ABSTRACT
Tumor-associated macrophages (TAM) play a controversial role in epithelial–mesenchymal transition (EMT) and prognosis of colorectal cancer (CRC). In particular, the microlocalization, polarization and prognostic impact of TAM in the immediate environment of invading CRC cells has not yet been established. To address this clinically relevant question, intraepithelial (iCD68) and stromal macrophages (sCD68), M1-macrophages (iNOS), M2-macrophages (CD163), cytokeratin-positive cancer cells (tumor buds) and expression of the anti-phagocytic marker CD47 were investigated in primary tumors of 205 well-characterized CRC patients. Cell-to-cell contacts between tumor buds and TAM were detected using high-resolution digital scans. The composition of the tumor microenvironment was analyzed with clinicopathological and molecular features. High CD68 counts predicted long term overall survival independent of microlocalization (iCD68 p=0.0016; sCD68 p=0.03), pT, pN, pM and post-operative therapy. CD68 infiltration correlated with significantly less tumor budding (iCD68 p=0.0066; sCD68 p=0.0091) and absence of lymph node metastasis (sCD68 p=0.0286). Cell-to-cell contact of sCD68 and invading cancer cells was frequent and ameliorated the detrimental prognostic effect of the tumor budding phenotype. Subgroup analysis identified long-term survival with CD47 loss and predominance of CD163+ M2 macrophages (p = 0.0366). CD163+ macrophages represented 40% of the total population, and positively correlated with total CD68 macrophage numbers (r[CD68/CD163] = 0.32; p = 0.0001). Strong CD163 infiltration predicted lower tumor grade (p = 0.0026) and less lymph node metastasis (p = 0.0056). This study provides direct morphological evidence of an interaction between TAM and infiltrating cancer cells. The prognostic impact of TAM is modulated by phenotype, microlocalization and the expression of anti-phagocytic markers in CRC.
Introduction
Tumor-immune cell interaction is an important field of research regarding prognosis in CRC. A shift of perspective away from the tumor alone toward an integrated analysis of pro- and antitumoral factors has led to a new road map for the understanding of cancer progression and the development of novel therapeutic approaches.Citation1 TAM assume a central role in the modulation of the tumor microenvironment and maintenance of the cancer stem cell niche and come in several different flavorsCitation2: M1 macrophages express pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-1 and IL-12 and have been assigned a high phagocytic activity and the capability to drive a potent antitumoral Th1 response.Citation3 M2 macrophages are characterized by expression of scavenger receptors such as CD163 and the ability to induce Th2 T-cell polarization and Foxp3-positive regulatory T cells.Citation4 Further, M2 macrophages may contribute to stromal remodeling, immune escape, cancer progression and metastasis.Citation5 The specific polarization of TAM may therefore skew the tumor microenvironment toward tumor rejection (M1) or favor immunotolerance, neoangiogenesis and stromal remodeling (M2).Citation6
Specific stromal gene expression signatures may render the tumor microenvironment permissible for single cancer cell invasion.Citation7 In CRC, the presence of single cancer cells or clusters of up to five cells in the tumor stroma (tumor budding) can be detected histologically and may be related to EMT.Citation8 This is supported by studies demonstrating the acquisition of a highly motile and invasive phenotype and the expression of markers related to the activation of Wnt-signaling in tumor budding cells.Citation8Citation9 In vitro studies have provided further evidence of a remarkable phenotypic plasticity of epithelial cancer cells in culture including the disintegration of cell–cell adhesions, loss of epithelial polarity, cytoskeletal remodeling and resolution of cell–matrix adhesion.Citation10 However, controversial data has been recently provided by genetic analyses that have assigned EMT-like gene expression signatures in CRC to cancer associated fibroblasts rather than invading cancer cells.Citation7,11 Conclusive evidence of EMT in primary CRC is therefore still lacking.
For CRC patients, the presence of tumor budding is associated with poor overall and disease-free survival, lymph node and distant metastasis and poor response to radio-chemotherapy.Citation12,13 Importantly, tumor progression through EMT may be supported by the tumor immunoenvironment: Transforming growth factor-β (TGFβ), produced by M2-polarized TAM can sustain tumor initiation and progression;Citation14 IL-11 and IL-6 emitted by cancer-associated fibroblasts and myeloid cells support CRC growth and survival.Citation15 A close interaction of infiltrating cancer cells with macrophage populations is also supported indirectly by the detection of tumor-loaded macrophages in the circulation of CRC patients.Citation16 However, the frequency of direct cell–cell interactions of macrophages and invading cancer cells in the tumor microenvironment of CRC has not been previously investigated. In particular, the impact of macrophage polarization on the formation of single cell invasion (tumor budding) in the tumor microenvironment remains to be addressed.
The balance of pro- and anti-phagocytic factors may further contribute to the susceptibility of cancer cells to immune-mediated destruction. CD47, a protein involved in self-/non-self-discrimination is broadly expressed on non-neoplastic tissues and mediates a “don't eat me signal” to tissue resident macrophages and infiltrating monocytes.Citation17 Loss of CD47 is frequent in epithelial and hematological malignancies and has been associated with a poor prognostic outcome.Citation18,Citation19 Further, CD47 has been associated with increased migratory capability of invading CRC cellsCitation20 and may represent a promising target for precision therapy.Citation19 This dual function makes CD47 a highly interesting biomarker for research in the context of the tumor budding phenotype and macrophage-mediated immune functions.
In the current study, we investigate the microlocalization and polarization of TAM in the tumor microenvironment of invading cancer cells. We identify direct cell-to-cell interactions by quantifying cellular contacts of TAM with tumor budding cells. Further, we evaluate the relationship of TAM infiltration and CD47 with clinicopathological and molecular features. Last, we define prognostic groups of CRC patients based on M1/M2 polarization and CD47 expression.
Results
Tumor buds assessed in the tissue microarray
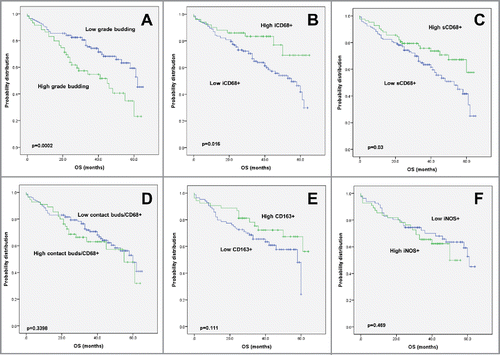
The association of tumor budding counts with clinicopathological features was tested using the tissue microarray approach. A high budding count as determined by tissue microarray analysis (Table S1) was associated with aggressive tumor features including a higher tumor grade (p < 0.0001), more advanced pT-stage (p = 0.0008), lymph node metastasis (p = 0.0015), lymphatic (p = 0.0001) and venous invasion (p = 0.0007). Patients with high budding counts per TMA spot had a significantly worse survival outcome (p = 0.0002) ().
Figure 1. Kaplan–Meier survival analysis, macrophage infiltrates and phenotypes. Survival curves of patients with primary CRC in correlation with (A) tumor budding, (B) iCD68 and (C) sCD68 counts. (D) Survival of CRC patients in correlation with the frequency of cell-to-cell contacts between tumor buds and CD68+ macrophages in the tumor microenvironment. Survival curves of CRC patients with primary CRC dependent on (E) CD163+ and (F) iNOS+ macrophage counts.
Intratumoral and stromal CD68+ counts
Next, we evaluated both iCD68+ and sCD68+ counts per tumor (). More frequent iCD68+ was correlated to significantly less tumor budding (p = 0.0066) and was more common in larger tumors (p = 0.0528). sCD68+ counts were linked to a larger tumor diameter (p = 0.0364), significantly less lymph node metastasis (p = 0.0286) and less tumor budding at the invasion front (p = 0.0091) (). Patients with elevated sCD68 counts less frequently received post-operative therapy (p = 0.0008). Higher counts of both iCD68 (p = 0.0016) and sCD68 (p = 0.03) were associated with an improved overall survival time (). Moreover, both iCD68 and sCD68 maintained their prognostic effect even after adjustment for pT, pN, pM and post-operative therapy (). In order to verify the robustness of the CD68 data by visual counting, all TMA slides were digitally analyzed using quantification software. Results are found in (Fig. S1). Excellent correlations were found between CD68 counting by eye and by software. Performance was similar for spots on TMA slide 1 (n = 317, r = 0.86) and on TMA slides 2–3 (n = 371, r = 0.88).
Figure 2. Representative images of macrophage infiltrates in the colorectal microenvironment (A) Intraepithelial and (B) stromal CD68+ macrophages (x100 each) (C) Direct cell-to-cell contacts between CD68+ macrophages and cytokeratin-positive tumor buds (x400) (D) Engulfment of cancer cells, as seen with pan-cytokeratin and CD68+ double immunohistochemistry (x400) (E) CD163+ M2 macrophage infiltrates (x100) (F) iNOS (brown) / MCT (red) double stain highlighting iNOS+/MCT− M1 macrophages (x100) (G) iNOS (brown) / CD163 (red) double stain showing co-existence of M1- and M2- polarized macrophages (x300) (H) Representative images from a tumor with a CD163+ (red) dominant M2-macrophage infiltrate and (I) from a tumor with an iNOS+ (brown) M1-macrophage infiltrate (CD163/iNOS double stain; x300).
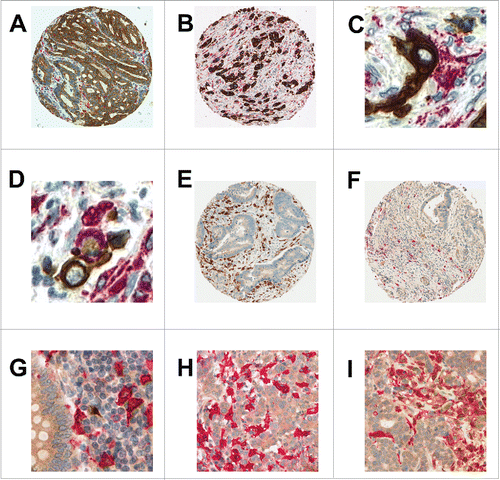
Table 1. Association of intratumoral (i) and stromal (s) CD68+ cells (normalized by stroma per punch) with clinicopathological and molecular features of CRC (n = 201).
Table 2. Multivariable Cox regression analysis of iCD68 and sCD68 counts in CRC adjusting for pT, pN, pM and post-operative therapy.
Contact between tumor buds and CD68+ macrophages
We observed direct cell-to-cell contacts between CD68+ macrophages and tumor buds in 63/201 (31%) of the cases evaluated (). Frequent contact between buds and CD68+ cells was found in tumors with higher grade (p = 0.0307), and lymph node metastasis (p = 0.0341), as well as in post-operatively untreated patients (p = 0.039), MMR-deficient cancers (p = 0.014) and those with BRAF mutations (p = 0.0109) (). There was no impact on survival (), suggesting a tempering of tumor aggression when direct contact between buds and CD68+ macrophages occurs. In addition, an engulfment of cancer cell fragments was frequently seen in 97.1% of cases using pan-cytokeratin and CD68+ double immunohistochemistry () but did not show an impact on clinicopathological features or survival.
Table 3. Association of CD68+ buds contact, stromal CD163 and iNOS (normalized by percent stroma per TMA spot) with clinicopathological features.
Predominant macrophage phenotype in colorectal cancers
We next assessed CD163+ and iNOS+/MCT− macrophages in the tumor microenvironment. shows immunohistochemistry stains for each marker as well as a combined CD163+/iNOS+ double staining, highlighting the minimal overlap between the two macrophage populations. The ratio of CD163+ to CD68+ indicates that 40% of all CD68+ macrophages were CD163+, whereas 60% were iNOS+ (p = 0.0019). Of note, iNOS expression was also noted in CRC cells () confirming previous observations.Citation21
Case-by-case analysis showed that there was a significant positive correlation between sCD68+ and CD163 (r = 0.32, p = 0.0001) but not between sCD68+ and iNOS (r = −0.05). Moreover, high CD163+ counts were associated with lower tumor grade (p = 0.0026), significantly less lymph node metastasis (p = 0.0056), and showed a trend toward less advanced pT-stage (p = 0.0641) and absence of lymphatic invasion (p = 0.0697). Patients with high CD163+ counts were less likely to require post-operative therapy (p = 0.0569) (). High CD163+ counts were linked to a KRAS wild-type genotype (p = 0.0068). Although not significant, a survival benefit can be seen from the Kaplan–Meier curve in (p = 0.111) in patients with high CD163+, but not iNOS.
CD47 on overall survival and correlation with macrophages in the tumor microenvironment
underlines data from 183 patients with evaluable expression for CD47 positive tumor cells. CD47 was expressed at high levels in 47% of cases under study (Fig. S2). Indeed, a significantly more frequent venous invasion (p = 0.0064) and tumor budding at the invasion front (p = 0.0491) is observed in patients with high CD47 expression. Although not reaching significance, distant metastatic disease (p = 0.0822) and lymphatic vessel invasion (p = 0.0868) were more common in CD47-expressing cancers.
Table 4. Association of CD47 expression with clinicopathological and molecular features (n = 182).
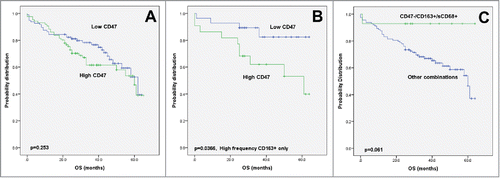
In univariate analysis, no association between CD47 and overall survival could be seen (). However, an effect modification by CD163 was observed. Namely, in tumors with a high CD163+ count only, CD47 expression had a major unfavorable effect on overall survival (p = 0.0366, ). In contrast, patients with a CD47 negative tumor in a CD163+ and sCD68+ microenvironment had an excellent prognosis ().
Figure 3. Kaplan–Meier survival analysis, CD47 expression. (A) Survival curves of patients with primary CRC in correlation with high or low CD47 expression. (B) Subgroup analysis of CD47 expression in tumors with strong CD163+ macrophage infiltration. (C) Subgroup analysis of patients with CD47 negative tumors and strong macrophage infiltration in comparison to all other marker combinations.
Discussion
Macrophages assume a controversial role in the tumor microenvironment as both pro- and antitumoral effects have been described. The present study investigated TAM microlocalization, polarization and expression of the anti-phagocytic molecule CD47 in the immediate environment of invading cancer cells. Based on a comprehensive histopathological analysis, we identify novel factors that may contribute to the differential prognostic impact of TAMs in CRC.
First, we investigated the microlocalization of CD68+ macrophages in the tumor microenvironment of CRC with a special focus on cellular contacts with infiltrating cancer cells. Strong sCD68 infiltration was correlated with favorable clinicopathological features including less lymph node metastasis and less tumor budding at the invasion front. Independently of microlocalization in the tumor stroma or cancer epithelium, higher counts of intratumoral macrophages were associated with an improved overall survival time. This data corroborates prognostic analyses on the favorable impact of overall CD68+ counts in CRC patients.Citation22,23 Interesting, however, is the absence of statistically significant differences for most of the prognostic factors associated with tumor budding in cases with strong cell–cell contact between macrophages and infiltrating cancer cells. This implies that macrophages may directly or indirectly counter cancer cell invasion in the tumor stroma. Indeed, Forssell and colleagues previously demonstrated that cellular contact between macrophages and tumor cells may inhibit cancer cell proliferation using functional assays but did not confirm the co-localization of TAM with invading cancer cells in the tumor microenvironment of CRC resection specimens.Citation22 In support of the in vitro observations, we now detected frequent cellular contacts between macrophages and infiltrating cancer cells in the stromal compartment. Further evidence for a close interaction of TAM with invading cancer cells is provided by the detection of tumor loaded macrophages in the tumor microenvironmentCitation16 and in blood samplesCitation24 of CRC patients. However, neither co-localization nor phagocytic uptake of tumor cell fragments provides further insight into mechanistic interaction. Validation of these observations in experimental studies is therefore recommended.
Second, we addressed the correlation of macrophage polarization with the tumor budding phenotype. We verified the mutual exclusivity of distinct CD163+ and iNOS positive populations using immunohistochemical double stains. For evaluation of M1 macrophages, a double staining technique using mast cell tryptase and iNOS was used. This was found to be highly beneficial for excluding iNOS-positive mast cells from analysis. Previous studies using iNOS as a marker have so far failed to take the potential contribution of mast cells to the iNOS-positive population into account.Citation25 This may explain why some divergent results have been published regarding the frequency and clinicopathological impact of the M1 and M2 phenotype in CRC. Interestingly, iNOS+ and CD163+ counts were strongly correlated in the tumor microenvironment, providing further evidence that TAM form a heterogeneous population that may change during tumor progression.Citation26 Importantly, iNOS-positive macrophage counts did not show an impact on clinicopathological features or survival in the present study. Instead, the positive associations of CD68+ infiltrates with favorable clinicopathological characteristics seem to be primarily driven by the presence of CD163+ macrophages. This counterintuitive finding is consistent with several published studies that have described a tendency toward more favorable outcome of CRC patients with increasing counts of M2 macrophages in the tumor microenvironment.Citation23,27 Based on mechanistic data, macrophage phenotypes may be highly plastic and modulated by pro-inflammatory cytokinesCitation26 and secreted factors from invading cancer cells.Citation28 As with regulatory T cells, the specific context, temporal kinetics and organ type may influence the prognostic impact of macrophage infiltrates in the immunoenvironment of solid tumors.Citation29 Mechanistic studies seem required to address the prognostic impact of TAM in CRC in the context of these variables. Further, a relatively low percentage of MMR-deficient tumors were detected in the present cohort (6.4%), which may limit the application of the present results to tumors with this molecular phenotype. We therefore recommend independent validation of the present data in CRC-patients with MMR-deficiency.
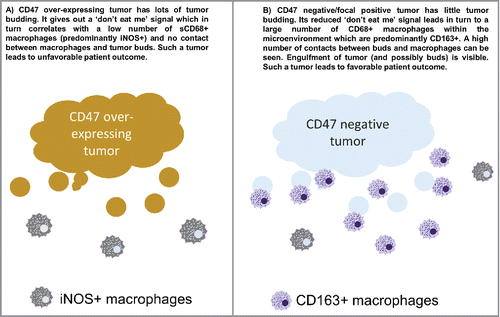
Last, we addressed the impact of the anti-phagocytic “don't eat me” molecule CD47 on macrophage populations, polarization and prognosis of CRC patients. As both tumor-related and host-related factors influence the prognosis of CRC patients, an integrative approach toward microenvironment studies may better capture the biological aggressiveness of CRC on a case-by-case basis. Based on the anti-phagocytic function of CD47, we hypothesized that tumors with an elevated CD47 expression would display decreased macrophage infiltration and an aggressive phenotype. In agreement with this, venous invasion and high grade tumor budding was frequently observed in CD47high tumors. Interestingly, CD47 expression also significantly modified the prognostic impact of TAM infiltration. In tumors with strong CD163 infiltration, CD47 expression had a major negative effect on survival, indicating that CD47+ tumor cells may be more resistant toward antitumoral effector mechanisms in a macrophage-rich tumor microenvironment [].
Conclusions
In summary, our results suggest a direct interaction of infiltrating cancer cells with TAM. Cellular contact and engulfment of tumor cell fragments is frequent in the microenvironment of CRC. Strong macrophage infiltration is an independent favorable prognostic indicator for CRC patients and may counter the aggressive tumor budding phenotype. This favorable prognostic impact of CD68+ infiltrates is maintained independent of macrophage polarization but is strongly modified by the expression of the anti-phagocytic molecule CD47 on colorectal tumors. Further experimental studies are recommended to decipher the mechanistic interaction of TAM with infiltrating cancer cells in the immunoenvironment.
Materials and methods
Patient cohort
Two hundred and one patients with primary CRC were entered into this study (Fig. S3). Patients were treated at the Fourth Department of Surgery, University of Athens, Greece between 2002 and 2007. Full histopathological re-review of tumor grade, histological subtype, pT, pN, pM, V and L classifications was conducted according to the TNM Classification of Malignant Tumors, 7th Edition (2009).Citation30 The total number of tumor buds in each TMA spot (diameter 0.6 mm) was counted using pan-cytokeratin stains.Citation31,32 The primary endpoint of interest was overall survival. Other clinical data retrieved from patient charts included age, tumor size, gender and post-operative therapy. No patients received any neoadjuvant treatment. Patient characteristics can be found in . This study was designed in accordance with the REMARK criteria.Citation33
Next-generation Tissue Microarray (ngTMA) construction
Tissue microarrays were constructed using the ngTMA approach.Citation34 For each patient, one hematoxylin and eosin (H&E) stained whole tissue slide containing representative regions of tumor center and invasion front was scanned (Pannoramic P250, 3DHistech, Budapest, Hungary). Using a tissue microarray annotation tool of 0.6 mm in diameter, slides were digitally annotated as follows: three regions of tumor center (blue); three regions of invasion front (yellow) and two regions of densest tumor budding (red), if available (Fig. S4).Citation35 Next, corresponding formalin-fixed (10% buffered formalin) paraffin-embedded tissue blocks were loaded into an automated tissue microarrayer (TMA Grandmaster, 3DHistech, Budapest, Hungary). The digital slides were aligned with the corresponding donor block. Annotated regions were cored from the donor block and transferred to the recipient ngTMA. Five different ngTMA blocks were produced resulting in 1608 spots for evaluation.
Immunohistochemistry
ngTMAs were sectioned at 3 µm. Immunohistochemistry was performed on an automated platform (Leica Bond RX, Leica Biosystems, Muttenz, Switzerland). Single immunohistochemistry for pan-cytokeratin, CD47, CD68, CD163 and iNOS were performed in addition to a double-immunohistochemistry for CD163 and iNOS, CD163 and CD68, iNOS and CD68, and iNOS and mast cell tryptase (MCT). iNOS/MCT double stains were used to support the differentiation of iNOS+/MCT+ mast cells from iNOS+/MCT−M1 macrophages. Details on antibodies and conditions are found in Table S2.
Normalization and scoring
Since each patient had multiple tumor cores taken from different regions within the tumor, the percentage of positive tumor cells across all cores was averaged. Both intraepithelial (i) and stromal (s) CD68+ macrophage counts were assessed. Cell–cell contacts between CD68+ macrophages and pancytokeratin-positive tumor budding cells in the stroma were quantified on high resolution digital scans (400x) using strict criteria: Cellular integrity, an identifiable nucleus and complete cytoplasmic (pancytokeratin, tumor buds) or membranous immunoreactivity (CD68) of each cell was required. A direct cell–cell linkage spanning at least one third of the cellular circumference was required to qualify for cellular contact between a tumor bud and stromal macrophages. For CD163 and iNOS, stromal infiltrates were counted on a numerical scale. Since a single tissue microarray spot may contain various degrees of stroma vs. tumor epithelial content, the percentage of stroma and tumor tissue per spot was recorded. Cell counts were normalized for tumor cell and stromal content of each spot. CD47 expression was scored by visual estimate as the percentage of tumor cells showing membranous reactivity in each spot; visual estimates were rounded to the nearest 5% and averaged for each case. Robustness of quantification procedures was assured by scoring of a subset of cases for each marker and marker combination by two independent observers (VHK and KC) in training sessions using a test TMA of 50 CRC cases. Disagreement was resolved using a multi-headed microscope preceding the application of the scoring combinations on the definite cohort. As CD68 was identified as an independent prognostic marker, inter-observer agreement was additionally assessed using HALO image analysis software (Indica Labs, Albuquerque, NM, USA) focusing on tumor spots (n = 688) distributed across three TMA slides. The algorithm was developed on slide 1 and applied to all three TMA slides (Fig. S1).
Assessment of KRAS, BRAF mutations and MMR status
BRAF (exon 15, V600E mutations) and KRAS (exon 2, codon 12 and 13) mutations were detected by pyrosequencing.Citation36 MMR status was based on expression of MLH1, MSH2, MSH6 and PMS2 by immunohistochemistry. Patients were considered MMR deficient when at least one protein was completely negative.
Statistical analysis
Spearman correlation coefficients (r) were used to determine the strength of the relationship between CD47, iCD68, sCD68, CD163 and iNOS. For consistency, scores were then dichotomized according to the mean into low and high groups. The Chi-Square test was used to determine the association of low and high cell counts with categorical variables and t-test for tumor size and age. Kaplan–Meier survival curves were plotted and survival time differences analyzed using the log-rank test. Multivariable Cox regression analysis was performed in order to determine the independent prognostic effect of the feature after adjustment for potential confounders. The proportional hazards assumption was verified. Hazard Ratios (HR) and 95% CI were used to determine effect size. Pearson's correlation coefficient was used to evaluate the strength of linear relationship between CD68 counts by a human observer and by software. All p values were two sided and considered significant when p < 0.05. Analyses were conducted on SPSS (V21) and SAS (V9.3, Cary, NC).
Ethics committee approval
The use of patient data has been approved by the local Ethics Committee at the University of Athens, Greece.
Disclosure of potential conflicts of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, royalties or patents received or pending.
Authors' contributions
VHK together with IZ conceived the study and study design, conducted the study, performed data interpretation and drafted the manuscript; IZ performed statistical analysis. KC scored immunohistochemistry, contributed to the study design and data interpretation, reviewed and approved the final manuscript. HD scored CD47 immunohistochemistry, critically reviewed and approved the final manuscript. LS performed digital image analysis, critically reviewed and approved the final manuscript. EK reviewed and categorized patient material and clinical data, reviewed and approved the final manuscript. AL contributed to the study design and data interpretation, critically reviewed and approved the final manuscript. All authors have reviewed and approved the final version to be published.
Supplemental_Files.zip
Download Zip (28.7 MB)Acknowledgments
The authors would like to express their sincere appreciation to Dr José Galvan, Dr Irene Centeno and Caroline Hammer from the Translational Research Unit, Institute of Pathology, University of Bern, Switzerland for excellent technical support and to Dr Christian Schürch for helpful discussion regarding the use of CD47 as a prognostic biomarker.
Funding
This study was kindly funded by the Bernese Cancer League and Oncosuisse (KFS 3294-08-2013).
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Raggi C, Mousa HS, Correnti M, Sica A, Invernizzi P. Cancer stem cells and tumor-associated macrophages: a roadmap for multitargeting strategies. Oncogene 2015; 35:671-82; PMID:25961921; http://dx.doi.org/10.1038/onc.2015.132
- Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med 2011; 9:216; PMID:22176642; http://dx.doi.org/10.1186/1479-5876-9-216
- Lau SK, Chu PG, Weiss LM. CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol 2004; 122:794-801; PMID:15491976; http://dx.doi.org/10.1309/QHD6YFN81KQ-XUUH6
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010; 11:889-96; PMID:20856220; http://dx.doi.org/10.1038/ni.1937
- Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol 2012; 2012:948098; PMID:22778768; http://dx.doi.org/10.1155/2012/948098
- Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, Sevillano M, Palomo-Ponce S, Tauriello DV, Byrom D et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet 2015; 47:320-9; PMID:25706628; http://dx.doi.org/10.1038/ng.3225
- Zlobec I, Lugli A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget 2010; 1:651-61; PMID:21317460; http://dx.doi.org/10.18632/oncotarget.199
- Bronsert P, Enderle-Ammour K, Bader M, Timme S, Kuehs M, Csanadi A, Kayser G, Kohler I, Bausch D, Hoeppner J et al. Cancer cell invasion and EMT marker expression: a three-dimensional study of the human cancer-host interface. J Pathol 2014; 234:410-22; PMID:25081610; http://dx.doi.org/10.1002/path.4416
- Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 2005; 24:5764-74; PMID:16123809; http://dx.doi.org/10.1038/sj.onc.1208927
- Isella C, Terrasi A, Bellomo SE, Petti C, Galatola G, Muratore A, Mellano A, Senetta R, Cassenti A, Sonetto C et al. Stromal contribution to the colorectal cancer transcriptome. Nat Genet 2015; 47:312-9; PMID:25706627; http://dx.doi.org/10.1038/ng.3224
- Lugli A, Karamitopoulou E, Zlobec I. Tumour budding: a promising parameter in colorectal cancer. Br J Cancer 2012; 106:1713-7; PMID:22531633; http://dx.doi.org/10.1038/bjc.2012.127
- Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R. Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol 2012; 25:1315-25; PMID:22790014; http://dx.doi.org/10.1038/modpathol.2012.94
- Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, Sevillano M, Nadal C, Jung P, Zhang XH et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell 2012; 22:571-84; PMID:23153532; http://dx.doi.org/10.1016/j.ccr.2012.08.013
- Putoczki TL, Thiem S, Loving A, Busuttil RA, Wilson NJ, Ziegler PK, Nguyen PM, Preaudet A, Farid R, Edwards KM et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell 2013; 24:257-71; PMID:23948300; http://dx.doi.org/10.1016/j.ccr.2013.06.017
- Faber TJ, Japink D, Leers MP, Sosef MN, von Meyenfeldt MF, Nap M. Activated macrophages containing tumor marker in colon carcinoma: immunohistochemical proof of a concept. Tumour Biol 2012; 33:435-41; PMID:22134871; http://dx.doi.org/10.1007/s13277-011-0269-z
- Chao MP, Weissman IL, Majeti R. The CD47-SIRPalpha pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol 2012; 24:225-32; PMID:22310103; http://dx.doi.org/10.1016/j.coi.2012.01.010
- Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr., van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009; 138:286-99; PMID:19632179; http://dx.doi.org/10.1016/j.cell.2009.05.045
- Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012; 109:6662-7; PMID:22451913; http://dx.doi.org/10.1073/pnas.1121623109
- Zhang Y, Sime W, Juhas M, Sjolander A. Crosstalk between colon cancer cells and macrophages via inflammatory mediators and CD47 promotes tumour cell migration. Eur J Cancer 2013; 49:3320-34; PMID:23810249; http://dx.doi.org/10.1016/j.ejca.2013.06.005
- Zafirellis K, Zachaki A, Agrogiannis G, Gravani K. Inducible nitric oxide synthase expression and its prognostic significance in colorectal cancer. APMIS 2010; 118:115-24; PMID:20132175; http://dx.doi.org/10.1111/j.1600-0463.2009.02569.x
- Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res 2007; 13:1472-9; PMID:17332291; http://dx.doi.org/10.1158/1078-0432.CCR-06-2073
- Algars A, Irjala H, Vaittinen S, Huhtinen H, Sundstrom J, Salmi M, Ristamaki R, Jalkanen S. Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer 2012; 131:864-73; PMID:21952788; http://dx.doi.org/10.1002/ijc.26457
- Japink D, Leers MP, Sosef MN, Nap M. CEA in activated macrophages. New diagnostic possibilities for tumor markers in early colorectal cancer. Anticancer Res 2009; 29:3245-51; PMID:19661342
- Edin S, Wikberg ML, Dahlin AM, Rutegard J, Oberg A, Oldenborg PA, Palmqvist R. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PloS one 2012; 7:e47045; PMID:23077543; http://dx.doi.org/10.1371/journal.pone.0047045
- Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, Olszewski MA. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio 2013; 4:e00264-13; PMID:23781069; http://dx.doi.org/10.1128/mBio.00264-13
- Nagorsen D, Voigt S, Berg E, Stein H, Thiel E, Loddenkemper C. Tumor-infiltrating macrophages and dendritic cells in human colorectal cancer: relation to local regulatory T cells, systemic T-cell response against tumor-associated antigens and survival. J Transl Med 2007; 5:62; PMID:18047662; http://dx.doi.org/10.1186/1479-5876-5-62
- Edin S, Wikberg ML, Rutegard J, Oldenborg PA, Palmqvist R. Phenotypic skewing of macrophages in vitro by secreted factors from colorectal cancer cells. PloS one 2013; 8:e74982; PMID:24058644; http://dx.doi.org/10.1371/journal.pone.0074982
- Banerjee A, Vasanthakumar A, Grigoriadis G. Modulating T regulatory cells in cancer: how close are we? Immunol Cell Biol 2013; 91:340-9; PMID:23567897; http://dx.doi.org/10.1038/icb.2013.12
- Sobin LH, Gospodarowicz MK, Wittekind C, International Union against Cancer. TNM classification of malignant tumours. Chichester, West Sussex, UK; Hoboken, NJ: Wiley-Blackwell, 2010.
- Karamitopoulou E, Zlobec I, Kolzer V, Kondi-Pafiti A, Patsouris ES, Gennatas K, Lugli A. Proposal for a 10-high-power-fields scoring method for the assessment of tumor budding in colorectal cancer. Mod Pathol 2013; 26:295-301; PMID:23018875; http://dx.doi.org/10.1038/modpathol.2012.155
- Horcic M, Koelzer VH, Karamitopoulou E, Terracciano L, Puppa G, Zlobec I, Lugli A. Tumor budding score based on 10 high-power fields is a promising basis for a standardized prognostic scoring system in stage II colorectal cancer. HumPathol 2013; 44:697-705; PMID:23159156; http://dx.doi.org/10.1016/j.humpath.2012.07.026
- Popat S, Houlston RS. Re: Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005; 97:1855; author reply -6; PMID:16368950; http://dx.doi.org/10.1093/jnci/dji445
- Zlobec I, Suter G, Perren A, Lugli A. A next-generation tissue microarray (ngTMA) protocol for biomarker studies. J Vis Exp 2014; 91:51893; 51893
- Zlobec I, Koelzer VH, Dawson H, Perren A, Lugli A. Next-generation tissue microarray (ngTMA) increases the quality of biomarker studies: an example using CD3, CD8, and CD45RO in the tumor microenvironment of six different solid tumor types. J Transl Med 2013; 11:104; PMID:23627766; http://dx.doi.org/10.1186/1479-5876-11-104
- Dawson H, Galvan JA, Helbling M, Muller DE, Karamitopoulou E, Koelzer VH, Economou M, Hammer C, Lugli A, Zlobec I. Possible role of Cdx2 in the serrated pathway of colorectal cancer characterized by BRAF mutation, high-level CpG Island methylator phenotype and mismatch repair-deficiency. Int J Cancer 2014; 134:2342-51; PMID:24166180; http://dx.doi.org/10.1002/ijc.28564