 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Hypoxia is a common feature in solid tumors that has been implicated in immune evasion. Previous studies from our group have shown that hypoxia upregulates the co-stimulatory receptor CD137 on activated T lymphocytes and on vascular endothelial cells. In this study, we show that exposure of mouse and human tumor cell lines to hypoxic conditions (1% O2) promotes CD137 transcription. However, the resulting mRNA is predominantly an alternatively spliced form that encodes for a soluble variant, lacking the transmembrane domain. Accordingly, soluble CD137 (sCD137) is detectable by ELISA in the supernatant of hypoxia-exposed cell lines and in the serum of tumor-bearing mice. sCD137, as secreted by tumor cells, is able to bind to CD137-Ligand (CD137L). Our studies on primed T lymphocytes in co-culture with stable transfectants for CD137L demonstrate that tumor-secreted sCD137 prevents co-stimulation of T lymphocytes. Such an effect results from preventing the interaction of CD137L with the transmembrane forms of CD137 expressed on T lymphocytes undergoing activation. Indeed, silencing CD137 with shRNA renders more immunogenic tumor-cell variants upon inoculation to immunocompetent mice but which readily grafted on immunodeficient or CD8+ T-cell-depleted mice. These mechanisms are interpreted as a molecular strategy deployed by tumors to repress lymphocyte co-stimulation via CD137/CD137L.
KEYWORDS:
Abbreviations
| sCD137 | = | soluble CD137 |
| CD137L | = | CD137 ligand |
| TM | = | transmembrane domain |
| N | = | Normoxia |
| H | = | Hypoxia |
Introduction
Malignant tissues deploy an array of mechanisms that interfere with potentially tumoricidal immune responses.Citation1,2 For these purposes, cancer cells exploit the natural mechanisms that prevent autoimmunity and excessive inflammation in the organism. Some of the immunosuppressive factors are soluble and reach distant haematopoietic and lymphoid organs, while others act locally in the malignant tissue. A link between hypoxia and immunomodulatory factors has become apparent over recent yearsCitation3 with important implications for cancer immune escape.
Co-stimulation and co-inhibition of T-cell activationCitation4 involves a set of functions that critically determines the outcome of T-cell-mediated antitumor responses. Immunomodulation with a therapeutic purpose can be achieved by disrupting the function of co-inhibitory receptorsCitation5,6 or by turning on the activity of co-stimulatory receptors.Citation7 Gene transfer of a co-stimulatory ligand to tumor cells renders such a cell line more immunogenic.Citation8-11 On the contrary, tumor cells frequently escape cancer by providing ligands for co-inhibitory receptors.Citation5,12
CD137 (4-1BB, Tnfsfr9) is a co-stimulatory member of the TNFR family discovered on T cells undergoing activation.Citation13 On lymphocytes, CD137 ligation contributes to enhance proliferation and effector functions, while importantly it prevents apoptosis.Citation14 Its expression was also found on activated NK cells,Citation15 where it enhances antibody-dependent cellular cytotoxicity.Citation16-18 Other leukocytes subsets gain CD137 expression upon activation,Citation19,20 but the functional significance of this finding is as yet unclear. Surprisingly, CD137 is also expressed on endothelial cells in the microcirculation of tumorsCitation21-23 and atherosclerotic lesionsCitation24 where it is instigated by hypoxia. Apart from the membrane attached form of CD137, a soluble form generated by alternative splicing has been identified.Citation25,26 Circulating sCD137 has been detected by ELISA in the serum and tumor homogenates of colorectal cancer patients undergoing surgery. The significance of this finding has not been established.Citation27
Agonist anti-CD137 antibodies frequently mediate tumor eradication in miceCitation28 and are being tested in humans with encouraging results in phase I/II trials (Clinical trial.gov NCT 01307267, NTC 0147121). Agonist antibodies engineered to be displayed on the membrane of tumor cells also dramatically enhance the immunogenicity of tumors,Citation11,29 as has also been observed with anti-CD137 agonist RNA aptamers targeted to the tumor cell surface.Citation30 The mechanism of action is mainly and ultimately dependent on the enhancement of cytotoxic T lymphocytes that destroy malignant lesions by direct cytotoxicityCitation28,31,32
Interestingly, CD137 expression is upregulated by hypoxia through HIF-1α indirectly mediated effectsCitation33 and as a result, CD137 is more prominently expressed on endothelial cells in tumor vasculature cellsCitation21and on tumor-infiltrating T lymphocytes.Citation33 While on lymphocytes agonist anti-CD137 mAb provide co-stimulation, on endothelial cells ligation results in an enhancement of adhesion and chemotactic functions for T-cell homing.Citation21
The only natural ligand known for CD137 is CD137L (4-1BBL, Tnfsf9).Citation34 This is expressed on activated dendritic cells, macrophages and B cells.Citation35 Upon ligation, it also mediates reverse signaling thus promoting inflammationCitation34 and when it is artificially expressed on tumor cells it enhances immunogenicity.Citation10,36
In this study, we report that hypoxia upregulates CD137 in a panel of mouse and human tumor cell lines. However, the predominant splicing form is soluble and able to bind to CD137L, thereby blocking its ability to provide co-stimulation to primed T lymphocytes. Accordingly, CD137-silenced tumor cells become more immunogenic upon grafting onto immunocompetent mice. These results contribute a novel and mischievous immunosuppressive mechanism cunningly deployed by tumors under hypoxia to counteract a pathway of T-cell co-stimulation.
Results
Retarded growth of CT26 colon cancer-derived tumors in CD137−/− mice
Reportedly, CD137−/− and CD137L−/− mice show a relative deficiency in the control of viral infections by CTL responses.Citation37,38 While performing experiments to examine if transplantable tumors would progress more aggressively and rapidly in CD137−/− mice, we noted a tendency toward the opposite outcome, since growth tended to be delayed several days and there were cases of spontaneous rejection.
As can be seen in Fig. S1A, the growth of transplanted syngeneic CT26 cells showed a surprising delay in CD137−/− mice. The sera of such tumor-bearing mice contained IgG antibodies directed to native CD137 as detected by indirect immunofluorescence on CD137-transfected 293T cells (Fig. S1B) and by ELISA on plate-absorbed recombinant CD137 (Fig. S1C). Since CD137−/− mice are not immunologically tolerant to CD137, they can be readily immunized by this antigen. Therefore, we serendipitously reached the conclusion that CT26 tumor cells had to somehow express CD137, resulting in tumor growth retardation and induction of anti-CD137 antibodies in CD137−/− mice. Indeed, transfer of sera containing CD137 antibodies from these CD137−/− tumor-bearing mice to WT mice grafted with CT26 tumors, retarded tumor growth and caused some complete rejections (Fig. S2).
Transplantable mouse tumor cell lines express CD137 under hypoxia
In previous studies, we have documented that hypoxia promoted CD137 expression in the case of both T lymphocytesCitation33 and endothelial cells in an HIF-1α-dependent fashion.Citation21 Therefore, we performed experiments to determine if hypoxia could induce CD137 on tumor cell lines raised from mouse tumors of different tissue origins.
shows that CD137 was upregulated at the mRNA level not only in CT26 cells, but also in EL-4, RENCA and B16 tumor cells. In this vein, a similar phenomenon has been observed in short-term passaged cell lines derived in our laboratory from spontaneous lung tumors (Azpilikueta A, Agorreta J et al, manuscript in preparation) indicating a more general trend.
Figure 1. CD137 mRNA expression is upregulated by hypoxia in mouse tumor cell lines. Quantitative RT-PCR assessment of CD137 mRNA expression under 21% and 1% O2 (hypoxia) in the indicated cell lines cultured under these conditions for 48h. A representative direct immunofluorescence staining for CD137 surface expression analyzed flow cytometry is presented in a corresponding histogram showing the discrepancy between the mRNA levels and the weak or dim surface protein staining. *p < 0.05, **p < 0.01.
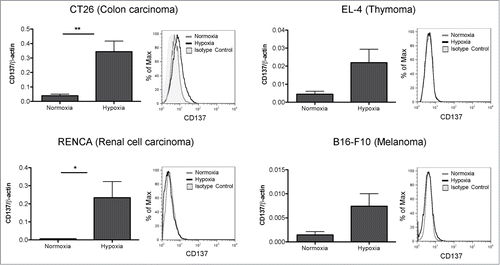
However, when analyzing the cell surface for the presence of CD137 by a sensitive flow cytometry assay, we were surprised by the weak surface levels detected in the case of CT26 cells and by the undetectable levels on the other cell lines tested ().
Hypoxia induces a soluble CD137 spliced form in tumor cells
A possible explanation for the absence of membrane staining was a predominance of the soluble alternative splicing form of CD137.Citation26 To address this issue, combinations of primers detecting the soluble and transmembrane CD137 isoforms were used () in quantitative RT-PCRs performed on the mRNA from the cell lines. shows that the transmembrane form is clearly in the minority as is shown by the electrophoresis migration of the amplified PCR products corresponding to the soluble and transmembrane forms (). Our findings in human cell lines derived from renal, lung, melanoma and hepatocellular tumors support a similar pattern of induction of sCD137 by hypoxia, indicating a mechanism conserved across species (Fig. S3).
Figure 2. Soluble form of CD137 predominates over membrane-attached forms. (A) Diagram representing the cDNA with the exons of mouse CD137 and RT-PCR products amplified with the indicated primer pairs. (B) Quantitative RT-PCR analyses using primers that amplify either transmembrane (primer pair 3–4) or total CD137 cDNA (primer pair 1–2) in corresponding cell lines. (C) RT-PCR products of total CD137 cDNA (primer pair 5–6) amplification showing that the soluble CD137 (sCD137) predominates over the transmembrane CD137 (tmCD137) form. (D) Sandwich ELISA assessment of the concentration of sCD137 in the tissue culture supernatants of the CT26 cell line. TM: transmembrane domain; MM: molecular weight marker; N: normoxia; H: hypoxia.
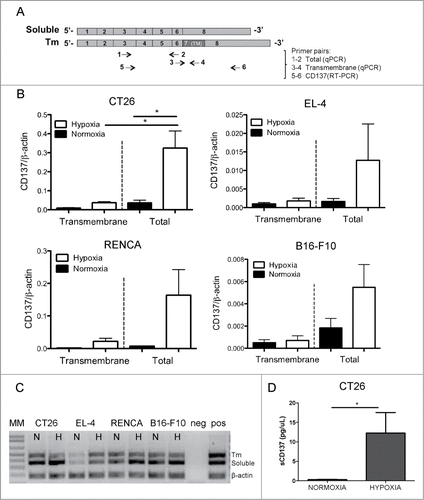
To ascertain as to whether sCD137 was indeed produced at the protein level, we set up a quantitative ELISA assay that clearly shows that the supernatant of CT26 cells contained higher concentrations of sCD137 when these cells were cultured under hypoxic conditions ().
Furthermore, we investigated if CD137 expression by tumor cells was taking place during in vivo tumor growth, given the fact that these tumors are hypoxic as we have recently reported.Citation21,33 shows that CD137 mRNA is expressed by CT26 cells grafted as tumors in CD137−/− mice. Using CD137−/− mice excludes contaminating mRNA from infiltrating lymphocytes or stromal cells. Again, total CD137 mRNA predominates over the transmembrane form.
Figure 3. Soluble CD137 is produced by in vivo grafted tumors. (A) CT26 tumors (10×10 mm in diameters) excised from CD137−/− mice were analyzed by quantitative RT-PCR for mCD137 encoding the transmembrane and the total CD137 isoforms. (B) The concentration of soluble CD137 (sCD137) assessed by ELISA in the sera of Rag−/− mice grafted with 5×105 CT26 cells for 21 days.
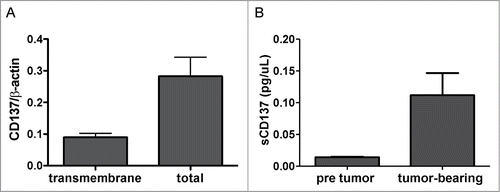
Consistent with these findings, the sera of immunoglobulin-deficient Rag2IL2Rγ−/− mice grafted with CT26 tumors contained readily detectable amounts of sCD137 by ELISA, that were undetectable prior to tumor grafting ().
Soluble CD137 produced by tumor cells binds to CD137L and blocks its co-stimulatory function
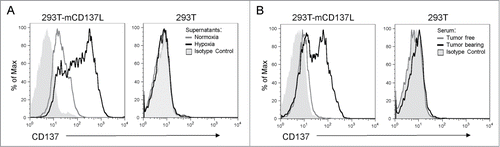
The functional role of sCD137 was hypothesized to be the blockade of the interactions of CD137L with membrane-bound co-stimulatory CD137. shows that the supernatant of CT26 cells cultured under hypoxia contained a sCD137 moiety that can be absorbed onto 293T cells transfected to express CD137L, but not onto their untransfected counterparts. Moreover, the sera of Rag2IL2Rγ−/− mice grafted with CT26 tumors contained a sCD137 form that was also able to bind to the CD137L transfectants, whereas it failed to bind to untransfected 293T cells. The sera from these mice prior to tumor grafting showed no signs of any such activity ().
Figure 4. Soluble CD137 produced by tumor cells binds to CD137 ligand. (A) Binding of sCD137 present in the supernatant of CT26 cells cultured under hypoxia to CD137 ligand (CD137L) transfected to 293T cells. Untransfected 293T were used as a specificity control and supernatants from normoxia and hypoxia cultured CT26 cells were tested without dilution. Binding was revealed by an anti-CD137 mAb which does not interfere with ligand binding (1D8 clone) (B) Similar experiment as in A performed with the serum of CT26-bearing Rag2IL2Rγ−/− mice as a source of sCD137 or with pre-tumor serum as a control.
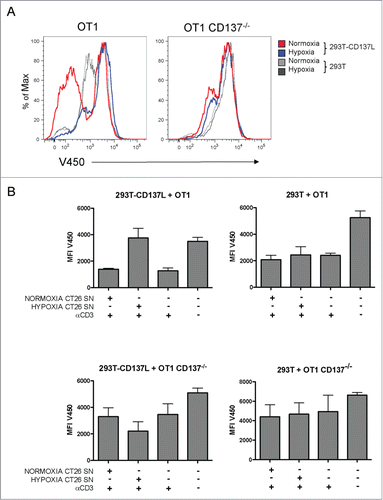
Binding of sCD137 to CD137L could functionally result in its blockade at the CD137 binding site, giving rise to a reduction in CD137L co-stimulatory activity. In assays of CD137L-293T cells co-cultured with primed CD137+CD8+ T cells, proliferation was assessed by Violet-450 dye dilution. In these assays, OT-1 TCR transgenic CD8+ T cells were used to ensure homogeneity and permit comparisons with identical TCR-transgenic lymphocytes obtained from CD137−/− double-transgenic mice. OT-1 lymphocytes were pre-activated for 48h with agonist anti-CD3ε mAb and then co-cultured with non-transfected and CD137L-transfected 293T cells. In this setting, the conditioned supernatant of hypoxia-treated CT26 cells inhibited proliferation while the supernatant of normoxic CT26 cells failed to inhibit proliferation (). As a control, it was observed that such an effect was not detectable when CD137−/− OT-1 lymphocytes were used, thus excluding CD137 unrelated effects of the supernatants in these co-cultures. shows representative histograms showing the degree of proliferation inhibition induced by conditioning these co-cultures with culture supernatants of hypoxic tumor cells. These T-cell proliferation experiments are summarized in .
Figure 5. Soluble CD137 in the supernatant of hypoxia-treated CT26 cells blocks CD137L-mediated T-cell costimulation. Cultures of CD8+-purified OT1 WT or OT1 CD137−/− T cells were stimulated with anti-CD3 mAb for 48h prior to seeding in co-cultures with 293T transfected or not with CD137L as indicated. T cells were pre-loaded with Violet 450 fluorescent dye and dilution of the fluorescent dye was used as a surrogate marker of T-cell proliferation. Cultures were conditioned with cell culture supernatants (1/16 diluted) of normoxia- or hypoxia-treated CT26 cells. (A) Representative flow cytometry histograms showing the extent of inhibition of proliferation by the sCD137-containing supernatants at 72h of coculture. (B) Pooled data of two independent experiments identically performed. MFI: mean fluorescence intensity; SN: supernatant.
Our interpretation of these results is that under hypoxia tumor cells produce an sCD137 moiety in order to block CD137L-mediated co-stimulation of primed CD137+ T lymphocytes, some of which are likely to be specific for tumor antigens.
CD137 silencing in hypoxic CT26 tumor cells renders them more immunogenic
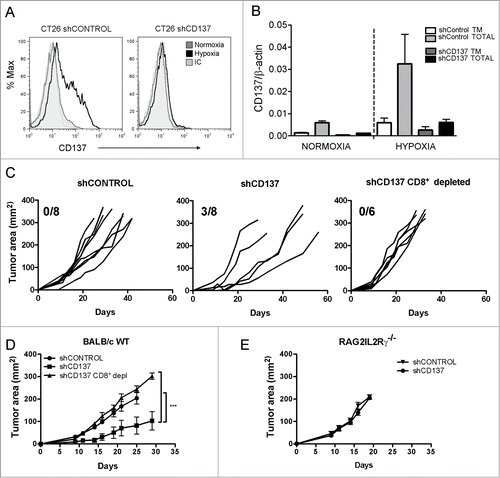
Polyclonal stable transfectants of a CD137 shRNA were generated in CT26 tumor cells by lentiviral transfection. Such transfectants were unable to upregulate surface CD137 in response to 1% O2 (). More importantly, CD137 RNA including the soluble form was almost completely silenced (). To study if CD137 loss results in higher immunogenicity under 1% O2, hypoxia pre-exposed shControl and shCD137 transfectants were inoculated subcutaneously in BALB/c WT mice and Rag2IL2Rγ−/− syngenic mice. As can be seen in , shCD137 transfectants progressed at slower pace and three out of eight mice underwent complete spontaneous rejections. By contrast, such tumors rapidly grafted and progressed in Rag2IL2Rγ−/−immunodeficient mice () or in immunocompetent BALB/c mice in which CD8+ T cells were selectively depleted with a specific mAb ().
Figure 6. CD137 silencing in CT26 tumor cells gives rise to more immunogenic variants. CT26 were stably transfected with lentiviral vectors to express a shRNA targeting CD137 (shCD137) or a scrambled control (shControl). (A) Transfectants were cultured for 36h in normoxia or 1% O2 and analyzed by flow cytometry to quantitatively determine the expression of surface CD137. (B) Quantitative RT-PCR analysis of total and transmembrane CD137 mRNA in the indicated transfectants under hypoxic or normoxic conditions as in A. (C) BALB/c wild type immunocompetent mice were subcutaneously inoculated with 5×105 cells of indicated transfectants and tumor sizes were individually followed over time. Tumor cells had been pre-exposed in every case to 1% O2 for 36h. The fraction of mice spontaneously rejecting their tumors is given in each graph. When indicated, BALB/c mice were mAb-depleted of CD8+T cells. (D) mean±SEM and Statistical comparisons of experiments in C whose results are representative of two independent experiments. (E) Tumor growth of the indicated transfectants in immunodeficient Rag2IL2Rγ−/− mice. Tm: transmembrane.
In conclusion, sCD137 expressed by CT26 tumor cells in response to hypoxia seems to be an important adaptive immune escape mechanism, at least in this tumor model.
Discussion
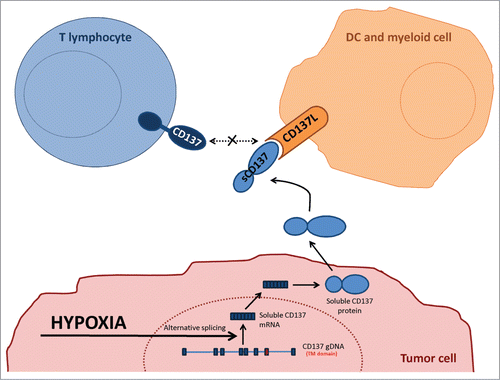
Tumor exploitation of immune system mechanisms to evade immune surveillance is currently considered a hallmark of cancer.Citation39 Importantly, tumors may induce immune escape mechanisms when undergoing immune attack or stress as previously described for instance in the case of adaptive acquisition of PD-L1Citation40,41 and IDO (indoleamine 2,3-dioxygenase) expression.Citation42 Tumors in mice and humans consistently show a degree of hypoxia at least in the core of primary or metastatic lesions. It is likely that hypoxia would be more pronounced under immune attack due to vascular disruption by secreted cytokines such as IFNγ and TNFα.Citation43 Evolutionary adaptation of tumors to cope with immunity ought to target the mechanisms that enhance the immune response.Citation1 In this study, we show that tumors can cunningly tamper with the CD137/CD137L co-stimulatory system which has the potential to elicit potent cytotoxic immune responses against cancer,Citation44 as schematized in .
Figure 7. Graphical interpretation of the experimental results. Schematic representation of the postulated mechanism underneath the experimental observations. TM: transmembrane; sCD137: soluble CD137.
Our results extend prior findings in the sense that CD137 expression is enhanced by hypoxia on T lymphocytes and endothelial cells in a HIF-1α-dependent fashion.Citation33 This feature is not exclusive to CD137 since it has also been observed with OX-40 (CD134), a close co-stimulatory relative of CD137 in the TNFR family, which is also clearly upregulated by hypoxia.Citation45
Expression of CD137 on grafted tumor cells is as intense as to be capable of inducing anti-CD137 antibodies in CD137−/− mice that do not appear in tolerized wild-type mice. The induction of antibodies in CD137−/− mice is so potent that it is convenient for raising monoclonal antibodies covering different epitopes on the CD137 molecule.
In an attempt to define a role for sCD137 in the tumor setting, we explored the possibility that it would block CD137L mediated co-stimulation. Indeed, our results clearly demonstrate the existence of such a mechanism, since we show that sCD137 produced in hypoxic cultures or by in vivo grafted tumor cells binds to CD137L and disrupts its co-stimulatory functions on primed CD8+ T lymphocytes. CD137-CD137L interactions are postulated to be important in immune synapses between antigen-presenting cells and T lymphocytesCitation46 and sCD137 may compete and disrupt such co-stimulatory events. In addition, some human tumor cell lines have been shown to weakly express CD137LCitation47 and the local presence of sCD137 would block its pro-immune functions.
Our observations have implications for cancer immunotherapy with anti-CD137 mAb, since the administered antibodies, in addition to signaling via CD137 on T and endothelial cells, should neutralize the sCD137 moieties which otherwise would obstruct CD137L functions. Moreover, such a mechanism helps to explain why single chain Fv anti-CD137 antibodies attached to the plasma membrane of tumorsCitation11,29 are more efficacious than the natural CD137 ligandCitation10,48,49 expressed at the same location. The explanation would be that CD137L is amenable to inhibition by sCD137, whereas agonist anti-CD137 antibodies are not. This concept could be relevant to refining reported attempts based on gene therapy with CD137LCitation48 or on the use of CD137L-Ig fusion proteins.Citation50
Our experiments with CD137-silenced variants of CT26 clearly demonstrate that these mechanisms are involved in immunoescape. When CT26 tumor cells could not upregulate CD137 in response to hypoxia, tumors were rejected or slowly progressed in contrast with control variants. This effect critically required CD8+ T cells.
Consistent with our conclusions and interpretations, sCD137 is also observed in patients suffering autoimmune conditions such as rheumatoid arthritis.Citation51 In this case, the production of sCD137 probably constitutes a negative feedback mechanism to attenuate damaging self-reactivity through CD137L blockade. Other possible mechanisms of sCD137 include reverse signaling via CD137L, but these would probably require sCD137 multimeric forms.Citation34
Further studies are warranted to rank the relative importance of this new mechanism among other immunosuppressive and tolerogenic mechanisms deployed by malignances.Citation5 The dependency of this immunosuppressive mechanism on hypoxia is quite intriguing and indicates a common theme,Citation3 according to which immune subversion can be enhanced when the tumor senses that it is under hypoxic stress. How splicing for the soluble form of CD137 is favored in the tumoral transcriptional scenario, or modulated by hypoxia, needs to be defined at the molecular level.
In our view, the aberrant secretion of sCD137 is clearly a simple and effective trick used by tumors to tackle a critical pathway of T-cell activation and memory differentiation.Citation52 In this sense, production of a blocking molecule for CD137L by hypoxic tumor cells most likely pursues immune escape.
Material and methods
Mice and cell lines
BALB/c wild-type mice (6–7 weeks old) were purchased form Harlan Laboratories. OT-1 and Rag2IL2Rγ−/− mice were purchased from the Jackson Laboratories and bred in our facilities. CD137−/− mice in BALB/c background have been previously described.Citation21 OT1 CD137−/−mice were bred in our laboratory by crossing OT-1 mice with CD137−/− mice in C57BL/6 background. All animal procedures were approved and conducted under institutional ethical committee guidelines (study number 137/12).
Mouse CT26 colon carcinoma, RENCA renal carcinoma, EL-4 thymoma and B16F10 melanoma cell lines were obtained from American Type Culture Collection (ATCC). AXBI human melanoma cells were derived at the clinical facility Erlangen from primary surgical samples and were used at early culture passages (kindly gift from Dr Kaempgen, Erlangen, Germany). A549 human lung cancer and HEPG2 hepatocellular carcinoma cells were obtained from ATCC. RCC10 renal cell carcinoma was kindly provided by Dr Luis del Peso (CSIC-UAM, Madrid, Spain).
Mouse cell lines were cultured in RPMI 1640 medium (Gibco) supplemented with 10% FBS (Sigma-Aldrich), 100IU/mL penicillin and 100ug/mL streptomycin (Gibco) and 5 × 10−5 M 2-mercaptoethanol (Gibco). Human cell lines were cultured in the same medium without 2-mercaptoethanol. To study hypoxia conditions, cell lines were cultured for 48h under 1% O2 atmosphere in the H35 Hypoxystation (Don Whitley) incubator.
Generation of stable transfectants
CD137 stable transfectants in 293T cells have been previously described.Citation53 To generate CD137L transfectants, plasmids pcDNA3-CD137L, was provided by Dr C. Smerdou (CIMA, Universidad de Navarra); pEZ-M02-CD137 (purchased from GeneCopoeia) were transfected using Lipofectamine (Invitrogen) into 293T cells cultured in p100 culture dishes (Corning) at a concentration of 2.5μg/dish. Following a 7-day culture, CD137L+ cells were sorted (FACS Aria II, BD Pharmigen) and those with the highest stable expression of the transgene were cloned and further expanded.
pGFP-C-shLenti vectors encoding CD137 and scramble siRNAs (purchased from Origene) were transiently transfected into 293T cell line with the lentiviral packaging plasmids pCMV-dR8.2 dvpr and pCMV-VSVG. The CT26 cell line was incubated 72h with the supernatant of packaging 293T containing lentiviral particles and brightly GFP-expressing cells were sorted by FACS and expanded in selection media containing puromycin (5μg/mL).
Tumor growth studies
Female BALB/c, CD137−/− or Rag2IL2Rγ−/− mice were inoculated subcutaneously in the flank with 5 × 105 CT26 wild-type cells or transfected variants in 50 μL of PBS. For experiments with shCD137 or shControl CT26, tumor cells were cultured prior to inoculation for 36h under hypoxic conditions. Mice and tumor size were monitored twice a week and mice were sacrificed when tumor areas reached 300mm2. For CD8+ depletion experiments, 200μg per dose of anti-CD8β mAb (clone H35-17-2) were given to mice on days –2, 0 and +3 with respect to the day of tumor cell inoculation. Completeness of depletion was checked by FACS on day +11 on peripheral blood lymphocytes.
CD137 expression
Total RNA was extracted from cell lines or tumors using a Maxwell 16LEV simplyRNA tissue kit (Promega). Reverse transcriptions were performed with M-MLV reverse transcriptase (Invitrogen) in the presence of RNAse OUT (Invitrogen). Real-time PCR was carried out with iQ SYBR green supermix in a iQ5 real time PCR detection system (Biorad). PCRs included primers for mouse CD137 cDNA (fw: 5′-AACATCTGCAGAGTGTGTGC-3′, rev: 5′-AGACCTTCCGTCTAGAGAGC-3′), mouse CD137 transmembrane cDNA (fw: 5′- AGAAGGACGTGGTGTGTGG-3′, rev: 5′-TAAGGACCTGCAAGGAGTGC-3′), human CD137 cDNA (fw: 5′-CACTCTGTTGCTGGTCCTCA-3′, rev: 5′-CACAGGTCCTTTGTCCACCT-3′) and human CD137 transmembrane cDNA (fw: 5′-GAAGGAGAGGGACGTGGTCT-3′, rev: 5′-GCGCAAGAAAGAAGGAGATG-3′). Data were normalized by comparison with levels of β-actin (mouse: fw: 5′-CGCGTCCACCCGCGAG-3′, rev: 5′- CCTGGTGCCTAGGGCG-3′; human: fw: 5′-AGCCTCGCCTTTGCCGA-3′, rev: 5′-CTGGTGCCTGGGGCG-3′). The expression of each transcript was represented according to this formula 2 ΔCt (Ct β-actin- Ct CD137).
We also carried out a semi-quantitative RT-PCR to amplify in the same assay the transmembrane and the soluble transcripts of mouse CD137 (fw: 5′-AACATCTGCAGAGTGTGTGC-3′, rev: 5′-GAGCTGCTCCAGTGGTCTTC-3′). The following program was used for the PCR: 94ºC for 5min, then 40 cycles: 94ºC for 45sec, Tm 64 ºC for 45sec, 72ºC 45sec in a 2720 Thermal Cycler (Applied Biosystems) with BioTaq DNA Polymerase (Bioline). Transmembrane isoform product length: 504 bp; soluble isoform product length: 369 bp.
Surface levels of CD137 in the cell lines were determined by direct immunofluorescence and flow cytometry. In every case, positive staining of activated T cells or a CD137 stable transfectant was used as a positive control. Antibodies used included anti-mouse CD137 PE and biotinylated (clone 17B5, Biolegend) and anti-human CD137 PE (clone 4B4-1, Biolegend), and Syrian Hamster IgG PE and biotinylated (Biolegend) and mouse IgG1 PE (Biolegend) as isotype-matched negative controls.
ELISA quantitation of soluble CD137
Protein levels of sCD137 were measured by employing a homemade sandwich ELISA. As capture antibody, plates were coated overnight with a monoclonal rat anti-mCD137 antibody (clone 2A, kindly provided by Dr Lieping Chen, Yale University New Haven, CT) at a concentration of 10μg/mL. After blocking with PBS supplemented with 10% FBS, samples were incubated for 2h at RT and plates were extensively washed with PBS 1% Tween. As detection antibody, a biotinylated monoclonal hamster anti-mCD137 (clone 17B5, Biolegend) was incubated at a concentration of 0.5μg/mL for 1h before washing and adding streptavidin-HRPO (at 1/250 dilution, DB Biosciences). Serial dilutions of mouse recombinant 4-1BB-Fc chimeric protein (R&D Systems) were used for the standard curve.
sCD137binding assays to CD137L
mCD137L stable transfectants in 293T cells were incubated with the serum of CT26-bearing Rag2IL2Rγ−/− mice or the culture supernatants of the indicated cell lines treated under hypoxia or normoxia conditions. The binding of the sCD137 present in the samples was detected on the cell surface by FACS analysis using an anti-CD137 monoclonal antibody that does not compete for the CD137L binding site (anti-CD137 PE clone 17B5, Biolegend).
Quantitation of anti-CD137 antibodies
Serial dilutions of the serum of CT26-bearing CD137−/− mice were incubated in ELISA plates coated with mouse recombinant 4-1BB-Fc chimeric protein (R&D Systems) and the antibodies against CD137 were detected and tittered using a goat anti-mouse IgG-HRP (Santa Cruz Biotechnology) according to the manufacturer's instructions.
These sera were also incubated with mouse CD137-293T transfectants and an anti-mouse IgG FITC secondary antibody (DakoCytomation) was employed to detect IgG antibodies captured at the cell surface CD137. Non-transfected 293T cells and pre-immune sera were used as controls for these indirect immunofluorescence assays developed by FACS.
Co-stimulation assays
CD8+ T lymphocytes were isolated from spleen of OT-1 and OT-1 CD137−/−transgenic mice using a magnetic isolation kit (mouse CD8+ T cells isolation kit, Miltenyi). CD8+ T cells were loaded with 0.5μM of Violet 450 fluorescent dye (Violate proliferation dye 450, BD Horizon) and activated with plate-bound anti-CD3ε (clone 145-2C11, Biolegend) for 48h. For co-culture assays, 1 × 105 irradiated (30000 Rads) mCD137L-293T or non-transfected 293T cells were cultured in 96 well plates with 2 × 105 pre-activated CD8+ OT-1 T cells for 72h adding tissue culture supernatants from the indicated cell lines as a source of tumor-derived sCD137. Relative fluorescence to assess fluorescent dye dilution resulting from proliferative activity was analyzed by FACS.
Statistics
Prism software (GraphPad Software) was used to analyze IgG titter, CD137 mRNA and protein expression by applying unpaired Mann Whitney test. For tumor growth studies, mean diameters of tumors over time were fitted using the following model: : *p < 0.05, **p < 0.01, ***p < 0.001.
Disclosure of potential conflicts of interest
IM is a consultant for Bristol-Myers Squibb, Merck Serono, Boehringer Ingelheim, Roche-Genentech, Takeda and Miltenyi Biotec. MJK is a Full time employee in Bristol-Myers Squibb.
Author contributions
Conception and design: SL, AP, IM, Development of methodology: SL, AP, EB, AA, AR, AMK, JIQ, AG, MRR and MAA, Acquisition of data: SL, AP, EB, AA, Provided critical reagents and transgenic mice: MJK, PB, Analysis and interpretation of data: SL, IM, AP, PB, MJK, JLP and AG, Writing, review and/or revision of the manuscript: SL, IM.
KONI_A_1062967_supplemental_material.zip
Download Zip (672.5 KB)Acknowledgments
We are grateful to Dr Paul Miller for English editing and to Dr Ana Rouzaut for fruitful scientific discussions and critical reading. Eneko Elizalde is acknowledged for excellent animal facility work.
Funding
Financial support was from MICINN (SAF2008-03294, SAF2011-22831 to IM). IM was also funded by the Departamento de Educación del Gobierno de Navarra and Departamento de Salud del Gobierno de Navarra, Redes temáticas de investigación cooperativa RETICC (RD06/0020/0065), European commission 7th framework program (ENCITE and IACT), and “UTE for project FIMA’’. PB is a recipient of a Miguel Servet contract from ISCIII. AM-K and SL are recipients of pre-doctoral scholarships from Ministerio de Economia.
References
- Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 2007; 25:267-96; PMID:17134371; http://dx.doi.org/10.1146/annurev.immunol.25.022106.141609
- Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Advances in immunology 2006; 90:51-81; PMID:16730261; http://dx.doi.org/10.1016/S0065-2776(06)90002-9
- Palazon A, Aragones J, Morales-Kastresana A, de Landazuri MO, Melero I. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin Cancer Res 2012; 18:1207-13; PMID:22205687; http://dx.doi.org/10.1158/1078-0432.CCR-11-1591
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 2013; 13:227-42; PMID:23470321; http://dx.doi.org/10.1038/nri3405
- Pardoll DM, The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:22437870; http://dx.doi.org/10.1038/nrc3239
- Perez-Gracia JL, Labiano S, Rodriguez-Ruiz ME, Sanmamed MF, Melero I. Orchestrating immune check-point blockade for cancer immunotherapy in combinations. Curr Opin Immunol 2014; 27:89-97; PMID:24485523; http://dx.doi.org/10.1016/j.coi.2014.01.002
- Melero I, Hirschhorn-Cymerman D, Morales-Kastresana A, Sanmamed MF, Wolchok JD. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res 2013; 19:1044-53; PMID:23460535; http://dx.doi.org/10.1158/1078-0432.CCR-12-2065
- Chen L, Ashe S, Brady WA, Hellstrom I, Hellstrom KE, Ledbetter JA, McGowan P, Linsley PS. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell 1992; 71:1093-102; PMID:1335364; http://dx.doi.org/10.1016/S0092-8674(05)80059-5
- Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science 1993; 259:368-70; PMID:7678351; http://dx.doi.org/10.1126/science.7678351
- Melero I, Bach N, Hellstrom KE, Aruffo A, Mittler RS, Chen L. Amplification of tumor immunity by gene transfer of the co-stimulatory 4-1BB ligand: synergy with the CD28 co-stimulatory pathway. Eur J Immunol 1998; 28:1116-21; PMID:9541607; http://dx.doi.org/10.1002/(SICI)1521-4141(199803)28:03%3c1116::AID-IMMU1116%3e3.0.CO;2-A
- Ye Z, Hellstrom I, Hayden-Ledbetter M, Dahlin A, Ledbetter JA, Hellstrom KE. Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nat Med 2002; 8:343-8; PMID:11927939; http://dx.doi.org/10.1038/nm0402-343
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8:793-800; PMID:12091876; http://dx.doi.org/10.1038/nm0902-1039c
- Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci U S A 1989; 86:1963-7; PMID:2784565; http://dx.doi.org/10.1073/pnas.86.6.1963
- Melero I, Murillo O, Dubrot J, Hervas-Stubbs S, Perez-Gracia JL. Multi-layered action mechanisms of CD137 (4-1BB)-targeted immunotherapies. Trends Pharmacol Sci 2008; 29:383-90; PMID:18599129; http://dx.doi.org/10.1016/j.tips.2008.05.005
- Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol 1998; 190:167-72; PMID:9878117; http://dx.doi.org/10.1006/cimm.1998.1396
- Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, Colevas AD, Weng WK, Clarke MF, Carlson RW et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest 2012; 122:1066-75; PMID:22326955; http://dx.doi.org/10.1172/JCI61226
- Kohrt HE, Colevas AD, Houot R, Weiskopf K, Goldstein MJ, Lund P, Mueller A, Sagiv-Barfi I, Marabelle A, Lira R et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest 2014; 124:2668-82; PMID:24837434; http://dx.doi.org/10.1172/JCI73014
- Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, Müller A, Pachynski R, Czerwinski D, Coutre S et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood 2011; 117:2423-32; PMID:21193697; http://dx.doi.org/10.1182/blood-2010-08-301945
- Zhang X, Voskens CJ, Sallin M, Maniar A, Montes CL, Zhang Y, Lin W, Li G, Burch E, Tan M et al. CD137 promotes proliferation and survival of human B cells. J Immunol 2010; 184:787-95; PMID:20008291; http://dx.doi.org/10.4049/jimmunol.0901619
- Lee SW, Park Y, So T, Kwon BS, Cheroutre H, Mittler RS, Croft M. Identification of regulatory functions for 4-1BB and 4-1BBL in myelopoiesis and the development of dendritic cells. Nat Immunol 2008; 9:917-26; PMID:18604213; http://dx.doi.org/10.1038/ni.1632
- Palazon A, Teijeira A, Martinez-Forero I, Hervas-Stubbs S, Roncal C, Penuelas I, Dubrot J, Morales-Kastresana A, Pérez-Gracia JL, Ochoa MC et al. Agonist anti-CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes. Cancer Res 2011; 71:801-11; PMID:21266358; http://dx.doi.org/10.1158/0008-5472.CAN-10-1733
- Broll K, Richter G, Pauly S, Hofstaedter F, Schwarz H. CD137 expression in tumor vessel walls. High correlation with malignant tumors. Am J Clin Pathol 2001; 115:543-9; PMID:11293902; http://dx.doi.org/10.1309/E343-KMYX-W3Y2-10KY
- Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St Croix B. Genes that distinguish physiological and pathological angiogenesis. Cancer cell 2007; 11:539-54; PMID:17560335; http://dx.doi.org/10.1016/j.ccr.2007.04.017
- Olofsson PS, Soderstrom LA, Wagsater D, Sheikine Y, Ocaya P, Lang F, Rabu C, Chen L, Rudling M, Aukrust P et al. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation 2008; 117:1292-301; PMID:18285570; http://dx.doi.org/10.1161/CIRCULATIONAHA.107.699173
- Kim JD, Kim CH, Kwon BS. Regulation of mouse 4-1BB expression: multiple promoter usages and a splice variant. Mol Cell 2011; 31:141-9; PMID:21347708; http://dx.doi.org/10.1007/s10059-011-0018-6
- Shao Z, Sun F, Koh DR, Schwarz H. Characterisation of soluble murine CD137 and its association with systemic lupus. Mol Immunol 2008; 45:3990-9; PMID:18640726; http://dx.doi.org/10.1016/j.molimm.2008.05.028
- Dimberg J, Hugander A, Wagsater D. Expression of CD137 and CD137 ligand in colorectal cancer patients. Oncol Rep 2006; 15:1197-200; PMID:16596186; http://dx.doi.org/10.3892/or.15.5.1197
- Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med 1997; 3:682-5; PMID:9176498; http://dx.doi.org/10.1038/nm0697-682
- Yang Y, Yang S, Ye Z, Jaffar J, Zhou Y, Cutter E, Lieber A, Hellström I, Hellström KE. Tumor cells expressing anti-CD137 scFv induce a tumor-destructive environment. Cancer Res 2007; 67:2339-44; PMID:17332366; http://dx.doi.org/10.1158/0008-5472.CAN-06-3593
- Pastor F, Kolonias D, McNamara JO, 2nd, Gilboa E. Targeting 4-1BB costimulation to disseminated tumor lesions with bi-specific oligonucleotide aptamers. Mol Ther 2011; 19:1878-86; PMID:21829171; http://dx.doi.org/10.1038/mt.2011.145
- Morales-Kastresana A, Catalan E, Hervas-Stubbs S, Palazon A, Azpilikueta A, Bolanos E, Anel A, Pardo J, Melero I. Essential complicity of perforin-granzyme and FAS-L mechanisms to achieve tumor rejection following treatment with anti-CD137 mAb. J Immunother Cancer 2013; 1:3; PMID:24764534; http://dx.doi.org/10.1186/2051-1426-1-3
- Lin GH, Liu Y, Ambagala T, Kwon BS, Ohashi PS, Watts TH. Evaluating the cellular targets of anti-4-1BB agonist antibody during immunotherapy of a pre-established tumor in mice. PloS one 2010; 5:e11003; PMID:20543982; http://dx.doi.org/10.1371/journal.pone.0011003
- Palazon A, Martinez-Forero I, Teijeira A, Morales-Kastresana A, Alfaro C, Sanmamed MF, Perez-Gracia JL, Peñuelas I, Hervás-Stubbs S, Rouzaut A et al. The HIF-1alpha hypoxia response in tumor-infiltrating T lymphocytes induces functional CD137 (4-1BB) for immunotherapy. Cancer Discov 2012; 2:608-23; PMID:22719018; http://dx.doi.org/10.1158/2159-8290.CD-11-0314
- Shao Z, Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J Leukoc Biol 2011; 89:21-9; PMID:20643812; http://dx.doi.org/10.1189/jlb.0510315
- Goodwin RG, Din WS, Davis-Smith T, Anderson DM, Gimpel SD, Sato TA, Maliszewski CR, Brannan CI, Copeland NG, Jenkins NA. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur J Immunol 1993; 23:2631-41; PMID:8405064; http://dx.doi.org/10.1002/eji.1830231037
- Guinn BA, DeBenedette MA, Watts TH, Berinstein NL. 4-1BBL cooperates with B7-1 and B7-2 in converting a B cell lymphoma cell line into a long-lasting antitumor vaccine. J Immunol 1999; 162:5003-10; PMID:10202049
- Kwon BS, Hurtado JC, Lee ZH, Kwack KB, Seo SK, Choi BK, Koller BH, Wolisi G, Broxmeyer HE, Vinay DS. Immune responses in 4-1BB (CD137)-deficient mice. J Immunol 2002; 168:5483-90; PMID:12023342; http://dx.doi.org/10.4049/jimmunol.168.11.5483
- Tan JT, Whitmire JK, Murali-Krishna K, Ahmed R, Altman JD, Mittler RS, Sette A, Pearson TC, Larsen CP. 4-1BB costimulation is required for protective anti-viral immunity after peptide vaccination. J Immunol 2000; 164:2320-5; PMID:10679066; http://dx.doi.org/10.4049/jimmunol.164.5.2320
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Flies DB, Sandler BJ, Sznol M, Chen L. Blockade of the B7-H1/PD-1 pathway for cancer immunotherapy. Yale J Biol Med 2011; 84:409-21; PMID:22180678
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Translat Med 2012; 4:127ra37; PMID:22461641; http://dx.doi.org/10.1126/scitranslmed.3003689
- Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest 2007; 117:1147-54; PMID:17476344; http://dx.doi.org/10.1172/JCI31178
- Yamaoka J, Kabashima K, Kawanishi M, Toda K, Miyachi Y. Cytotoxicity of IFN-gamma and TNF-alpha for vascular endothelial cell is mediated by nitric oxide. Biochem Biophys Res Commun 2002; 291:780-6; PMID:11866433; http://dx.doi.org/10.1006/bbrc.2002.6487
- Vinay DS, Kwon BS. Immunotherapy of cancer with 4-1BB. Mol Cancer Therapeutics 2012; 11:1062-70; PMID:22532596; http://dx.doi.org/10.1158/1535-7163.MCT-11-0677
- Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol 2013; 14:1173-82; PMID:24076634; http://dx.doi.org/10.1038/ni.2714
- Nam KO, Kang H, Shin SM, Cho KH, Kwon B, Kwon BS, Kim SJ, Lee HW. Cross-linking of 4-1BB activates TCR-signaling pathways in CD8+ T lymphocytes. J Immunol 2005; 174:1898-905; PMID:15699116; http://dx.doi.org/10.4049/jimmunol.174.4.1898
- Salih HR, Kosowski SG, Haluska VF, Starling GC, Loo DT, Lee F, Aruffo AA, Trail PA, Kiener PA. Constitutive expression of functional 4-1BB (CD137) ligand on carcinoma cells. J Immunol 2000; 165:2903-10; PMID:10946324; http://dx.doi.org/10.4049/jimmunol.165.5.2903
- Xu DP, Sauter BV, Huang TG, Meseck M, Woo SL, Chen SH. The systemic administration of Ig-4-1BB ligand in combination with IL-12 gene transfer eradicates hepatic colon carcinoma. Gene Ther 2005; 12:1526-33; PMID:15973445; http://dx.doi.org/10.1038/sj.gt.3302556
- Martinet O, Ermekova V, Qiao JQ, Sauter B, Mandeli J, Chen L, Chen SH. Immunomodulatory gene therapy with interleukin 12 and 4-1BB ligand: long- term remission of liver metastases in a mouse model. J Natl Cancer Institute 2000; 92:931-6; PMID:10841829; http://dx.doi.org/10.1093/jnci/92.11.931
- Meseck M, Huang T, Ma G, Wang G, Chen SH, Woo SL. A functional recombinant human 4-1BB ligand for immune costimulatory therapy of cancer. J Immunother 2011; 34:175-82; PMID:21304403; http://dx.doi.org/10.1097/CJI.0b013e318206dac1
- Michel J, Langstein J, Hofstadter F, Schwarz H. A soluble form of CD137 (ILA/4-1BB), a member of the TNF receptor family, is released by activated lymphocytes and is detectable in sera of patients with rheumatoid arthritis. Eur J Immunol 1998; 28:290-5; PMID:9485208; http://dx.doi.org/10.1002/(SICI)1521-4141(199801)28:01%3c290::AID-IMMU290%3e3.0.CO;2-S
- Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol 2003; 3:609-20; PMID:12974476; http://dx.doi.org/10.1038/nri1148
- Martinez-Forero I, Azpilikueta A, Bolanos-Mateo E, Nistal-Villan E, Palazon A, Teijeira A, Perez-Chacon G, Morales-Kastresana A, Murillo O, Jure-Kunkel M et al. T cell costimulation with anti-CD137 monoclonal antibodies is mediated by K63-polyubiquitin-dependent signals from endosomes. J Immunol 2013; 190:6694-706; PMID:23690480; http://dx.doi.org/10.4049/jimmunol.1203010
