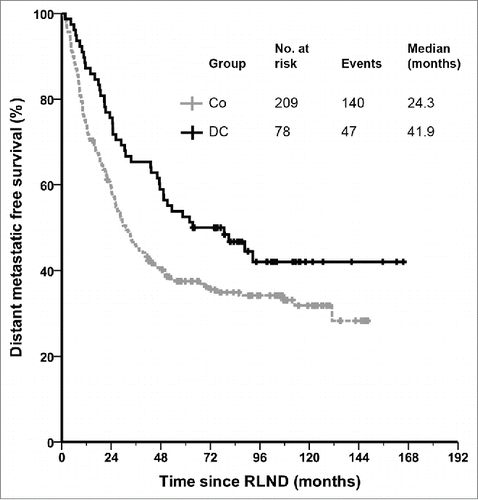
Kaplan–Meier survival analysis demonstrates a significant difference in OS in favor of the DC vaccinated patients compared to matched controls (). The median OS increased more than two-fold in patients who received adjuvant DC vaccination as compared with that of the matched controls, from 31.0 (95% CI 23.6–38.5) to 63.6 mo (95% CI 24.5–102.7; p = 0.018). The 1-, 2- and 5-years survival rates were 87%, 76% and 53% for the DC vaccinated patients and 74%, 59% and 38% for the controls (p = 0.018; p = 0.009; p = 0.008, respectively). For the time to distant metastasis a trend was seen in favor of the DC vaccinated patients, with a median of 41.9 (95% CI 32.3–51.4) vs. 24.3 mo in the control group (95% CI 18.9–29.7; p = 0.081; ).
Univariate and multivariate analyses on overall survival
Cox regression analysis was used to predict prognostic factors of OS in all patients. Four baseline characteristics were predictors of OS (p < 0.05): age, ulceration, N substage and site of RLND. Furthermore, adjuvant DC vaccination was also a significant positive predictor of OS (Supplementary Table 1). To further investigate whether adjuvant DC vaccination is an independent prognostic factor for OS, the multivariate Cox proportional hazards model was applied to the significant variables of the univariate analysis. The multivariate analysis, in both forward and backward model, revealed that ulceration (HR 1.57; 95%CI 1.15–2.13; p = 0.004; Wald 8.2), N substage (HR 1.23; 95%CI 1.17–1.30; p < 0.001; Wald 66.9), and adjuvant DC vaccination (HR 0.59; 95%CI 0.42–0.84; p = 0.003; Wald 8.9) were independent predictors of OS.
Discussion
Adjuvant treatment with DC vaccination in patients with regional metastasized melanoma, who are at high risk for recurrence of disease even after RLND, results in a significant benefit on OS compared to matched controls, with only minimal toxicity. This is the first study on a large cohort with sufficiently long follow-up to draw any conclusions on the clinical outcome in melanoma patients with regional metastasis treated with adjuvant DC vaccination.
In distant metastatic melanoma patients we have shown that the presence of tumor-specific T cells is a positive predictive factor for OS and functionality correlates with survival.Citation27,28 However, the presence of tumor-specific T cells after DC vaccination might also represent the patients with a more potent immune system and therefore longer survival regardless of treatment. In this study we demonstrate that in melanoma patients with regional metastasis the presence of functional tumor-specific T cells after adjuvant DC vaccination is also positively correlated with survival, which provides further evidence that activation of the immune system against melanoma cells by DC vaccination plays a pivotal role in its clinical efficacy. This is further substantiated by the finding that functional tumor-specific T cells are more frequently found in melanoma patients with regional metastasis (71%), in comparison to patients with distant metastasis (23%).Citation27 This might partially be explained by that the patients with regional metastasis received more vaccinations and more delayed-type hypersensitivity (DTH) skin tests. Additionally, the greater efficacy of DC vaccination in the adjuvant setting, eradicating residual micrometastasis if present, and higher frequencies of function tumor-specific T cells may also be caused by less tumor burden and therefore less tumor-mediated immune suppression compared to metastatic disease.Citation29
Unfortunately, almost all clinical trials with DC vaccines are conducted in university hospitals that usually do not have the funds to run extensive randomized trials. As personalized cellular products are not commonly produced by pharmaceutical companies, randomized trials with DC vaccines are scarce. In the absence of a randomized study, this study used matched controls for survival analysis. To limit selection bias, we matched patients on N substage, which is the major prognostic factor in regionally spread melanoma.Citation4,30 As DC vaccination was commenced within 2 mo after RLND and relapse within 2 mo from surgery is rare, we believe this minimized the risk of selection bias. Furthermore, patients who did not complete their first cycle of vaccination were not excluded from the analyses. Comparison of baseline characteristics indicate that the controls used in this study were representative of the adjuvant treated patients. However, a small difference was present in the higher incidence of nodal metastasis in the cervical region in control patients. Since cervical metastasis may have longer disease-specific survival after RLND compared to patients with axillae and groin metastasis,Citation31 it is unlikely this would give a survival benefit for the DC vaccinated patients. Furthermore, in the multivariate analysis the site of RLND did not maintain a significant effect on OS. Adjuvant radiotherapy andIFN-α given to several patients in the control group did not show a statistically significant impact on OS in the univariate analysis. The absent or only minimal effect on OS is supported by literature.Citation3,12,Citation15
Potential time bias could occur due to the influence on OS of recent developments in the treatment of patients with distant metastasis, mostly with the introduction of ipilimumab. A small difference was seen in treatment received in patients who developed metastatic disease during follow-up. This discrepancy may be caused by the duration of follow-up, which was longer in DC vaccinated patients, and the completeness of registration; numerous control patients returned to their local hospital after RLND for follow-up and data on further systemic treatment might not be accurately traced. Although referring hospitals were contacted to gather information, these data may be less robust. However, most patients who developed distant metastasis did so before ipilimumab and BRAF inhibitors were widely used and only received dacarbazine or no systemic treatment at all. In addition, when the 4 ipilimumab-treated patients were excluded from the analyses, this had only little effect on the median OS (58.7 mo) and remained statistically significant from matched controls (p = 0.027). The larger difference in OS compared to distant metastatic free survival benefit of DC vaccination compared to controls, might also be partially explained by the less robust data from the controls as death is a more accurate to trace event than the development of distant metastasis. Furthermore, in multiple studies with immunotherapy, both with DC vaccination as with immune checkpoint inhibitors, OS benefit is often more pronounced than the effect on progresson-free survival. This widespread phenomenon might be explained by a treatment-induced slowing of tumor growth, without establishing an equilibrium in the tumor microenvironment, which is maintained after documented progression of disease. We believe that the findings of our study are important to both the research and clinical community, as with nowadays targeted therapies and checkpoint inhibitors, it will be difficult to analyze the effect of vaccination in itself without being obscured by perhaps many subsequent immune interventions.
Despite proper matching, the literature shows that historical controls generally have worse clinical outcome compared to randomized controls.Citation32 For example, Canvaxin, a polyvalent vaccine, showed promising results in a phase II trial but failed to meet phase III trial endpoints. Still, our controls had a similar 5-year survival rate as a comparable group of melanoma patients after RLND reported in recent literature.Citation33
A randomized clinical trial with DC vaccination in the adjuvant setting is needed to prospectively validate the efficacy of DC-vaccines in the adjuvant treatment following RLND for melanoma. The introduction of adjuvant DC vaccination after RLND would not interfere with the current standard of care, especially in Europe where IFN-α is not recommended. Recent data on the randomized trial with adjuvant ipilimumab are widely debated since the first results were shown.Citation34 The effect on RFS is indisputable, but toxicity is high (42% grade 3/4 adverse events). Therefore, it is questionable if adjuvant ipilimumab will become the standard of care after RLND in patients with regional metastasis.
In conclusion, adjuvant treatment with DC vaccination after RLND in patients with regional metastasis of melanoma is safe and results in a favorable OS compared to matched controls. Importantly, DC vaccination is well tolerated and clearly less toxic than adjuvant IFN-α or ipilimumab. These results suggest that DC vaccination has efficacy as adjuvant treatment of melanoma, and provide further support to test this in a prospective randomized clinical trial.
Patients and Methods
Patient characteristics
We retrospectively analyzed a cohort of 78 melanoma patients with histologically proven regional metastasis without evidence of distant metastasis, who were enrolled in our DC vaccination studies between December 1999 and February 2009. Patients received adjuvant DC vaccination within 2 mo from RLND. WHO performance status was 0 or 1. Additional inclusion criteria include HLA-A*02:01 phenotype and melanoma expressing the melanoma-associated antigens gp100 (compulsory) and tyrosinase (non-compulsory). Patients with serious concomitant disease or a recent history of second malignancy were excluded. The studies were approved by the appropriate Medical Ethical Review Board and written informed consent was obtained from all patients.
Treatment schedule
All patients were vaccinated with cytokine-matured monocyte-derived autologous DCs loaded with tumor-associated antigens of gp100 and tyrosinase according to a schedule of three biweekly vaccinations. In absence of disease recurrence, patients received a maximum of two maintenance cycles at 6-mo intervals. Differences in protocols included the route of administration, method of antigen loading and combined treatment with low-dose IL-2 (). Patients received a maximum of 10 × 106 DCs intradermal, 15 × 106 intranodal or 20 × 106 intravenous per vaccination. For the exact details regarding the vaccination protocols we refer to these individual studies.Citation35-38
Dendritic cell vaccine
Monocytes were enriched from leukapheresis products by plastic adherence of peripheral blood mononuclear cells or by counterflow centrifugation using Elutra-cell separator (Gambro BCT) and single-use, functionally sealed disposable Elutra sets, as described before.Citation39 Monocytes were cultured in the presence of IL-4 (500 U/mL), GM-CSF (800 U/mL) (both Cellgenix) and KLH (10 μg/mL, Calbiochem). DCs were matured with autologous monocyte-conditioned medium (30%, v/v) supplemented with prostaglandin E2 (10 μg/mL, Pharmacia & Upjohn) and 10 ng/mL TNF-α (Cellgenix) for 48 h.Citation40 This procedure gave rise to mature DCs meeting the release criteria.Citation24 DCs were pulsed with 2 gp100-derived peptides and a tyrosinase-derived peptide or electroporated with mRNA encoding full-length gp100 or tyrosinase protein.Citation41-43 These melanoma-associated tumor-antigens were selected as they are widely expressed on primary melanoma and melanoma metastasis and have shown to induce functional cytotoxic T cells.Citation41,44 Cells were resuspended in 0.1 mL for injection.Citation45
Skin-test infiltrating lymphocyte analyses
Skin tests were performed within two weeks after each vaccination cycle as described previously.Citation27,28 Briefly, DCs loaded with either gp100, tyrosinase or both epitopes were injected intradermally in the skin of the back of the patient. After 48 h, punch biopsies (6 mm) were taken. Half of the biopsy was manually cut and cultured in RPMI-1640 containing 7% human serum and IL-2 (100 U/mL). After 2–4 weeks of culturing, skin-test infiltrating lymphocytes were stained with tetrameric-MHC complexes containing the gp100 or tyrosinase epitopes and functionality was tested. Functional tumor-specific T cells were defined by binding of tetrameric-MHC complexes for at least one of the epitopes and either the production of cytokines or cytotoxic activity in response to tumor antigen stimulation.
Matched controls
Matched controls were obtained from a database from the Erasmus MC Cancer Institute, an academic hospital comparable to Radboudumc were patients were not included in DC vaccination trials. This database consists of 563 melanoma patients who had undergone RLND between 1982 and 2010. Preoperative imaging and follow-up was performed according to the Dutch national guidelines. Controls were matched to study patients on the basis of N substage according to AJCC criteria and on timeframe (1992–2009). If more than 3 matches were found in the database for one study object, age and sex were used to select the closest matches.
Statistical analysis
OS was calculated from the date of RLND to death using the Kaplan–Meier method and compared using the log-rank test. Statistical significance of baseline characteristics was evaluated using chi-square tests. The univariate analysis was performed by the Cox proportional hazards model. Multivariate survival analysis was carried out using the Cox proportional hazards model with stepwise elimination of significant univariate parameters with forward and backward stepwise methods. Ulceration was assumed absent if not reported in the pathology report. P values less than 0.05 were considered significant. SPSS version 20.0 software (SPSS Inc.) was used for statistical analysis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publishers website.
Supplementary_Table_1._Univariate_analyses_-_overall_survival.docx
Download MS Word (21.2 KB)Acknowledgments
The authors thank the involved technicians Nicole Scharenborg, Annemiek de Boer, Mandy van de Rakt, Michel Olde Nordkamp, Christel van Riel, Marieke Kerkhoff, Jeanette Pots, Rian Bongaerts and Tjitske Duiveman-de Boer and Charlotte Oude Ophuis and Stijn van der Ploeg for their assistance with data collection.
Funding
This work was supported by grants from the Dutch Cancer Society (Grants KUN2006–3699, KUN2010–4722, KUN2009–4402 and KUN2009–4580) and the Nijmeegs Offensief tegen Kanker foundation, C.G.F. received the Netherlands Organization for Scientific Research Spinoza award. J.d.V. received NWO-Vici-918.146.55. The author(s) have no proprietary or commercial interest in any materials discussed in this article.
References
- Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol 2004; 150:179-85; PMID:14996086; http://dx.doi.org/10.1111/j.1365-2133.2004.05708.x
- Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol 2009; 27:3-9; PMID:19095149; http://dx.doi.org/10.1016/j.clindermatol.2008.09.001
- Agrawal S, Kane JM, Guadagnolo BA, Kraybill WG, Ballo MT. The benefits of adjuvant radiation therapy after therapeutic lymphadenectomy for clinically advanced, high-risk, lymph node-metastatic melanoma. Cancer 2009; 115:5836-44; PMID:19701906; http://dx.doi.org/10.1002/cncr.24627
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27:6199-206; PMID:19917835; http://dx.doi.org/10.1200/JCO.2009.23.4799
- Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross MI, Kirkwood JM et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001; 19:3622-34; PMID:11504744
- van Akkooi AC, Bouwhuis MG, de Wilt JH, Kliffen M, Schmitz PI, Eggermont AM. Multivariable analysis comparing outcome after sentinel node biopsy or therapeutic lymph node dissection in patients with melanoma. Br J Surg 2007; 94:1293-9; PMID:17702089; http://dx.doi.org/10.1002/bjs.5814
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A Jr, Kirkwood JM et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001; 19:3635-48; PMID:11504745
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507-16; PMID:21639808; http://dx.doi.org/10.1056/NEJMoa1103782
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Robert C, Thomas L, Bondarenko I, O'Day S, M DJ, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517-26; PMID:21639810; http://dx.doi.org/10.1056/NEJMoa1104621
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015; 372:30-9; PMID:25399551; http://dx.doi.org/10.1056/NEJMoa1412690
- Burmeister BH, Henderson MA, Ainslie J, Fisher R, Di Iulio J, Smithers BM, Hong A, Shannon K, Scolyer RA, Carruthers S et al. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol 2012; 13:589-97; PMID:22575589; http://dx.doi.org/10.1016/S1470-2045(12)70138-9
- Kalialis LV, Drzewiecki KT, Klyver H. Spontaneous regression of metastases from melanoma: review of the literature. Melanoma Res 2009; 19:275-82; PMID:19633580; http://dx.doi.org/10.1097/CMR.0b013e32832eabd5
- Eggermont AM, Suciu S, Santinami M, Testori A, Kruit WH, Marsden J, Punt CJ, Sales F, Gore M, Mackie R et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet 2008; 372:117-26; PMID:18620949; http://dx.doi.org/10.1016/S0140-6736(08)61033-8
- Wheatley K, Ives N, Hancock B, Gore M, Eggermont A, Suciu S. Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat Rev 2003; 29:241-52; PMID:12927565; http://dx.doi.org/10.1016/S0305-7372(03)00074-4
- Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015; 16:522-30; PMID:25840693; http://dx.doi.org/10.1016/S1470-2045(15)70122-1
- Verma S, Quirt I, McCready D, Bak K, Charette M, Iscoe N. Systematic review of systemic adjuvant therapy for patients at high risk for recurrent melanoma. Cancer 2006; 106:1431-42; PMID:16511841; http://dx.doi.org/10.1002/cncr.21760
- Hersey P, Coates AS, McCarthy WH, Thompson JF, Sillar RW, McLeod R, Gill PG, Coventry BJ, McMullen A, Dillon H et al. Adjuvant immunotherapy of patients with high-risk melanoma using vaccinia viral lysates of melanoma: results of a randomized trial. J Clin Oncol 2002; 20:4181-90; PMID:12377961; http://dx.doi.org/10.1200/JCO.2002.12.094
- Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med 2011; 364:2119-27; PMID:21631324; http://dx.doi.org/10.1056/NEJMoa1012863
- Adema GJ, Hartgers F, Verstraten R, de Vries E, Marland G, Menon S, Foster J, Xu Y, Nooyen P, McClanahan T et al. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature 1997; 387:713-7; PMID:9192897; http://dx.doi.org/10.1038/42716
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392:245-52; PMID:9521319; http://dx.doi.org/10.1038/32588
- Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med 1999; 5:405-11; PMID:10202929; http://dx.doi.org/10.1038/7403
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007; 449:419-26; PMID:17898760; http://dx.doi.org/10.1038/nature06175
- Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med 2004; 10:475-80; PMID:15122249; http://dx.doi.org/10.1038/nm1039
- Lesterhuis WJ, Aarntzen EH, De Vries IJ, Schuurhuis DH, Figdor CG, Adema GJ, Punt CJ. Dendritic cell vaccines in melanoma: from promise to proof? Crit Rev Oncol Hematol 2008; 66:118-34; PMID:18262431; http://dx.doi.org/10.1016/j.critrevonc.2007.12.007
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Ding S, Byrd DR, Cascinelli N, Cochran AJ, Coit DG, Eggermont AM et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol 2010; 28:2452-9; PMID:20368546; http://dx.doi.org/10.1200/JCO.2009.27.1627
- Aarntzen EH, Bol K, Schreibelt G, Jacobs JF, Lesterhuis WJ, van Rossum MM, Adema GJ, Figdor CG, Punt CJ, de Vries IJ. Skin-test infiltrating lymphocytes early predict clinical outcome of dendritic cell based vaccination in metastatic melanoma. Cancer Res 2012: 72:6102-10; PMID:23010076; http://dx.doi.org/10.1158/0008-5472.CAN-12-2479
- de Vries IJ, Bernsen MR, Lesterhuis WJ, Scharenborg NM, Strijk SP, Gerritsen MJ, Ruiter DJ, Figdor CG, Punt CJ, Adema GJ. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J Clin Oncol 2005; 23:5779-87; PMID:16110035; http://dx.doi.org/10.1200/JCO.2005.06.478
- Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res 2007; 13:5256-61; PMID:17875753; http://dx.doi.org/10.1158/1078-0432.CCR-07-0892
- van Akkooi AC, Bouwhuis MG, van Geel AN, Hoedemaker R, Verhoef C, Grunhagen DJ, Schmitz PI, Eggermont AM, de Wilt JH. Morbidity and prognosis after therapeutic lymph node dissections for malignant melanoma. Eur J Surg Oncol 2007; 33:102-8; PMID:17161577; http://dx.doi.org/10.1016/j.ejso.2006.10.032
- Wevers KP, Bastiaannet E, Poos HP, van Ginkel RJ, Plukker JT, Hoekstra HJ. Therapeutic lymph node dissection in melanoma: different prognosis for different macrometastasis sites? Ann Surg Oncol 2012:19:3913-8; PMID:22588472; http://dx.doi.org/10.1245/s10434-012-2401-8
- Sacks H, Chalmers TC, Smith H Jr. Randomized versus historical controls for clinical trials. Am J Med 1982; 72:233-40; PMID:7058834; http://dx.doi.org/10.1016/0002-9343(82)90815-4
- Morton DL, Hsueh EC, Essner R, Foshag LJ, O'Day SJ, Bilchik A, Gupta RK, Hoon DS, Ravindranath M, Nizze JA et al. Prolonged survival of patients receiving active immunotherapy with Canvaxin therapeutic polyvalent vaccine after complete resection of melanoma metastatic to regional lymph nodes. Ann Surg 2002; 236:438-48; discussion 48-9; PMID:12368672; http://dx.doi.org/10.1097/00000658-200210000-00006
- Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM et al. Ipilimumab versus placebo after complete resection of stage III melanoma: initial efficacy and safety results from the EORTC 18071 phase III trial. ASCO Meeting Abstracts 2014; 32:LBA9008.
- Lesterhuis WJ, Schreibelt G, Scharenborg NM, Brouwer HM, Gerritsen MJ, Croockewit S, Coulie PG, Torensma R, Adema GJ, Figdor CG et al. Wild-type and modified gp100 peptide-pulsed dendritic cell vaccination of advanced melanoma patients can lead to long-term clinical responses independent of the peptide used. Cancer Immunol Immunother 2011; 60:249-60; PMID:21069321; http://dx.doi.org/10.1007/s00262-010-0942-x
- Lesterhuis WJ, de Vries IJ, Schreibelt G, Lambeck AJ, Aarntzen EH, Jacobs JF, Scharenborg NM, van de Rakt MW, de Boer AJ, Croockewit S et al. Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients. Clin Cancer Res 2011; 17:5725-35; PMID:21771874; http://dx.doi.org/10.1158/1078-0432.CCR-11-1261
- Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, Schreibelt G, Mus R, De Wilt JH, Haanen JB et al. Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res 2013; 73:19-29; PMID:23087058; http://dx.doi.org/10.1158/0008-5472.CAN-12-1127
- Aarntzen EH, Schreibelt G, Bol K, Lesterhuis WJ, Croockewit AJ, de Wilt JH, van Rossum MM, Blokx WA, Jacobs JF, Duiveman-de Boer T et al. Vaccination with mRNA-electroporated dendritic cells induces robust tumor antigen-specific CD4+ and CD8+ T cells responses in stage III and IV melanoma patients. Clin Cancer Res 2012; 18:5460-70; PMID:22896657; http://dx.doi.org/10.1158/1078-0432.CCR-11-3368
- Berger TG, Feuerstein B, Strasser E, Hirsch U, Schreiner D, Schuler G, Schuler-Thurner B. Large-scale generation of mature monocyte-derived dendritic cells for clinical application in cell factories. J Immunol Methods 2002; 268:131-40; PMID:12215381; http://dx.doi.org/10.1016/S0022-1759(02)00189-8
- de Vries IJ, Adema GJ, Punt CJ, Figdor CG. Phenotypical and functional characterization of clinical-grade dendritic cells. Methods Mol Med 2005; 109:113-26; PMID:15585917
- Topalian SL, Gonzales MI, Parkhurst M, Li YF, Southwood S, Sette A, Rosenberg SA, Robbins PF. Melanoma-specific CD4+ T cells recognize nonmutated HLA-DR-restricted tyrosinase epitopes. J Exp Med 1996; 183:1965-71; PMID:8642306; http://dx.doi.org/10.1084/jem.183.5.1965
- Li K, Adibzadeh M, Halder T, Kalbacher H, Heinzel S, Muller C, Zeuthen J, Pawelec G. Tumour-specific MHC-class-II-restricted responses after in vitro sensitization to synthetic peptides corresponding to gp100 and Annexin II eluted from melanoma cells. Cancer Immunol Immunother 1998; 47:32-8; PMID:9755876; http://dx.doi.org/10.1007/s002620050501
- de Vries IJ, Lesterhuis WJ, Scharenborg NM, Engelen LP, Ruiter DJ, Gerritsen MJ, Croockewit S, Britten CM, Torensma R, Adema GJ et al. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res 2003; 9:5091-100; PMID:14613986
- Bakker AB, van der Burg SH, Huijbens RJ, Drijfhout JW, Melief CJ, Adema GJ, Figdor CG. Analogues of CTL epitopes with improved MHC class-I binding capacity elicit anti-melanoma CTL recognizing the wild-type epitope. Int J Cancer 1997; 70:302-9; PMID:9033632; http://dx.doi.org/10.1002/(SICI)1097-0215(19970127)70:3%3c302::AID-IJC10%3e3.0.CO;2-H
- Schuurhuis DH, Lesterhuis WJ, Kramer M, Looman MG, van Hout-Kuijer M, Schreibelt G, Boullart AC, Aarntzen EH, Benitez-Ribas D, Figdor CG et al. Polyinosinic polycytidylic acid prevents efficient antigen expression after mRNA electroporation of clinical grade dendritic cells. Cancer Immunol Immunother 2009; 58:1109-15; PMID:19018531; http://dx.doi.org/10.1007/s00262-008-0626-y