ABSTRACT
Dendritic cell (DC) vaccines were generated by apoptotic squamous cell carcinoma (SCC) cells induced by 5-aminolevulinic acid (ALA)-mediated photodynamic therapy (PDT). ALA-PDT-DC vaccine inhibited the growth of SCC in mice, indicating that immunogenic apoptotic cells can activate an effective antitumor adaptive immunity and lead to a DC vaccine-based cancer immunotherapy.
Auto-commentary
Failure of the host immune system for cancer immunosurveillance is recognized as one of the key factors for both cancer occurrence and metastasis.Citation1 Ideal anticancer therapies are expected to not only kill tumor cells directly, but also induce systemic antitumor immunity. The majority of current anticancer regimens mediate killing of the target cells by activating apoptosis.Citation2 In a ‘classical’ sense, apoptosis is often considered to be an immunologically ‘silent’ or even an immunosuppressive cell death process.Citation3,4 However, recent studies indicated that some cancer therapies, such as chemotherapy, radiotherapy, and hypericin-mediated PDT, could lead to apoptosis in an immunogenic fashion and these dying tumor cells often release or expose damage-associated molecule patterns (DAMPs) as ‘immunogenic signals’, inducing an effective antitumor immune response.Citation4,5
To induce effective antitumor immune responses, killed cells must be distinguished from normal cell death processes, and recognized as the ‘altered selves’ by the immune cells that provide innate immunity. DCs are the major link between the innate and adaptive immune systems, considered as the most professional antigen-presenting cells (APCs), since they are crucial in the uptake, transport, processing, and presentation of antigens to T cells, leading to induction of tumor-specific immune responses.Citation6,7 It was recently reported that DC-based vaccines obtained through stimulation of DCs by ex vivo prepared tumor antigens enhanced therapeutic antitumor immune responses.
Topical ALA-mediated PDT, ALA-PDT, is a novel therapeutic modality widely used to treat actinic keratosis, Bowen's Disease, superficial skin SCC, and other cancerous and precancerous skin diseases with the advantages of minimal invasiveness, great aesthetic outcomes, low morbidity, minimal functional disturbance, and high-level tolerance.Citation8
We studied the effect of ALA-PDT on host immune system.Citation9 ALA-PDT induced apoptosis, inhibited SCC growth, and reduced tumor volume. The numbers of DCs, CD4+ and CD8+ T cells distributed in the tumor tissues increased gradually after ALA-PDT. In addition, there was a marked increase in TNF-α expression after treatment.Citation9
We further investigated the effect of ALA-PDT-induced apoptotic tumor cells on potentiating maturation of DCs.Citation10 We observed morphology maturation of DCs potentiated by ALA-PDT-treated apoptotic tumor cells, including enlargement of dendrites and increase of lysosomes. These changes made DCs capable of capturing and processing tumor antigens. Moreover, ALA-PDT-treated apoptotic tumor cells induced phenotypic maturation of DCs, including upregulation of MHC class II and co-stimulatory CD80 and CD86 molecules. The surface expression of these molecules was higher than that induced by freeze-thawed tumor cells. We also evaluated the pattern of certain cytokines including the secretion of IFNγ, IL-12, and IL-10 and found that DCs exposed to ALA-PDT-treated SCC cells displayed a distinguished pattern of functional activation characterized by IFNγhigh, IL-12high, and IL-10absent. Interestingly, LPS and especially freeze-thawed cells stimulated the production of IL-10. Our study found that PDT-induced apoptotic tumor cells were more effective in potentiating functional maturation of DCs (enhanced capability to secret IFNγ and IL-12 and to induce T cell proliferation) than PDT-treated or freeze/thaw-treated necrotic tumor cells, consistent with previous reports by other researchers.
We also developed a DC vaccine using ALA-PDT-treated apoptotic cells and used the DC vaccine against SCC tumors in mice.Citation10 The observed protection against tumor growth by the DC vaccine at the challenge site showed successful priming of the adaptive immune system. In contrast, although freeze-thawed SCC cells were able to activate DCs to express IFNγ and IL-12, which are critical to the development of a cellular immune response, they failed to generate an effective DC vaccine to resist tumor challenge.Citation10 This may be due to the fact that F/T-DCs simulated production of IL-10, which suppresses immune responses.
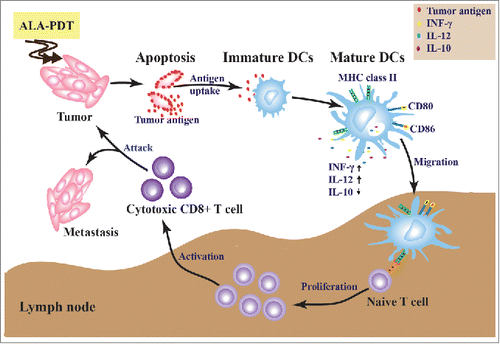
Our findings indicated a strong antitumor immunity induced by the ALA-PDT-DC vaccine, stimulated by immunogenic apoptotic cancer cells. Its mechanism, using ALA-PDT-treated apoptotic cells as sources of tumor antigens, is shown in . ALA-PDT-induced immunogenic tumor cells stimulated the maturations of DCs, including morphology maturation (enlargement of dendrites and increase of lysosomes), phenotypic maturation (upregulation of surface expression of MHC-II, DC80, and CD86), and functional maturation (enhanced capability to secrete IFNγ and IL-12, and to induce T cell proliferation and activation). The mature DCs worked as tumor vaccines to prevent tumor growth.
Figure 1. Antitumor immune responses induced by ALA-PDT-treated immunogenic apoptotic cells. ALA-PDT-treated apoptotic cells work as sources of tumor antigens to stimulate the maturations of DCs. T cell immunity is initiated by the mature DCs, preventing tumor growth.
Our study may lead to an improved treatment modality against metastatic cancers.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
The authors thank Janet S. Clark for clerical and editorial assistance.
Funding
This work was supported by National Natural Science Foundation of China (81272990, 81472538, 81472796), the Key Project of Science and Technology of Shanghai (11ZR1432800), the Key Project of Shanghai Municipal Commission of Health and Family Planning (20124034), and by the US National Institutes of Health (R21 EB0155091–01).
References
- Chow MT, Möller A, Smyth MJ. Inflammation and immune surveillance in cancer. Semin Cancer Biol 2012; 22:23-32; PMID:22210181; http://dx.doi.org/10.1016/j.semcancer.2011.12.004
- Nicholson DW. From becch to clinic with apoptosis-based therapeutic agents. Nature 2000; 407:810-6; PMID:11048733; http://dx.doi.org/10.1038/35037747
- Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol 2009; 9:353-63; PMID:19365408; http://dx.doi.org/10.1038/nri2545
- Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010 Jan; 1805(1):53-71; PMID:19720113; http://dx.doi.org/10.1016/j.bbcan.2009.08.003
- Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012; 12(12):860-75; PMID:23151605; http://dx.doi.org/10.1038/nrc3380
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12(4):265-77; PMID:22437871; http://dx.doi.org/10.1038/nrc3258
- Ma Y, Shurin GV, Peiyuan Z, Shurin MR. Dendritic cells in the cancer microenvironment. J Cancer 2013; 4(1):36-44; PMID:23386903; http://dx.doi.org/10.7150/jca.5046
- Wang XL, Wang HW, Guo MX, Xu SZ. Treatment of skin cancer and pre-cancer using topical ALA-PDT - a single hospital experience. Photodiagnosis Photodyn Ther 2008; 5:127-33; PMID:19356643; http://dx.doi.org/10.1016/j.pdpdt.2008.05.003
- Wang HW, Li JJ, Lv T, Tu QF, Huang Z, Wang XL. Therapeutic and immune effects of 5-aminolevulinic acid photodynamic therapy on UVB-induced squamous cell carcinomas in hairless mice. Exp Dermatol 2013; 22(5):262-3; PMID:23528211; http://dx.doi.org/10.1111/exd.12132
- Ji J, Fan ZX, Zhou FF, Wang XJ, Shi L, Zhang HY, Wang PR, Yang DG, Zhang LL, Chen WR, Wang XL. Improvement of DC vaccine with ALA-PDT induced immunogenic apoptotic cells for skin squamous cell carcinoma. Oncotarget 2015 Jul 10; 6(19):17135-46; PMID:25915530.
