ABSTRACT
Toll-like receptor (TLR) agonists are potent immunostimulatory agents that have demonstrated great potential for cancer immunotherapy. We have genetically-engineered tumor-specific T cells to deliver and secrete the TLR5 ligand (TLR5L) flagellin to the tumor site to provide costimulation for antitumor immune activity. We found that TLR5L-secreting T cells offered a therapeutic benefit by altering several aspects including augmenting T cell effector function and expansion as well as reshaping the tumor microenvironment toward one that enhances antitumor T cell responses.
The field of cancer immunotherapy is rapidly evolving, resulting in the generation of novel approaches to stimulate anticancer immune responses. TLRs are pattern recognition receptors that play a critical role in activating the immune system. Due to their potent immunostimulatory capabilities, several TLR agonists are approved for the treatment of various malignancies with many others currently under clinical investigation.Citation1 The majority of studies regarding the role of TLR agonists as immune adjuvants focus on their ability to stimulate innate immune cells, such as dendritic cells, which in turn activate antitumor T cell responses. However, of particular interest to our group has been the ability of TLR agonists to directly costimulate cytotoxic T cell responses.
Studies from several groups have demonstrated that TLR engagement on activated T cells increases their cytolytic function, proliferation, survival, and is associated with enhanced antitumor activity.Citation2,3 However, the costimulatory effects on T cells depend on concurrent TLR and T cell receptor stimulation. To ensure that sufficient levels of TLR agonists reach the tumor site, TLR agonists need to be administered systemically at high doses, which can result in the excessive release of pro-inflammatory factors. Although intratumoral injection can enhance efficacy, this approach may not be feasible in patients with disseminated metastatic disease. Furthermore, TLRs can be expressed on many different cell types, including tumor cells, and TLR stimulation under certain conditions can lead to pro-tumorigenic effects.Citation4 The pleotropic effects of TLR stimulation on T cells and cancer cells was recently summarized.Citation5
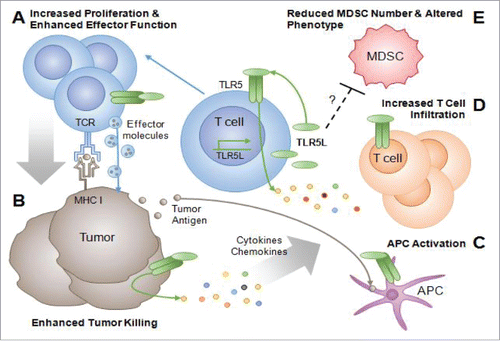
We tested the hypothesis that tumor-reactive T cells engineered to secrete the TLR5L flagellin would serve as a continuous source of T cell costimulation within the tumor and augment antitumor activity.Citation6 Flagellin was chosen because it is one of the only protein TLRLs that can be encoded by DNAs; most other TLR agonists are nucleic acids or molecular compounds that cannot be encoded on a gene vector. T cells engineered to secrete the TLR5L increased T cell proliferation, survival, and cytotoxicity of tumor cells in human and murine in vitro models (). TLR5L-secreting T cells delayed tumor growth in mice and improved survival relative to mice treated with control T cells. Increased antitumor activity in xenogeneic models was associated with elevated levels of several cytokines and chemokines (i.e., CX3CL1 and CCL22). Interestingly, TLR5 engagement on human melanoma cells also induced various chemokines, such as CCL2 and CXCL1-3, which we presume further prompted the influx of tumor-reactive T cells and antigen presenting cells (APC) (). Importantly, the therapeutic benefits of T cell-guided delivery of TLR5L extended beyond the local stimulation of T cells. Intratumoral delivery of the TLR5L by T cells reshaped the tumor environment toward one that reinvigorated antitumor immune responses. Mice treated with TLR5L–producing T cells were characterized by a reduction in the number of T cells expressing exhaustion markers as well as increased MHC I expression on tumor cells. Unexpectedly, we found that TLR5L also reduced the number of CD11b+Gr1high splenic and tumor myeloid-derived suppressor cells (MDSCs) (). Moreover, cells from the tumors of mice treated with TLR5L–producing T cells demonstrated an increase in the expression of MHC I, MHC II, and CD86, a phenotype associated with a more mature and less suppressive cell types. The reduction of CD11b+Gr1high cells was linked to reduced CXCL5 levels in the serum, a factor known to be involved in the generation and migration of MDSCs. These studies highlight for the first time the use of tumor-reactive T cells as delivery vehicles to provide a continuous source of a TLR agonist to reshape the tumor environment and enhance antitumor responses.
Figure 1. TLR5L–secreting T cells costimulate T cell responses and alter the tumor microenvironment. (A) Tumor reactive T cells migrate to the tumor where they secrete TLR5L, leading to increased T cell proliferation and survival. (B) TLR5-stimulated T cells exhibit increased tumor cell lysis. This process releases tumor antigens, making them available for uptake and presentation by APC. TLR5 engagement on APCs further potentiates their ability to cross-present antigen (C). Tumor cells themselves also respond to TLR5 engagement, releasing various cytokines and chemokines. The production of cytokines and chemokines by TLR-stimulated T cells and tumor cells results in the recruitment and activation of (C) APC and endogenous T cells to the tumor, which propagate antitumor immune responses (D). (E) TLR5L–secreting T cells reduce the number of CD11b+Gr1high MDSCs in tumor-bearing mice. Furthermore, intratumoral delivery of TLR5L by T cells results in the induction of MHC I, MHC II, and CD86 on CD11b+Gr1high cells, a phenotype associated with a more mature and less T cell suppressive cell types. Whether the changes in MDSC numbers or phenotype occur via TLR5 engagement or occur as a result of other factors induced by TLR5L are unknown.
The use of TLR5 agonists in cancer immunotherapy has also been emphasized by other groups. Leigh et al. developed an optimized TLR5 agonist, CBLB502, for the treatment of lymphoma in pre-clinical models.Citation7 They demonstrated that the antitumor effects were mediated by natural killer (NK) and CD8+ T cells and were dependent on perforin. In their model, the TLR5L acted upon CD11b+ CD11c+ APC which stimulated T cell antitumor immunity. Garaude et al. reported that tumor cells engineered to express flagellin functioned as a potent vaccine capable of controlling EL4 thymoma and B16 melanoma tumors in mice.Citation8 In addition to signaling through TLR5, Garaude demonstrated that flagellin contributed to antitumor responses by activating another class of pattern recognition receptors, NOD-like receptors (NLRs). While we attribute the antitumor activity of TLR5L to stimulating TLR5 on various cell types, we consider that the costimulatory effects of TLR5L may also involve engagement of NLRs on APC.
Exploiting the tumor-trafficking capabilities of T cells to selectively deliver drugs to tumors is a promising approach for localizing drugs to tumor sites. In addition to TLR agonists, T cells could be engineered to express a wide array of immune mediators to reshape the tumor microenvironment. Recent advances would allow for tighter control of gene expression by genetically engineering proteins under the control of T cell activation-specific promoters, such as NFAT.Citation9 Clinical applications could include the engineering of T cells to co-express both the TLRL (or other immune modulator) along with tumor-specific T cell receptors, chimeric antigen receptors, or in combination with systemic immunotherapies such as immune checkpoint blockade. These studies, along with other recently published data, support the development of T cells as delivery vehicles to boost antitumor immune responses.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by the NIH National Cancer Institute (NCI) R01CA140917, the University of Maryland Marlene and Stewart Greenebaum Cancer Center and National Institute of Allergy and Infectious Diseases (NIAID) T32AI007540.
References
- Vacchelli E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: toll-like receptor agonists for cancer therapy. Oncoimmunology 2013; 2:e25238; PMID:24083080; http://dx.doi.org/10.4161/onci.25238
- Geng D, Zheng L, Srivastava R, Velasco-Gonzalez C, Riker A, Markovic SN, Davila E. Amplifying TLR-MyD88 signals within tumor-specific T cells enhances antitumor activity to suboptimal levels of weakly immunogenic tumor antigens. Cancer Res 2010; 70:7442-54; PMID:20807806; http://dx.doi.org/10.1158/0008-5472.CAN-10-0247
- Xiao H, Peng Y, Hong Y, Huang L, Guo ZS, Bartlett DL, Fu N, Munn DH, Mellor A, He Y. Local administration of TLR ligands rescues the function of tumor-infiltrating CD8 T cells and enhances the antitumor effect of lentivector immunization. J Immunol 2013; 190:5866-73; PMID:23610140; http://dx.doi.org/10.4049/jimmunol.1203470
- Lu H. TLR agonists for cancer immunotherapy: tipping the balance between the immune stimulatory and inhibitory effects. Front Immunol 2014; 5:83; PMID:24624132; http://dx.doi.org/10.3389/fimmu.2014.00083
- Kaczanowska S, Joseph AM, Davila E. TLR agonists: our best frenemy in cancer immunotherapy. J Leukoc Biol 2013; 93:847-63; PMID:23475577; http://dx.doi.org/10.1189/jlb.1012501
- Geng D, Kaczanowska S, Tsai A, Younger K, Ochoa A, Rapoport AP, Ostrand-Rosenberg S, Davila E. TLR5 ligand-secreting T cells reshape the tumor microenvironment and enhance antitumor activity. Cancer Res 2015; 75:1959-71; PMID:25795705; http://dx.doi.org/10.1158/0008-5472
- Leigh ND, Bian G, Ding X, Liu H, Aygun-Sunar S, Burdelya LG, Gudkov AV, Cao X. A flagellin-derived toll-like receptor 5 agonist stimulates cytotoxic lymphocyte-mediated tumor immunity. PLoS One 2014; 9:e85587; PMID:24454895; http://dx.doi.org/10.1371/journal.pone.0085587
- Garaude J, Kent A, van RN, Blander JM. Simultaneous targeting of toll- and nod-like receptors induces effective tumor-specific immune responses. Sci Transl Med 2012; 4:120ra16; PMID:22323829; http://dx.doi.org/10.1126/scitranslmed.3002868
- Zhang L, Morgan RA, Beane JD, Zheng Z, Dudley ME, Kassim SH, Nahvi AV, Ngo LT, Sherry RM, Phan GQ et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin Cancer Res 2015; 21:2278-88; PMID:25695689; http://dx.doi.org/10.1158/1078-0432.CCR-14-2085
