ABSTRACT
Poly(I:C) acts on dendritic cells to induce potent antitumor effects through the production of cytokines/interferons, activation of natural killer cells and proliferation of cytotoxic T lymphocytes. In some tumor or myeloid lineages, poly(I:C) seemed to induce necroptosis in concert with a pan-caspase inhibitor by directly acting on toll-like receptor (TLR) 3 in both in vivo and in vitro models.
Introduction
RNA governs many basic functions for maintaining life. It is crucial for transcribing and translating genetic information. It plays a fundamental biological role in the nucleus and cytoplasm. Double-stranded (ds) or stem-structured RNA derived from virus-infected cells or exosomes have been shown to release and act on neighboring cells. RNA can be an intercellular mediator that modulates cellular functions in an extrinsic fashion.Citation1 Furthermore, cytoplasmic RNA sensors, such as the retinoic acid-inducible gene I and melanoma differentiation-associated protein 5 (MDA5), mediate viral RNA recognition in cells. Hence, mammalian myeloid cells and most tumor cells express TLR3 and cytoplasmic RNA sensors for detecting abnormal structured RNA.Citation1
Macrophage cell death has been found to occur through RNA sensor signaling as in the case of tumor necrosis factor (TNF)-α or death receptors.Citation2 The receptor interacting protein (RIP)1/RIP3 pathway participates in evoking cell death signaling. Apoptosis or necroptosis occurs depending on the presence or absence of caspase-8 activity, respectively.Citation2 During viral infection, bystander cell death may reflect not only simple direct infection but also the action of the mediators involved in releasing RNA. It has been reported that RNA pattern-sensing in dendritic cells (DCs) results in the activation of the immune system, including T and natural killer (NK) cells.Citation3,4 Here, we summarize the role of cellular RNA release with respect to tumor cell death and tumor-shrinkage as shown in an in vivo study by Takemura and collaborators.Citation3
RNA-mediated tumor necroptosis occurs in immunocompromised mice
RNA-dependent cell death can be assessed by the exogenous addition of poly(I:C), which is a synthetic dsRNA-mimic. We examined the RNA-dependent cell death of mouse tumor cell lines in the initial study and the following conclusions were obtained in vivo using RNA-sensitive tumor-implanted mice.Citation3 Mouse colon carcinoma CT26 cells expressed high level of the TLR3 protein and produced type I interferon (IFN) in a TLR3/TIR domain-containing adaptor molecule 1 (TICAM1)-dependent manner in response to polyI:C. In the in vitro assay, cell death was induced in CT26 cells stimulated with zVAD, which is a pan-caspase inhibitor. Cell death was also induced by poly(I:C) via the production of TLR3/TICAM1-reactive oxygen species without production of TNF-α and IFNγ, which are inducers of necroptosis.Citation2 Cell death did not occur in cells with RIP3 knockdown or cells that were treated with necrostatin-1, which is a RIP1 inhibitor.Citation2 This indicated that poly(I:C)-induced cell death in CT26 cells was occurring from necroptosis. In the in vivo mouse model, using depletion antibodies showed that poly(I:C) induced regression of CT26 tumor growth mainly occurred through the actions of NK cells and cytotoxic T lymphocytes (CTLs). In the absence of NK cells and CTLs, zVAD enhanced the suppressive effect of poly(I:C) on tumor growth. Furthermore, zVAD and poly(I:C) together significantly decreased tumor volume in CT26 tumor-bearing Rag2−/−/Jak3−/− double knockout mice, which lack T, B and NK cells, without an increase in the production of TNF-α, IFN-β and IFNγ as compared with the poly(I:C) only animals. Expression of necroptosis indicators such as RIP1, RIP3 and mixed lineage kinase domain-like protein mRNA,Citation2 and TdT- mediated dUTP nickend labeling (TUNEL)-positive cells were increased among tumor cells isolated from poly(I:C) and zVAD-treated mice. These results suggest that poly(I:C) directly affects tumor cells, independently of effector cells and cytokines, and reduces tumor growth ().
Figure 1. PolyI:C induces tumor necroptosis via the TLR3 -TICAM1 pathway. When CT26 cells are treated with pan-caspase inhibitor, zVAD, the interaction of TICAM1 and RIP3 is enhanced in response to poly(I:C), leading to cell death by necroptosis. In the absence of immune cells, this necroptosis pathway contributes to tumor regression in vivo. Necroptosis is induced in macrophages stimulated with poly(I:C).Citation2 Poly(I:C) also acts on DCs and macrophages to elicit effective CTL/NK activation and modify tumor microenvironment, respectively, as previously reported.Citation4-6 The antitumor response of TLR3 appears to be cell type-specific. Notably, tumor-infiltrated macrophages harbor all the signal axes inducible by poly(I:C)/TLR3 as illustrated. The functional alteration of macrophages may be associated with epigenetic status of the cells in inflammatory environment.
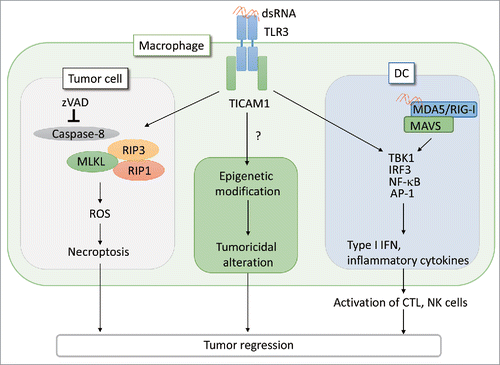
Injection of poly(I:C) robustly activates both the innate and acquired immunity of the host. This includes activation of DCs, macrophages, NK cells and induction of CTLs all of which lead to modification of the tumor microenvironment and inhibition of tumor growth.Citation1 The activation mechanisms of effector cells in response to poly(I:C)/TLR3 has been well studied by us and other groups ().Citation5,6 In addition, poly(I:C) potently activates NK cells and CTLs via type I IFN production in stromal cells in a MDA5/mitochondrial antiviral-signaling protein (MAVS)-dependent manner.Citation1 CD8α+ DCs, which highly express TLR3, are activated in response to poly(I:C) injection to promote cross-priming of CTLs in a TLR3/TICAM1-dependent manner.Citation4,6 Poly(I:C) induces activation of NK cells via expression of IRF3-dependent NK-activating molecule on DCs.Citation5 Moreover, Poly(I:C) also acts on tumor-associated macrophages (TAM) and myeloid-derived suppressor cells (MDSC), which suppress the antitumor response to assist tumor growth.Citation7,8 Tumor-infiltrating TAMs rapidly produce TNF-α to induce necrosis in tumor cells in response to poly(I:C) injection.Citation7 MDSCs produce type I IFN to prime NK cells via the MDA5/MAVS pathway in stimulation with poly(I:C).Citation8 Thus, poly(I:C) not only activates host effector cells, which directly regress tumor cells, but it also modulates suppressor myeloid cells to support antitumor immunity resulting in the conversion of tumor supportive TAMs and MDSCs to antitumor effector cells.Citation7,8 The macrophage functional conversion presumably occurs secondary to epigenetic alteration due to TICAM-1 output (). The results from the Takemura's studyCitation3 points out the third effect of RNA on tumor microenvironment: direct action of RNA on tumor cell TLR3 causes tumor necroptosis and shrinkage independently of the immune effectors or tumor-infiltrating cells.
In many cases, the TLR3 response of tumor cells does not induce necroptosis by RIP1/RIP3 signaling. This is because in most tumors the caspase is functioning properly. If DCs, immune activation is executed by RNA recognition and tumor mass is reduced by immune cytolysis to mask necroptosis-mediated tumor shrinkage. Hence, RNA/TLR3-dependent tumor regression might be observable when the immune system functions incompletely in patients.
In general, tumor immunity and progression induced by the tumor microenvironment and inflammatory conditions define the status of tumor growth,Citation9,10 which involves epigenetic alteration of the cells. The finding that poly(I:C) induces obvious tumor regression in immunocompromised mice suggests that the release of exosomes or tumor RNAs triggers a mediator that acts on the tumor extracellularly. If the immune system is fully functional, this type of tumor cell death and shrinkage cannot be detected. The RNA-mediated tumor necroptosis constitutes a defense system as a backup for antitumor immunity, which was revealed in the study using immunocompromised mice.Citation3
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Seya T, Shime H, Takaki H, Azuma M, Oshiumi H, Matsumoto M. TLR3/TICAM-1 signaling in tumor cell RIP3-dependent necroptosis. Oncoimmunology 2012; 1:917-23; PMID:23162759; https://doi.org/10.4161/onci.21244
- Jouan-Lanhouet S, Riquet F, Duprez L, Vanden Berghe T, Takahashi N, Vandenabeele P. Necroptosis, in vivo detection in experimental disease models. Semin Cell Dev Biol 2014; 35:2-13; PMID:25160988; https://doi.org/10.1016/j.semcdb.2014.08.010
- Takemura R, Takaki H, Okada S, Shime H, Akazawa T, Oshiumi H, Matsumoto M, Teshima T, Seya T. PolyI:C-induced, TLR3/RIP3-dependent necroptosis backs up immune effector-mediated tumor elimination in vivo. Cancer Immunol Res 2015; 3(8):902-14.PMID:25898986; https://doi.org/10.1158/2326-6066.CIR-14-0219.
- Azuma M, Ebihara T, Oshiumi H, Matsumoto M, Seya T. Cross-priming for antitumor CTL induced by soluble Ag + polyI:C depends on the TICAM-1 pathway in mouse CD11c(+)/CD8α(+) dendritic cells. Oncoimmunology 2012; 1:581-92; PMID:22934250; https://doi.org/10.4161/onci.19893
- Ebihara T, Azuma M, Oshiumi H, Kasamatsu J, Iwabuchi K, Matsumoto K, Saito H, Taniguchi T, Matsumoto M, Seya T. Identification of a polyI:C-inducible membrane protein that participates in dendritic cell-mediated natural killer cell activation. J Exp Med 2010; 207:2675-87; PMID:21059856; https://doi.org/10.1084/jem.20091573
- Azuma M, Takeda Y, Nakajima H, Sugiyama H, Ebihara T, Oshiumi H, Matsumoto M, Seya T. TLR3-derived IL-12 induction by BATF3 fundamentally supports to drive Ag-specific antitumor T cell responses in CD8a+ dendritic cells. Cancer Res 2015; (in press)
- Shime H, Matsumoto M, Oshiumi H, Tanaka S, Nakane A, Iwakura Y, Tahara H, Inoue N, Seya T. Toll-like receptor 3 signaling converts tumor-supporting myeloid cells to tumoricidal effectors. Proc Natl Acad Sci U S A 2012; 109:2066-71; PMID:22308357; https://doi.org/10.1073/pnas.1113099109
- Shime H, Kojima A, Maruyama A, Saito Y, Oshiumi H, Matsumoto M, Seya T. Myeloid-derived suppressor cells confer tumor-suppressive functions on natural killer cells via polyinosinic: polycytidylic acid treatment in mouse tumor models. J Innate Immun 2014; 6:293-305; PMID:24192491; https://doi.org/10.1159/000355126
- Galluzzi L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial Watch: Experimental Toll-like receptor agonists for cancer therapy. Oncoimmunology 2012; 1:699-716; PMID:22934262; https://doi.org/10.4161/onci.20696
- Matsumoto M, Tatematsu M, Nishikawa F, Azuma M, Ishii N, Morii-Sakai A, Shime H, Seya T. Defined TLR3-specific adjuvant that induces NK and CTL activation without significant cytokine production in vivo. Nat Commun 2015; 6:6280; PMID:25692975; https://doi.org/10.1038/ncomms7280
