Abstract
Therapeutic anti-glycan antibodies for cancer treatment are limited, in spite of the differential glycophenotype of cancer cells and associated biology. We have generated anti-Le glycan antibodies with clinical potential and multifaceted functionality. Increased understanding of all aspects of glycomic research will ensure the continued development of glycan-targeted immunotherapeutics.
Post-translational glycosylation of proteins and lipids has important functional consequences for mammalian cells.Citation1 The considerable complexity of the glycome is a direct result of the variety of glycosidic linkages between monosaccharides giving rise to wide-ranging structural diversity. It has been estimated that over 50 % of proteins are glycosylated. In mammalian cells, the glycolipid composition of biomembranes ranges from less than 5% to 20% of the membrane lipids.
Recent advances in cancer cell glycomics have highlighted the differential glycan make-up of tumor cells versus their normal counterparts.Citation2 These transformation-associated glycosylation changes constitute: (i) increased branching (N-glycans), (ii) higher density (O-glycans), (iii) incomplete synthesis, (iv) neosynthesis, and (v) increases in sialylation and fucosylation, arising mainly from the genetic and epigenetic dysregulation of the biosynthetic enzymes.Citation3 Importantly, the altered glycophenotype is intricately linked to the majority of cancer cell biology hallmarks: proliferative signaling, inducing angiogenesis, activating invasion and metastasis, and resisting cell death.Citation4 Targeting the tumor glycan signature thus provides an attractive strategy for immunotherapy.
Developing glycan-specific antibodies for cancer immunotherapy has been challenging as glycans are poor immunogens often resulting in weak affinity IgM antibodies with limited clinical value.Citation5 We have developed a successful method to raise high-affinity antibodies against cancer cell glycans with therapeutic potential.
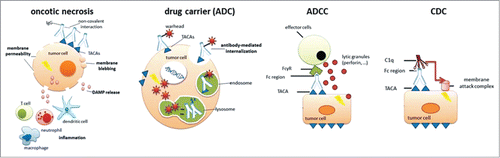
Through immunizations with cancer cell plasma membrane glycolipid extracts we recently generated two anti-glycan antibodies recognizing a unique subset of lewis (Le) glycans.Citation6 Le glycans are formed by the sequential addition of fucose onto oligosaccharide precursors on glycoproteins as well as glycolipids through the concerted action of a set of glycosyltransferases. Type I chains (containing Galβ(1→3)GlcNAc) form Lea and Leb, whereas type II chains (containing Galβ(1→4)GlcNAc) form Lex and Ley.Citation7 Our antibodies bound avidly (subnanomolar Kd) to LecLex- and Lea-containing glycoproteins and glycolipids on a wide range of tumor cells and tissues, the combination of which led to multimodal functionality such as: (i) atypical direct cell killing, (ii) glycoepitope-density dependent cellular internalization with lysosomal delivery of cargo (ADC), and (iii) sound immune effector functions (ADCC and CDC) (Fig. 1), culminating in significant survival benefit in a colorectal cancer xenograft model.
The direct cell killing in particular is appealing as it was associated with rapid cellular aggregation coinciding with propidium iodide uptake (reflecting cellular permeability), and the formation of membrane pores of heterogeneous sizes, ultimately leading to cell death via a mechanism resembling oncotic necrosis. Although the finer details of the molecular mechanisms underlying this membrane-centered cytotoxicity remain to be elucidated, it bore no resemblance to classical apoptosis in that there was little evidence of caspase activation or DNA fragmentation. A limited subset of other anti-glycolipid antibodies has been shown to induce a similar mode of cell killing, notably a number of ganglioside antibodies that are currently undergoing clinical evaluation such as racotumomab (anti-NeuGcGM3) for the treatment of non-small cell lung cancer NCT01460472, NCT01240447) and a number of pediatric tumors (NCT01598454) and KW2871 (anti-GD3) for the treatment of metastatic melanoma (NCT00679289). In the context of B-cell lymphoma, type II anti-CD20 antibodies such as tositumomab, are equally effective at mediating homotypic cell adhesion-associated direct cell killing in a caspase-independent manner.Citation8 This antibody-induced ‘programmed cell death’ was dependent on actin remodeling and involved lysosomal destabilization.
The cell death resulting from antibody-induced pore formation may constitute a form of immunogenic cancer cell death (ICD) through the release of cellular content, including damage-associated molecular patterns (DAMPS), into the extracellular space.Citation9,10 This may amplify the antibody-mediated effects, counteracting the immunosuppressive microenvironment, potentially resulting in long-lasting antitumor immunity. It remains to be seen whether this will translate into improved efficacy of anti-glycan antibodies in vivo.
Anti-glycan antibodies can conceivably be used in a number of alternative settings for instance as carriers for drug conjugates or in a bispecific format to redirect T-cells. In all these settings absence of cross-reactivity with normal healthy tissues is warranted in order to avoid off-target toxicity. The immunohistochemical analysis of our Le antibodies revealed strong staining of a large percentage of tumor tissues covering colorectal, gastric, pancreatic, non-small cell lung, and ovarian cancer tissues, combined with no cross-reactivity with most normal tissues including lung, liver (parenchyma), heart, brain, and kidney. We observed some (low to moderate) normal cross-reactivity (mainly with a subset of gastrointestinal tissues), but it is unclear whether this represents the same target as the tumor tissues.
As our antibodies bind glycoproteins as well as glycolipids, we have initiated studies using genetically engineered cell lines that express Lea glycan either on proteins or on lipids in order to delineate which target recognition drives the observed direct cytotoxic effects. Preliminary results suggest that lipid binding instigates the direct cell killing, as cells expressing the glycoepitope predominantly on glycoproteins are refractory. Interference with ‘glycosynapse’ formation, for instance through glycolipid internalization or via a direct physical effect on the membrane could be one way in which glycolipid-binding antibodies initiate the events culminating in cell death. Ongoing studies also encompass the immunogenic potential of this form of cell death through the combination of syngeneic mouse models in immunocompetent mice using a vaccination strategy.
In spite of the many advantages of targeting glycans in cancer and recent advances in cancer cell glycomics, the number of cancer glycan-specific antibodies with clinical potential is limited. The glycomic research field is nonetheless expanding, with ongoing efforts encompassing: (i) biomarker discovery and validation through improving the methodology for quantitative glycan profiling of tumor tissues, (ii) increased understanding of glycosylation dysregulation during oncogenesis, and (iii) development of novel glycan immunization strategies, all of which will aid the development of glycan targeting cancer therapeutics.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by a Developmental Pathway Funding Scheme from the MRC (MR/M015564/1, LG Durrant).
References
- Dalziel M, Crispin M, Scanlan CN, Zitzmann N, Dwek RA. Emerging principles for the therapeutic exploitation of glycosylation. Science 2014; 343:1235681; PMID:24385630; http://dx.doi.org/10.1126/science.1235681
- Drake RR. Glycosylation and cancer: moving glycomics to the forefront. Adv Cancer Res 2015; 126:1-10; PMID:25727144; http://dx.doi.org/10.1016/bs.acr.2014.12.002
- Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer 2005; 5:526-42; PMID:16069816; http://dx.doi.org/10.1038/nrc1649
- Hakomori SI, Cummings RD. Glycosylation effects on cancer development. Glycoconj J 2012; 29:565-6; PMID:22996057; http://dx.doi.org/10.1007/s10719-012-9448-4
- Rabu C, McIntosh R, Jurasova Z, Durrant L. Glycans as targets for therapeutic antitumor antibodies. Future Oncol 2012; 8:943-60; PMID:22894669; http://dx.doi.org/10.2217/fon.12.88
- Chua JX, Vankemmelbeke M, McIntosh RS, Clarke PA, Moss R, Parsons T, Spendlove I, Zaitoun AM, Madhusudan S, Durrant LG. Monoclonal Antibodies Targeting LecLex-Related Glycans with Potent Antitumor Activity. Clin Cancer Res 2015; 21(13):2963-74; http://dx.doi.org/10.1158/1078-0432.CCR-14-3030
- Stanley P, Cummings RD. Structures Common to Different Glycans. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, eds. Essentials of Glycobiology: Cold Spring Harbor, NY, 2009.
- Alduaij W, Ivanov A, Honeychurch J, Cheadle EJ, Potluri S, Lim SH, Shimada K, Chan CH, Tutt A, Beers SA et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood 2011; 117:4519-29; PMID:21378274; http://dx.doi.org/10.1182/blood-2010-07-296913
- Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012; 12:860-75; PMID:23151605; http://dx.doi.org/10.1038/nrc3380
- Kepp O, Senovilla L, Kroemer G. Immunogenic cell death inducers as anticancer agents. Oncotarget 2014; 5:5190-1; PMID:25114034