ABSTRACT
Thymidylate synthase (TS) is a tumor-associated enzyme critical for DNA replication and main 5′-fluorouracil (5′-FU) target. TSPP/VAC1 is a multi-arm trial phase-Ib trial program aimed to investigate the toxicity and biomodulatory activity of a poly-epitope-peptide vaccine to TS (TSPP) in cancer patients (pts). Here, we present the results of the TSPP/VAC1/arm C trial aimed to evaluate TSPP in combination with chemo-immunotherapy in pretreated metastatic colo-rectal cancer (mCRC) pts. Twenty-nine pts, 14 males and 15 females, received poly-chemotherapy with gemcitabine [GEM; 1,000 mg/sqm, day-1], oxaliplatin [OX; 80 mg/sqm, day-2], levofolinate [100 mg/sqm, days 1–2], bolus/infusional 5′-FU [400 mg/800 mg/sqm, days 1–2], sargramostim [50 μg, days 3–7/q30], and interleukin-2 [sc. 0.5 MIU twice a day, days 8–14/18–30] [GOLFIG-regimen]. Seventeen pts received sc. TSPP injections at escalating dosage [3 pts, 100 µg (DL-1); 3 pts, 200 µg (DL-2) and 11pts, 300 µg (DL-3)] one week after each chemotherapy cycle (concomitant module), while 10 out 12 pts received TSPP (300 µg) after 12 GOLFIG courses [dose level (DL)-0] (sequential module). TSPP MTD was not achieved. Adverse events consisted in swelling/erythema at injection sites (17 cases), G1–2 haematological (16 cases) and gastro-enteric events (12), fever, rhinitis, conjunctivitis, and poly-arthralgia and rise in auto-antibodies [ANA, ENA, c-ANCA, p-ANCA in the DL1–3 pts]. Both treatment-modules showed immunomodulating and antitumor activity (disease-control-rate, DL1–3 and DL0 were 70.6% and 83.3%, respectively) with a better survival recorded in the second group [median OS DL1–3 vs. DL0 = 8 vs. 16 mo, p = 0.049]. The promising long-term survival produced by the sequential treatment module deserves further phase II evaluation.
Introduction
Active specific immunotherapy (ASI) is a re-emerging anticancer treatment strategy and immune-checkpoint regulator monoclonal antibodies (mAbs) and tumor-associate-antigen (TAA)-specific vaccine formulations are currently under active investigation in cancer pts.Citation1-5 In the last 15 years, several attempts have been made to combine different therapeutic modalities with ASI in the treatment of solid malignancies in order to improve its antitumor efficacy. Radio- and chemo-immunotherapy have produced promising results in both preclinical and clinical studies.Citation6-11
We generated and characterized new vaccine constructs able to direct an effective immune-response to molecular structures which are over-expressed in cancer cells and, at the same time, are critical for their growth, survival, and diffusion, as the Epidermal growth Factor Receptor [EGFR], the parathyroid hormone related peptide [PTHrP] and TS.Citation6-9 TS, in particular, is a cancer-associated target enzyme, inhibited by several anticancer drugs (antimetabolites) such as 5′-FU, a cytotoxic drug which is a basic component of different regimens for the treatment of mCRC and other common malignancies.Citation10-12 5′-FU is a pro-drug, that upon transformation to 5′-FdUMP, binds and permanently inhibits the catalytic site of TS in the presence of levo-folinate. TS is responsible for deoxy-uridine monophosphate (dUMP) methylation to thymidylate, a crucial component for DNA synthesis and repair, whose expression is S phase specific, and is often over-expressed in tumor tissue. TS is an mRNA binding protein which auto-regulates its expression depending on the intracellular levels of metabolites, cofactors, and substrates. Therefore, cancer cell exposure to 5′-FU, by inducing thymidylate depletion, promotes transient TS over-expression within 24/48 h in the surviving cells. TS-overexpression in tumor tissue, is finally predictive of 5′-FU resistance and poor prognosis in mCRC pts.Citation10-12 We previously demonstrated that TS is a potential candidate target for ASI, and identified 3 TS-derived epitopes with HLA-A(*)02.01 amino-acid anchorage motifs (TS-1, TS-2, and TS-3), recognized by human cytotoxic- T-lymphocytes (CTLs).Citation8 We also characterized a 27-mer peptide, designated as TSPP, which combines the amino-acidic sequences of these peptides.Citation9 We showed that TSPP elicits a powerful multi-epitope specific CTL response with antitumor activity in vitro.Citation9,13 and was safe and immunogenic in HHD mice (transgenic for the expression of HLA-A(*)02.01 and human CD8α). In this murine model, TSPP vaccination prevented and/or eradicated tumors after injection of syngeneic EL4/HHD lymphoma cells.Citation9,13 As an additional finding, TSPP antitumor activity, was greatly enhanced when used in combination with 5′-FU-based chemotherapy.Citation9,13 Indeed, we observed the better results when TSPP immunizations were weekly administered in alternate combination with the GOLF poly-chemotherapy,Citation13 a previously characterized multi-drug regimen with GEM, OX, levofolinic acid (LF) and infusional 5′-FU.Citation14,15 5′-FdUMP binds TS and promotes its ubiquitination and proteolysis by the proteasome system.Citation12 TS processing by the proteasomes, in turn, promotes the assembling of class-I HLA/TS-epitope peptides which are subsequently transported to the membrane becoming available for recognition by specific CTL precursors.Citation9,13 Additionally, thymidine depletion in cancer cells also promotes TS over-expression, thus augmenting the amounts of TS epitopes available for CTL recognition. We already demonstrated that tumor cell exposure to GOLF regimen increases the expression of multiple TAA (like TS, CEA, and EGFR), enhances the expression of danger signals and “eat-me” molecules, and induces immunogenicity in colon and breast cancer cell models in vitro.Citation9,13,14,16-19 These preclinical findings provided a clear rationale to investigate the GOLF regimen in combination with an immune-adjuvant IG-1 cytokine regimen (sc. Interleukin (IL)-2 and sc. Granulocyte-Macrophage colony-stimulating-factor(GM-CSF) (GOLFIG chemo-immunotherapy regimen) in colon cancer pts and successively, in combination with TSPP-based immunotherapy. The GOLFIG regimen has been already evaluated in mCRC pts, resulting safe, with significant immune-modulating effects and powerful antitumor activity.Citation15,20,21 On these bases, we designed a multi-arm phase Ib trial program (TSPP/VAC-1) to investigate the toxicity and immune-biological activity of TSPP vaccination in cancer pts. Each arm of the program was defined on a dose-finding setting, and aimed to test TSPP vaccination alone (Arm A) or associated with GM-CSF and IL-2 (Arm B) or in combination with the GOLFIG regimen (Arm C). The results obtained in the first two arm trials suggested that TSPP is safe, produces self-limiting auto-immunity signs, is able to generate a TS-specific CTL response with significant rise in serum auto-antibodies (AAbs) such as anti-nuclear Abs (ANA); extractable nuclear antigen Abs (ENA); anti-proteinase-3 anti-neutrophil cytoplasmic Abs (P-ANCA), and anti-myeloperoxidase anti-neutrophil cytoplasmic Abs (C-ANCA)].Citation22
In the present study, we report the results concerning the arm C trial of the TSPP/VAC-1 program. This part of the study was planned to enroll pretreated mCRC pts, who received TSPP vaccination concomitantly (alternate weeks) or sequentially (after 12 GOLFIG courses) to the GOLFIG chemo-immunotherapy.
Patient and methods
The TSPP/VAC-1 is a multi-arm phase Ib trial program designed to test the toxicity and immunological activity of TSPP, a new poly-epitope cancer vaccine to TS, in different therapeutic conditions and in advanced cancer pts. The protocol consisted of three parallel and independent trial arms designed to test TSPP vaccination alone (Arm A) and in combination with immune-adjuvant IG-1 regimen (Arm B) and in combination with GOLFIG chemo-immunotherapy regimen (Arm C). The latter trial arm was reserved to mCRC pts only and evaluated the effects of peptide vaccination, administered concomitantly with the GOLFIG regimens (DL1–3), and/or sequentially (DL0) after 10/12 courses of this regimen.
Ethical considerations and study design
The study was designed according to good clinical practice (GCP) recommendations, authorized by the Italian National Institute of Health (Istituto Superiore di Sanità) and by the Italian Ministry of Health, approved by the University of Siena Ethical Committee Board (equivalent to Human Subject Committee of Investigational Review Board) and registered with the TSPP/VAC-1 code, Eudract: # 2009–016897–33. This phase Ib study was planned as a dose escalation trial program, in three parallel and independent arms (A, B, and C). TSPP dose-escalation was planned according to the Fibonacci's series. The first cohort of pts received 100 µg of peptide (DL1), the second, 200 µg (DL2), and the third 300 µg (DL3), every 21 d new pts could be enrolled in higher dose level cohorts, only if no Grade IV event were demonstrated in pts treated with lower doses. Pts assigned to the arm A received treatment with vaccine peptide alone, those of arm B received TSPP and sc. GM-CSF (Sargramostim/Leukine→, Berlex, USA) (50 µg days 1–5) and sc. IL-2 (Proleukin→, Novartis, Switzerland) (0.5 MIU bi-daily, days 6–15), while those of arm C/DL1–3 received peptide vaccination 7 d after the beginning of the chemo-immunotherapy cycle with gemcitabine 1 g/sqm on the day 1, oxaliplatin 85 mg/sqm on the day 2, Levofolinic acid 100 mg/sqm on the days 1 and 2, bolus 5′-FU 400 mg/sqm on days 1 and 2, and infusional 5′-FU 800 mg/sqm on days 1 and 2, every 15 d these pts also received sc. GM-CSF (50 µg days 3–7) and sc. recombinant (r) IL-2 (Aldesleukine, 0.5 MIU bi-daily on days 8–14 and 17–29). Pts in arm C/DL0 were aimed to receive TSPP vaccination alike arm B, after they had received 12 GOLFIG courses.Citation15,23
TSPP vaccine
TSPP (YMIAHITGLFLDSLGFSTTLGDAHIYL) was synthesized and characterized by good manufacturing practice (GMP) procedures by the American Peptide Ltd (Rockville, MD, USA). The aseptic vial filling process was performed by the Pharmacy of the Azienda Ospedaliera Universitaria Senese, which also performed the stability study, endotoxin evaluation, and chemical related toxicity analysis of the product. TSPP was dissolved in DMSO (1.5 mg peptide in 1 mL DMSO). The exact peptide dose (100, 200, or 300 µg), was diluted with PBS in a volume of 250 µL, and then 1:2 diluted with Montanide ISA 720 VG ST (Seppic, Milan, Italy) as adjuvant. The final volume of the vaccine was 500 µL/dose.
Patients
The study-arm-C trial was planned to evaluate the immunological activity and toxicity of TSPP in combination with GOLFIG chemo-immunotherapy, in mCRC pts, who had received at least two standard treatment lines. The primary endpoints of the study were the identification of the maximal tolerated dose (MTD) and most effective biological dose (MEBD) of TSPP peptide, by evaluating the frequency of adverse events per dose level and predefined immune-biological events in three cohorts of three pts each. Evidence of antitumor activity was a secondary endpoint. The inclusion criteria were the following: written informed consent, histological diagnosis of malignant disease, at least two previous chemotherapy lines for advanced disease, measurable disease (according to WHO tumor response criteria), ECOG performance status ≤2, normal renal and hepatic functions, white blood cell count ≥2,500/mm3, hemoglobin levels ≥9 g/dL, platelet cell count ≥100,000/mm3, and normal heart function. The exclusion criteria were the following: any major organ failure, unstabilized central nervous system involvement, second malignancies, active infectious disease, major inflammatory and autoimmune rheumatic diseases, and acquired immune-suppression. Treatment allocation was not masked. Standard clinical and laboratory evaluation (clinical history, physical examination, blood count and chemistry, serum dosage of C-reactive protein (CRP), erythrocyte-sedimentation rate (ESR), lactate dehydrogenase (LDH), rheumatoid factor, carcinoembryonic antigen (CEA) and CA19.9 assays, chest X-rays, and ultrasound abdominal scans, were performed at baseline and repeated every 6 weeks. Pts' sera were tested for ANA by IFA, (starting dilution 1:160) (SSA HEp2000, ImmunoConcepts), EliASymphony screening (Thermo Fisher Scientific), and further ELiA tests were performed for single ANA specificities; ANCA; p-ANCA; C-ANCA ENA were tested on Phadia250 instrument; ANCA were measured by indirect immunofluorescence using INOVA substrate. Contrast CT scans were scheduled every three months. Pts enrolled in the arm C/DL1–3 received combined TSPP/GOLFIG treatment for a maximal of 12 cycles, then those who did not progress, received TSPP vaccination every 21 d at the dosage of 300 µg until disease progression, occurrence of unacceptable toxicity, clinical judgment, or withdrawal of consent. Any further treatment decision after disease progression was left to the physician in charge. Adverse events, toxicity, and treatment response were evaluated according to WHO classification.
ELISA assays and multiplex analysis
Serum levels of Interferon(IFN)γ, Tumor necrosis factor (TNF)-α, IL10, IL4, IL12, IL10, and IL17 cytokines were measured using Bio-Plex human cytokine multiplex kits (Bio-Rad Inc., Hercules, CA). The fluorescence intensity of the beads was measured by using the Bio-Plex array reader. Bio-Plex Manager software with five-parametric-curve fitting was used for data analysis. Serum levels of IL6 and IL8 were measured by using human cytokine ELISA kits and analyzed according to the manufacturer's protocol (Bio-Rad Inc., Hercules, CA).
Fluorescence-activated cell sorting analysis of patient peripheral blood mononuclear cells (PBMC)
The pts' PBMCs were purified by Ficoll-Hypaque (Celbio S.P.A., Italy) gradient separation of buffy coats of heparinized blood samples and analyzed by Fluorescence-activated cell sorting (FACS) analysis, as described in previous studies.Citation24,25
PBMC were stained with different pools of labeled mAbs (CD4V450, CD45RAPE, CD62LFITC, CCR7PE-Cy7, PharMingen;CD8+PerCPCy 5.5, CD45ROAPC, CD3FITC, CD19FITC, CD14FITC, CD11cAPC, CD16PE, Rat IgG2aFITC, Mouse IgG1, Becton Dickinson, Italy; CD56 APC, Mouse IgG2a APC Immunotools, DE; CD15 PE ABCam, UK; CD25 PE, FoxP3 FITC, eBioscence, UK) and examined by a FACScalibur BD instrument. Ki67 positive cells and regulatory-T-cells (Tregs) were analyzed after intracellular immune-staining according to the manufacturer's protocol (eBioscience). Cytofluorimetric analysis was carried out by using the FlowJo→software.
Statistical analysis
The statistical analysis was carried out by the GraphPadInstat 3.2 statistical software. The results were expressed as the mean −/− standard deviation (SD) of four determinations made in three different experiments, and analyzed by the two-tail Student's t-test. A p value of 0.05 or less was considered statistically significant. Kaplan Meier descriptive statistics was performed by the use of SPSS statistical package.
Results
Demographics, chemo-immunotherapy, peptide vaccination and dose- escalation
Twenty-nine pts with mCRC, 14 males and 15 females, with a good performance status (ECOG 0–1), who had received at least two treatment lines (standard poly-chemotherapy −/− cetuximab and/or bevacizumab) signed an informed consent and were enrolled between May 2011 and July 2013 (). According to the pre-established platform (see pts and methods), all of them received GOLFIG poly-chemo-immunotherapy. Seventeen pts, received sc. TSPP vaccination at escalating dosage [three pts entered the DL-1; 3, the DL-2; 11, the DL-3 cohort] on biweekly bases, starting one week after each chemotherapy cycle (concomitant treatment). Twelve pts received GOLFIG chemo-immunotherapy alone (DL0) for 12 courses and then maintenance therapy including TSPP vaccination every three weeks. Two of them were excluded from the study, due to early disease progression and decline in performance status. Maintenance therapy included TSPP vaccination [(300 µg on the day 1 every three weeks) sc.GM-CSF (50 µg at day, days 1–5 every three weeks), sc.rIL2 (0,5 MIU twice a day, days 6–15 every three weeks).
Table 1. Patients' characteristics. Mut: mutated; Wt: wild type; MAINT: number of vaccine maintenance therapy cycle; PD: disease progression; SD: stable disease; PR: partial response.
Antitumor activity—Within the 17 pts, who received the concomitant TSPP/GOLFIG treatment (DL1–3), we recorded progression of disease (PD) in 9 (52.9%), a partial response (PR) in 3 (17.64%), and stable disease (SD) in 5 (29.4%) subjects, including four pts (CLCDL2; SACDL2; BGCDL3; MUCDL3) (23.5%) surviving over 12 mo. On the other hand, in the 12 pts who were enrolled in the sequential treatment group, we recorded two PDs occurring before the planned TSPP vaccination, four PRs (25%), and six SDs (50%). Seven (63.6%) of these pts (SRCDL0, BLDL0, MLDL0, PGDL0, MACDL0, PACDL0, DLCDL0) survived more than 12 mo. ()
Although this trial was not designed on a comparative setting in term of antitumor activity, we performed an exploratory descriptive Kaplan Meyer and a Log-Rank test to compare PFS and OS in pts who received concomitant or sequential treatments, finding a better performance in term of OS in the latter group (). In our series, the Univariate analysis did not find any significant correlation of k-ras mutational status and HLA-A2+ haplotype with either PFS (wt-k-ras vs. k-ras mut status, p: 0.063; HLA-A2+ vs. HLA.A2− haplotype, p: 0.682) or OS (wt-k-ras vs. k-ras mut status p: 0.203; HLA-A2+ vs. HLA.A2− haplotype, p: 0.601) of these pts (data not shown in figure).
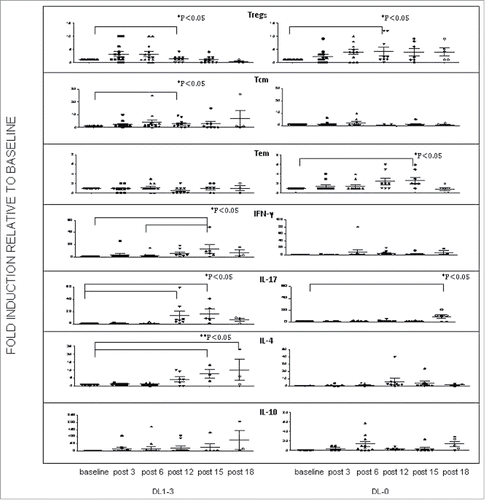
Figure 1. The upper figures (first three rows) represent the fold change modulation relative to baseline values of plotted percentages of peripheral Treg, Tcm, Tem of the pts enrolled in the trial and who received the concomitant (DL 1 -3) (left panels) and sequential (DL-0) treatment modules (right panels). Statistical significance was calculated by performing the one-way Anova analysis of variance, post-test Bonferroni with 95% IC. The bottom figures (last four rows) represent the fold change modulation relative to baseline values of serum level of IFNg, IL-17, IL-4, and IL10 of the pts who received the concomitant (DL1 -3) (left panels) or the sequential treatment modules (DL0) (right panels). Statistical significance was calculated by performing the one-way Anova analysis of variance, post-test Bonferroni with 95% IC. Post-3, i6, i12, i15,and i18 represent the number of treatment courses received by the pts. Pts in the DL1 -3 group received concomitant TSPP vaccination and GOLFIG chemo-immunotherapy and then TSPP maintenance starting after 12 courses (Post-12). Pts in the DL0 group received GOLFIG chemo-immunotherapy alone and then TSPP maintenance starting after 12 courses (Post-12).
Toxicity profile
The treatment was safe also in this setting of advanced and largely pre-treated pts. There were no lethal adverse events and there was no statistical correlation between frequency of adverse events and TSPP dosage or absence of concomitant TSPP vaccination (DL0). Similarly, there was no significant difference when the two groups of pts, receiving concomitant (DL1–3) or sequential TSPP/GOLFIG treatment (DL0) were compared. The majority of adverse events were strictly related to the poly-chemotherapy and consisted in moderate (Grade I–II) hematologic (anemia and thrombocytopenia) and gastro-enteric (mucositis) toxicity. A reaction to oxaliplatin was observed in four pts, who continued the treatment without this drug. There was no TSPP dose-dependent difference in adverse event frequency (). However, it should be underlined that pts who received a concomitant TSPP/GOLFIG treatment showed higher frequency of grade 1–2 asthenia, fever, poly-arthralgia, and biochemical signs of hypothyroidism (six cases) (rise in TSH levels).()
Table 2. Adverse events. DL: TSPP dose level.
Twelve out of 17 pts who received concomitant treatment and eight pts who received TSPP maintenance showed a local reaction at the vaccine injection site, which reflected the occurrence of delayed hypersensitivity with appearance of erythematous reactions with swelling and induration still present 72–96 h later. TSPP vaccination, therefore, resulting safe and well tolerated did not increase the frequency of grade 3–4 adverse events associated with GOLFIG regimen. Finally, the frequency of adverse events per dose level did not allow to identify an MTD for TSPP (DL0–3) in combination with GOLFIG poly-chemo-immunotherapy.()
Immunobiological monitoring
Hemocytometric analysis—GOLFIG poly-chemotherapy induced a slight but progressive decline in neutrophil counts, which was not affected by TSPP vaccination (). We also detected a progressive rise in lymphocyte, monocyte, and eosinophil counts which, again, was not influenced by concomitant TSPP vaccination. When the hemocytometric monitoring curves of pts in DL1–3 and DL0 groups were compared, we were unable to demonstrate any statistical difference in lymphocyte (p = 0.605), monocyte (p = 0.807) and eosinophil (p = 0.199) count monitoring.()
Table 3. Treatment groups.
Serologic analysis of systemic inflammatory markers—An analysis of serological inflammatory markers revealed a trend to progressive decline in CRP and an increase in ESR in both group. Pts who received the sequential treatment (DL0), however, showed lower levels of inflammatory markers CRP (p = 0.041) and ESR (p < 0.001) at baseline and along the treatment.()
Serologic analysis of auto-antibodies
The serum monitoring of AAbs potentially indicative of autoimmunity, showed a progressive and significant increase in ENA, p-ANCA, and c-ANCA only in the group of pts who received the concomitant treatment (DL1–3) and no change in the other group (DL0). Differences in ANA, ENA, c-ANCA, and p-ANCA values between the concomitant and sequential treatment groups resulted statistically significant (p values for ANA and ENA, c-ANCA, p-ANCA value curves were 0.049, 0.0345, 0.0163, and 0.0412, respectively) ( and ). Our analysis did not reveal particular changes in other AAbs such as ASMA, ANCA, and anti-thyroid Abs.
Flow cytometry
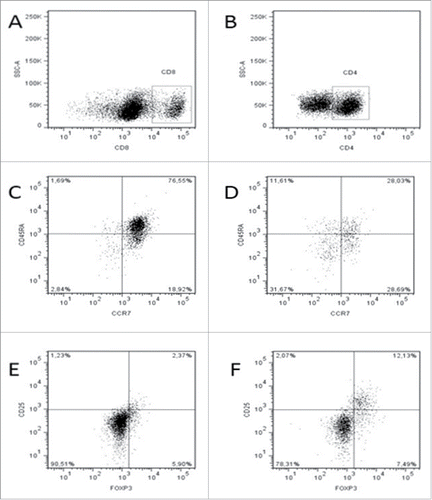
Flow cytometry analysis performed on the peripheral blood of the pts receiving the concomitant and sequential treatment modules did not show differences and changes in the percentages of peripheral CD3+CD4+ (DL1–3 vs. DL0 group; p = 0.22) and CD3+CD8+ CTLs (DL1–3 vs. DL0 group; p = 0.145). A further analysis of these T cells, similarly, did not show differences in the percentages of CD3+CD4+CD45−memory T cells (DL1–3 vs. DL0 group; p = 0.301). Both group of pts receiving the concomitant and sequential treatment module showed an early increase in peripheral CD4+CD25+FoxP3+ Tregs which was lost on the long-term (). There was no difference between the two groups of pts (DL1–3 vs. DL0, p= 0.21) suggesting that this effect was related to the presence of GM-CSF and IL-2 in the GOLFIG regimen and not to TSPP administration. The group of pts receiving the concomitant treatment module instead, showed an increase in central memory (CD8+CD45RA−CCR7+) T cells (Tcm), while that receiving the sequential module showed an progressive increase in effector memory T cells (Tem) (CD8+CD45RA−CCR7−).()
Figure 2. Kaplan Meyer descriptive for statistics Panels (A)–(B) represent median PFS (A) and OS (B) in pts enrolled in the concomitant (DL1–3) and sequential (DL0) treatment modules. Median PFS, DL1–3 vs. DL0: 3 i/i 0.738 (95% IC: 2.55–5.44) mo vs. Seven i/i 0.818 (95% IC:4.64–7.85), p= 0.340; median OS, DL1–3 vs. DL0: 8 i/i 0.038 (95% IC: 6.13–12.69) mo vs. 16 i/i 1.67 (95% IC: 10.17–17.05) mo; p D 0.049 by log rank test. Panels (C)–(E) represent peripheral blood cell counts in mCRC pts enrolled in the TSPP/VAC1 arm-C trial, at baseline and before each treatment course. Lymphocyte counts (C), monocyte counts (D), eosinophil counts (E). Results are expressed as a number of cells £ 102/sqmm i/standard error. Panels (F)–(H) represent serum auto-antibodies in mCRC pts enrolled in the TSPP/VAC1 arm-C trial, at baseline and before each treatment course. ENA (F), c-ANCA (G), and p-ANCA (H). Results are expressed as a units/mLi/istandard error. Symbols represent the groups of pts who received TSPP vaccination as concomitant (&) or sequential treatment modules (). Pts in the DL1–3 group received concomitant TSPP vaccination and GOLFIG chemo-immunotherapy and then TSPP maintenance starting after 12 courses (Post-12). Pts in the DL0 group received GOLFIG chemo-immunotherapy alone and then TSPP maintenance starting after 12 courses (Post-12). Statistical significance (p <0 .05) with the co-respective baseline value is represented by an asterisk.
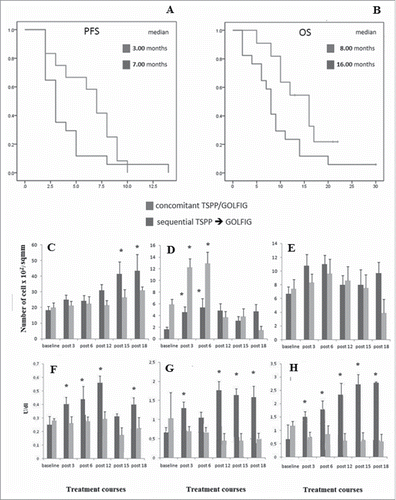
Figure 3. Flow cytometry analysis: Central memory T cells (TCM;CD45RA−CCR7+) and effector memory T cells (TEM; CD45RA−CCR7−) gated on CD3+CD8+ lymphocytes (A) of representative patient PBMCs (DL3), isolated at the baseline (C) and after six treatment lines (D) Regulatory T cells (Treg; CD25+Foxp3+) gated on CD3+CD4+ lymphocytes (B) of representative patient PBMCs, isolated at the baseline (E) and after six treatment lines (F).
Finally, it was also recorded a trend to increase in peripheral natural killer (NK) cells (CD3−CD56+CD16+) in both groups of pts, which achieved statistical significance only in the DL0 group. Differences between the two pts' groups were not significant (DL1–3 vs. DL0, p = 0.49) (). The increase in this cell subpopulation seems to be related to the presence of immune-adjuvant cytokines in the GOLFIG regimen and not to the TSPP vaccination.
Cytokine analysis
A cytokine analysis performed on the serum of these pts showed a completely different profile between the two groups of pts receiving concomitant and sequential treatment module. Our analysis showed no changes in TNFα, IL12 levels (data not shown), IL6 and IL8 () while, a significant and progressive increase in IFNγ and IL-4 was observed in the pts who received the concomitant treatment (DL1–3) (). In the same group, there was also a trend increase in IL10, which did not achieve statistical significance probably, for the small sample of pts. We finally, observed a significant increase in IL17 levels during the maintenance treatment in both group of pts which seems strictly related to the TSPP vaccination (). The ability of TSPP vaccination to increase the precursors frequency of TSPP specific CTL precursors was demonstrated by IFNγ ELISPOT assay in the previous study and was not correlated with either dosage of combined treatment [22 and data not shown].
Discussion
Our results confirm the safety and immune-modulating activities of TSPP in concomitant and sequential combination with GOLFIG chemo-immunotherapy. The MTD of this peptide could not be identified, while its MEBD was recognized at the dosage of 300 µg which was associated with the highest frequency of biomodulatory effects and longer survival. TSPP vaccination was associated to a high frequency of local immune-reactions at the vaccine injection site, rhinitis, conjunctivitis, and poly-arthralgia as shown in the previous study.Citation22 TSPP vaccination did not increase the frequency and severity of the adverse events associated with the GOLFIG chemo-immunotherapy, which occurred with the same frequency in the groups of pts who received either the concomitant or the sequential treatment modules. On the whole, the frequency of adverse events was similar to that reported in previous clinical trials investigating the GOLFIG regimen alone in pretreated mCRC pts.Citation15,19,20 The results of the present study suggest that the TSPP vaccine in combination with GOLFIG poly-chemotherapy is a feasible treatment for pretreated mCRC pts. The sequential treatment appeared to be more active than the concomitant in terms of OS (p = 0.049), and in both cases, it was not affected by k-ras mutational status and HLA-A2 haplotype . These results appear intriguing, although with significant limitation related to the small number of enrolled pts. We have previously demonstrated that the GOLFIG regimen is a very active treatment, that enhances TS specific CTL precursors and promotes an effective anticancer CTL response correlated with the occurrence of autoimmunity and longer survival in mCRC pts.Citation20,21 These features lead us to believe that the GOLFIG regimen, which combines poly-chemotherapy and immune-adjuvant cytokines, when supported by TSPP vaccination, in addition to a significant reduction of tumor burden, could also promote a mechanism of antigen cascade and cross-presentation triggering an efficient specific immune-response with poly-antigen specificity.
In the present study, TSPP vaccination given concomitantly with GOLFIG treatment was associated with systemic and clinical signs of immune-activation. This assumption was supported by the finding of a coordinate and progressive rise in lymphocytes, monocytes, and appearance of AAbs such as ANA, ENA pANCA e cANCA which are associated with the most common autoimmune-diseases. Consistent with the last finding, we also detected clinical signs of autoimmunity like poly-artharalgia, hypothyroidism, rhinitis, conjunctivitis, and fever in some pts. Some of these events were sporadically observed also in pts who received GOLFIG chemo-immunotherapy alone, as shown in previous studies,Citation20,21,26 and similarly, in the pts enrolled in the DL0 group of the present trial, prior receiving TSPP vaccination. By comparing the concomitant with sequential treatment groups, we also recorded a significant difference in term of systemic inflammatory status with much higher serum level of CRP e ESR in the first group. This finding could be indicative of chronic inflammation which, in turn, may affect the immunological activity of TSPP vaccination as described in literature.Citation27-31 The reasons explaining the difference in inflammatory status in the two groups of pts are not clear. It could be either related to an effect of the combined treatment or just to a specific feature of the pts enrolled in the study and deserves to be further investigated with a more homogenous patient population. In line with previous results performed on mCRC pts who had received the GOLFIG regimen, we also detected an increase in Tregs in both patient groups which is lost on the long term. The treatment associated-rise in Tregs, as reported by other authors,Citation32 can be considered as an inhibitory feedback response to an efficient antigen specific immune-reaction in order to avoid dangerous over-reactions and auto-immunity, which mainly occur in a chronic inflammatory status under selective cytokine pressure, or dependent by the IL-2 administration.Citation27-32 In both groups of pts, Treg rise was however, lost after the first 10–12 treatment courses, as probably caused by GOLFIG regimen.Citation15,20 This finding, together with lower systemic inflammatory status, could contribute to explain the more significant antitumor activity of TSPP vaccine in the sequential treatment group (DL0). In fact, these pts received vaccination when the Tregpopulation is exhausted and therefore, unable to affect the immunological activity of TSPP.Citation27,28,32 The results of the immunological analysis, which showed a general treatment-associated increase in peripheral lymphocyte counts, provides a further support to this hypothesis. In this context, there was no difference in the percentages of CD3+CD4+ or CD3+CD8+ T cells, however, in the DL0 group we found an increase of the Tem subset during TSPP vaccination (started after 12 GOLFIG courses), which was not detected in the concomitant treatment group. This is a very efficient CTL subset which derives by Tcmmaturation in the presence of immune-specific micro-environmental conditions (cytokines, chemokines, adhesion molecules, etc.).Citation33-37 This hypothesis is supported in our pts, by the data concerning the treatment-related increase of IL-17, a cytokine which is able to sustain a local immune-reaction in the tumor site. In our pts, we also detected a treatment-related increase of IL-4 and trend increase of IL-10, which could be related to Treg stimulation and/or of an intrinsic ability of TSPP vaccine to promote a Th2 humoral response as confirmed by a systemic progressive increase of AAbs in these pts. Additionally, we also found a significant increase in IFNγ, a Th1 cytokine, which is strongly involved in the antigen specific CTL response in the DL1–3 group pts. A last consideration concerns the expansion of peripheral NKs in both groups of pts during the first 10 treatment courses. This finding, which seems to be sustained by GM-CSF and IL-2 cytokines, both present in the GOLFIG regimen, suggests that this treatment is a valid support to TSPP vaccination. NK cells in fact, together with an innate ability to kill metastatic cancer cells, are also able to produce IFNγ and enhance the antigen presenting ability of peripheral DCs.Citation38 We can conclude that TSPP-vaccination in combination with the GOLFIG chemo-immunotherapy is safe and immune-biologically active in pretreated mCRC pts. We have also provided preliminary evidence of a promising antitumor effect in both groups of pts receiving either the concomitant or the sequential treatment. The latter group of pts in particular, showed an unexpected PFS and OS median of 7 and 16 mo, respectively, which resulted significantly much longer than that reported in other clinical trials investigating newest drugs in pre-treated mCRC pts, which rarely show a median PFS and OS longer of 2 and 6 mo, respectively. We believe that our results provide the rationale to design further trials in mCRC pts aimed to investigate the antitumor activity of TSPP vaccine in pts who have received a frontline chemo-immunotherapy line.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Acknowledgment
We wish to thank the head of nurse team, Dr G.Fruscoloni; the distinguished nurse specialists M. Bianchi, N. Cicatelli, S. Bacci, D. Graziuso, and L. Spito; and the medical staff and residents who offered the best care and management to trial patients Finally, we wish to thank all patients and their families who enthusiastically participated to the study.
Funding
This study was supported by the Italian Ministry of Health “Ricerca finalizzata 2010 “RF-2010–2313550 and the “Associazione culturale Federico II,” Siena.
References
- Franks HA, Wang Q, Patel PM. New anticancer immunotherapies. Anticancer Res 2012 Jul; 32(7):2439-53; PMID:22753700
- Mellstedt H, Vansteenkiste J, Thatcher N. Vaccines for the treatment of non-small cell lung cancer: investigational approaches and clinical experience. Lung Cancer 2011 Jul; 73(1):11-7 Epub 2011 Apr 6. Review; PMID:21474197; http://dx.doi.org/10.1016/j.lungcan.2011.02.023
- Buonaguro L, Petrizzo A, Tornesello ML, Buonaguro FM. Translating tumor antigens into cancer vaccines. Clin Vaccine Immunol 2011 Jan; 18(1):23-34 Epub 2010 Nov 3. Review; PMID:21048000; PMID:24316553; http://dx.doi.org/10.1128/CVI.00286-10
- Baxevanis CN, Perez SA, Papamichail M. Cancer immunotherapy. Crit Rev Clin Lab Sci 2009; 46(4):167-89 Review; PMID:19650714; http://dx.doi.org/10.1080/10408360902937809
- Mocellin S, Pilati P, Nitti D. Peptide-based anticancer vaccines: recent advances and future perspectives. Curr Med Chem 2009; 16(36):4779-96 Review; PMID:19929787; http://dx.doi.org/10.2174/092986709789909648
- Scardino A, Alimandi M, Correale P, Smith SG, Bei R, Firat H, Faure O, Graf-Dubois S, Marrocco J, Chouaib S et al. A polyepitope DNA vaccine targeted to HER2/ErbB-2 elicits a broad range of human and murine CTL effectors to protect against tumor challenge. Cancer Res 2007 Jul 15; 67(14):7028-36; PMID:17638916; http://dx.doi.org/10.1158/0008-5472.CAN-06-3998
- Francini G, Scardino A, Kosmatopoulos K, Lemonnier FA, Campoccia G, Sabatino M, Pozzessere D, Petrioli R, Lozzi L, Neri P et al. High-affinity HLA-A(*)02.01 peptides from parathyroid hormone-related protein generate in vitro and in vivo antitumor CTL response without autoimmune side effects. J Immunol 2002 Nov 1; 169(9):4840-9; PMID:12391194; http://dx.doi.org/10.4049/jimmunol.169.9.4840
- Correale P, Sabatino M, Cusi MG, Micheli L, Nencini C, Pozzessere D, Petrioli R, Aquino A, De Vecchis L, Turriziani M et al. In vitro generation of cytotoxic T lymphocytes against HLA-A2.1-restricted peptides derived from human thymidylate synthase. J Chemother 2001 Oct; 13(5):519-26; PMID:11760216; http://dx.doi.org/10.1179/joc.2001.13.5.519
- Correale P, Del Vecchio MT, Di Genova G, Savellini GG, La Placa M, Terrosi C, Vestri M, Urso R, Lemonnier F, Aquino A et al. Five-fluorouracil-based chemotherapy enhances the antitumor activity of a thymidylate synthase-directed polyepitopic peptide vaccine. J Natl Cancer Inst 2005 Oct 5; 97(19):1437-45; PMID:16204693; http://dx.doi.org/10.1093/jnci/dji188
- Rustum YM. Thymidylate synthase: a critical target in cancer therapy? Front Biosci 2004 Sep 1; 9:2467-73. Review; PMID:15353299; http://dx.doi.org/10.2741/1408
- Van Der Wilt CL, Peters GJ. New targets for pyrimidine antimetabolites in the treatment of solid tumours. One: Thymidylate synthase. Pharm World Sci 1994 Apr 15; 16(2):84-103; PMID:7518280; http://dx.doi.org/10.1007/BF01880660
- Ligabue A, Marverti G, Liebl U, Myllykallio H. Transcriptional activation and cell cycle block are the keys for 5-fluorouracil induced up-regulation of human thymidylate synthase expression. PLoS One 2012; 7(10):e47318. Epub 2012 Oct 9; PMID:23056627; http://dx.doi.org/10.1371/journal.pone.0047318
- Correale P, Del Vecchio MT, La Placa M, Montagnani F, Di Genova G, Gori Savellini G, Terrosi C, Mannucci S, Giorgi G, Francini G et al. Chemotherapeutic drugs may be used to enhance the killing efficacy of human tumor antigen peptide specific CTLs. J Immunother 2008; 31:132-47; PMID:18481383; http://dx.doi.org/10.1097/CJI.0b013e31815b69c8
- Correale P, Cusi MG, Del Vecchio MT, Aquino A, Prete SP, Tsang KY, Micheli L, Nencini C, La Placa M, Montagnani F et al. Dendritic cell-mediated cross-presentation of antigens derived from colon carcinoma cells exposed to a highly cytotoxic multidrug regimen with gemcitabine, oxaliplatin, 5-fluorouracil, and leucovorin, elicits a powerful human antigen-specific CTL response with antitumor activity in vitro. J Immunol 2005 Jul 15; 175(2):820-8; PMID:16002679; http://dx.doi.org/10.4049/jimmunol.175.2.820
- Correale P, Cusi MG, Tsang KY, Del Vecchio MT, Marsili S, Placa ML, Intrivici C, Aquino A, Micheli L, Nencini C et al. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer patients. J Clin Oncol 2005 Dec 10; 23(35):8950-8. Epub 2005 Aug 1; PMID:16061910; http://dx.doi.org/10.1200/JCO.2005.12.147
- Correale P, Aquino A, Giuliani A, Pellegrini M, Micheli L, Cusi MG, Nencini C, Petrioli R, Prete SP, De Vecchis L et al. Treatment of colon and breast carcinoma cells with 5-Fluorouracil enhances expression of carcinoembryonic antigen and to HLA-A(*)02.01 restricted, CEA-peptide-specific cytotoxic T cells in vitro. Int J Cancer 2003 Apr 10; 104(4):437-45; PMID:12584740; http://dx.doi.org/10.1002/ijc.10969
- Correale P, Marra M, Remondo C, Migali C, Arcuri FP, Carducci A, Loiacono L, Abruzzese A, Tagliaferri P, Caraglia M. Cytotoxic drugs up-regulate Epidermal Growth Factor Receptor (EGFR) expression in colon cancer cells and enhance their susceptibility to EGFR-targeted Antibody-Dependent-Cell-mediated-Cytotoxicity (ADCC). Eur J Cancer Jun 2010; 46(9):1703-11, IF= 4.454; PMID:20399639; http://dx.doi.org/10.1016/j.ejca.2010.03.005
- Correale P, Botta C, Cusi MG, Del Vecchio MT, De Santi M, Gori Savellini G, Bestoso E, Apollinari S, Ginanneschi C, Mannucci S et al. Cetuximab −/− chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int J Cancer 2012 Apr 1; 130(7):1577-89 Epub 2011 Aug 16; PMID:21618510; http://dx.doi.org/10.1002/ijc.26181
- Botta C, Correale P. Cetuximab-based immune-chemotherapy enhances antigen presentation and cytotoxic T cell activity in colorectal cancer patients. J Immunotherapy 2012 Jun; 35(5):440-7; PMID:22576349; http://dx.doi.org/10.1097/CJI.0b013e31825943aa
- Correale P, TagliaferriP, Fioravanti A, Del VecchioMT, RemondoC, Montagnani F, RotundoMS, Ginanneschi C, Martellucci I, Francini E et al. Immunity feedback and clinical outcome in colon cancer patients undergoing chemo-immunotherapy with Gemcitabine + FOLFOX followed by subcutaneous Granulocyte Macrophage-Colony Stimulating Factor and Aldesleukine (GOLFIG-1 trial). Clin Cancer Res 2008; 14(13):4192-9; PMID:18593999; http://dx.doi.org/10.1158/1078-0432.CCR-07-5278
- Correale P, Botta C, Rotundo MS, Guglielmo A, Conca R, Licchetta A, Pastina P, Bestoso E, Ciliberto D, Cusi MG et al. Immune-boost with gemcitabine, oxaliplatin, levofolinate, 5-flurouracil, granulocyte/macrophage colony-stimulating-factor (GM-CSF) and aldesleukine (GOLFIG) enhances progression-free and overall-survival over FOLFOX chemotherapy in metastatic colorectal cancer patients: GOLFIG-2 multi-centric open label randomized phase III trial. J Immunother 2014 Jan; 37(1):26-35; PMID:24316553; http://dx.doi.org/10.1097/CJI.0000000000000004
- Cusi MG, Botta C, Pastina P, Rossetti MG, Dreassi E, Guidelli GM, Fioravanti A, Martino EC, Gandolfo C, Pagliuchi M, Basile A, Carbone SF, Ricci V, Micheli L, Tassone P, Tagliaferri P, Pirtoli L, Correale P. Phase I trial of thymidylate synthase poly-epitope peptide (TSPP) vaccine in advanced cancer patients. Cancer Immunol Immunother. 2015 Sep; 64(9):1159-73; PMID:26031574; http://dx.doi.org/10.1007/s00262-015-1711-7.
- CorrealeP, Campoccia G, Tsang KY, Micheli L, CusiG, Sabatino M, BruniG, SestiniS, PetrioliR, Pozzessere D et al. Recruitment of dendritic cells and enhanced antigen specific immune-reactivity in cancer patients treated with hrGM-CSF (molgramostim) and hr IL-2: results from a Phase Ib Clinical Trial. Eur J Cancer 2001; 37 (7):892-902; PMID:11313178; http://dx.doi.org/10.1016/S0959-8049(01)00063-6
- Czerkinsky CC, Nilsson LA, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Met 1983; 65 (1-2):109-21; PMID:6361139; http://dx.doi.org/10.1016/0022-1759(83)90308-3
- Moodie Z, Price L, Gouttefangeas C, Mander A, Janetzki S, Lower M, Welters MJ, Ottensmeier C, van der Burg SH, Britten CM. Response definition criteria for ELISPOT assays revisited. Cancer Immunol Immunother 2010; 59 (10):1489-501; PMID:20549207; http://dx.doi.org/10.1007/s00262-010-0875-4
- Correale P, Bertoldi I, Miracco C, FioravantiA, Francini G. Occurrence of discoid lupus erythematousus in a good responder colon carcinoma patient receiving chemo-immunotherapy. J Chemother. 2008 Apr; 20(2):278-81. IF= 0.922; PMID:18467257; http://dx.doi.org/10.1179/joc.2008.20.2.278
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454 (7203):436-44; PMID:18650914; http://dx.doi.org/10.1038/nature07205
- Predina J, Eruslanov E, Judy B, Kapoor V, Cheng G, Wang LC, Sun J, Moon EK, Fridlender ZG, Albelda S et al. Changes in the local tumor microenvironment in recurrent cancers may explain the failure of vaccines after surgery. Proc Natl Acad Sci USA 2013; 110 (5):E415-424; PMID:23271806; http://dx.doi.org/10.1073/pnas.1211850110
- Botta C, Barbieri V, Ciliberto D, Rossi A, Rocco D, Addeo R, Staropoli N, Pastina P, Marvaso G, Martellucci I et al. Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non-small cell lung cancer patients. Cancer Biol therapy 2013; 14 (6):469-75; PMID:23760488; http://dx.doi.org/10.4161/cbt.24425
- Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC cancer 2013; 13:158; PMID:23530866; http://dx.doi.org/10.1186/1471-2407-13-158
- Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014; 106 (6):dju124; PMID:24875653; http://dx.doi.org/10.1093/jnci/dju124
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008; 133 (5):775-87; PMID:18510923; http://dx.doi.org/10.1016/j.cell.2008.05.009
- Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. Eur J Immunol 2009; 39 (8):2076-82; PMID:19672903; http://dx.doi.org/10.1002/eji.200939722
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999; 99 (1):23-33; PMID:10520991; http://dx.doi.org/10.1016/S0092-8674(00)80059-8
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004; 22:745-63; PMID:15032595; http://dx.doi.org/10.1146/annurev.immunol.22.012703.104702
- Correale P, Rotundo MS, Botta C, Del Vecchio MT, Ginanneschi C, Licchetta A, Conca R, Apollinari S, De Luca F, Tassone P et al. Tumor infiltration by T lymphocytes expressing chemokine receptor 7 (CCR7) is predictive of favorable outcome in patients with advanced colorectal carcinoma. Clin Cancer Res 2012; 18 (3):850-7; PMID:22142823; http://dx.doi.org/10.1158/1078-0432.CCR-10-3186
- Correale P, Rotundo MS, Botta C, Del Vecchio MT, Tassone P, Tagliaferri P. Tumor infiltration by chemokine receptor 7 (CCR7)(+) T-lymphocytes is a favorable prognostic factor in metastatic colorectal cancer. Oncoimmunol 2012; 1 (4):531-2; PMID:22754775; http://dx.doi.org/10.4161/onci.19404
- Terrazzano G, Carbone E. NK cells blur the frontier between innate and acquired immunity. Front Immunol 2012; 3:400; PMID:23293641; http://dx.doi.org/10.3389/fimmu.2012.00400
