ABSTRACT
Colorectal cancer (CRC) develops through a multistep process and is modulated by inflammation. However, the inflammatory pathways that support intestinal tumors at different stages remain incompletely understood. Interleukin (IL)-33 signaling plays a role in intestinal inflammation, yet its contribution to the pathogenesis of CRC is unknown.
Using immunohistochemistry on 713 resected human CRC specimens, we show here that IL-33 and its receptor ST2 are expressed in low-grade and early-stage human CRCs, and to a lesser extent in higher-grade and more advanced-stage tumors. In a mouse model of CRC, ST2-deficiency protects from tumor development. Moreover, bone marrow (BM) chimera studies indicate that engagement of the IL-33/ST2 pathway on both the radio-resistant and radio-sensitive compartment is essential for CRC development. Mechanistically, activation of IL-33/ST2 signaling compromises the integrity of the intestinal barrier and triggers the production of pro-tumorigenic IL-6 by immune cells.
Together, this data reveals a tumor-promoting role of IL-33/ST2 signaling in CRC.
Abbreviations
| AOM | = | azoxymethane |
| BM | = | bone marrow |
| CLN | = | caudal lymph nodes |
| CRC | = | colorectal cancer |
| DSS | = | dextran sodium sulfate |
| FFPE | = | formalin-fixed paraffin-embedded |
| H&E | = | hematoxylin and eosin |
| IEC | = | intestinal epithelial cell |
| IHC | = | immunohistochemistry |
| IL | = | interleukin |
| IBD | = | inflammatory bowel diseases |
| LPS | = | lipopolysaccharide |
| TMA | = | tissue microarray |
| UICC | = | Union for International Cancer Control |
| WT | = | wild-type |
Introduction
Despite substantial improvements in treatment and prognosis during the past decades, CRC remains one of the most common human malignancies with lethal outcome in a considerable number of cases.Citation1 Various genetic alterations in key cellular pathways that underlie colon tumorigenesis have been identified.Citation2,3 There is now compelling evidence that intestinal tumorigenesis is greatly promoted by chronic inflammation that follows such genetically-driven tumor-initiating events.Citation4 For example, patients suffering from inflammatory bowel diseases (IBD) have an increased risk to develop colorectal carcinomas.Citation5,6 Several pro-inflammatory cytokines including TNFα and IL-6 are critically involved in the pathogenesis of IBD.Citation7 Similarly, serum IL-6 levels in CRC patients are associated with advanced-stage cancer, and increased blood concentration of IL-6 is an independent adverse prognostic marker of survival.Citation8 In addition, immune infiltrates within colorectal tumors can negatively or positively influence patient survival, potentially due to their distinct secretion patterns of cytokines.Citation9 Therefore, it is critical to determine the impact of specific cytokines on CRC development, progression and patient survival.
Recently, the cytokine IL-33 has been described to fulfill important functions in the intestine, including regulation of barrier permeability and healingCitation10 as well as immunity against helminth infection.Citation11 IL-33 protein is constitutively expressed mainly in the nucleus of endothelial cells, epithelial barrier tissues and myofibroblasts,Citation12-14 where it is complexed with chromatin and may modulate gene expression.Citation15 Upon tissue stress or damage, IL-33 is released as an alarmin and binds to a heterodimeric receptor complex consisting of ST2 (IL-1RL1) and IL-1 receptor accessory protein to promote inflammation. ST2 is expressed on the surface of a variety of cells, including epithelial cells, stromal cells and immune cells. A soluble isoform of ST2 (sST2) might either function as a decoy receptor or might extend the half-life of circulating IL-33.Citation12,16
Mucosal IL-33 is increased in patients with active ulcerative colitis (UC),Citation14,16–18 where it is highly expressed in subepithelial myofibroblasts below UC lesions.Citation14,19 Levels of IL-33 and sST2 are elevated in sera of UC and Crohn's disease (CD) patients.Citation16 While human studies have established a consistent association between intestinal inflammation and expression of IL-33 or ST2 in IBD patients, mouse models of IBD addressing the function of the IL-33/ST2 pathway have shown a more complex picture. Genetic ablation of IL-33Citation20 or ST2Citation10 ameliorated clinical symptoms and intestinal inflammation in the early phase of dextran sodium sulfate (DSS) colitis. At the same time, and in apparent contrast, Il33−/− mice displayed delayed resolution of DSS-dependent tissue damageCitation20 and administration of exogenous IL-33 ameliorated chronic DSS colitis.Citation21 Therefore, the IL-33/ST2 pathway may exert multiple functions in intestinal disorders.
Since IL-33 has a profound impact on inflammatory pathologies of the intestine, and since inflammation drives increased proliferation and reduced apoptosis in the intestinal epithelium,Citation4 we sought to investigate the role of IL-33/ST2 signaling in intestinal tumorigenesis. Analysis of two independent patient cohorts using human tissue microarrays (TMAs) revealed strong expression of both IL-33 and ST2 in less advanced and low-grade (G1-2) CRCs, suggesting a carcinogenic role of the IL-33/ST2 axis predominantly in early-stage carcinogenesis. IL-33 was also consistently expressed in colonic adenomas. Mechanistic investigations using the azoxymethane (AOM)/DSS model of CRC in mice indicated that genetic blockade of the IL-33/ST2 pathway significantly prevents tumor formation. In addition, activation of the IL-33/ST2 pathway induces leakiness of the intestinal barrier and production of IL-6 by immune cells, both known CRC promoting factors. In summary, our data provides strong evidence for a critical functional involvement of IL-33/ST2 signaling in intestinal tumorigenesis.
Results
IL-33 and ST2 are expressed in early-stage colorectal tumors
IL-33 has been functionally implicated in inflammatory disorders,Citation12 while only few studies so far have reported a clear contribution of IL-33 to cancers. To investigate a potential association between IL-33 and cancers of different organs, we screened a database with expression data from a variety of cancer cell lines. Among the different types of cancers, elevated transcript levels of IL33 were measured in a greater proportion of established cell lines originating from the large intestine compared to cells from other organs (Fig. S1A). Moreover, in silico analysis of a gene expression library from tissue biopsies or resections from the large intestine revealed increased IL33 transcript levels in primary tumor samples compared with healthy samples (p < 0.01) (Fig. S1B). This data raised the possibility of a specific involvement of IL-33 in CRC pathogenesis.
To investigate a potential role of IL-33 during CRC development and progression, we performed immunohistochemistry (IHC) for IL-33 on two independent CRC cohorts with a total of 713 patients sampled on TMAs. Only IL-33 staining on intestinal epithelial cells (IECs) was taken into account for the analysis. Endothelial cells were consistently found to show positive IL-33 staining and therefore served as an internal positive control for IL-33 staining ( and Fig. S2A). Both CRC cohorts were combined for statistical analysis. One of the cohorts also included 11 patients suffering from two synchronous colorectal carcinomas with different locations in the colon or rectum. These patients were excluded from the statistical analysis.
Figure 1. Representative picture of TMA cores showing healthy mucosa, low-grade and high-grade adenocarcinomas, respectively. Sections were stained for (A) IL-33 or (B) ST2. Scale bars: overview: 100 µm; inlay 25 µm.
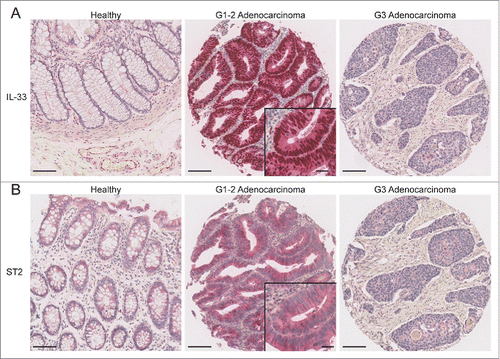
Compared with healthy mucosa where it was not detected, IL-33 expression was detected in a significant number of adenomas and low-grade adenocarcinomas (G1–2; p = 0.0003) ( and Fig. S2B). IL-33-expressing tumors were significantly associated with an early pT stage (pT1–2; p = 0.0199) and showed a tendency to have earlier Union for International Cancer Control (UICC) stages (p = 0.0723) (). In addition, IL-33 was expressed in a higher proportion of low-grade than high-grade tumors (G3) ().
Table 1. Association of clinicopathological features and IL-33
One of the 11 patients with two synchronous carcinomas was diagnosed with two tumors in the sigmoid colon. Interestingly, one carcinoma was an early-stage tumor (pT1) and strongly expressed IL-33 in all tumor cells, whereas the other carcinoma had a higher tumor stage (pT3) and was completely negative for IL-33. Similarly, ST2 was strongly expressed in this patient's pT1 tumor, but not in the synchronous pT3 tumor (data not shown).
Next we studied ST2 expression on the TMAs by IHC. As expected, ST2 expression was detected in endothelial cells, myofibroblasts and infiltrating immune cells (Fig. S2C and D). Similar to IL-33, only ST2 expression in the intestinal epithelium was counted in our analysis (), and like IL-33, ST2 was expressed and associated with earlier UICC stages (p = 0.0074) and lower tumor grade (G1–2; p = 0.0028) (). In addition, ST2-positive cancers showed less frequent vascular invasion (p = 0.0467) or lymph node metastases (p = 0.0052). ST2-negative cancers were mostly located in the right hemicolon, whereas ST2-positive tumors were more frequently located on the left side, including rectum (p = 0.0102).
Table 2. Association of clinicopathological features and ST2
It is of note that IL-33 or ST2 expressions were not associated with overall patient survival. However, epithelial IL-33 and ST2 expression correlated with low-grade and early-stage tumors (Table S1). This suggests that the IL-33/ST2 pathway is functionally active in these tissues.
Taken together, we conclude that IL-33 is specifically upregulated in CRC. IL-33/ST2-positive CRCs are significantly associated with an early tumor stage, and IL-33 and ST2 do not determine patient survival. Hence, our data implies that the IL-33/ST2 pathway is effectively engaged particularly in adenomas and low-grade / early-stage tumors.
Ablation of IL-33/ST2 signaling prevents CRC tumorigenesis
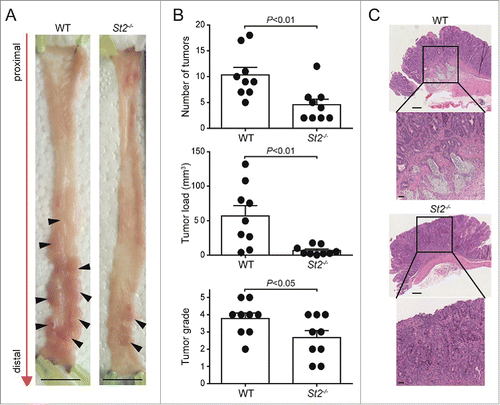
To address the role of IL-33/ST2 signaling for tumorigenesis in vivo, we used a murine model of CRC and injected wild-type (WT) and St2−/− mice twice with the procarcinogen AOM, followed by three rounds of DSS feeding. AOM-induced tumors typically follow the adenoma-carcinoma sequence of malignant transformation while recapitulating several molecular alterations similar to human CRC (reviewed inCitation22,23). However, metastasis has not been reported for C57BL/6 mice treated with AOM/DSS. Therefore, the AOM/DSS model of experimental CRC is reflective of the early-stage events leading to colorectal tumorigenesis in patients. We found that St2−/− mice were significantly protected from AOM/DSS-triggered CRC as they displayed fewer tumors than WT counterparts (p = 0.0064) ( and B). Tumor load was also lower in St2−/− mice (p = 0.0019) (). Furthermore, WT mice showed higher-grade tumors (p = 0.0489) including some cases of invasive carcinomas which were absent in St2−/− animals ( and C). This result was confirmed by endoscopic analysis of tumor-bearing mice over time, where large tumors became detectable earlier in WT compared with St2−/− mice (Fig. S3).
Figure 2. Genetic disruption of the IL-33/ST2 axis protects mice from AOM/DSS-induced CRC. (A) Macroscopic pictures of colonic tumors in representative WT and St2−/− mice. Black arrowheads indicate single tumors. (B) Reduction in number of tumors, tumor load and tumor grade in St2−/− compared with WT mice. (C) Representative hematoxylin and eosin (H&E) sections displaying the most advanced tumor grades in WT and St2−/− groups. Scale bars: overview: 200 µm; inlay 50 µm. Data represent means ± SEM; n = 9 samples per group. Statistical analyses were performed using Student t test (tumor number and severity) or Mann–Whitney test (tumor load).
Interestingly, transcript levels of Il33 and St2 progressively increased over time in whole colon tissue of mice treated with AOM/DSS (Fig. S4A and B, respectively).
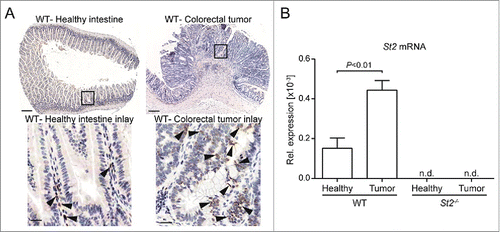
As for biopsies of early-stage CRCs in patients, IL-33 protein expression was increased in mouse tumors in comparison to healthy and/or tumor-free tissue, and mainly restricted to IECs ( and Fig. S4C). In addition, St2 was upregulated at the mRNA level in tumors versus adjacent tumor-free colons (). Finally, we assessed the expression of selected target genes in size-matched tumors of WT and St2−/− mice vs. adjacent, tumor-free colon. These genes were either biomarkers for known biological functions of IL-33 such as Mpo for neutrophil influx,Citation24 the macrophage marker Arg1, or known IL-33 target genes that were reported to be important for inflammation-driven tumorigenesis.Citation25–28 Transcription of chemokine genes Cxcl1, Cxcl2 and Ccl2 and cytokine genes Il6, Tnf, Il11, Il1b and Il17a was increased in tumors versus control tissue, yet independently of IL-33/ST2 signaling (Fig. S5A–H). Similarly, there was no significant difference in the expression of Mpo, Nos2 and Arg1 (Fig. S5I and K). Since there were no differences in the analyzed transcript levels between WT and St2−/− tumors at a late time point of our model (10 weeks post induction), these results suggest that the observed tumorigenic effects are independent of the above IL-33-dependent inflammatory target genes and their mechanisms at the analyzed time point of the AOM/DSS model.
Figure 3. IL-33 and St2 expression are induced in CRC in mice. (A) IHC for IL-33 of healthy and tumor WT intestinal tissue. Scale bars: overview: 200 µm; inlay 25 µm. (B) Increased St2 transcript levels in WT tumor vs. adjacent tumor-free tissue. Data represent means ± SEM; n = 9 samples per group; n.d., non-detected. Statistical analysis was performed using paired t test.
Taken together, our data indicates that the IL-33/ST2 pathway is preferentially upregulated in colonic tumors and contributes to CRC development.
Pleiotropic function of IL-33/ST2 signaling in CRC tumorigenesis
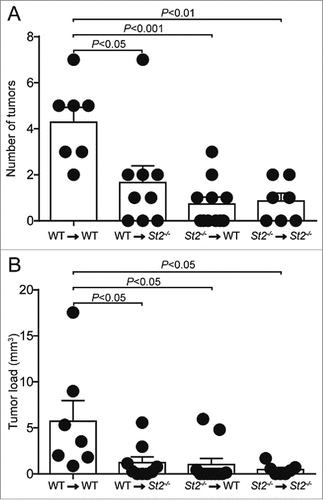
Next we generated sets of reciprocal BM chimeric mice to assess the relative contribution of the radio-resistant/stroma vs. the radio-sensitive/hematopoietic compartment to IL-33/ST2-dependent CRC development. Overall, we observed fewer and smaller tumors in chimeric mice, likely due to antibiotic treatment during BM reconstitution, as reported by others.Citation29 Yet we found that chimeras with disrupted IL-33/ST2 signaling either in the stroma or the hematopoietic system developed fewer tumors and showed lower tumor load compared with control mice that were sufficient for IL-33/ST2 signaling in both compartments (). Further, our results suggest that both compartments contributed to tumor formation through an ST2-dependent mechanism. Therefore, engagement of the IL-33/ST2 pathway on both radio-resistant and hematopoietic cells critically supports CRC development.
Figure 4. IL-33/ST2 signaling on both the radio-resistant and radio-sensitive compartment supports CRC development. (A) Number of tumors and (B) tumor load was analyzed in the indicated sets of chimeric mice. Data are means ± SEM and representative of one experiment; n = 7–10 samples per group. Statistical analyses were performed using one-way ANOVA with Bonferroni post-test.
IL-33/ST2 signaling promotes epithelial leakiness and IL-6 production
Evidence from chimeric mice led us first to assume a direct pro-tumorigenic effect of the IL-33/ST2 pathway on radio-resistant colonic epithelial cells. ST2 is expressed by several human colon cancer cell lines (Fig. S6A). However, stimulation of ST2-expressing HT-29, Caco-2 and LS 174T cells with IL-33 did not modulate their proliferation rate (Fig. S6B, C and D), even when cultured with reduced serum concentrations (data not shown). These results argued against a direct role of IL-33 in the regulation of IEC proliferation in CRC.
Therefore we analyzed whether IL-33 modulated other parameters of the colonic epithelium. A recent study suggested that IL-33 regulates intestinal permeability,Citation10 and CRC tumorigenesis is associated with a deterioration of the epithelial barrier.Citation30 To address how IL-33/ST2 signaling may affect intestinal barrier function in inflammation-associated CRC, we generated BM chimeric mice that expressed ST2 selectively on radio-resistant cells and treated them with one cycle of DSS. Compared to St2−/−-to-St2−/− chimeric mice, we found in these chimeras an increased translocation of bacteria from the intestine, as indicated by presence of bacterial 16S rRNA in liver and higher lipopolysaccharide (LPS) levels in sera (Fig. S7, ). This demonstrated that IL-33/ST2 signaling on the radio-resistant compartment during colonic inflammation compromises the integrity of the intestinal barrier, a physiologic parameter suggested to contribute to CRC.Citation30
Figure 5. Engagement of the IL-33/ST2 signaling compromises the integrity of the intestinal barrier and stimulates the production of pro-tumorigenic IL-6. Indicated groups of BM chimeric mice were challenged with DSS and (A) LPS and (B) IL-6 were measured in the serum; n = 5–9 samples. (C) IL-33 stimulated Il6 expression in lamina propria (LP) and caudal lymph node (CLN) immune cells from DSS-treated WT mice. For LP, cells were pooled from 5 donors and analyzed. Three independent experiments were performed, each represented by a dot. For CLN, cells from four individual mice were analyzed. (D) Serial sections of low-grade human colonic adenocarcinomas were stained for IL-6 or CD11c. Scale bars: representative overview: 100 µm; inlay 25 µm. Data are means ± SEM and show one representative from two independent experiments. Statistical analyses were performed using Student t test (A, B) and paired Student t test (C).
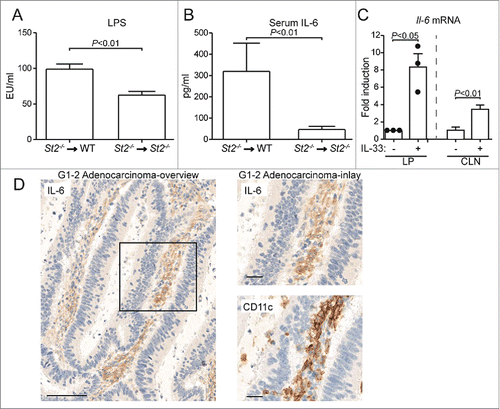
In line with increased serum LPS levels, we found elevated IL-6 in the serum of DSS-treated St2−/−-to-WT chimeric mice that have intact IL-33/ST2 signaling in their radio-resistant compartment (). This was of particular importance for our study because IL-6 is a key cytokine contributing to CRC pathogenesis in humans and mice.Citation7,8,27
In line with previous studies,Citation10,20 we found that the IL-33/ST2 pathway accelerated acute colitis that preceded tumor development in the AOM-DSS model (Fig. S8). Therefore, it is conceivable that the IL-33/ST2-dependent inflammation of the intestinal epithelium enhances the formation of CRC. However, enhanced colitis was also found if only the radio-resistant compartment was ST2-competent (Fig. S8C), yet these chimeras were protected from tumorigenesis later on (see ). This suggests that IL-33 contributes to CRC beyond the promotion of early inflammation, by an additional effect on stromal cells.
In the following, we studied IL-33-driven inflammatory changes enhancing CRC. Results from BM chimeras indicated that the IL-33/ST2 pathway is engaged on the radio-sensitive compartment to drive tumorigenesis. To assess whether activation of the IL-33/ST2 pathway on intestinal hematopoietic cells may contribute to the observed induction of IL-6, we isolated intestinal leukocytes from DSS-treated mice, stimulated them ex vivo with IL-33, and measured Il6 transcription. We found that flow cytometry-sorted CD45+ hematopoietic cells from the lamina propria upregulated Il6 expression upon IL-33 stimulation. An IL-33-dependent increase of Il6 transcript levels was also detected in immune cells from colon-draining lymph nodes (). Therefore, we conclude that IL-33 signaling elicits IL-6 from intestinal immune cells.
As for IL-33, stronger IL-6 immunoreactivity was observed in adenomas and low-grade tumors of patients than in healthy intestine or higher-grade CRCs. This IL-6 staining colocalized with CD11c-positive cells (Fig. S9, ), indicating the possible presence of CD11c+ dendritic cells that produce IL-6 in early-stage cancer lesions. Additionally, recombinant IL-6 promoted a dose-dependent phosphorylation of the oncogenic transcription factor STAT3 in HT-29 and Caco-2 cells (Fig. S10A and B). This activation is an important step regulating the proliferation and survival of IECs in vivo.Citation27
Collectively, our findings suggest that IL-33/ST2 signaling on radio-resistant cells compromises the intestinal barrier during intestinal inflammation. This facilitates the translocation of microbial products to the circulation, which correlates with elevated systemic IL-6 levels. In addition, activation of the IL-33/ST2 pathway in hematopoietic cells of the intestine leads to local induction of IL-6. Since IL-6 has the potential to activate intestinal epithelial cells, we propose that IL-33/ST2 signaling may contribute to colorectal tumorigenesis in part through IL-6.
Discussion
Progression of CRC is dependent on proliferative and survival signals from the tumor environment, including immune cells.Citation4,31 Cytokines, in particular, affect CRC development by their effect on angiogenesis and cancer cell survival.Citation31 Both its preferential expression in epithelial barriers and its functional involvement in intestinal diseases suggested a role for IL-33 in CRC.
Here, we present evidence that both IL-33 and ST2 proteins are expressed in colonic tumors in humans, with a preference in lower grade and stage tumors. We also show that IL-33/ST2 signaling critically contributes to intestinal tumorigenesis in mice, possibly by inducing the pro-tumorigenic cytokine IL-6 (). IL-33 has a dual function, both as an inhibitor of transcription when residing in the nucleus,Citation32 or as a pro-inflammatory cytokine upon release.Citation12 Through analysis of St2−/− mice, we focused on the latter, extracellular role of IL-33.
Figure 6. Model for the role of the IL-33/ST2 pathway in CRC. (A) Stress/damage drives the production and release of IL-33 which binds to its receptor ST2 on immune effector cells and (B) drives the production of pro-tumorigenic factors, including IL-6. (1) IL-33 also binds to ST2 on IECs, (2) which decreases the tightness of the intestinal barrier and (3) promotes the translocation of intestinal bacteria or bacterial products via the blood stream. Bacteria-derived molecules such as LPS enter otherwise sterile compartments and engage innate immune receptors such as Toll-like receptors (TLRs) (4) to trigger the production of pro-inflammatory cytokines, including IL-6. It is not clear whether ST2+ immune cells also express TLRs. (5/C) IL-6 acts on IECs to promote tumor development via the phosphorylation of the oncogenic transcription factor STAT3.
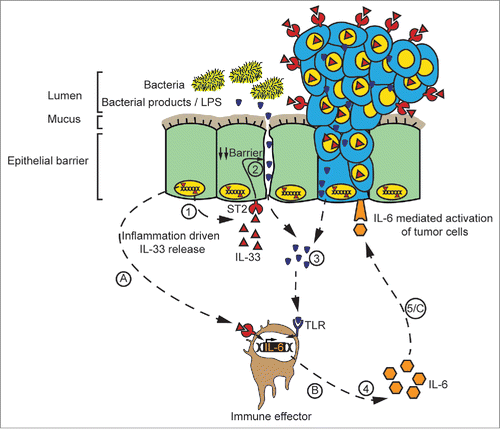
A functional contribution of the IL-33/ST2 pathway to carcinogenesis has so far only been analyzed for few types of solid tumors, such as head-and-neck squamous cell carcinoma.Citation33 Elevated serum IL-33 was suggested as a negative prognostic biomarker for lungCitation34 and gastric cancer.Citation35 Our findings reveal that IL-33 and ST2 are expressed to a lower extent in advanced tumors compared to early tumors, yet this difference is not absolute. Further, our data indicates that IL-33 and ST2 expression have no prognostic value in CRC. Such cancer type-specific differences might be linked to their particular micro-environments. Indeed, intestinal tumors are directly exposed to the gut microbiota because tumor development compromises the intestinal epithelial barrier, as evidenced by the presence of bacterial 16S rRNA in early human adenomas.Citation30 IL-33 may get induced in the mucosa as a reaction to bacterial-derived products,Citation13 its release may weaken the intestinal mucosa even more, and instigate a tissue-destructive feed-forward loop (). In addition, some IL-33-dependent cytokines like IL-6 may not only contribute to antibacterial defense but promote tumor development at the same time.
Our results show that IL-33 and ST2 are expressed predominantly in low-grade tumors, possibly as an amplifier of the low-level inflammation within tumors.Citation20,30 The reduced expression of IL-33 and ST2 in poorly differentiated human adenocarcinomas may also support the notion that expression of these proteins may be restricted to cells in the diseased intestinal mucosa that have preserved some epithelial characteristics. However, the IL-33/ST2 axis may possibly also play a role in higher-grade and more advanced-stage tumors, as there were for instance one third of CRCs with higher UICC stage showing ST2 expression versus two thirds not showing ST2 expression.
Our data suggests that activation of the IL-33/ST2 pathway may contribute to tumorigenesis by stimulating or amplifying inflammation in early stages of the AOM/DSS model. Consistent with this interpretation, both transcript and protein levels of Il33 have been reported to be transiently upregulated in the colon of DSS-treated mice at the peak of acute colitis, and after removal of DSS to rapidly decline almost to background levels.Citation10,20 This may explain the unchanged IL-33 expression levels on day 20, i.e. ten days after the first cycle of DSS in our AOM/DSS protocol. More sustained colonic IL-33 expression is observed as tumors become established in this model. However, this later increase of IL-33 might be less important for inflammatory aspects of tumorigenesis, because in established tumors of the AOM/DSS model we could not detect ST2-dependent selective up- or downregulation of several inflammatory transcripts.
Our data also suggests that the inflammatory infiltrate within tumors is distinct in different stages of CRC development, and further investigations will help identify these stage-specific molecular crosstalks of tumors and the tumor microenvironment.
Our results indicate that BM chimeric mice with ST2 expression restricted to their radio-resistant stromal cells develop fewer and smaller tumors than WT-to-WT chimeras, although they show strong clinical symptoms during acute DSS treatment. This suggests a stromal cell-intrinsic tumorigenic mechanism that depends on ST2 and that is distinct from ST2-mediated regulation of intestinal permeability. The nature of this mechanism is yet to be characterized.
A recent study by Liu et al. reported that IL-33 expression in a xenotransplant model of human SW620 CRC cells into nude mice promoted lung metastasis.Citation36 However, we did not find any significant association between IL-33/ST2 expression in primary tumors and metastasis to lymph nodes in CRC patients. Furthermore, combined positive expression of ST2 and IL-33 negatively correlated with venous invasion in our cohort. Hence, our investigation focused on CRC tumorigenesis rather than on metastasis, in line with a recent report describing elevated IL33 and ST2 transcript levels in colorectal adenomas.Citation37
Our findings indicate that engagement of IL33/ST2 signaling stimulates the production of tumor-promoting IL-6, at least during the inflammatory phase of the AOM/DSS model. It is likely that other pro-tumorigenic cytokines besides IL-6 are secreted after ST2 activation and it remains to be investigated whether IL-33/ST2 engagement in colorectal tumors induces additional pro-tumorigenic mediators.
Besides stimulating the production of pro-tumorigenic IL-6, activation of the IL-33/ST2 axis may also lead to an accumulation of myeloid-derived suppressor cellsCitation38,39 or promote regulatory T cell (Treg) functionCitation40 to negatively control the antitumor response. Indeed, high frequency of tumor-infiltrating FOXP3+ cells has been associated with early pT-stage tumors in a large cohort of mismatch-repair proficient CRC patients.Citation41 In light of our data indicating overexpression of ST2/IL-33 in pT1-2 tumors, it may be relevant to address whether Tregs exert an IL-33/ST2 signaling-dependent suppressive function in CRC lesions.
Our TMA-based approach allowed large scale analysis of CRC cases; however the size of the tissue cores limited this analysis to the study of IL-33/ST2 expression in IECs. Therefore, this technique did not allow us to examine how expression of IL-33 or ST2 by other cell types is associated with CRC stages and grades. Our functional data from mouse experiments shows that immune cells in the tumor environment respond to IL-33 by secreting pro-tumorigenic IL-6. Further studies are needed to dissect the role of non-epithelial stromal cells such as myofibroblasts for IL-33/ST2-dependent CRC.
In conclusion, our study demonstrates that activation of the IL-33/ST2 pathway on epithelial and immune cells critically modulates colon tumorigenesis. Since IL-33 and ST2 expression patterns are temporally and spatially restricted during CRC progression in patients, it is conceivable that this pathway preferentially drives early-stage events of colon carcinogenesis. Therefore, a therapeutic inhibition of the IL-33/ST2 axis might curtail CRC progression, particularly when applied at an early stage of tumor development.
Methods
Patient selection
Formalin-fixed paraffin-embedded (FFPE) CRC resections of 713 patients diagnosed throughout a 19-year period (1993–2011) at two different institutions in Switzerland (Institute of Surgical Pathology Zurich and Institute of Pathology Liestal) were examined. The Zurich CRC cohort was described beforeCitation42 and consisted of 253 patients. The Liestal CRC cohort consisted of 471 patients. The study was approved by the Cantonal Ethics Committees of Zurich and Basel. Clinicopathological patient characteristics are shown in Table S2. In addition, the Liestal cohort contained 11 patients with synchronous CRCs. In these 11 patients, two tumors at different positions in the colon were diagnosed at the same time point and were both included in the TMA. CRCs with pre-operative treatment were excluded from the analysis.
Construction of tissue microarrays and scoring for IL-33 and ST2
All tumors were re-evaluated by at least two board-certified pathologists (K.D.M., T.T., G.C., A.W.) according to WHO criteria. Two TMAs of each CRC cohort were constructed as described previously.Citation43 The Liestal cohort was stained for both IL-33 and ST2, the Zurich cohort for IL-33. Only nuclear IL-33 immunohistochemical staining in epithelial cells was scored as positive and endothelial cells served as internal control for IL-33 positivity. The percentages of IL-33- and ST2-positive epithelial cells in patient samples were recorded by two board-certified pathologists (K.D.M. and G.C.) in a blinded manner.
Immunohistochemistry of human tissue
Immunohistochemistry of TMA sections was performed as previously described.Citation10 Briefly, sections were pre-incubated on the BondMax system (Leica) in Bond Epitope Retrieval Solution 2 (Leica, AR9640) (pH 9.0) for 30 min at 95°C, and then stained either for IL-33 (1:400, goat anti-human IL-33 IgG, R&D systems, AF3625), or for ST2 (1:400, rabbit anti-human ST2, Sigma Aldrich, PRS3363). The Bond Polymer Refine Red Detection kit (Leica, DS9390) was used for detection of both IL-33 and ST2.
Alternatively, serial full tissue sections of FFPE resection specimens were cut and stained either for CD11c (1:100, clone 5D11, Leica, PA0554) after pre-treatment in Tris buffer at 95°C for 40 min or for IL-6 (1:3000, Abcam, ab9324) after pre-treatment in Tris buffer at 95°C for 20 min. Specific binding of primary antibodies was visualized using a polymer-based visualization system with horseradish peroxidase as the enzyme (Envision+; Dako, K4006) and 3,3′-diaminobenzidine as the chromogen. Slides were scanned using an Aperio Scanscope (Leica).
Induction and assessment of intestinal tumors in mice
All animal experiments were performed in accordance with Swiss Federal regulations and were approved by the Cantonal Veterinary Office. C57BL/6J mice were purchased from Jackson Laboratories and subsequently bred in-house. Il1rl1−/− (St2−/−) mice were previously describedCitation44 and backcrossed on a C57BL/6J background.
As a model for CRC induction, we used the AOM (Sigma, A5486)/DSS (MP Biomedicals, 0216011090) model as previously described.Citation45 Seven days after injection with AOM, mice were given 1% DSS in the drinking water for up to 5 d, followed by 7 d of water. After this, mice received a second injection of AOM, followed by 2 cycles of 1% DSS and water. Unless otherwise indicated, mice were sacrificed 70 d after the first injection of AOM.
For matching of commensal communities between experimental groups before AOM/DSS treatment, mice were co-housed for 3–4 weeks or soiled bedding was exchanged between cages for a similar duration. Also, soiled bedding was exchanged at least weekly and for the entire duration of an experiment.
Macroscopic tumors were counted by two independent observers. Tumor load was measured as previously described,Citation22 and gene expression level was performed. Tumors were graded by a board-certified pathologist (A.L.), in a blinded manner. Criteria for grading are listed in Supplementary Material and Methods.
Evaluation of barrier permeability
Animals were given DSS in the drinking water for 5 d followed by 3 d of water. Serum, lymph nodes, spleen, liver and colon were taken to analyze barrier integrity. LPS in the serum of animals was measured using the Pierce LAL Chromogenic Endotoxin Quantitation Kit (Thermo Scientific, 88282) according to the manufacturer's protocol. 16S rRNA and IL-6 were measured through qPCR and ELISA respectively.
Isolation and ex vivo stimulation of immune cells
Mice were treated with DSS and immune cells were harvested after homogenization of caudal lymph node (CLN) or after purification of CD45+ cells by flow cytometry from single-cell suspensions of the colonic lamina propria. Cells were then stimulated ex vivo with 100 ng/ml recombinant murine IL-33 (Peprotech, 210-33). Thirty hours later, cells were harvested for qPCR analysis.
Statistical analysis
In a first step, clinicopathological features of both patient cohorts were evaluated. Since no statistically significant differences in the distribution of relevant prognostic parameters were identified, the two cohorts were pooled. Next, a random subset of 30% of all patients (n = 217) was selected. Receiver Operating Characteristic (ROC) curve analysis was performed to identify the optimal immunohistochemical cut-off score for IL-33 and ST2 best discriminating between patients who are deceased or censored/alive. The reliability of the cut-off scores was determined by 500 bootstrapped resamples of the data. The final cut-off score of 5% for IL-33 and 30% for ST2 was assigned. Next, the two cut-off scores were applied to the entire cohort of patients and associations with clinicopathological features and survival time was analyzed. Categorical variables were analyzed using the Chi-Square or Fisher's Exact test, while continuous variables with Wilcoxon's Rank Sum Test. Kaplan–Meier curves and log-rank test for univariate survival analysis was performed and Cox regression models, after verification of the proportional hazards model were also analyzed. Missing data was considered to be missing at random. Analyses were carried out using SAS V9.4 (The SAS Institute).
For in vivo and in vitro studies, statistical evaluations were performed using GraphPad Prism v.5.04 for Windows (GraphPad Software). Only statistically significant differences are indicated in the figures.
Disclosure of potential conflicts of interest
G.R., S.J., J.R.M. T.B. and T.J. are employees of Novartis Pharma AG. The other authors disclose no potential conflicts of interest.
1062966_supplemental_files.pdf
Download PDF (1.6 MB)Acknowledgments
We thank Regula Stuber as well as the team of the Translational Research Unit for their excellent technical support. We thank Christoph Müller and Mario Noti for critical comments.
Funding
This work was supported by grants from the Swiss National Science Foundation (310030_138188), the Bern University Research Foundation (to P.K.), the Foundation Johanna Duermueller-Bol (to P.K. and L.M) and by a Boehringer-Ingelheim Fonds fellowship (to L.M.).
References
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014; 383:1490-502; PMID:24225001; http://dx.doi.org/10.1016/S0140-6736(13)61649-9
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. The New England journal of medicine 1988; 319:525-32; PMID:2841597; http://dx.doi.org/10.1056/NEJM198809013190901
- Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol 2011; 6:479-507; PMID:21090969; http://dx.doi.org/10.1146/annurev-pathol-011110-130235
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883-99; PMID:20303878; http://dx.doi.org/10.1016/j.cell.2010.01.025
- Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med 1990; 323:1228-33; PMID:2215606; http://dx.doi.org/10.1056/NEJM199011013231802
- Jess T, Gamborg M, Matzen P, Munkholm P, Sorensen TI. Increased risk of intestinal cancer in Crohn's disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol 2005; 100:2724-9; PMID:16393226; http://dx.doi.org/10.1111/j.1572-0241.2005.00287.x
- Mudter J, Neurath MF. Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis 2007; 13:1016-23; PMID:17476678; http://dx.doi.org/10.1002/ibd.20148
- Belluco C, Nitti D, Frantz M, Toppan P, Basso D, Plebani M, Lise M, Jessup JM. Interleukin-6 blood level is associated with circulating carcinoembryonic antigen and prognosis in patients with colorectal cancer. Ann Surg Oncol 2000; 7:133-8; PMID:10761792; http://dx.doi.org/10.1007/s10434-000-0133-7
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/10.1126/science.1129139
- Sedhom MA, Pichery M, Murdoch JR, Foligne B, Ortega N, Normand S, Mertz K, Sanmugalingam D, Brault L, Grandjean T et al. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut 2013; 62:1714-23; PMID:23172891; http://dx.doi.org/10.1136/gutjnl-2011-301785
- Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol 2008; 180:2443-9; PMID:18250453; http://dx.doi.org/10.4049/jimmunol.180.4.2443
- Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol 2010; 10:103-10; PMID:20081870; http://dx.doi.org/10.1038/nri2692
- Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, Girard JP. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol 2012; 188:3488-95; PMID:22371395; http://dx.doi.org/10.4049/jimmunol.1101977
- Kobori A, Yagi Y, Imaeda H, Ban H, Bamba S, Tsujikawa T, Saito Y, Fujiyama Y, Andoh A. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol 2010; 45:999-1007; PMID:20405148; http://dx.doi.org/10.1007/s00535-010-0245-1
- Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A 2007; 104:282-7; PMID:17185418; http://dx.doi.org/10.1073/pnas.0606854104
- Pastorelli L, Garg RR, Hoang SB, Spina L, Mattioli B, Scarpa M, Fiocchi C, Vecchi M, Pizarro TT. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci U S A 2010; 107:8017-22; PMID:20385815; http://dx.doi.org/10.1073/pnas.0912678107
- Beltran CJ, Nunez LE, Diaz-Jimenez D, Farfan N, Candia E, Heine C, Lopez F, Gonzalez MJ, Quera R, Hermoso MA. Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflamm Bowel Dis 2010; 16:1097-107; PMID:20014018; http://dx.doi.org/10.1002/ibd.21175
- Seidelin JB, Bjerrum JT, Coskun M, Widjaya B, Vainer B, Nielsen OH. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol Lett 2010; 128:80-5; PMID:19913053; http://dx.doi.org/10.1016/j.imlet.2009.11.001
- Sponheim J, Pollheimer J, Olsen T, Balogh J, Hammarstrom C, Loos T, Kasprzycka M, Sorensen DR, Nilsen HR, Kuchler AM et al. Inflammatory bowel disease-associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am J Pathol 2010; 177:2804-15; PMID:21037074; http://dx.doi.org/10.2353/ajpath.2010.100378
- Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, Nambu A, Abe T, Kiyonari H, Matsumoto K et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A 2010; 107:18581-6; PMID:20937871; http://dx.doi.org/10.1073/pnas.1003059107
- Grobeta P, Doser K, Falk W, Obermeier F, Hofmann C. IL-33 attenuates development and perpetuation of chronic intestinal inflammation. Inflamm Bowel Dis 2012; 18:1900-9; PMID:22508383; http://dx.doi.org/10.1002/ibd.22900
- Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc 2007; 2:1998-2004; PMID:17703211; http://dx.doi.org/10.1038/nprot.2007.279
- Rosenberg DW, Giardina C, Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis 2009; 30:183-96; PMID:19037092; http://dx.doi.org/10.1093/carcin/bgn267
- Alves-Filho JC, Sonego F, Souto FO, Freitas A, Verri WA, Jr., Auxiliadora-Martins M, Basile-Filho A, McKenzie AN, Xu D, Cunha FQ et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med 2010; 16:708-12; PMID:20473304; http://dx.doi.org/10.1038/nm.2156
- Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJ et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest 2012; 122:3127-44; PMID:22922255; http://dx.doi.org/10.1172/JCI61067
- Gao Y, Li X, Yang M, Zhao Q, Liu X, Wang G, Lu X, Wu Q, Wu J, Yang Y et al. Colitis-accelerated colorectal cancer and metabolic dysregulation in a mouse model. Carcinogenesis 2013; 34:1861-9; PMID:23615396; http://dx.doi.org/10.1093/carcin/bgt135
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009; 15:103-13; PMID:19185845; http://dx.doi.org/10.1016/j.ccr.2009.01.001
- Pollheimer J, Bodin J, Sundnes O, Edelmann RJ, Skanland SS, Sponheim J, Brox MJ, Sundlisaeter E, Loos T, Vatn M et al. Interleukin-33 drives a proinflammatory endothelial activation that selectively targets nonquiescent cells. Arterioscler Thromb Vasc Biol 2013; 33:e47-55; PMID:23162017; http://dx.doi.org/10.1161/ATVBAHA.112.253427
- Klimesova K, Kverka M, Zakostelska Z, Hudcovic T, Hrncir T, Stepankova R, Rossmann P, Ridl J, Kostovcik M, Mrazek J et al. Altered gut microbiota promotes colitis-associated cancer in IL-1 receptor-associated kinase M-deficient mice. Inflamm Bowel Dis 2013; 19:1266-77; PMID:23567778; http://dx.doi.org/10.1097/MIB.0b013e318281330a
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012; 491:254-8; PMID:23034650; http://dx.doi.org/10.1038/nature11465
- Klampfer L. Cytokines, inflammation and colon cancer. Curr Cancer Drug Targets 2011; 11:451-64; PMID:21247378; http://dx.doi.org/10.2174/156800911795538066
- Ali S, Mohs A, Thomas M, Klare J, Ross R, Schmitz ML, Martin MU. The dual function cytokine IL-33 interacts with the transcription factor NF-kappaB to dampen NF-kappaB-stimulated gene transcription. J Immunol 2011; 187:1609-16; PMID:21734074; http://dx.doi.org/10.4049/jimmunol.1003080
- Chen SF, Nieh S, Jao SW, Wu MZ, Liu CL, Chang YC, Lin YS. The paracrine effect of cancer-associated fibroblast-induced interleukin-33 regulates the invasiveness of head and neck squamous cell carcinoma. J Pathol 2013; 231:180-9; PMID:23775566; http://dx.doi.org/10.1002/path.4226
- Hu LA, Fu Y, Zhang DN, Zhang J. Serum IL-33 as a diagnostic and prognostic marker in non- small cell lung cancer. Asian Pac J Cancer Prev 2013; 14:2563-6; PMID:23725175; http://dx.doi.org/10.7314/APJCP.2013.14.4.2563
- Sun P, Ben Q, Tu S, Dong W, Qi X, Wu Y. Serum interleukin-33 levels in patients with gastric cancer. Dig Dis Sci 2011; 56:3596-601; PMID:21643739; http://dx.doi.org/10.1007/s10620-011-1760-5
- Liu X, Zhu L, Lu X, Bian H, Wu X, Yang W, Qin Q. IL-33/ST2 pathway contributes to metastasis of human colorectal cancer. Biochem Biophys Res Commun 2014; 453(3):486-92; PMID:25280997; http://dx.doi.org/10.1016/j.bbrc.2014.09.106
- Cui G, Qi H, Gundersen MD, Yang H, Christiansen I, Sorbye SW, Goll R, Florholmen J. Dynamics of the IL-33/ST2 network in the progression of human colorectal adenoma to sporadic colorectal cancer. Cancer Immunol Immunother 2014; 64(2):181-90; PMID:25324197; http://dx.doi.org/10.1007/s00262-014-1624-x
- Huang JR, Tsai YC, Chang YJ, Wu JC, Hung JT, Lin KH, Wong CH, Yu AL. alpha-Galactosylceramide but not phenyl-glycolipids induced NKT cell anergy and IL-33-mediated myeloid-derived suppressor cell accumulation via upregulation of egr2/3. J Immunol 2014; 192:1972-81; PMID:24465013; http://dx.doi.org/10.4049/jimmunol.1302623
- Jovanovic IP, Pejnovic NN, Radosavljevic GD, Pantic JM, Milovanovic MZ, Arsenijevic NN, Lukic ML. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int J Cancer 2014; 134:1669-82; PMID:24105680; http://dx.doi.org/10.1002/ijc.28481
- Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 2014; 513(7519):564-8; PMID:25043027; http://dx.doi.org/10.1038/nature13577
- Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L, Tornillo L. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer 2010; 126:2635-43; PMID:19856313; http://dx.doi.org/10.1002/ijc.24989
- Rossle M, Sigg M, Ruschoff JH, Wild PJ, Moch H, Weber A, Rechsteiner MP. Ultra-deep sequencing confirms immunohistochemistry as a highly sensitive and specific method for detecting BRAF V600E mutations in colorectal carcinoma. Virchows Arch 2013; 463:623-31; PMID:24085553; http://dx.doi.org/10.1007/s00428-013-1492-3
- Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998; 4:844-7; PMID:9662379; http://dx.doi.org/10.1038/nm0798-844
- Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med 2000; 191:1069-76; PMID:10727469; http://dx.doi.org/10.1084/jem.191.6.1069
- De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, Signori E, Fazio VM. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog 2011; 10:9; PMID:21483655; http://dx.doi.org/10.4103/1477-3163.78279
