ABSTRACT
The prognosis of high-risk neuroblastoma (NB) remains poor, although immunotherapies with anti-GD2 antibodies have been reported to provide some benefit. Immunotherapies can be associated with an IFNγ storm that induces in tumor cells the “adaptive immune resistance” characterized by the de-novo expression of Programmed Death Ligands (PD-Ls). Tumor cells can also constitutively express PD-Ls in response to oncogenic signaling. Here, we analyze the constitutive and the inducible surface expression of PD-Ls in NB cells. We show that virtually all HLA class Ipos NB cell lines constitutively express PD-L1, whereas PD-L2 is rarely detected. IFNγ upregulates or induces PD-L1 both in NB cell lines in vitro and in NB engrafted nude/nude mice. Importantly, after IFNγ stimulation PD-L1 can be acquired by NB cell lines, as well as by metastatic neuroblasts isolated from bone marrow aspirates of high-risk NB patients, characterized by different MYCN amplification status. Interestingly, in one patient NB cells were poorly responsive to IFNγ stimulation, pointing out that responsiveness to IFNγ might represent a further element of heterogeneity in metastatic neuroblasts. Finally, we document the presence of lymphocytes expressing the PD-1 receptor in NB-infiltrated bone marrow of patients. PD-1pos cells are mainly represented by αβ T cells, but also include small populations of γδ T cells and NK cells. Moreover, PD-1pos T cells have a higher expression of activation markers. Overall, our data show that a PD-L1-mediated immune resistance mechanism occurs in metastatic neuroblasts and provide a biological rationale for blocking the PD-1/PD-Ls axis in future combined immunotherapeutic approaches.
Introduction
Neuroblastomas (NB) are extra-cranial neuroectodermal tumors that account for 15% of all childhood cancer deaths. Different prognostic factors are critical for identifying high-risk NB and guiding therapeutic choices. These factors include age, stage and amplification of MYCN (MYCNampl), the major oncogenic driver.Citation1 High-risk patients present with metastatic disease (stage 4 or M) at diagnosis and have a grim prognosis due to resistance to conventional therapies and early relapse, which not only occur at the primary tumor site but frequently arises in the bone marrow.Citation2,3
Natural Killer (NK) cells, when appropriately activated, are capable of killing NB cells. This has been demonstrated, in vitro and in animal models, using as targets long-term cultured NB cell lines as well as bone marrow-infiltrating neuroblasts isolated from stage M patients,Citation4–6 although the latter appear to be more resistant to NK-mediated killing as compared to cell lines.Citation4 The degree of susceptibility to the NK-mediated cytolytic activity relies on both the repertoire and the surface density of ligands expressed on NB cell surface. In particular, neuroblasts lack HLA class I molecules or show a level of their expression insufficient to generate signals turning off NK-cell function via the inhibitory killer-cell immunoglobulin-like receptors (KIRs).Citation7 Vice versa, tumor cells can express different ligands engaging receptors that trigger the NK cytolytic machinery and the release of immunostimulatory cytokines, such as IFNγ. These include UL16-binding proteins (ULBP)-2 and ULBP-3, ligands of NKG2D, and Poliovirus Receptor (PVR, CD155) and Nectin-2 (CD112) that are recognized by DNAM-1.Citation8,9
Clinical evidences show that the immune system is unable to guarantee a long-lasting control of the disease and, in particular, an efficient NK-mediated destruction of NB fail to occur in vivo, suggesting the existence of mechanisms allowing tumor evasion of host immunity. For example, in some patients, metastatic neuroblasts lack the expression of PVR, and its absence correlates with poor susceptibility to NK-mediated killing.Citation10 Moreover, in all patients, tumor cells stably express B7-H3,Citation11 a transmembrane surface glycoprotein endowed with pro-tumoral propertiesCitation12,13 that, interacting with an (still unknown) inhibitory receptor, is capable of limiting both NK and T cell-mediated cytolytic activity.Citation11,14 B7-H3 is considered an unfavorable prognostic factor in both hematological malignanciesCitation15 and solid tumors, including NB,Citation16-22 and clinical trials with a fully human antibodyCitation23 are ongoing.
An additional mechanism of escape could be the exploitation by tumors of the immune checkpoints, inhibitory pathways that physiologically maintain self-tolerance and limit the duration and amplitude of immune responses, thus minimizing tissue damage.Citation24,25 One possible pathway is represented by the PD-1/PD-Ls axis. Programmed cell death 1 (PD-1, CD279) is an inhibitory receptor, mainly expressed by αβ and γδ T cells. Interestingly however, some reports demonstrated the expression of PD-1 also in activated NK cells.Citation26,27 Most data on PD-1 functions are referred to αβ T cells, where PD-1 has been demonstrated to switch off the T cell function mostly in peripheral tissues. Indeed, unlike CTLA-4, PD-1 is expressed during the late phase of T cell activation and, upon engagement with its ligands, it inhibits kinases involved in T cell activation.Citation28 In γδ T cells, TCR triggering might partially overcome the inhibitory effect mediated by PD-1. In particular, while proliferation rate might be affected by PD-1 engagement, slight differences in either cytokine production or cytotoxicity were observed in γδ T cells interacting with PD-L1pos or PD-L1neg tumors.Citation29 PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273) are members of the B7 family and represent ligands of PD-1.Citation30 PD-L2 expression is mainly restricted to antigen presenting cells (APC), whereas PD-L1 is expressed in several normal tissues.Citation24,25 Interestingly, certain tumors have been shown to express PD-L1 and its interaction with the receptor has been suggested to play a crucial role in immune evasion.Citation24,31
In full agreement with preclinical experimental data, combined therapies that include the blockade of the PD-1/PD-L1 pathway resulted in long-term responses in patients with advanced melanoma and, very recently, two anti-PD-1 antibodies obtained FDA approval.Citation25 Interestingly, clinical responses were observed also in patients affected, at the time of therapeutic decision, by PD-L1neg melanoma and carcinomas. In this context, it has been shown that tumor cells can upregulate PD-L1 surface density upon stimulation with IFNγ and TNF-α,Citation24,32 cytokines that are released by T lymphocytes, NK cells and macrophages during effective Th1-polarized antitumor responses.
In the present study, we analyzed the constitutive and the inducible expression of PD-Ls in human NB cell lines and ex vivo isolated neuroblasts, and evaluated PD-1 expression on lymphocytes in tumor-infiltrated bone marrow aspirates.
Results
Analysis of the constitutive expression of PD-L1 and PD-L2 in NB cell lines
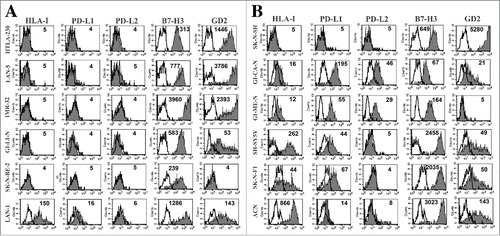
HLA class I and PD-Ls might be key regulators of both NK- and T cell-mediated immune surveillance. We analyzed their constitutive surface expression in twelve human NB cell lines characterized by either the presence or the absence of MYCN amplification (MYCNampl) ( and Fig. S1). Although, HLA-I was mainly detected on non-MYCNampl cell lines, exceptions to the rule existed. Indeed, MYCNampl LAN-1 cells expressed significant levels of HLA-I molecules and, conversely, non-MYCNampl SK-N-SH cells consistently lacked their expression. Similarly, when analyzing PD-L1 expression, it appeared mostly restricted to non-MYCNampl cells. However, it was detectable in MYCNampl LAN-1 cells and absent in non-MYCNampl SK-N-SH cells ( and Fig. S1). Thus, as for HLA-I, in the NB cell lines analyzed, the constitutive expression of PD-L1 did not appear to strictly correlate with MYCN status. However, HLA-I positive cell lines co-expressed in all instances PD-L1, thus suggesting a possible link in the capability to express these molecules. The constitutive expression of PD-L2 was rarely detected and it was restricted to GICAN and GIMEN, non-MYCNampl HLA-Ipos PD-L1pos cell lines. It is of note that HLA-Ipos PD-L1pos NB cell lines, although expressing one or more ligand for the DNAM-1 and NKG2D activating NK (and T) cell receptorsCitation4,33 (Fig. S2) shared the expression of B7-H3 (), a ligand involved in modulation of NK and T cell-mediated cytotoxicity whose expression has been shown to correlate with a worse prognosis in different tumor histotypes, including NB.Citation4
Figure 1. Analysis of the constitutive expression of PD-L1 and PD-L2 in NB cell lines. Representative cytofluorimetric analysis of the expression of PD-L1, PD-L2 and HLA-I in MYCNampl (panel A) and non-MYCNampl (panel B) NB cell lines. B7-H3 and GD2 are shown for comparison. White profiles refer to cells incubated with isotype-matched controls. Values inside each histogram indicate the Median Fluorescence Intensity (MFI).
Cytokines-mediated induction or upregulation of PD-Ls in NB cell lines
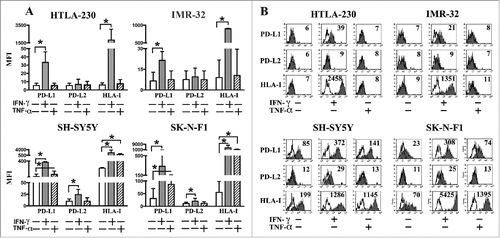
Representative MYCNampl or non-MYCNampl cell lines were cultured in the presence of IFNγ or TNF-α. As shown in , in MYCNampl (HTLA-230, IMR-32) cells, IFNγ induced the de novo surface expression of HLA-I and PD-L1 molecules, while it did not promote that of PD-L2. On the other hand, in non-MYCNampl cell lines (SH-SY5Y and SK-N-F1), it induced both expression of PD-L2 and upregulation of HLA-I and PD-L1. The capability of TNF-α to modulate ligands expression was reduced as compared to that of IFNγ. Indeed, the two representative MYCNampl cell lines were totally unresponsive to TNF-α, preserving their PD-L1neg PD-L2neg HLA-Ineg phenotype. Moreover, in non-MYCNampl cell lines, TNF-α conditioning resulted in a smaller increase of HLA-I and PD-L1, as compared to those observed with IFNγ ().
Figure 2. PD-L1 and PD-L2 expression in INFγ− or TNF-α−treated NB cell lines. Panel A: cytofluorimetric analysis of the expression of PD-L1, PD-L2 and HLA-I in representative MYCNampl (HTLA-230, IMR-32) and non-MYCNampl (SH-SY5Y and SK-N-F1) cell lines cultured for 48 h either in the absence (white bars) or in the presence of IFNγ (gray bars) or TNF-α (striped bars). Mean of MFI and 95% confidence intervals are indicated. *p < 0 .05. Panel B: Representative cytofluorimetric analysis of PD-L1, PD-L2 and HLA-I expression in untreated or cytokine-treated NB cell lines. White profiles refer to cells incubated with isotype-matched controls. Values inside each histogram indicate the MFI.
Interestingly, in MYCNampl cells the de novo expression of PD-L1 showed a kinetics even more rapid than that of HLA-I (Fig. S3). Indeed, the maximal PD-L1 expression was observed at 24 h after IFNγ stimulation (mean of fold increase = 6.4), with no significant increase (or decrease) at later time, whereas HLA-I expression was significantly increased after 24 h (mean of fold increase = 96.6) but reached the highest level of expression at 48 h (mean of fold increase = 329.7). On the other hand, in non-MYCNampl cells the kinetics of upregulation of these molecules was comparable, with a maximal expression at 24 h. Regarding PD-L2, it was undetectable at any time in MYCNampl, whereas it was acquired by non-MYCNampl cells, and progressively increased until 48 h (Fig. S3).
IFNγ induces PD-L1 expression in a human NB mouse model
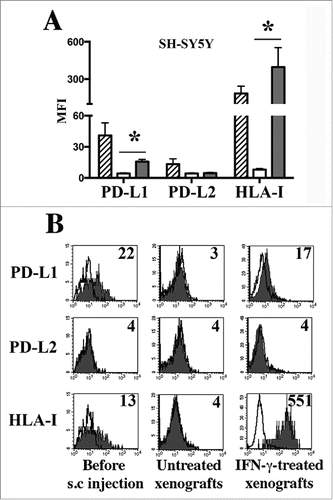
In order to verify whether NB cells could acquire PD-Ls in an in vivo setting, we used an animal model based on the subcutaneous injection of the human (HLA-Ipos PD-L1pos PD-L2neg) SH-SY5Y NB cell line in immunodeficient mice (see Materials and Methods for more details). This model allowed a rapid quantification of the tumor burden and an easy access for the intra-tumor injection of IFNγ, with minimal suffering of the animal, when compared with the invasive surgery required to treat orthotopic tumor models. At the end of the treatments, tumors were removed and single cell suspensions of NB cells from untreated or IFNγ-treated xenografts were analyzed for ligands expression, gating on cells expressing the GD2pos B7-H3pos phenotype ( and Fig. S4A). Remarkably, the engraftment resulted in NB cells lacking PD-L1 and HLA-I expression, while preserving their original GD2pos B7-H3pos phenotype. Importantly, in line with data obtained in vitro (), intra-tumor injection of IFNγ restored the surface expression of both PD-L1 and HLA-I (). On the other hand, no significant changes were observed in terms of PD-L2 expression ().
Figure 3. PD-L1 expression in human NB xenografts. Panel A: Cytofluorimetric analysis of PD-L1, PD-L2 and HLA-I expression in the SH-SY5Y cell line just before subcutaneous (s.c.) injection in animals (stripped bars), and in xenografts derived from untreated (white bars) or IFNγ-treated mice (gray bars). Mean of MFI, 95% confidence intervals and significance are indicated. *p < 0 .05. Panel B: Representative cytofluorimetric analysis of PD-L1, PD-L2 and HLA-I in the SH-SY5Y cells before s.c. injection, in untreated or IFNγ-treated xenografts. White profiles refer to cells incubated with isotype-matched controls. Values inside each histogram indicate the MFI.
Analysis of the PD-Ls and PD-1 expression in high-risk NB patients
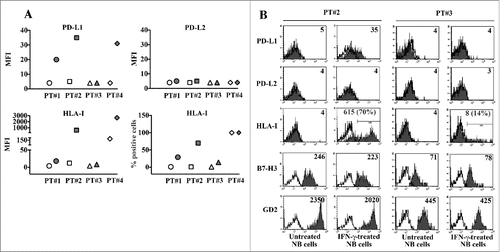
We analyzed the constitutive and inducible expression of PD-Ls in neuroblasts purified from bone marrow aspirates of patients with stage M NB ( and Fig. S4B). GD2pos B7-H3pos neuroblasts did not constitutively express PD-L1 and PD-L2. In all patients analyzed, neuroblasts maintained their PD-L2-negative phenotype even in the presence of IFNγ. On the contrary, IFNγ stimulation induced the expression of PD-L1 in three out four patients analyzed, whose MYCN status were amplified (PT#1), gain (PT#2) or non-amplified (PT#4) (). Responsiveness to the cytokine was confirmed by the de novo expression or upregulation of HLA-I expression, which reached the highest median intensity in neuroblasts from the non-MYCNampl PT#4. Interestingly, PT#3, who was characterized as PT#2 by a MYNCgain status, was poorly responsive to IFNγ conditioning. Indeed, upon cytokine stimulation, neuroblasts did not show any induction of PD-L1 expression, and that of HLA-I molecules was restricted to a very small percentage of cells ().
Figure 4. Analysis of the constitutive and inducible PD-L1 and PD-L2 expression in neuroblasts from NB patients. Panel A: Neuroblasts from (CD45-depleted) bone marrow aspirates of stage M patients (GD2pos B7-H3pos), untreated (white symbol) or treated with IFNγ over 5 d (gray symbol) were analyzed by flow cytometry for the expression of the indicated molecules. Raw values are plotted. Patient 1 (PT#1) (MYCNampl), Patient 2 (PT#2) (MYCNgain), Patient 3 (PT#3) (MYCNgain), Patient 4 (PT#4) (non-MYCNampl). MFI or percentage of positive cells are indicated. Panel B: Representative cytofluorimetric analysis of PD-L1, PD-L2 and HLA-I expression in IFNγ-responsive (PT#2) and IFNγ-unresponsive (PT#3) neuroblasts. White profiles refer to cells incubated with isotype-matched controls. Values inside each histogram indicate the MFI and, in brackets, the percentage of positive cells.
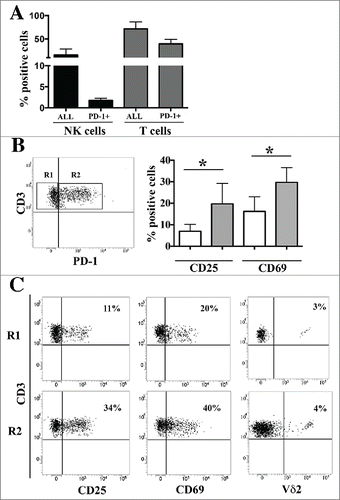
We analyzed PD-1 expression on NK and T lymphocytes in NB-infiltrated bone marrow aspirates. T cells represented the large majority (70%) of lymphocytes at this site. Among NK lymphocytes, a small percentage (up to 2%) of PD-1pos cells were detected. On the other hand, about 40% of CD3pos cells expressed PD-1 (). PD-1pos CD3pos lymphocytes showed a significant higher percentage of CD25pos and CD69pos cells, as compared to the PD-1 negative counterpart (). Interestingly, PD-1pos T cells also included a subset of γ/δ Τ cells ().
Figure 5. Surface expression of PD-1 in lymphocytes from NB-infiltrated bone marrow aspirates of patients with stage M. Panel A: Bone marrow infiltrating NK and T cells were analyzed by multicolor flow cytometry. The percentage of the two lymphocyte populations and of the PD-1pos subsets is shown. Mean and 95% confidence intervals are indicated. Panel B: Gating strategy (left) and CD25 and CD69 expression (right) in CD3pos PD-1neg (R1, white bars) and CD3pos PD-1pos (R2, gray bars) T cells. Mean of percentage of positive cells, MFI and 95% confidence intervals are indicated. * p < 0 .05. Panel C: Representative cytofluorimetric analysis of CD25, CD69 and Vδ2 expression in CD3pos PD-1neg (R1) and CD3pos PD-1pos (R2) T cells. Percentage of positive cells and MFI are indicated.
Discussion
An important challenge for pediatric oncologists is represented by stage M NBs, a disease often refractory to standard therapies, that frequently shows recurrence or progression.Citation3 Current therapeutic strategies are based on risk factors that do not take into consideration the expression, in tumor cells, of surface molecules crucial for the recognition by the immune system, probably underestimating a further element of heterogeneity existing among patients. Phenotypic and functional analysis showed that infiltrating neuroblasts can either lack or express low levels of HLA-I,Citation11 thus representing non-optimal targets for HLA-restricted T cell-based immunotherapies. In these cases, tumor cells can display susceptibility to NK-mediated killing, which, however, in some patient, is limited by the absence on tumor cells of key ligands for activating NK receptors.Citation10 Moreover, in all patients, NB cells constitutively and stably express at the cell surface B7-H3, a molecule endowed, not only with an immune-regulatory activity that limits T and NK cell-mediated killing, but also with direct tumor promoting properties.Citation4 The characterization of the biological features of B7-H3, together with its poor expression in most normal tissues, is recently driving novel immunotherapeutic approaches targeting this tumor-associated marker.Citation23,25,34
Here we show that, together with the constitutive expression of B7-H3, NB cells can exploit inducible members of the B7 family to regulate key effectors of the immune system. Indeed, the surface expression of PD-Ls, and in particular of PD-L1 can be induced in NB cells by inflammatory cytokines such as INFγ and TNF-α. As demonstrated in other tumors,Citation35 in NB cells, INFγ stimulation is more potent than TNF-α. INFγ induced the expression of PD-L1 in various MYCNampl and non-MYCNampl NB cell lines in vitro as well as in an animal tumor model based on the use of SH-SY5Y cells. Different from most NB cell lines (including the prototypic MYCNampl HTLA-230), which require orthotopic models for in vivo growth, the SH-SY5Y cell line has the ability to both respond to IFNγ stimulation and grow subcutaneously. The use of an orthotopic mouse model as a new experimental approach could be considered in future investigations.
Importantly, INFγ also induced the expression of PD-L1 in freshly-isolated metastatic neuroblasts from patients, which did not present detectable levels of this molecule at the cell surface. PD-L1 expression occurs independently of the MYCN amplification status, whereas it is apparently coordinated with that of HLA-class I molecules. Moreover, the INFγ-mediated de novo induction of PD-L1 shows a more rapid kinetics as compared to HLA-I molecules, thus suggesting that in an inflammatory microenvironment PD-L1 could prematurely limit the activity of T lymphocytes and precede the acquisition of HLA-I levels optimal for the KIR-mediated inhibition of NK cell functions. This observation is in line with data recently published by Boes M. et al., who shows that in NB cell lines stimulated by poly(I:C), an agonist of Toll-Like Receptor 3 (TLR3), the kinetic of PD-L1 expression is more rapid than that of HLA-I.Citation36 Importantly, they also demonstrate that the antibody-mediated blocking of PD-L1 increases the T-cell stimulatory properties of poly(I:C)-stimulated NB cell lines.Citation36
B7-H3 is considered a negative prognostic factor in different solid tumors, whereas the analysis of PD-L1 expression gave conflicting results.Citation37–42 No strict correlation has been demonstrated between PD-L1 mRNA levels in carcinomas and survival.Citation35 Accordingly, in human NB primary tumors the levels of PD-L1 expression did not show correlation with overall and relapse-free survival (Fig. S5). In this context, further analysis of NB specimens will allow to validate the relevance of epigenetic mechanisms regulating PD-Ls expression at post-transcriptional levels.Citation43,44 Other studies reported a better survival in patients carrying PD-L1pos tumors that were characterized by elevated lymphocyte infiltration, and the presence of infiltrating PD-1pos T cells has been considered a favorable prognostic marker.Citation45 Both conditions reflect the existence of effective antitumor responses, but also highlight the opposite, beneficial versus detrimental, effects of an inflammatory/Th1-polarized microenvironment. Indeed, the release of large amounts of INFγ by activated T and NK cells amplifies the immune responses, but also forces the expression of PD-L1, which could result in premature inhibition of lymphocyte proliferation and effector function.Citation46 Overall, our present data emphasize the complexity of the PD-1/PD-L1 axis and suggest that, in NB, PD-L1 might be considered a MYCN-independent “tumor-inflammatory” prognostic factor rather than simply a tumor-associated marker.
The susceptibility of NB cells to INFγ stimulation supports the usefulness of the blockade of the PD-1/PD-L1 pathway in combined therapeutic approaches aimed at achieving long-lasting remission in patients with high-risk NB. Indeed, both conventional and innovative therapeutic protocols generate an inflammatory microenvironment capable of influencing PD-L1 expression in tumor cells. It is of note that INFγ is highly represented in the serum of tumor patients suffering of the Cytokine Release Syndrome (CRS), which frequently occurs with targeted immunotherapeutic approaches.Citation47 These include the use of antibodies directed against tumor-associated antigens and adoptive therapies with chimeric antigen receptor (CAR)-modified T cells. The Ab-mediated targeting of the oncofetal antigen GD2 has been already included in the standard cure of high-risk NBCitation3 and approaches based on the infusion of CAR-T cells specific for GD2 are tested in preclinicalCitation48,49 and clinicalCitation50 studies.
Notably, metastatic neuroblasts appear to display variability in the response to INFγ stimulation. In particular, in one patient analyzed in our study (PT#3), tumor cells were very poorly responsive to the cytokine and did not express detectable levels of PD-L1 upon cytokine stimulation. More information about the frequency of IFNγ resistant NB will derive from the analysis of cohort of patients larger than that used in the present study. In this context, it is of note that NB is a rare pediatric disease and both the number and the volume of samples are scarce. Since the beginning of our study, we received 19 bone marrow aspirates from NB patients (Table S1). However, 12 samples were NB free and in 3 cases, due to the poor tumor infiltration (<1 % of total cells), purified NB cells were insufficient to perform in vitro IFNγ stimulation. Thus, although NB resistance to IFNγ needs to be supported by the analysis of a wider number of patients, susceptibility or resistance to INFγ stimulation by tumor cells might be taken into consideration when selecting patients for PD-1/PD-L1 blocking approach. On the other hand, it is conceivable that also NB patients characterized by PD-L1 negative tumors might benefit from the block of the PD-1/PD-L1 axis. Indeed, both PD-L1 and PD-L2 expression is induced in APC including macrophages and dendritic cells and, together with chronic antigen exposure, play a role in exhaustion of the immune response.Citation25,51 Thus, interfering with the PD-1/PD-Ls immune checkpoint might also reactivate the crosstalk between APC and lymphocytes. According to this hypothesis, clinical responses with anti-PD-1 antibodies have been observed also in patients whose tumors were considered negative for PD-L1 expression.Citation52 In this context, the analysis of bone marrow aspirates from stage M patients showed the presence of infiltrating NK and T lymphocytes and, although T cells were clearly more represented, a PD-1pos cell subset was detectable in both lymphocyte populations. Interestingly, PD-1pos T cells also included γδ T cells, which, lacking HLA-I restriction, might be involved in the early phase of endogenous or adoptive immune responses against NB.Citation53,54
In conclusion, our study provides a biological rationale for considering blocking the PD-1/PD-L1 axis as an additional immunotherapeutic approach in combined therapies of high-risk NB patients. Such therapies might include also targeting of B7-H3, an immunomodulatory pro-tumoral molecule that shows a striking stability at the NB cell surface.
Materials and methods
Neuroblastoma cell lines
GI-LI-N, GI-ME-N and GI-CA-N NB cell lines were established at the Laboratory of Oncology, Giannina Gaslini Institute, Genova, Italy; SH-SY5Y, SK-N-F1, IMR-32, LAN-1, LAN-5, SK-N-BE-(2) and SK-N-SH cell lines were purchased from Banca Biologica and Cell Factory (IRCCS Azienda Ospedaliera Universitaria San Martino-IST, Genova, Italy). NB cell lines are periodically checked for MYCN amplification by fluorescence in situ hybridization analysis. HTLA-230 and ACN were kindly provided by Dr. E. Bogenmann (Children’s Hospital Los Angeles, Los Angeles, CA) and by the late Dr S Carrel, respectively.Citation55
NB cell lines were cultured in the presence of RPMI 1640 medium supplemented with 10% heat inactivated FCS (Sigma-Aldrich), 50 mg/mL streptomycin, 50 mg/mL penicillin and 2 mM glutamine (henceforth referred to complete medium). The NB cell lines used in this study were checked for morphology, proliferation rate and mycoplasma contamination, after thawing and within four passages in culture.
Neuroblastoma patients
After informed consent, bone marrow was aspirated from iliac crests of children diagnosed with stage M NB and admitted at the Oncology Unit of the Giannina Gaslini Institute (Table S1). Diagnosis and staging were performed according to the INRG-SS.Citation1 The study was approved by the Istituto Giannina Gaslini Ethics Committee and the procedures were in accordance with the Helsinki Declaration of 1975. NB were purified from bone marrow aspirates as previously described.Citation11
Mouse tumor model
All animals were purchased from Harlan Laboratories (Harlan Italy, S.Pietro al Natisone, Italy) and housed under specific pathogen-free conditions. Experiments involving animals were reviewed and approved by the Licensing and Ethical Committee of IRCCS Azienda Ospedaliera Universitaria San Martino – IST (Genova, Italy), and by the Italian Ministry of Health. In vivo experiments were performed with three mice for group. 2 × 107 SH-SY5Y cells were subcutaneously injected in the mid-dorsal region of five-week-old female nude/nude mice, as previously described.Citation56 Tumors were allowed to grow for 3 weeks, and then intratumorally treated with 300 ng/mL of IFNγ in complete medium, every day for 2 d. Control mice received complete medium alone. 24 h after the end of treatments, mice were sacrificed by cervical dislocation after being anesthetized with xilezine (Xilor 2%, Bio98 Srl, Milan, Italy), NB tumors removed, immersed in complete medium, and minced by an homogenizer at 4°C. The single cell suspensions were subjected to erythrocytes lysis (1.54 M NH4Cl; 99.8 mM KHCO3; 0.988 mM EDTA), and washed in PBS before the cytofluorimetric analysis performed gating GD2pos, B7-H3pos cells.
Cytofluorimetric analysis, IFNγ and TNF-α treatment
For one-color cytofluorimetric analysis (FACSCalibur Becton Dickinson & Co, Mountain View, CA) cells were stained with the appropriate mAbs followed by Phycoerythrin (PE)-conjugated isotype-specific goat anti-mouse second reagent (Southern Biotechnology Associated, Birmingham, AL).Citation11 On every experimental session, the flow cytometer performances were monitored, the reproducibility of the fluorescence intensity was aligned by calibrated microsfere (Becton Dickinson & Co, Mountain View, CA) and isotype matched antibodies were used as controls. For multicolor cytofluorimetric analysis of bone marrow samples, NK or T cells were gated by physical parameters and the combined use of anti-CD56, anti-CD3, anti-CD19, anti-CD45 mAbs. The analyses were performed on FACSVerse (Becton Dickinson & Co) and data were analyzed by FacsSuite software 1.0.5 version.
For cytokine stimulation NB cell lines or freshly-isolated neuroblasts were seeded at 200.000 cells/well in round flat bottom plates and cultured (for 2 or 5 days, respectively) in the presence of TNF-α or INFγ (PeproTech, Rock Hill, NJ) at the final concentration of 100 ng/mL. Cytofluorimetric analysis was performed by gating GD2pos, B7-H3pos cells.
Monoclonal antibodies
A6136 (IgM, anti-HLA class-I), M5B14 (IgM) and NE97 (IgG2b) (anti-B7-H3), M5A10 (IgG1, anti-PVR), U191 (IgM, anti-Nectin-2), KRA236 (IgG1, anti-CD226), c227 (IgG1, anti-CD69), MAR93 (IgG1, anti-CD25) and BAB281 (IgG1, anti-NKp46) mAbs were produced in our lab. Anti-PD-L1.3.1 (IgG1, anti-PD-L1), anti-PD-L2 (IgG1, anti-PD-L2) and anti-PD-1 mAbs were produced in D. Olive’s lab, Anti-ULPB2 (165903, IgG2a) and anti-ULBP3 (166510, IgG2a) mAbs (SantaCruz biotechnology, inc). Anti-GD2 mAb (14.G2a, IgG2A), anti-CD16-PerCPCy5.5 and anti-CD45-V500 (BD Bioscience PharMingen, San Diego, CA). Anti-CD56-PC7 (C218 clone) (Beckman Coulter, Immunotech, Marseille, France); anti-CD45-FITC anti-CD3-VioGreen, anti-CD19-VioBlue, anti-Vdelta1-VioBlue, anti-Vdelta2-APC and anti-TCRab-APC-Vio770 mAbs (Miltenyi Biotec, Bergisch Gladbach, Germany); Goat anti-mouse isotype specific secondary reagents (anti-IgG1-APC-Cy7 and anti-IgG2B-PE) (Southern Biotec). The KL247 (IgM, anti-NKp46), DF200 (IgG1 anti-KIRs) and AZ158 (IgG2a, anti-KIRs) mAbs produced in our lab were used as isotype-matched controls.
PD-L1 gene expression analysis in NB patients
Correlation of overall and relapse-free survival of NB patients and levels of PD-L1 expression were obtained from the Versteeg database containing whole-genome sequence data from 88 human NB primary tumors,Citation57 evaluated using R2 Genomics Analysis and Visualization Platform (http://hgserver1.amc.nl/cgi-bin/r2/main.cgi).
Statistical analysis
Wilcoxon–Mann–Whiteny p value test (non-parametric significance test) was employed. The statistical level of significance (p) is indicated. Graphic representation and statistical analysis were performed using the PASW Statistic version 20.0 software (formerly SPSS Statistics) (IBM, Milan Italy) and GraphPad Prism 6 (GraphPad Software La Jolla, CA).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Material.docx
Download MS Word (832.1 KB)Acknowledgments
We thank Dr. G. Reggiardo (Medi Service, Genova, Italy) for help in statistical analysis, Dr A. Garaventa (Istituto G. Gaslini, Genova) for providing neuroblastoma BM aspirates. We apologize to the colleagues whose work we could not cite because of space constraints.
Funding
This work was supported by Associazione Italiana per la Ricerca sul Cancro (A.I.R.C.) (Investigator Grant 15704 and special project 5×1000 9962), Ministero dell’Istruzione, dell’Università e della Ricerca (M.I.U.R) (PRIN 20103FMJEN) and Ministero della Salute (5 × 1000 e Ricerca Corrente) and Equipe FRM DEQ20140329534. F. Bellora is recipient of a fellowship awarded by A.I.R.C. (special project 5×1000 9962).
References
- Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 2009; 27:289-97; PMID:19047291; http://dx.doi.org/10.1200/JCO.2008.16.6785
- Park JR, Eggert A, Caron H. Neuroblastoma: biology, prognosis, and treatment. Pediatr Clin North Am 2008; 55:97-120, x; PMID:18242317; http://dx.doi.org/10.1016/j.pcl.2007.10.014
- Cheung NK, Dyer MA. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer 2013; 13:397-411; PMID:23702928; http://dx.doi.org/10.1038/nrc3526
- Bottino C, Dondero A, Bellora F, Moretta L, Locatelli F, Pistoia V, Moretta A, Castriconi R. Natural Killer Cells and Neuroblastoma: tumor recognition, escape mechanisms and possible novel immunotherapeutic approaches. Front Immunol 2014; 5:56; PMID:24575100; http://dx.doi.org/10.3389/fimmu.2014.0005
- Castriconi R, Dondero A, Cilli M, Ognio E, Pezzolo A, De Giovanni B, Gambini C, Pistoia V, Moretta L, Moretta A et al. Human NK cell infusions prolong survival of metastatic human neuroblastoma-bearing NOD/scid mice. Cancer Immunol Immunother 2007; 56:1733-42; PMID:17426969; http://dx.doi.org/10.1007/s00262-007-0317-0
- Valteau-Couanet D, Leboulaire C, Maincent K, Tournier M, Hartmann O, Benard J, Beaujean F, Boccaccio C, Zitvogel L, Angevin E. Dendritic cells for NK/LAK activation: rationale for multicellular immunotherapy in neuroblastoma patients. Blood 2002; 100:2554-61; PMID:12239169; http://dx.doi.org/10.1182/blood.V100.7.2554
- Falco M, Moretta L, Moretta A, Bottino C. KIR and KIR ligand polymorphism: a new area for clinical applications? Tissue Antigens 2013; 82:363-73; PMID:24498992; http://dx.doi.org/10.1111/tan.12262
- Bottino C, Castriconi R, Moretta L, Moretta A. Cellular ligands of activating NK receptors. Trends Immunol 2005; 26:221-6; PMID:15797513; http://dx.doi.org/10.1016/j.it.2005.02.007
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331:44-9; PMID:21212348; http://dx.doi.org/10.1126/science.1198687
- Castriconi R, Dondero A, Corrias MV, Lanino E, Pende D, Moretta L, Bottino C, Moretta A. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res 2004; 64:9180-4; PMID:15604290; http://dx.doi.org/10.1158/0008-5472.CAN-04-2682
- Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa AR, Negri F, Conte R, Corrias MV, Moretta L et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A 2004; 101:12640-5; PMID:15314238; http://dx.doi.org/10.1073/pnas.0405025101
- Lemke D, Pfenning PN, Sahm F, Klein AC, Kempf T, Warnken U, Schnölzer M, Tudoran R, Weller M, Platten M et al. Costimulatory protein 4IgB7H3 drives the malignant phenotype of glioblastoma by mediating immune escape and invasiveness. Clin Cancer Res 2012; 18:105-17; PMID:22080438; http://dx.doi.org/10.1158/1078-0432.CCR-11-0880
- Yuan H, Wei X, Zhang G, Li C, Zhang X, Hou J. B7-H3 over expression in prostate cancer promotes tumor cell progression. J Urol 2011; 186:1093-9; PMID:21784485; http://dx.doi.org/10.1016/j.juro.2011.04.103
- Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Schnölzer M, Tudoran R, Weller M, Platten M et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol 2003; 4:899-906; PMID:12925852; http://dx.doi.org/10.1038/ni967
- Hu Y, Lv X, Wu Y, Xu J, Wang L, Chen W, Li J, Zhang S, Qiu H. Expression of costimulatory molecule B7-H3 and its prognostic implications in human acute leukemia. Hematology 2014; 20(4):187-95; PMID:25130683; http://dx.doi.org/10.1179/1607845414Y.0000000186
- Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res 2007; 67:7893-900; PMID:17686830; http://dx.doi.org/10.1158/0008-5472.CAN-07-1068
- Gregorio A, Corrias MV, Castriconi R, Dondero A, Mosconi M, Gambini C, Moretta A, Moretta L, Bottino C. Small round blue cell tumours: diagnostic and prognostic usefulness of the expression of B7-H3 surface molecule. Histopathology 2008; 53:73-80; PMID:18613926; http://dx.doi.org/10.1111/j.1365-2559.2008.03070.x
- Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, Thompson RH, Boorjian SA, Dong H, Leibovich BC et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res 2008; 14:5150-7; PMID:18694993; http://dx.doi.org/10.1158/1078-0432.CCR-08-0536
- Sun TW, Gao Q, Qiu SJ, Zhou J, Wang XY, Yi Y, Shi JY, Xu YF, Shi YH, Song K et al. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunol Immunother 2012; 61:2171-82; PMID:22729558; http://dx.doi.org/10.1007/s00262-012-1278-5
- Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, Scardino PT, Sharma P, Allison JP. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A 2007; 104:19458-63; PMID:18042703; http://dx.doi.org/10.1073/pnas.0709802104
- Wang J, Chong KK, Nakamura Y, Nguyen L, Huang SK, Kuo C, Zhang W, Yu H, Morton DL, Hoon DS. B7-H3 associated with tumor progression and epigenetic regulatory activity in cutaneous melanoma. J Invest Dermatol 2013; 133:2050-8; PMID:23474948; http://dx.doi.org/10.1038/jid.2013.114
- Wang L, Zhang Q, Chen W, Shan B, Ding Y, Zhang G, Cao N, Liu L, Zhang Y. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PloS One 2013; 8:e70689; PMID:23940627; http://dx.doi.org/10.1371/journal.pone.0070689
- Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, Burke S, Ciccarone V, Li H, Yang Y et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res 2012; 18:3834-45; PMID:22615450; http://dx.doi.org/10.1158/1078-0432.CCR-12-0715
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:22437870; http://dx.doi.org/10.1038/nrc3239
- Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: what's here, what's next? Curr Opin Immunol 2015; 33C:23-35; http://dx.doi.org/10.1016/j.coi.2015.01.006
- Benson DM, Jr., Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010; 116:2286-94; PMID:20460501; http://dx.doi.org/10.1182/blood-2010-02-271874
- Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N, Viaud S, Ryffel B, Yagita H, Kaplanski G et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res 2011; 71:5393-9; PMID:21724589; http://dx.doi.org/10.1158/0008-5472.CAN-11-0993
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192:1027-34; PMID:11015443; http://dx.doi.org/10.1084/jem.192.7.1027
- Iwasaki M, Tanaka Y, Kobayashi H, Murata-Hirai K, Miyabe H, Sugie T, Toi M, Minato N. Expression and function of PD-1 in human gammadelta T cells that recognize phosphoantigens. Eur J Immunol 2011; 41:345-55; PMID:21268005; http://dx.doi.org/10.1002/eji.201040959
- Ghiotto M, Gauthier L, Serriari N, Pastor S, Truneh A, Nunes JA, Olive D. PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1. Int Immunol 2010; 22:651-60; PMID:20587542; http://dx.doi.org/10.1093/intimm/dxq049
- Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 2012; 1:1223-5; PMID:23243584; http://dx.doi.org/10.4161/onci.21335
- Wilke CM, Wei S, Wang L, Kryczek I, Kao J, Zou W. Dual biological effects of the cytokines interleukin-10 and interferon-gamma. Cancer Immunol Immunother 2011; 60:1529-41; PMID:21918895; http://dx.doi.org/10.1007/s00262-011-1104-5
- Raffaghello L, Prigione I, Airoldi I, Camoriano M, Levreri I, Gambini C, Pende D, Steinle A, Ferrone S, Pistoia V. Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma. Neoplasia 2004; 6:558-68; PMID:15548365; http://dx.doi.org/10.1593/neo.04316
- Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, Humm JL, Xu H, Wolden SL, Souweidane MM et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neuro Oncol 2010; 97:409-18; PMID:19890606; http://dx.doi.org/10.1007/s11060-009-0038-7
- Ritprajak P, Azuma M. Intrinsic and extrinsic control of expression of the immunoregulatory molecule PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncol 2015; 51:221-8; PMID:25500094; http://dx.doi.org/10.1016/j.oraloncology.2014.11.014
- Boes M, Meyer-Wentrup F. TLR3 triggering regulates PD-L1 (CD274) expression in human neuroblastoma cells. Cancer Lett 2015; 361:49-56; PMID:25697485; http://dx.doi.org/10.1016/j.canlet.2015.02.027
- Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007; 104:3360-5; PMID:17360651; http://dx.doi.org/10.1073/pnas.0611533104
- Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010; 116:1757-66; PMID:20143437; http://dx.doi.org/10.1002/cncr.24899
- Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005; 11:2947-53; PMID:15837746; http://dx.doi.org/10.1158/1078-0432.CCR-04-1469
- Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res 2007; 13:709s-15s; PMID:17255298; http://dx.doi.org/10.1158/1078-0432.CCR-06-1868
- Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 2006; 108:19-24; PMID:16530813; http://dx.doi.org/10.1016/j.acthis.2006.01.003
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37; PMID:22461641; http://dx.doi.org/10.1126/scitranslmed.3003689
- Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Ann Rev Pathol 2014; 9:287-314; PMID:24079833; http://dx.doi.org/10.1146/annurev-pathol-012513-104715
- Gong AY, Zhou R, Hu G, Li X, Splinter PL, O'Hara SP, LaRusso NF, Soukup GA, Dong H, Chen XM. MicroRNA-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in cholangiocytes. J Immunol 2009; 182:1325-33; PMID:19155478; http://dx.doi.org/10.4049/jimmunol.182.3.1325
- Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 2013; 73:128-38; PMID:23135914; http://dx.doi.org/10.1158/0008-5472.CAN-12-2606
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annual Rev Immunol 2008; 26:677-704; PMID:18173375; http://dx.doi.org/10.1146/annurev.immunol.26.02-1607.090331
- Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J 2014; 20:119-22; http://dx.doi.org/10.1097/PPO.00000-00000000035
- Nishio N, Diaconu I, Liu H, Cerullo V, Caruana I, Hoyos V, Bouchier-Hayes L, Savoldo B, Dotti G. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res 2014; 74:5195-205; PMID:25060519; http://dx.doi.org/10.1158/0008-5472.CAN-14-0697
- Singh N, Liu X, Hulitt J, Jiang S, June CH, Grupp SA, Barrett DM, Zhao Y. Nature of tumor control by permanently and transiently modified GD2 chimeric antigen receptor T cells in xenograft models of neuroblastoma. Cancer Immunol Res 2014; 2:1059-70; PMID:25104548; http://dx.doi.org/10.1158/2326-6066.CIR-14-0051
- Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011; 118:6050-6; PMID:21984804; http://dx.doi.org/10.1182/blood-2011-05-354449
- Balkhi MY, Ma Q, Ahmad S, Junghans RP. T cell exhaustion and Interleukin 2 downregulation. Cytokine 2015; 71:339-47; PMID:25516298; http://dx.doi.org/10.1016/j.cyto.2014.11.024
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014; 20:5064-74; PMID:24714771; http://dx.doi.org/10.1158/1078-0432.CCR-13-3271
- Di Carlo E, Bocca P, Emionite L, Cilli M, Cipollone G, Morandi F, Raffaghello L, Pistoia V, Prigione I. Mechanisms of the antitumor activity of human Vgamma9Vdelta2 T cells in combination with zoledronic acid in a preclinical model of neuroblastoma. Mol Ther 2013; 21:1034-43; PMID:23481325; http://dx.doi.org/10.1038/mt.2013.38
- Airoldi I, Bertaina A, Prigione I, Zorzoli A, Pagliara D, Cocco C, Meazza R, Loiacono F, Lucarelli B, Bernardo ME, et al. gammadelta T cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-alphabeta+/CD19+ lymphocytes. Blood 2015; 125(15):2349-58; PMID:25612623; http://dx.doi.org/10.1182/blood-2014-09-599423
- Corrias MV, Scaruffi P, Occhino M, De Bernardi B, Tonini GP, Pistoia V. Expression of MAGE-1, MAGE-3 and MART-1 genes in neuroblastoma. Int J Cancer 1996; 69:403-7; PMID:8900375; http://dx.doi.org/10.1002/(SICI)1097-0215(19961021)69:5%3c403::AID-IJC9%3e3.0.CO;2-9
- Pastorino F, Loi M, Sapra P, Becherini P, Cilli M, Emionite L, Ribatti D, Greenberger LM, Horak ID, Ponzoni M. Tumor regression and curability of preclinical neuroblastoma models by PEGylated SN38 (EZN-2208), a novel topoisomerase I inhibitor. Clin Cancer Res 2010; 16:4809-21; PMID:20702613; http://dx.doi.org/10.1158/1078-0432.CCR-10-1354
- Molenaar JJ, Koster J, Zwijnenburg DA, van Sluis P, Valentijn LJ, van der Ploeg I, Hamdi M, van Nes J, Westerman BA, van Arkel J et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 2012; 483:589-93; PMID:22367537; http://dx.doi.org/10.1038/nature10910
