ABSTRACT
We recently reported that in multiple myeloma increased Th22 cell frequencies correlate with poor prognosis. Here we show that within the same patients' cohort Th17 cells associate with bone disease and not with prognosis. Thus, we propose that Th22 and Th17 cells play non-redundant roles in multiple myeloma and constitute independent therapeutic targets.
Abbreviations
| BM | = | bone marrow |
| IL | = | interleukin |
| ISS | = | International Staging System |
| MGUS | = | monoclonal gammopathy of undetermined significance |
| MM | = | multiple myeloma |
| MSC | = | mesenchymal stromal cell |
| OPG | = | osteoprotegerin |
| PB | = | peripheral blood |
| RANKL | = | receptor activator of nuclear factor kappa-B ligand |
| SMM | = | smoldering multiple myeloma |
| TGF | = | transforming growth factor |
| Th | = | T helper |
| TNF | = | tumor necrosis factor |
| Treg | = | T regulatory |
Multiple myeloma (MM) is a clonal plasma cell disorder that is the second most common hematological malignancy and still remains an incurable disease with poor survival.Citation1 In most cases, it arises from monoclonal gammopathy of undetermined significance (MGUS) or smoldering MM (SMM). MGUS and SMM are characterized by the presence of M-protein and bone marrow (BM) plasmacytosis but lack the distinctive clinical manifestations of full-blown MM, including hypercalcemia, renal insufficiency, anemia, and bone disease.Citation1
The causes of MM remain largely unknown.Citation2 In addition to the genetic alterations, the biology and progression of MM are strictly dependent on its microenvironment (i.e., the BM). Cross-talks among tumor cells, resident accessory cells and leukocytes within the BM create redundant mechanisms at the basis of tumor growth, osteolysis, neoangiogenesis, multi-drug resistance, relapse and immune evasion.Citation2
MM patients at diagnosis are generally responsive to therapy.Citation1 However, despite recent improvements in disease management, most patients eventually relapse and become refractory to treatment, due to both changes in the tumor biology and development of aggressive, drug-resistant phenotypes. A major advance in the definition of prognostic markers in MM was achieved with the introduction of the International Staging System (ISS), which combines serum β2-microglobulin and albumin levels to produce at diagnosis 3 risk groups (stages I, II and III) with a median overall survival of 62, 45 and 29 months, respectively.Citation3 This classification is still the best index used in the clinical practice to stratify patients according to their prognosis.
The profile of cytokines released by tumor-infiltrating CD4+ T helper (Th) cells has been shown to impact on cancer prognosis with opposite anti-tumor or pro-tumor effects, depending on the distinctive Th cell subset.Citation4 Th1 cells promote tumor rejection through secretion of interferon (IFN)-γ, Th2 cells primarily favor tumor progression through secretion of interleukin (IL)-4, IL-5 and IL-13, while the role Th17 cells that secrete IL-17 and IL-22 is more debated and appears to be tumor-dependent.Citation4 More recently, an additional Th cell subset (i.e., Th22 cells), whose characteristics are discussed below, has been studied in several tumor models,Citation5 among which MM,Citation6 collectively suggesting a correlation with poor prognosis and tumor-promoting functions.
As Th17 and Th22 cells share common and distinct features and have been both implicated in MM, here we will focus on these two Th cell subsets by describing their general common and distinctive features and by discussing their role in MM clinical correlates within the same cohort of MM patients.
Th22 and Th17 cells: common and distinct features
Th22 cells have been originally identified as a new Th cell subset associated to skin disorders and characterized by the secretion of IL-22 in the absence of IL-17.Citation7,8 Th22 cells are distinct from Th1, Th2 and Th17 cellsCitation7 and co-produce IL-13 and tumor necrosis factor (TNF)-α.Citation8 Th22 and Th17 cells share similarities, but they are distinguished by a number of important differences as well. Similar to Th17 cells, Th22 cells express IL-22, CCR4 and CCR6 but in contrast they also express CCR10.Citation7,8 In addition, Th22 cells do not express the Th17 markers IL-23R and CD161 and they do not secrete IL-17, IL-17F, IL-26 and CCL20.Citation9 CD4+ T cells differentiate into Th22 cells in the presence of IL-6 and TNF-α, whereas Th17 polarization in humans requires IL-1β, IL-6, IL-21, and IL-23 and, to a minor extent, tumor growth factor (TGF)-β; this latter cytokine inhibits Th22 differentiation.Citation7 Th17 differentiation is transcriptionally controlled by RORγt, RORα, AHR and IRF4. AHR is also a master regulator of Th22 differentiation,Citation7 although additional distinctive intracellular molecules for this subset are still being investigated.
From a functional point of view, both Th17 and Th22 subsets are involved in mucosal immunity, inflammation and autoimmune disorders.Citation10 Th22 cells are recruited to surface barriers where they contribute to host defense against microbial pathogens, promote wound healing and favor tissue remodeling.Citation11 Th17 cells take part in responses against specific fungi and extracellular bacteria.Citation9 In addition, Th22 and Th17 cytokines may cause pathogenic effects. Th22 cells are mostly involved in inflammatory skin disorders, like psoriasis, atopic eczema and allergic contact dermatitis.Citation12 Th17 cells promote autoimmune diseases, including rheumatoid arthritis, multiple sclerosis, inflammatory bowel disorders and psoriasis as well.Citation13 Moreover, as reported above, Th17 and, more recently, Th22 cells have been studied in the context of several tumors.Citation5,14
Th17 cells in MM
In the last years, several groups have investigated the role of Th17 cells in MM, where the peculiar BM cytokine milieu (e.g., IL-6, TGF-β, IL-1β, IL-23) can favor Th17 differentiation and/or expansion. The profile of cytokines released (i.e., IL-17 alone or in association with IFN-γ in the case of poly-functional Th17-Th1 cells) has been linked to one or more aspects of MM pathology, such as increase in osteoclastogenesis, promotion of MM cell growth and inhibition of immune function.Citation15-17 A pro-osteoclastogenic activity of Th17 cells had been first described in the context of autoimmune arthritis,Citation18 where arthritogenic Th17 cells are recruited to the inflamed tissues through a CCL20-CCR6 axis.Citation19 A similar mechanism of bone erosion has been hypothesized in MM, where IL-17 mediates up-regulation by BM cells of the expression of the receptor activator of NF-κB ligand (RANKL), which is a differentiation factor for osteoclasts and whose natural soluble antagonist is osteoprotegerin (OPG).Citation17 Interestingly and in agreement with the arthritis model,Citation19 MM patients with but not without osteolytic lesions showed significantly higher levels of BM plasma CCL20 and CCL20 expression by osteoblasts.Citation20
Bone disease is a prevalent cause of morbidity in MM affecting more than 80% of patients with clinical manifestations comprising bone pain, pathologic fractures, spinal cord compression and hypercalcemia.Citation1 The increase in bone catabolism results from imbalanced osteoclast and osteoblast activity that is mediated by factors released upon the interaction between MM cells and BM-mesenchymal stromal cells (MSCs) and other cell types in the BM microenvironment, some of which stimulate osteoclast formation (e.g., RANKL, IL-6, IL-1, MIP-1α, SDF-1α) and some other inhibit osteoblast activity (e.g., DKK-1, TGF- β, IL-3, HGF).Citation21,22 In agreement with a role for IL-17 in bone disease in MM, immune cells, among which Th17 cells, have been shown to influence bone homeostasis in many diseases by perturbing the RANKL and OPG levels as well as the osteoclast/osteoblast ratio.Citation23 Importantly, univariate analyses in MM patients with bone disease showed that factors mediating bone destruction and levels of cytokines that selectively induce and maintain the Th17 phenotype tightly correlated with the extent of bone disease.Citation17
The enhancement of osteoclastogenesis seems to be the most peculiar role of Th17 cells in MM. In addition to the correlation with the extent of bone disease,Citation17 it has been recently reported that primary immature dendritic cells in MM are prone to osteoclast-like trans-differentiation after IL-17 stimulation.Citation24 Other roles have been ascribed to IL-17, such as promotion of MM cell growth/survival, which is supported by the expression of the IL-17 receptor on MM cell lines and primary tumors,Citation15,25 and inhibition of immune cell functions.Citation15 However, no direct correlation between Th17 cell numbers and disease prognosis and/or severity has been provided. T regulatory cells (Tregs) are often studied in relation to Th17 cells, as their differentiation is mutually controlled, and the Treg/Th17 balance is considered to be a marker of immunoregulatory function.Citation10 A skew towards an increase of the Treg/Th17 cell ratio in MM has been associated with an immunosuppressive stateCitation26,27 and, accordingly, long-term survival in MM has been correlated with a favorable Treg/Th17 balance.Citation26,28
Th22 cells in MM
We recently addressed the frequency and function of Th22 cells in MM.Citation6 We found that Th22 (i.e., IL-22+IL-17−IL-13+) cells are significantly increased in peripheral blood (PB) and BM of ISS stage III and relapsed/refractory MM patients compared with healthy donors and patients with asymptomatic (i.e., MGUS and SMM) or ISS stage I/II disease. Importantly, the percentage of Th22 cells in ISS stage III MM patients is significantly higher compared with patients with ISS stage I/II, demonstrating that in MM increased frequency of Th22 cells associates with poor prognosis.Citation6 In support of our findings, increased IL-22 serum levels were also recently detected in MM patients with active disease.Citation29 Mechanistically, we demonstrated how the Th22 prototypical cytokines IL-22 and IL-13 exert both direct and indirect tumor-promoting functions in MM.Citation6 IL-22 directly increases tumor cell growth and resistance to drug-induced cell death on MM plasma cells by binding to its receptor IL22RA1, which is aberrantly expressed in a fraction of primary tumors. IL-13 augments adhesive molecule expression and IL-6 secretion by BM-MSCs, which are responsive to IL-13 as other cells of stromal/fibroblastic origin,Citation30 indirectly fostering MM cell proliferation. Interestingly, IL-13-driven up-regulation of adhesion molecules on BM-MSCs, by strengthening cell-cell interactions between MM and BM stromal cells, might be also involved in other mechanisms of MM progression, such as multi-drug resistance and immune evasion, leading the way to further studies.
Th17 and Th22 cells within the same cohort of MM patients
Data reported above point to an association between Th17 cells and bone disease and between Th22 cells and disease prognosis, respectively. To confirm the distinctive roles of Th22 and Th17 cells in MM, we took advantage of our previously described cohort of MM patientsCitation6 and compared the frequency of the two Th cell subsets in relation to the clinical status and the presence of bone lesions.
Thirty-seven MM patients, among whom 23 at diagnosis with different ISS stages and 14 with relapsed/refractory disease,Citation6 were stratified in two categories based on the absence (N) or the presence (Y) of bone disease, detected by one or more diagnostic techniques (i.e., X-ray, nuclear magnetic resonance, computerized tomography scan and positron emission tomography). The analysis of the frequency of Th17 (i.e., IL-17+) cells was performed by ex-vivo intracellular cytokine staining on PB and BM CD3+-gated cells, as described in Di Lullo G et al.Citation6
In agreement with the work of Dhodapkar KM et al.,Citation16 we found that IL-17+ T cells were significantly increased in BM compared to PB in paired samples of both asymptomatic (i.e., MGUS and SMM) and symptomatic patients (). However, when considering the total IL-17 producing CD3+ cells in PB and BM of the subjects enrolled in the study, we did not find any significant difference among the categories in both PB () and BM (), suggesting that the frequency of Th17 cells in MM does not correlate with disease prognosis. Indeed, at difference with Th22 cells whose frequency correlates with the clinical status (), the frequency of Th17 cells did not significantly change in relation to the ISS stage or in relapsed/refractory disease (). These data are in agreement with Bryant C et al.,Citation28 who also did not find a significant increase of Th17 cells in the blood of MM patients compared with normal controls and in partial agreement with the results from Prabhala RH et al.,Citation15 who found a significantly elevated frequency of Th17 cells in PB but not in BM of MM patients compared with healthy controls.
Figure 1. Percentage of Th17 cells in healthy donors and asymptomatic and symptomatic MM patients. (A) Analysis of IL-17+ cells of total CD3+ cells in paired samples of PB and BM mononuclear cells. Significant values calculated with the Wilcoxon matched-pairs signed rank test were indicated as: *p < 0.05 and **0.001< p <0.01. (B) Percentage of IL-17+ cells of total CD3+ cells in PB in different categories of subjects: Healthy donors (n = 15), MGUS+SMM (n = 11), Diagnosis (n = 20), Refractory/Relapse (n = 9). (C) Percentage of IL-17+ cells of total CD3+ cells in BM in different categories of subjects: Healthy donors (n = 4); MGUS+SMM (n = 10), Diagnosis (n = 19), Refractory/Relapse (n = 14). (B–C) No significant differences were observed between the different categories of subjects tested.
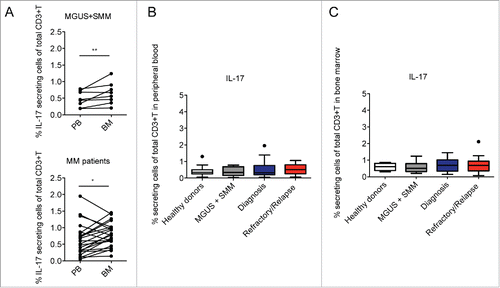
Figure 2. Percentage of Th22 cells and Th17 cells in relation to the clinical status and the presence or the absence of bone disease. Tukey plots of cumulative results from intracellular staining analyses of IL-22, IL-17 and IL-13 expression by CD3+ T cells in BM aspirates. (A–B) Percentage of Th17 (i.e., IL-17+) cells (A) and Th22 (i.e., IL-22+IL-17−IL-13+) cells (B) in the BM of MM patients classified according to clinical status as: ISS Stage I+II (n = 12), Stage III (n = 7) and relapsed/refractory (Rel/Ref) (n = 14). (C–D) Percentage of Th17 (C) and Th22 (D) cells in the BM of MM patients grouped according to absence (N) (n = 11) or presence (Y) (n = 18) of bone disease. Responses significantly different by Mann Whitney U test are indicated as: *p < 0.05 and **0.001 < p < 0.01; ns: not significant.
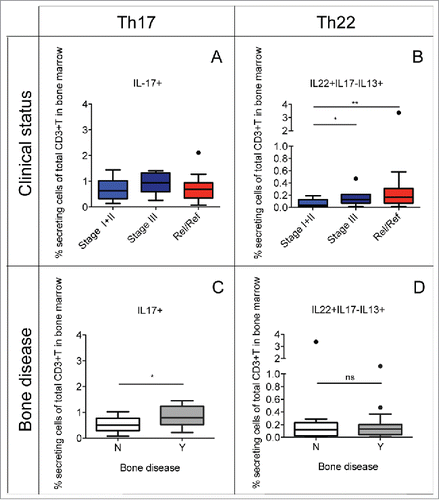
As Th17 cells are increased in the BM compared with PB of MM patients () and Th17 cells in the BM have been associated with bone disease in MM,Citation17 we compared the frequency of Th17 and Th22 cells in the BM of MM patients stratified based on the absence (N) or the presence (Y) of bone disease. Indeed, we found that Th17 cells were significantly increased in patients with bone lesions compared with patients without bone lesions (), whereas no difference was observed for Th22 cells (). As Th17 cells comprise a subset of IL-17+IL-22+ T cells, we performed our analyses also on separate IL-17+IL-22- and IL-17+IL-22+ Th17 subsets with similar results to those obtained with total Th17 cells (data not shown). This suggests that possibly other co-released cytokines/factors might be relevant in determining the different effects on the clinical parameters (i.e., especially on disease prognosis) of Th17 and Th22 cells, such as IL-13 in the case of Th22 cells. Collectively, these data confirm the role for Th17 cells in the mechanisms of bone damage whereas Th22 cells are apparently unrelated to bone erosion.
To further understand the impact of Th22 and Th17 cells on the two defined disease outcomes, we performed fitted logistic univariate and multivariate analysis models taking into consideration the clinical status (categorized as stage I+II or stage III+relapsed/refractory) and bone disease (categorized as Yes or No), respectively. As covariates we considered, all as continuous variables, the frequency of Th17 cells, the frequency of Th22 cells and the value of lactate dehydrogenase (LDH). In univariate analysis, we confirmed that increased frequency of Th22 cells increases the probability to belong to the category of patients with stage III+relapsed/refractory (p < 0.05) and increased frequency of Th17 cells increases the probability to belong to the category of patients with bone disease (p < 0.05). In multivariate analysis when the clinical status was the considered outcome, we found that increased frequency of Th22 cells increases the probability to belong to the category of patients with stage III+ relapsed/refractory (p value around 7%) while increased frequency of Th17 cells did not. On the contrary when bone disease was the considered outcome, we found that increased frequency of Th17 cells increases the probability to belong to the patients with bone disease (p value around 4%) while increased frequency of Th22 cells did not. Collectively and notwithstanding the limitation of the small sample size for these types of analysis, these results support our conclusions.
Conclusions
By comparing the role of Th22 and Th17 cells within the same cohort of MM patients, we found that increased levels of Th22 cells in the BM associate with a poor prognosis whereas increased levels of Th17 cells associate with the development of bone disease. These results demonstrate significantly different and mutually exclusive associations for these Th cell subsets with the indicated MM clinical correlates. Interestingly, non-redundant roles for Th17 and Th22 cell subsets have been also described in atopic allergic diseases where Th17 cells exert pro-inflammatory functions by attracting neutrophils, whereas Th22 cells activate protective and regenerative epithelial cell responses.Citation12 Future studies are worthwhile for better defining the distinctive roles of these two Th cell subsets in non-neoplastic as well as neoplastic diseases, especially in view of therapeutic interference with their functions. In the case of MM, IL-17 should be targeted by therapies focusing on the reduction of bone disease, whereas the Th22-prototype cytokines IL-22 and IL-13 should be targeted by therapies interfering with the IL-22/IL-22RA1 and the IL-13/IL-13RA1 axes with the aims of reducing the tumor burden and re-establishing sensitivity to drug-induced cell death, thus hampering the onset of drug-resistance.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgement
We thank Anna Maria Paganoni (Laboratory for Modeling and Scientific Computing-MOX, Politecnico di Milano) for assistance in performing the univariate and multivariate analyses.
Funding
This work was supported by the Italian Association for Cancer Research (AIRC, IG-11353 and IG-11340). Giulia Di Lullo is recipient of a fellowship from the Fondazione Umberto Veronesi.
References
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011; 364:1046-60; PMID:21410373; http://dx.doi.org/10.1056/NEJMra1011442
- Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer 2007; 7:585-98; PMID:17646864; http://dx.doi.org/10.1038/nrc2189
- Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, et al. International staging system for multiple myeloma. J Clin Oncol 2005; 23:3412-20; PMID:15809451; http://dx.doi.org/10.1200/JCO.2005.04.242
- Protti MP, Monte LD, Lullo GD. Tumor antigen-specific CD4+ T cells in cancer immunity: from antigen identification to tumor prognosis and development of therapeutic strategies. Tissue Antigens 2014; 83:237-46; PMID:24641502; http://dx.doi.org/10.1111/tan.12329
- Lim C, Savan R. The role of the IL-22/IL-22R1 axis in cancer. Cytokine Growth Factor Rev 2014; 25:257-71; PMID:24856143; http://dx.doi.org/10.1016/j.cytogfr.2014.04.005
- Di Lullo G, Marcatti M, Heltai S, Brunetto E, Tresoldi C, Bondanza A, Bonini C, Ponzoni M, Tonon G, Ciceri F, et al. Th22 cells increase in poor prognosis multiple myeloma and promote tumor cell growth and survival. Oncoimmunology 2015; 4:e1005460; PMID:26155400; http://dx.doi.org/10.1080/2162402X.2015.1005460
- Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol 2009; 10:857-63; PMID:19578369; http://dx.doi.org/10.1038/ni.1767
- Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol 2009; 10:864-71; PMID:19578368; http://dx.doi.org/10.1038/ni.1770
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 2007; 8:950-7; PMID:17676044; http://dx.doi.org/10.1038/ni1497
- Akdis M, Palomares O, van de Veen W, van Splunter M, Akdis CA. TH17 and TH22 cells: a confusion of antimicrobial response with tissue inflammation versus protection. J Allergy Clin Immunol 2012; 129:1438-49; quiz50-1; PMID:22657405; http://dx.doi.org/10.1016/j.jaci.2012.05.003
- Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Annu Rev Immunol 2015; 33:747-85; PMID:25706098; http://dx.doi.org/10.1146/annurev-immunol-032414-112123
- Souwer Y, Szegedi K, Kapsenberg ML, de Jong EC. IL-17 and IL-22 in atopic allergic disease. Curr Opin Immunol 2010; 22:821-6; PMID:21087848; http://dx.doi.org/10.1016/j.coi.2010.10.013
- Benham H, Norris P, Goodall J, Wechalekar MD, FitzGerald O, Szentpetery A, Smith M, Thomas R, Gaston H. Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res Ther 2013; 15:R136; PMID:24286492; http://dx.doi.org/10.1186/ar4317
- Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Front Immunol 2014; 5:276; PMID:24987392; http://dx.doi.org/10.3389/fimmu.2014.00276
- Prabhala RH, Pelluru D, Fulciniti M, Prabhala HK, Nanjappa P, Song W, Pai C, Amin S, Tai YT, Richardson PG, et al. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood 2010; 115:5385-92; PMID:20395418; http://dx.doi.org/10.1182/blood-2009-10-246660
- Dhodapkar KM, Barbuto S, Matthews P, Kukreja A, Mazumder A, Vesole D, Jagannath S, Dhodapkar MV. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17-1 cells) enriched in the bone marrow of patients with myeloma. Blood 2008; 112:2878-85; PMID:18669891; http://dx.doi.org/10.1182/blood-2008-03-143222
- Noonan K, Marchionni L, Anderson J, Pardoll D, Roodman GD, Borrello I. A novel role of IL-17-producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood 2010; 116:3554-63; PMID:20664052; http://dx.doi.org/10.1182/blood-2010-05-283895
- Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 2006; 203:2673-82; PMID:17088434; http://dx.doi.org/10.1084/jem.20061775
- Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med 2007; 204:2803-12; PMID:18025126; http://dx.doi.org/10.1084/jem.20071397
- Giuliani N, Lisignoli G, Colla S, Lazzaretti M, Storti P, Mancini C, Bonomini S, Manferdini C, Codeluppi K, Facchini A, et al. CC-chemokine ligand 20/macrophage inflammatory protein-3alpha and CC-chemokine receptor 6 are overexpressed in myeloma microenvironment related to osteolytic bone lesions. Cancer Res 2008; 68:6840-50; PMID:18703490; http://dx.doi.org/10.1158/0008-5472.CAN-08-0402
- Giuliani N, Rizzoli V, Roodman GD. Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition. Blood 2006; 108:3992-6; PMID:16917004; http://dx.doi.org/10.1182/blood-2006-05-026112
- Galson DL, Silbermann R, Roodman GD. Mechanisms of multiple myeloma bone disease. Bonekey Rep 2012; 1:135; PMID:23951515; http://dx.doi.org/10.1038/bonekey.2012.135
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 2007; 7:292-304; PMID:17380158; http://dx.doi.org/10.1038/nri2062
- Tucci M, Stucci S, Savonarola A, Ciavarella S, Cafforio P, Dammacco F, Silvestris F. Immature dendritic cells in multiple myeloma are prone to osteoclast-like differentiation through interleukin-17A stimulation. Br J Haematol 2013; 161:821-31; PMID:23594390; http://dx.doi.org/10.1111/bjh.12333
- Sun Y, Pan J, Mao S, Jin J. IL-17/miR-192/IL-17Rs regulatory feedback loop facilitates multiple myeloma progression. PLoS One 2014; 9:e114647; PMID:25489847; http://dx.doi.org/10.1371/journal.pone.0114647
- Favaloro J, Brown R, Aklilu E, Yang S, Suen H, Hart D, Fromm P, Gibson J, Khoo L, Ho PJ, et al. Myeloma skews regulatory T and pro-inflammatory T helper 17 cell balance in favor of a suppressive state. Leuk Lymphoma 2014; 55:1090-8; PMID:23865833; http://dx.doi.org/10.3109/10428194.2013.825905
- Braga WM, Atanackovic D, Colleoni GW. The role of regulatory T cells and TH17 cells in multiple myeloma. Clin Dev Immunol 2012; 2012:293479; PMID:22489248; http://dx.doi.org/10.1155/2012/293479
- Bryant C, Suen H, Brown R, Yang S, Favaloro J, Aklilu E, Gibson J, Ho PJ, Iland H, Fromm P, et al. Long-term survival in multiple myeloma is associated with a distinct immunological profile, which includes proliferative cytotoxic T-cell clones and a favourable Treg/Th17 balance. Blood Cancer J 2013; 3:e148; PMID:24036947; http://dx.doi.org/10.1038/bcj.2013.34
- Tsirakis G, Pappa CA, Kolovou A, Kokonozaki M, Neonakis I, Alexandrakis MG. Clinical significance of interleukin-22 in multiple myeloma. Hematology 2015; 20:143-7; PMID:25055724; http://dx.doi.org/10.1179/1607845414Y.0000000182
- Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Canonica GW, Jasmin C, Azzarone B. Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. Implication in asthma. J Clin Invest 1998; 101:2129-39; PMID:9593769; http://dx.doi.org/10.1172/JCI741
