ABSTRACT
Neoplastic cells can escape immune control leading to cancer growth. Regulatory T cells (Treg), myeloid-derived suppressor cells (MDSC) and tumor-associated macrophages (TAM) are crucial in immune escape. TAM are divided based on their immune profile, M1 are immunostimulatory while M2 are immunosuppressive. Research so far has mainly focused on the intratumoral behavior of these cells. This study, on the other hand, explored the systemic changes of the key metabolites [IL-4 (interleukin), IL-13, arginase, IL-10, VEGF-A (vascular endothelial growth factor), CCL-2 (chemokine (C-C) motif ligand 2) and TGF-β (transforming growth factor)] linked to Treg, MDSC and TAM during the course of the disease in ovarian and fallopian tube cancer patients. Serum samples were therefore analyzed at diagnosis, after (interval)-debulking surgery and after chemotherapy (paclitaxel–carboplatin). We also determined galectin-1 (gal-1), involved in angiogenesis and tumor-mediated immune evasion. We found significantly lower levels of IL-10, VEGF-A, TGF-β and arginase and higher levels of gal-1 after chemotherapy compared to diagnosis. After debulking surgery, a decrease in IL-10 was significant. Gal-1 and CCL-2 appeared independent prognostic factors for progression-free and overall survival (OS) (multivariate analysis). These results will help us in the decision making of future therapies in order to further modulate the immune system in a positive way.
Abbreviations
| CBA | = | Cytometric Bead Array |
| CCL-2 | = | chemokine (C-C) motif ligand 2 |
| CD | = | cluster of differentiation |
| DAMPs | = | damage-associated molecular patterns |
| ELISA | = | Enzyme-Linked Immuno Sorbent Assay |
| FIGO | = | International Federation of Gynecology and Obstetrics |
| Gal-1 | = | galectin-1 |
| IDO | = | indoleamine 2,3 dioxygenase |
| IL | = | interleukin |
| iNOS | = | inducible nitric oxide synthase |
| MDSC | = | myeloid-derived suppressor cells |
| MHC | = | major histocompatibility complex |
| OS | = | overall survival |
| PFS | = | progression-free survival |
| STIC | = | serous tubal intraepithelial carcinomas |
| TAA | = | tumor associated antigens |
| TAM | = | tumor-associated macrophages |
| TGF-β | = | transforming growth factor β |
| Treg | = | regulatory T cells |
| VEGF | = | vascular endothelial growth factor |
Introduction
Ovarian cancer is the second most frequent pelvic gynecological cancer and the most common cause of gynecological cancer-associated death among women.Citation1 In most women, the disease is diagnosed in an advanced stage, which correlates with a poor prognosis and a high recurrence risk. The standard of care remains debulking surgery in combination with platin-based chemotherapy. This consists of either primary debulking surgery and adjuvant chemotherapy or neoadjuvant chemotherapy followed by interval debulking surgery, depending on FIGO stage and predictive factors concerning residual macroscopic disease after surgery.Citation2 Tubal cancer on the other hand, is very rare with an incidence of 0.41 cases per 100,000 women in the US. Since the discovery of the serous tubal intraepithelial carcinomas (STIC) and a recent review discovering only few differences between primary fallopian tube cancer and primary ovarian cancer, tubal cancer was and still is treated like ovarian cancer (For a review see refs 3–4).
Current evolutions in anticancer research have confirmed that the immune system can control cancer. If cells transform into (pre-) cancerous cells the host responds to the expressed tumor antigens and damage-associated molecular patterns (DAMPs) with an innate and adaptive immune response. This often leads to elimination of the neoplastic cells or to equilibrium. In this situation, tumor cells are not eliminated by the immune system, but reside in a dormant state.Citation5,6 Due to the continuous immune pressure, more immune-resistant tumor cells will arise. A myriad of events will occur: (1) tumor associated antigens (TAA) and major histocompatibility complex (MHC) molecules are lost; (2) chronic inflammation at the tumor site leads to continuous activation of peripheral T cells and induces the development of Treg. In the tumor microenvironment, certain chemokines such as CCL-2 and CCL-22 lead to the trafficking of Treg, MDSC and monocytes into the tumor. Further expansion of the Treg population is enhanced (5) through the presence of several immunosuppressive factors such as indoleamine 2,3 dioxygenase (IDO) and transforming growth factor β (TGF-β); (3) MDSC accumulate in the tumor microenvironment through the presence of VEGF, CCL-2, TGF-β and other chemokines;Citation7,8 (4) monocytes infiltrate into the tumor and differentiate into TAM. Initially, they will present an M1 phenotype (CD86+, MHCII+), leading to antitumor immunity by initiating the adaptive immune response. Once hypoxia and immunosuppression take the upper hand, there is a switch to the M2 phenotype (CD163+, CD206+). Although this creates new points of action for immunotherapy, this switch will lead to further immunosuppression and promotion of tumor growth, through the production of several immunosuppressive cytokines, such as interleukin (IL)-4, IL-10, IL-13, VEGF, CCL-2 and TGF-β.Citation10 In the end, this combination will result in a strong immune suppressive environment, leading to immune escape. Tumor cells can proliferate and the tumor becomes clinically apparent.
Until now, ovarian cancer research has primarily focused on tumor tissue, with a large focus on genetic changes. Moreover, immunological changes so far have only been studied in tumor tissue. Nevertheless, since ovarian cancer is a widespread metastatic disease, one can appreciate that the analysis of the systemic immune changes is crucial. One way to look at the changes in the immune suppressive milieu is by looking at the metabolites produced by tumor cells and immune suppressive cells. gives an overview on what is currently known about a selection of them. Additionally, we analyzed gal-1, a glycan-binding protein. It has a natural immunosuppressive function and a pivotal role in the maintenance of self-tolerance and T cell homeostasis. Via interaction with β-galactoside expressing glycoproteins on the T cell surface, gal-1 can negatively regulate T cell survival, antagonize T cell signaling and block pro-inflammatory cytokine secretion.Citation11 Furthermore, gal-1 blunts T cell responses via promoting accumulation and expansion of Tregs.Citation12 It is overexpressed by numerous malignant cell types, including ovarian cancer, by activated vascular endothelial cells, by normal activated T cells and by Treg. In anti-VEGF refractory tumors, gal-1 has been documented to bind VEGF receptor 2 and to maintain angiogenesis.Citation13 The role of gal-1 has been studied in ovarian cancer and is associated with a poor prognosis and it accelerates the proliferation and invasive capacity of the tumor cells.Citation14
Table 1. Overview on immunologic metabolites that can be detected in serum.
Results
Patient characteristics
An overview of the patient characteristics and outcome is given in and . The majority (90%) was diagnosed with serous ovarian carcinoma at an advanced stage (FIGO stage IIIC and IV) and 79% of patients had one or more relapses. The median follow up time was 47 months. The median PFS was 16 months, the median OS was 50 months (). We can therefore conclude that our study population was a representative group.
Figure 1. Survival of ovarian cancer patients included in the study group (n = 80). (A) Progression-free survival (months); (B) Overall survival (months). Kaplan–Meier estimate; 95% confidence interval.
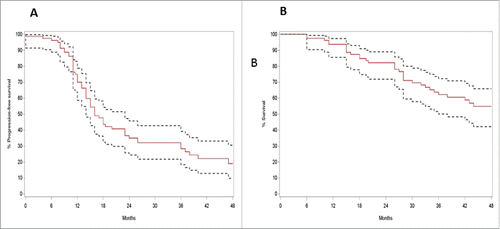
Table 2. Overview on patient characteristics (n = 80).
Immunosuppression at diagnosis of ovarian cancer patients versus healthy controls
First, we compared the metabolite values between naïve samples (diagnosis of ovarian cancer without invasive procedure, most commonly by diagnosis at ultrasound) (n = 32) and samples taken after diagnostic laparoscopy (n = 23). There were no significant differences in the values between these two time points (). Therefore, we will combine the two groups in further analyses and we will refer to them as one group “at diagnosis.” In case we had patients with measurements at both occasions, the average value was used (this was the case in five patients). Two metabolites (TGF-β and arginase) could not be measured in two samples (naïve and laparoscopy) because of the small sample volume.
Table 3. Overview on the presence of metabolites in serum of patients with ovarian cancer at different time points during the course of the disease (comparison of cohorts of patient samples, n=135).
Serum samples from 50 patients “at diagnosis” were compared with serum samples from 10 healthy donors. IL-10 (p < 0.001) and TGF-β (p = 0.021) were significantly higher in patients compared to controls. We could not observe a decrease change of gal-1 with increasing age of healthy controls (p = 0.135).Citation15
Immunosuppression in ovarian cancer patients at diagnosis vs. after three chemotherapy cycles
A total of 37 patients received three cycles of paclitaxel–carboplatin and three patients received three cycles of carboplatin in monotherapy. We found significant lower levels of IL-10 (p < 0.001), VEGF (p = 0.040), TGF-β (p < 0.001) and arginase (p < 0.001) and higher levels of gal-1 (p = 0.016) after chemotherapy compared to diagnosis (). After exclusion of the seven patients who received AMG 386 or placebo together with carboplatin–paclitaxel in study (BGOG-ov7), statistical results did not change (data not shown). After exclusion of patients treated with carboplatin only (since this is not the standard of care in ovarian cancer treatment), IL-10, TGF-β, arginase and gal-1 kept their statistical significance.
Immunosuppression in ovarian cancer patients at diagnosis versus after (interval) debulking surgery
We obtained 15 samples after primary debulking surgery and 19 samples after interval debulking surgery. In two serum samples, arginase and TGF-β could not be analyzed, because the sample volume was not sufficient. In both patient groups, a decreased level of IL-10 (p < 0.001) was demonstrated compared to patients measured at diagnosis.
Longitudinal evolutions in metabolite values
Of 40 patients, we gathered more than one sample during their disease course, enabling us to measure longitudinal evolutions in metabolite values. The composition of the groups is presented in . We can discriminate three groups: group 1/17 samples from patients at diagnosis and after three cycles of paclitaxel–carboplatin. Here, we found significant lower levels of IL-10 (p = 0.0005), VEGF (p = 0.0079), TGF-β (p 0.0092), arginase (p = 0.0093) and CCL-2 (p = 0.0093). There was a trend for increasing gal-1 levels (p = 0.0797); group 2/11 and seven samples from patients at diagnosis and respectively after primary debulking surgery and interval debulking surgery. Comparable to the whole group of samples, IL-10 showed decreased levels (p = 0.0049 and p = 0.0781); group 3/from four patients we gathered measurements taken after treatment (one patient after primary debulking and adjuvant chemotherapy, one patient after three cycles of neoadjuvant chemotherapy, after interval debulking and after three cycles of adjuvant chemotherapy and two patients after adjuvant chemotherapy) and at recurrence. No systematic differences in metabolite values were found between these two groups.
Table 4. Overview on the presence of metabolites in serum of patients with ovarian cancer at different time points during the course of the disease (comparison of consecutive samples taken from the same patient).
Immunosuppressive metabolites and tumor grade
Metabolite values at diagnosis did not differ significantly between high grade and low grade ovarian cancers.
Progression free and overall survival
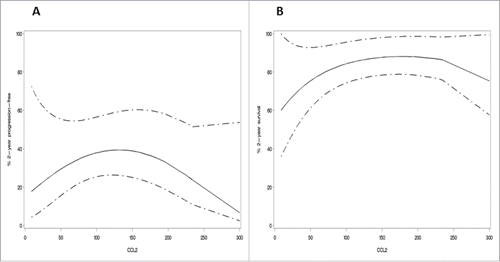
The association between metabolite values and PFS and OS was studied in a multivariable (including FIGO stage and residual disease after cytoreductive surgery as prognostic variables) analysis. Gal-1 and CCL-2 appeared to be independent prognostic factors for both PFS and OS. In detail, higher values of gal-1 were associated with an increased risk of progression (p = 0.0293) and death (p = 0.0096). For CCL-2, a quadratic effect appeared, implying that both lowest and highest values of CCL-2 were associated with increased risk of progression (p = 0.0294) and death (p = 0.0377) ().
Figure 2. Quadratic effect of CCL-2 expression in ovarian cancer patients at diagnosis. Values of CCL-2 in relation to the progression-free survival (A) and the overall survival (B). The graph (univariate analysis) shows a quadratic effect of CCL-2 values in ovarian cancer patients at diagnosis. This implicates that both low and high levels of CCL-2 are associated with worse prognosis of ovarian cancer patients. Predicted two-year survival; 95% confidence interval.
Discussion
The role of the immune system in the development and recurrence of cancer is crucial. In ovarian cancer, studies so far have investigated the intratumoral presence of immune suppressive cells. This study is the first one to suggest an important systemic role for Treg, MDSC and TAM, based on the presence of their metabolites in serum allowing us to gain insight in overall immunosuppression. Moreover, we could demonstrate that conventional standard therapies (radical debulking surgery and paclitaxel–carboplatin based chemotherapy) significantly reduce these metabolite levels and that gal-1 and CCL-2 independently worsened the PFS and OS.
As demonstrated in , the existing immunological studies in ovarian cancer are scarce, do not cover the total immune suppressive repertoire and are limited in sample size (mean 61.5, range 16–130 patients). However, our results certainly confirm previous findings: decrease of IL-10 after cytoreductionCitation16 and an increase of IL-10, TGF-β and arginase in ovarian cancer patients at diagnosis.Citation17,18 In contrast to reported findings on VEGF, we could not correlate the presence of VEGF to prognosis nor did we see an increase after surgery.Citation19–23
We found that gal-1 serum levels increased after three cycles of paclitaxel–carboplatin. Similar finding have already been described for glioblastoma, where gal-1 expression increased in endothelial and glioma cells after radiotherapy and after treatment with temozolomide.Citation24,25 This seems contradictory, however, in lung and ovarian cancer, gal-1 overexpression appears to promote chemotherapy resistance and downregulation of gal-1 expression can sensitize tumor cells to platin-based chemotherapy.Citation14,26,27 In ovarian cancer, gal-1 could possibly mediate these effects through activation of the H-Ras/Raf-1/ERK pathway.Citation14 The group of Le Mercier et al. suggested that increased gal-1 levels therefore seem to be representative of defense mechanisms against cytotoxic drugs, such as chemotherapy, and that gal-1 could consequently be of major importance in chemotherapy resistance.Citation24 Both our results in gal-1 (increase after chemotherapy and being an independent prognostic factor) support this theory.
Literature provides mixed data about CCL-2 levels in the serum of ovarian cancer patients. Compared to healthy controls, both lower levelsCitation28,29 as higher levels of CCL-2 Citation30,31 are reported. Some studies claim that higher levels are associated with advanced disease.Citation30,31 In our study population, we showed that both the lowest as well as the highest serum levels of CCL-2 were independently associated with a poor prognosis. A possible explanation might lay in the findings that CCL-2 can act dichotomously. In a mammary carcinoma model for example, Li et al. found that CCL-2 seemed to stimulate immunosurveillance of developing malignancies and metastatic cells. However, after a long-term inhibition of CCL-2 they observed an increase of metastatic burden. On the other hand, CCL-2 also appeared to enhance the progression of primary lesions that had already reached a “critical mass”.Citation32 This finding might explain the measurements of CCL-2 in our study, however, it also implies cautiousness when it should be used in a diagnostic or therapeutic setting.
This is—to the best of our knowledge—the first study in serum that explores the different aspects of immune suppression at diagnosis and after standard treatment in ovarian cancer patients. The next step to study the systemic changes in the immune system in ovarian cancer is a prospective inclusion of ovarian cancer patients from the moment of diagnosis until palliation, not only at the serum level but also at the cellular level. This type of study will be able to reveal what type of immune suppressive cells/systemic immune suppression will be most crucial during what point in the disease course. Hopefully, this insight can help us to better optimize and time the best therapy at the best moment in the future.
Materials and methods
Serum samples
After approval of the local ethical committee, a total of 135 serum samples, obtained in 80 patients with the histopathological diagnosis of ovarian/tubal cancer, were analyzed. Samples were collected from 2010–2014, after written informed consent. They were gathered at diagnosis (n = 32), after diagnostic laparoscopy (n = 23), after primary debulking (n = 15) [all without macroscopic tumor post-surgery], after three neoadjuvant cycles of paclitaxel–carboplatin (n = 40), after interval debulking (n = 19) [17][17 had no macroscopic remaining tumor post-surgery, two had an unresectable metastasis of 1–2cm post-surgery] and at diagnosis of recurrent disease (n = 6). In seven patients, neoadjuvant paclitaxel–carboplatin was given in the BGOG-OV7 study, implying that the chemotherapy was associated with the simultaneous administration of AMG386 (a selective angiopoietin-1/-2 neutralizing peptibody) or placebo. At present, the study has not been unblinded yet. Samples after laparoscopy, chemotherapy, debulking or interval debulking were collected respectively 13, 33, 26.5 and 21 d (median) after surgery/chemotherapy. Of 40 patients, two or more consecutive samples were available. In addition, serum was collected prospectively after approval of the local ethical committee from 10 healthy age-matched controls, without ovarian pathology.
Serum was collected in BD Vacutainer® Serum Tubes containing silica (ref 369032 and 367896, BD) and kept at 4°C until centrifugation. Samples were centrifuged at 2700–3000 rpm during 10 min. This was done in the majority of samples within 48 h after prelevation. However, 12 samples (8%) could only be processed 3–8 d after prelevation (mean 4.5 d). Resulting serum was collected and stored in aliquots at −80°C until further analysis.
Cytometric bead assay (CBA)
All serum samples were analyzed on the presence of IL-4, IL-10, IL-13, IL-17, IFNγ, VEGF-A, TGF-β and CCL-2 by the use of CBA flex sets (ref respectively 558272, 558274, 558450, 562151, 561515, 558336, 560429, 558287—BD), according to the firms' guidelines in 96-well plates. Samples were acidified prior to the analysis for TGF-β; samples (except for TGF-β) were used undiluted. Samples were analyzed by the LSR Fortessa flow cytometer (BD). Analysis was performed by FLOWJO software.
Enzyme-linked immunosorbent assay (ELISA)
All serum samples were analyzed for the presence of gal-1 by ELISA (anti-gal-1 from R&D, ref AF1152 and a biotinylated goat antihuman gal-1 antibody (R&D with ref BAF1152)). Our protocol was published earlier.Citation15
Arginase-1 activity assay
Arginase-1 was determined to give an impression of MDSC and TAM activity. L-arginine is a substrate for two enzymes, iNOS (that generates nitric oxide) and arginase-1 (that converts L-arginine in urea and L-ornithin). MDSC show an increased activity of arginase-1 and iNOS, resulting in a relative depletion of L-arginine in the micro-environment and a relative increase in NO. This results in the inhibition of T cell proliferation and function. In all serum samples, arginase-1 activity was measured, through determination of the urea content using the QuantiChrom™ Arginase Assay Kit (ref DARG-200—Bioassay Systems) following the manufacturer's protocol.
Statistical methodology
Normality was assessed by visual inspection of the histograms of metabolite values. The Mann–Whitney U test was used to compare metabolite values between two groups of patients evaluated at different measurement occasions. The Wilcoxon signed-rank test was used to analyze evolutions of metabolites within subsets of patients with longitudinal measurements. The Cox proportional hazard model was used to analyze the association between metabolite values at diagnosis and progression-free survival (PFS) and OS. Both linear and quadratic trends were tested.
All statistical tests are two-sided and a 5% significance level is assumed for all tests. A large number of statistical tests was performed. Given the exploratory nature of this study, no correction for multiple testing was applied. All analyses have been performed using SAS software, version 9.4 of the SAS System for Windows.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1111505_Supplement.zip
Download Zip (67.5 KB)Funding
This research was supported by the Olivia Hendrickx Research Fund and the Amgen Leerstoel of the KU Leuven. An Coosemans and Tina Verschuere are supported by the Fund for Scientific Research Flanders (FWO). Sven Seys is supported by a PDM mandate of the KU Leuven.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9-29; PMID:24399786; http://dx.doi.org/10.3322/caac.21208
- Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ, Panici PB et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010; 363:943-53; PMID:20818904; http://dx.doi.org/10.1056/NEJMoa0908806
- Chene G, Dauplat J, Radosevic-Robin N, Cayre A, Penault-Llorca F. Tu-be or not tu-be: that is the question…about serous ovarian cancer carcinogenesis. Crit Rev Oncol Hematol 2013; 88:134-43; PMID:23523591; http://dx.doi.org/10.1016/j.critrevonc.2013.03.004
- Sorensen RD, Schnack TH, Karlsen MA, Hogdall CK. Serous ovarian, fallopian tube and primary peritoneal cancers: a common disease or separate entities – a systematic review. Gynecol Oncol 2015; 136:571-81; PMID:25615934; http://dx.doi.org/10.1016/j.ygyno.20-15.01.534
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565-70; PMID:21436444; http://dx.doi.org/10.1126/science.1203486
- Baert T, Timmerman D, Vergote I, Coosemans A. Immunological parameters as a new lead in the diagnosis of ovarian cancer. Facts Views Vis Obgyn 2015; 1:67-72; PMID:25897373
- Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14:1014-22; PMID:24048123; http://dx.doi.org/10.1038/ni.2703
- Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J 2010; 16:348-53; PMID:20693846; http://dx.doi.org/10.1097/PPO.0b013e3181eb3358
- Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer 2013; 13:739-52; PMID:24060865; http://dx.doi.org/10.1038/nrc3581
- Noy R, Pollard JW. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014; 41:49-61; PMID:25035953; http://dx.doi.org/10.1016/j.immuni.2014.06.010
- Rabinovich GA, Ilarregui JM. Conveying glycan information into T cell homeostatic programs: a challenging role for galectin-1 in inflammatory and tumor microenvironments. Immunol Rev 2009; 230:144-59; PMID:19594634; http://dx.doi.org/10.1111/j.1600065X.2009.00787.x
- Ito K, Stannard K, Gabutero E, Clark AM, Neo S-Y, Onturk S, Blanchard H, Ralph SJ. Galectin-1 as a potent target for cancer therapy: role in the tumor microenvironment. Cancer Metastasis Rev 2012; 31:763-78; PMID:22706847; http://dx.doi.org/10.1007/s10555-012-9388-2
- Croci DO, Cerliani JP, Dalotto-Moreno T, Méndez-Huergo SP, Mascanfroni ID, Dergan-Dylon S, Toscano MA, Caramelo JJ, Garcia-Vallejo JJ, Ouyang J et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell 2014; 156:744-58; PMID:24529377; http://dx.doi.org/10.1016/j.cell.2014.01.043
- Zhang P, Shi B, Zhou M, Jiang H, Zhang H, Pan X, Gao H, Sun H, Li Z. Galectin-1 overexpression promotes progression and chemoresistance to cisplatin in epithelial ovarian cancer. Cell Death Dis 2014; 5:e991; PMID:24407244; http://dx.doi.org/10.1038/cddis.2013.526
- Verschuere T, Van Woensel M, Fieuws S, Lefranc F, Mathieu V, Kiss R, Van Gool SW, De Vleeschouwer S. Altered galectin-1 serum levels in patients diagnosed with high-grade glioma. J Neurooncol 2013; 115:9-17; PMID:23824536; http://dx.doi.org/10.1007/s11060-013-1201-8
- Napoletano C, Bellati F, Landi R, Pauselli S, Marchetti C, Visconti V, Sale P, Liberati M, Rughetti A, Frati L et al. Ovarian cancer cytoreduction induces changes in T cell population subsets reducing immunosuppression. J Cell Mol Med 2010; 14:2748-59; PMID:19780872; http://dx.doi.org/10.1111/j.1582-4934.2009.00911.x
- Nowak M, Glowacka E, Szpakowski M, Szyllo K, Malinowski A, Kulig A, Tchorzewski H, Wilczynski J. Proinflammatory and immunosuppressive serum, ascites and cyst fluid cytokines in patients with early and advanced ovarian cancer and benign ovarian tumors. Neuro Endocrinol Lett 2010; 31:375-83; PMID:20588232.
- Nishio H, Yaguchi T, Sugiyama J, Sumimoto H, Umezawa K, Iwata T, Susumu N, Fujii T, Kawamura N, Kobayashi A et al. Immunosuppression through constitutively activated NF-κB signalling in human ovarian cancer and its reversal by an NF-κB inhibitor. Br J Cancer 2014; 110:2965-74; PMID:24867687; http://dx.doi.org/10.1038/bjc.2014.251
- Svendsen MN, Werther K, Nielsen HJ, Kristjansen PEG. VEGF and tumour angiogenesis. Impact of surgery, wound healing, inflammation and blood transfusion. Scand J Gastroenterol 2002; 37:373-9; PMID:11989825; http://dx.doi.org/10.1080/003655202317315971
- Bandiera E, Franceschini R, Specchia C, Bignotti E, Trevisiol C, Gion M, Pecorelli S, Santin AD, Ravaggi A. Prognostic significance of vascular endothelial growth factor serum determination in women with ovarian cancer. ISRN Obstet Gynecol 2012; 2012:245756; PMID:22792477; http://dx.doi.org/10.5402/2012/245756
- Mahner S, Woelber L, Eulenburg C, Schwarz J, Carney W, Jaenicke F, Milde-Langosch K, Lueller V. TIMP-1 and VEGF-165 serum concentration during first-line therapy of ovarian cancer patients. BMC Cancer 2010; 10:139; PMID:20388222; http://dx.doi.org/10.1186/1471-2407-10-139
- Rudlowski C, Pickart a-K, Fuhljahn C, Friepoertner T, Schlehe B, Biesterfeld S, Schroeder W. Prognostic significance of vascular endothelial growth factor expression in ovarian cancer patients: a long-term follow-up. Int J Gynecol Cancer 2006; 16 Suppl 1:183-9; PMID:16515588; http://dx.doi.org/10.1111/j.1525-1438.2006.00307.x
- Yu L, Deng L, Li J, Zhang Y, Hu L. The prognostic value of vascular endothelial growth factor in ovarian cancer: a systematic review and meta-analysis. Gynecol Oncol 2013; 128:391-6; PMID:23142075; http://dx.doi.org/10.1016/j.ygyno.2012.11.002
- Le Mercier M, Lefranc F, Mijatovic T, Debeir O, Haibe-Kains B, Bontempi G, Decaestecker C, Kiss R, Mathieu V. Evidence of galectin-1 involvement in glioma chemoresistance. Toxicol Appl Pharmacol 2008; 229:172-83; PMID:18313712; http://dx.doi.org/10.1016/j.taap.2008.01.009
- Strik HM, Schmidt K, Lingor P, Tönges L, Kugler W, Nitsche M, Rabinovich GA, Bähr M. Galectin-1 expression in human glioma cells: modulation by ionizing radiation and effects on tumor cell proliferation and migration. Oncol Rep 2007; 18:483-8; PMID:17611674; http://dx.doi.org/10.3892/or.18.2.483
- Kim H-J, Jeon H-K, Cho YJ, Park YA, Choi J-J, Do I-G, Song SY, Lee YY, Choi CH, Kim TJ et al. High galectin-1 expression correlates with poor prognosis and is involved in epithelial ovarian cancer proliferation and invasion. Eur J Cancer 2012; 48:1914-21; PMID:22386573; http://dx.doi.org/10.1016/j.ejca.2012.02.005
- Chung LY, Tang SJ, Sun GH, Chou TY, Yeh TS, Yu SL, Sun KH. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clin Cancer Res 2012; 18:4037-47; PMID:22696230; http://dx.doi.org/10.1158/1078-0432.CCR-11-3348
- Falcão-Júnior JO, Teixeira-Carvalho A, Cândido EB, Lages EL, Ferreira Freitas GG, Lamaita RM, Freire Bonfim LP, Borges Salera R, Traiman P P, da Silva-Filho AL. Assessment of chemokine serum levels in epithelial ovarian cancer patients. Tumori 2013; 99:540-4; PMID:24326845; http://dx.doi.org/10.1700/1361.15108
- Gorelik E, Landsittel DP, Marrangoni AM, Modugno F, Velikokhatnaya L, Winans MT, Bigbee WL, Herberman RB, Lokshin AE. Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiol Biomarkers Prev 2005; 14:981-7; PMID:15824174; http://dx.doi.org/10.1158/1055-9965.EPI-04-0404
- Hefler L, Tempfer C, Heinze G, Mayerhofer K, Breitenecker G, Leodolter S, Reinthaller A, Kainze C. Monocyte chemoattractant protein-1 serum levels in ovarian cancer patients. Br J Cancer 1999; 81:855-9; PMID:10555758; http://dx.doi.org/10.1038/sj.bjc.6690776
- Tsai-Turton M, Santillan A, Lu D, Bristow RE, Chan KC, Shih IM, Roden RB. p53 autoantibodies, cytokine levels and ovarian carcinogenesis. Gynecol Oncol 2009; 114:12-7; PMID:19398128; http://dx.doi.org/10.1016/j.ygyno.2009.03.028
- Li M, Knight DA, A Snyder L, Smyth MJ, Stewart TJ. A role for CCL2 in both tumor progression and immunosurveillance. Oncoimmunology 2013; 2:e25474; PMID:24073384; http://dx.doi.org/10.4161/onci.25474
- Wang HW, Joyce JA. Alternative activation of tumor-associated macrophages by IL-4: Priming for protumoral functions. Cell Cycle 2010; 9:4824-35; PMID:21150330; http://dx.doi.org/10.4161/cc.9.24.14322
- Landskron G, De La Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014; 2014:149185; PMID:24901008; http://dx.doi.org/10.1155/2014/149185.
- Fujisawa T, Joshi BH, Puri RK. IL-13 regulates cancer invasion and metastasis through IL-13Rα2 via ERK/AP-1 pathway in mouse model of human ovarian cancer. Int J Cancer 2012; 131:344-56; PMID:21858811; http://dx.doi.org/10.1002/ijc.26366
- Zhang X, Weng W, Xu W, Wang Y, Yu W, Tang X, Ma L, Pan Q, Wang J, Sun F. Prognostic significance of interleukin 17 in cancer: a meta-analysis. Int J Clin Exp Med 2014; 7:3258-69; PMID:25419357
- Yao F, Yan S, Wang X, Shi D, Bai J, Li F, Sun B, Qian B. Role of IL-17F T7488C polymorphism in carcinogenesis: a meta-analysis. Tumor Biol 2014; 35:9061-8; PMID:24913709; http://dx.doi.org/10.1007/s13277-014-2171-y
- Lan C, Huang X, Lin S, Huang H, Cai Q, Lu J, Liu J. High density of IL-17-producing cells is associated with improved prognosis for advanced epithelial ovarian cancer. Cell Tissue Res 2013; 352:351-9; PMID:23397428; http://dx.doi.org/10.1007/s00441-013-1567-0
- Block MS, Maurer MJ, Goergen K, Kalli KR, Erskine CL, Behrens MD, Oberg AL, Knutson KL. Plasma immune analytes in patients with epithelial ovarian cancer. Cytokine 2015; 73:108-13; PMID:25743245; http://dx.doi.org/10.1016/j.cyto.2015.01.035
- Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 2007; 109:1568-73; PMID:17023580; http://dx.doi.org/10.1182/blood-2006-06-031856
- Chang CI, Liao JC, Kuo L. Macrophage arginase promotos tumor cell growth and suppresses nitric-mediated tumur cytotoxicity. Cancer Res 2001; 61:1100-6; PMID:11221839
- Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Philips GM et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014; 513:559-63; PMID:25043024; http://dx.doi.org/10.1038/nature13490
- Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JA, Tamagnone L, Mazzone M. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell 2013; 24:695-709; PMID:24332039; http://dx.doi.org/10.1016/j.ccr.2013.11.007
- Yigit R, Massuger LFAG, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol 2010; 117:366-72; PMID:20144842; http://dx.doi.org/10.1016/j.ygyno.2010.01.019
- Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci 2011; 7:651-8; PMID:21647333; http://dx.doi.org/10.7150/ijbs.7.651
- Komiyama S, Kurahashi T, Ishikawa M, Tanaka K, Komiyama M, Mikami M, Udagawa Y. Expression of TGFβ1 and its receptors is associated with biological features of ovarian cancer and sensitivity to paclitaxel/carboplatin. Oncol Rep 2011; 25:1131-8; PMID:21249319; http://dx.doi.org/10.3892/or.2011.1151
- Tas F, Karabulut S, Serilmez M, Ciftci R, Duranyildiz D. Clinical significance of serum epithelial cell adhesion molecule (EPCAM) and vascular cell adhesion molecule-1 (VCAM-1) levels in patients with epithelial ovarian cancer. Tumor Biol 2013; 1:1-8; PMID:24307621; http://dx.doi.org/10.1007/s13277-013-1401-z
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009; 29:313-26; PMID:19441883; http://dx.doi.org/10.1089/jir.2008.0027
- Zhang J, Patel L, Pienta KJ. Targeting chemokine (C-C motif) Ligand 2 (CCL2) as an example of translation of cancer molecular biology to the clinic. Prog Mol Biol Transl Sci 2010; 95:31-53; PMID:21075328; http://dx.doi.org/10.1016/B978-0-12-385071-3.00003-4
- Getter MA, Bui-Nguyen TM, Rogers LM, Ramakrishnan S. Chemotherapy induces macrophage chemoattractant protein-1 production in ovarian cancer. Int J Gynecol Cancer 2010; 20:918-25; PMID:20683396; http://dx.doi.org/10.1111/IGC.0b013e3181e5c442
- Fader AN, Rasool N, Vaziri SAJ, Kozuki T, Faber PW, Elson P, Biscotti CV, Michener CM, Rose PG, Rojas-Espaillat L et al. CCL2 expression in primary ovarian carcinoma is correlated with chemotherapy response and survival outcomes. Anticancer Res 2010; 30:4791-8; PMID:21187454
- Duan Z, Feller AJ, Penson RT, Chabner BA, Seiden MV. Discovery of differentially expressed genes associated with paclitaxel resistance using cDNA array technology: Analysis of interleukin (IL) 6, IL-8, and monocyte chemotactic protein I in the paclitaxel-resistant phenotype. Clin Cancer Res 1999; 5:3445-53; PMID:10589757
- Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer 2013; 13:871-82; PMID:24263190; http://dx.doi.org/10.1038/nrc3627
- Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 2014; 26:605-22; PMID:25517747; http://dx.doi.org/10.1016/j.ccell.2014.10.006
- Kandukuri SR, Rao J. FIGO 2013 staging system for ovarian cancer: what is new in comparison to 1988 staging system? Curr Opin Obstet Gynecol 2015; 27:48-52; PMID:25490382; http://dx.doi.org/10.1097/GCO.0000000000000135
