ABSTRACT
Natural killer (NK) cells are increasingly used in clinical studies in order to treat patients with various malignancies. The following review summarizes platform lectures and 2013–2015 consortium meetings on manufacturing and clinical use of NK cells in Europe and United States. A broad overview of recent pre-clinical and clinical results in NK cell therapies is provided based on unstimulated, cytokine-activated, as well as genetically engineered NK cells using chimeric antigen receptors (CAR). Differences in donor selection, manufacturing and quality control of NK cells for cancer immunotherapies are described and basic recommendations are outlined for harmonization in future NK cell studies.
Introduction
Significant progress has been made in NK-cell based therapies in haploidentical stem cell transplantation (haploSCT)Citation1 or in the non-transplant settingCitation2 since NK cells contribute to the graft versus leukemia/tumor (GvL/GvT) effect with general no or at least less graft vs. host disease (GvHD) compared to allogeneic T cells.Citation3,4 Based on platform lectures on the clinical workshop at the international NK2013 meeting in Heidelberg, Germany, which was the 14th meeting of the Society for Natural Immunity, the formation of an international NK cell working group was announced to address harmonization on (i) donor selection, (ii) manufacturing and (iii) quality control (QC) for the clinical use of NK cells in Europe and United States. Based on regular consortium meetings in 2013–2015 including joint sessions of the European Bone Marrow Transplantation (EBMT) and the International Society of Cellular Therapy (ISCT), the group provided an overview of recent pre-clinical and clinical results in NK-cell based donor lymphocyte infusions (NK-DLI) including unstimulated, cytokine-activated, as well as genetically engineered NK cells using CARs. In the latter case, both gene-transduced primary human NK cells and the NK92 cell line were efficiently redirected against malignancies as reviewed by group membersCitation5,6 recently.
Reports on clinical NK cell therapies
Eleven speakers presented data on the NK2013 meeting in two clinical sessions chaired by Andrea Velardi, Perugia/Italy and Rupert Handgretinger, Tuebingen/Germany.
Ulrike Koehl summarized a phase I/II study (Switzerland/Germany) in 25 patients with high-risk malignancies treated with 0.5–4.5×107/kg freshly purified or IL-2-activated (1000 U/mL) NK-DLI on days +3, +40, +100 post haploSCT.Citation1,7,8 No signs of GvHD occurred, if residual T cells were <25 × 103/kg. Elevated sMICA levels in patient's plasma correlated with impaired NK-cell cytotoxicity, but high dose of IL-2 activated NK-DLI could restore NKG2D-mediated-cytotoxicity by scavenging of sMICA.Citation9,10 Activated NK-DLI was also more resistant against mycophenolate mofetil, which was used as immunosuppressive therapy in haploSCT with CD3/CD19 depleted grafts, compared to unstimulated NK-DLI.Citation11 Finally, a strongly increased selective anticancer activity using CAR-modified NK92 cells redirected against ErB2/HER2pos breast cancer and GD2pos Neuoblastoma was presented.Citation12,13
Christian Kalberer reported on different NK-DLI studies at the Stem Cell Transplantation unit, Kantonsspital in BaselCitation1 and on a large-scale NK cell expansion protocol yielding sufficient numbers for multiple infusions to patients with haematological malignancies.Citation14 Purity of CD56+CD3− NK cells was>94% with less than 0.01% T cell contamination.Citation15 Currently in trials with activated NK-DLI post haploSCT in AML and Multiple Myeloma patients several applications with 8.5×109–44×109 NK-DLI were given. NK cells were expanded in air-permeable bags with serum-free culture medium supplemented with GMP-grade IL-2/IL-15, human AB+ serum, anti-CD3 antibody and irradiated autologous cells achieving expansion rates of up to 76-fold. Aliquots with 1.0×108 cells/kg were cryopreserved.
Evren Alici showed data on ex-vivo-expanded NK cells from both healthy donors and patients.Citation16,17 Comparing bags, an automated bioreactor and flask-based culture systems, significant expansion of NK cells was obtained with all systems under feeder-free GMP conditions. However, the bioreactor yielded in a product highly enriched in NK cells (mean: 9.8 × 109) and improved cytotoxicity. Alici also presented data on NK cells transduced with a membrane bound form of IL-6 fused with NKp30 NK cell activation receptor as a control reaching transduction rates of 18% and 29%, respectively. Lytic activity of the CAR-expressing cells against malignant Namalwa B cells was 80% compared to 55% for untransduced cells at 10:1 ratio. Finally, Alici showed his genetic and functional screening method about the complex network of interactions between the patient´s autologous NK cells and the respective tumor cell.
Jan Spanholtz gave a talk on NK cell immunotherapy against AML using CD34+-derived NK cells.Citation18 In order to obtain large numbers of functional NK cells, he used an efficient cytokine-based culture system for ex vivo expansion and differentiation of NK cells from umbilical cord blood (UCB).Citation19,20 The expansion in a bioreactor yielded more than 2,000-fold expansion, generating doses of more 1×1010 NK cells and a purity of >90%.Citation21 These NK cell products are currently used for immunotherapy in elderly AML patients not eligible for transplantation.Citation22
Dean Lee reported on clinical translation of ex vivo NK cell expansion with membrane-bound IL-21. He developed a system for ex vivo expansion of NK cells that supports greater than 30,000-fold expansion in 3 weeks, enabling a single donor phlebotomy to yield cell doses of 50–100 times greater than that achievable by apheresis and CD3-depletion.Citation23,24 The method generates NK cells with reduced senescence, high cytotoxicity, serial killing ability, and endogenous cytokine production for improved survival, proliferation, and function.Citation25 This has been translated to GMP-compliant procedures and clinical trials are under way to apply this approach to autologous, allogeneic and UCB NK-DLI in transplant and non-transplant settings.Citation26
Jeffrey Miller presented data on haploidentical NK-DLI with exogenous IL-2 to treat patients with AML, NHL and ovarian cancer.Citation2,27,28 In vivo persistence of NK cells 7 d after infusion, and successful in vivo expansion at day 14 (100 NK-DLI/µL) correlated with leukemia clearance. Expansion of host Tregs was associated with lack of NK cells. He also demonstrated data on the use of bispecific killer engagers (BIKEs), which are able to impart antigen-specific selectivity. A BIKE created from single chain Fv (scFv) specific for CD16 on NK cells fused by a linker to a scFv against CD33 on AML targets can create an immune synapse and trigger CD16 on NK cells to kill primary AML.Citation29 Finally, Miller suggested that NK-DLI will be most effective if given with optimal cytokines (IL-15) to induce in vivo expansion and agents to enhance target specificity.Citation30
Mark Lowdell reported on two-stage priming of allogeneic NK cells for patients with AML.Citation31 Resting NK cells require a “priming” and “triggering” process. While NK-sensitive tumors provide both signals, NK-resistant tumors evade lysis by failure to prime. M. Lowdell showed data on a tumor cell line (CTV-1) that primes resting NK cells but fails to trigger lysis.Citation32,33 These tumor-activated NK cells (TaNK) then retain the ability to lyse NK-resistant leukaemias.Citation34 He created a GMP-compliant manufacturing process for TaNK cells as cellular medicines and designed a clinical trial to determine the toxicity of infusions of TaNK cells from related haploidentical donors in a cohort of eight patients with AML at different disease stages.Citation31
Lutz Uharek showed data on early adoptive transfer of allogeneic NK-DLI among a prospective phase I/II trial. Twenty-five patients with AML, ALL, CML, Hodgkin's disease and MDS received a mean of 9.8 × 106 CD56+CD3− NK-DLI/kg at day +2 post haploSCT. NK-DLI showed promising survival rates in patients lacking other treatment options.Citation35,36 Best results were achieved in patients with AML in remission, but responses were also seen in patients with refractory disease.
Hans Klingemann reported on the highly cytotoxic NK-92 cell line as an alternative option for cancer treatment. Phase I trials showed that irradiated NK-92 cell infusions were well tolerated up to a tested dose range of 1 × 1010/m2.Citation5,37,38 Some clinically responses were seen in patients with advanced lung cancer, melanoma and lymphoma. NK-92 cell expansion was performed in VueLife bags or in G-Rex bioreactors for an ongoing trial.Citation39,40 Cells were shipped in fresh IL-2 at room temperature.Citation41,42 Finally, Klingemann presented data on the generation of several NK-92 CAR variantsCitation5,6,13 and postulated combination with checkpoint inhibitors to further improve efficacy.Citation43
Antonio Curti showed data on 14 elderly patients with high-risk AML receiving purified CD56+CD3− NK-DLI from haploidentical KIR-ligand mismatched donors.Citation44 The median number of infused NK cells was 2.74×106/Kg.Citation45 No NK cell-related toxicity, including GVHD, was observed. Two patients in molecular relapse achieved molecular CR lasting 9 mo for both patients. 7/12 patients in morphological CR, are disease-free (median 28 mo; range 9–63). After NK-DLI, donor NK cells were found in the peripheral blood of all evaluable patients (peak value on day 10). They were also detected in the bone marrow in some cases (peak value on day 5). In addition, a rise in IL-15 serum level was followed by increase in donor chimerism.
Wing Leung presented his approach to optimize NK-DLI for childhood malignancies.Citation46 The first step is donor selection including high resolution KIR typing. Clinical results were summarized to underscore the importance of KIR and HLA in allogeneic NK-DLI. Next, novel techniques were presented, including newer cytokines (IL-12, IL-15, IL-18, IL-21), artificial presenting cells, antibodies, immunocytokines, and CAR to optimize NK cell numbers, purity and potency. For clinical application, careful selection of patient populations and timing of NK-DLI were outlined. Data were reported on the use of NK-DLI for the induction of remission in patients with refractory leukemia, for consolidation after SCT in patients with poor-prognosis diseases, and for the replacement of SCT in hematologic malignancies with high-risk features in clinical remission.Citation47,48
Donor Selection
Typing of KIR, HLA, and FcγR are important for donor selection because their polymorphisms affect NK cell function and thereby the clinical outcomes of NK cell therapy. The KIR gene family is as highly polymorphic as the HLA family; therefore, tissue typing for these two families of genes have evolved substantially over the years () and even high-resolution analysis of KIR repertoire is investigated on NK cell subsets.Citation49 There are three levels of KIR typing.
Figure 1. Evolution of tissue typing. Typing of KIR and HLA are important for donor selection because their polymorphisms affect NK cell function. HLA typing is done at a resolution that allows discernment of the KIR ligand groups in HLA-A, B, and C. KIR typing involves three levels: genotyping for gene content and B-scoring, phenotyping for gene expression, and allelotyping for allele polymorphisms.
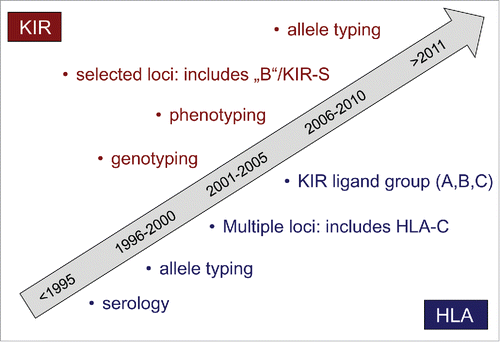
The first level of KIR typing is genotyping to assess the gene content.Citation47 For example, approximately 95% of individuals have inhibitory KIR2DL1, 50% have KIR2DL2, 85% have KIR2DL3, and 95% have KIR3DL1. For activating receptors, KIR2DS1 is present in approximately 35% of individuals, KIR2DS2 in 50%, and KIR3DS1 in 35%.
Based on the genotyping results, individuals may be classified on the basis of activating KIR content (KIR “A” vs. “B”), or on the basis of inhibitory KIR. KIR2DL3 is a hallmark gene in the centromeric (Cen) motif of “A” haplotypes, and it segregates as allele with KIR2DL2 which is a hallmark gene of “B”-haplotypes. Similarly, the telomeric motifs of “A” haplotypes typically contain KIR3DL1, which segregates as allele with KIR3DS1 in Tel-B motifs. Therefore, a simple 4-gene typing may be used clinically to classify the donors asCen-A/A(KIR2DL2−KIR2DL3+),Cen-A/B(KIR2DL2+KIR2DL3+), Cen-B/B(KIR2DL2+KIR2DL3−),Tel-A/A(KIR3DS1−KIR3DL1+),Tel-A/B(KIR3DS1+KIR3DL1+),orTel-B/B(KIR3DS1+KIR3DL1−). The B-score is the sum of the number of Cen-B and Tel-B gene-content motifs (range 0–4).Citation46,50 If two or more similar donors are available, those with Cen-B and Tel-B genotypes with highest B-score are favored.Citation51-54
Preferable donors on the basis of inhibitory KIR are those which possess a KIR for which the cognate ligand is absent in the recipient (i.e., receptor-ligand mismatch according to the Memphis model)Citation48 in particular when the donors also possess the corresponding ligand themselves (i.e., is “licensed”, per the ligand mismatch according to the Perugia model).Citation3
The second level of KIR typing is phenotyping by flow cytometry to measure the number of KIR mismatched NK cells and by quantitative PCR to measure the frequency of gene expression.Citation55 There is considerable heterogeneity in gene expression among normal donors, with more than 10-fold difference commonly observed. Because the size of receptor-ligand mismatched NK cells in the donors corresponds to the alloreactivity of their NK cells against target cells without the cognate ligands, the donors with the largest number by phenotyping are preferable.
The third level of KIR typing is allelotyping. Different alleles of a KIR gene have different functional properties. For example, KIR3DL1*004 is not expressed on NK cell surface and cannot educate NK cells. In comparison to KIR2DL1 alleles that have cysteine at position 245 in the transmembrane domain (KIR2DL1-C245), KIR2DL1 alleles that have arginine at the same position (KIR2DL1-R245) have stronger licensing capability, longer durability of surface expression after ligand interaction, and more recruitment of Src-homology-2 domain-containing protein tyrosine phosphatase 2 and β-arrestin 2.Citation56 Patients who received a donor haematopoietic graft containing KIR2DL1-R245 had better survival and lower cumulative incidence of disease progression than those patients who received a donor graft that contained only KIR2DL1-C245.Citation51 Patients who received a KIR2DL1-R245–positive graft with HLA-C receptor-ligand mismatch had the best survival. Therefore, donors with stronger KIR alleles should be chosen if available.
Finally, typing of FcγR polymorphisms is essential, in particular when NK cell therapy is used in combination with antibodies in which ADCC is a key mechanism of action. FcγRIIIa of 158V alleles binds IgG1 better than does those encoded by 158F alleles, resulting in enhanced activation of NK cells and better ADCC.Citation57
Clinical-grade purification and ex vivo expansion of NK cells
Preclinical NK-cell studies demonstrated that it is possible to produce a sufficient number of NK-DLI with effective tumor cytotoxicity. This has led to the translation of several preclinical NK-cell studies into GMP quality clinical-scale manufacturing of NK-DLI that are used in clinical trials. Both autologous and allogeneic NK-cell products are manufactured and have been used against numerous types of malignancies such as melanoma, leukemia and various types of carcinomas ().Citation14,16,17,21,39,58-70
Table 1. Expansion protocol for manufacturing of NK cell products.
Even though manufacturing of NK-DLI is quite variable, the infusion of NK-DLI is well tolerated by patients. While febrile reactions are the most commonly observed complications, in general NK-DLI have a promising safety profile and most importantly they have an encouraging therapeutic effect that has been observed in some studies. More specifically, NK-DLI administered to patients with myeloid neoplasms appear to be the most effective therapy among many very early (Phase I/II) clinical trials.Citation1,2,71,72 While most studies so far have used fresh, immunomagnetically isolated NK cells, some early clinical studies were performed with in-vitro activated and expanded NK-DLI ().Citation8,27,37,58,61,63,73-80
Table 2. In vitro-activated and expanded NK cells for clinical trials.
Even though promising results regarding safety, viability and antitumor responses have been reported, differences in NK-DLI manufacturing used in several clinical trials makes it challenging to determine the preferred source of NK-DLI and expanding conditions. While most of these studies using activated and expanded NK cells did not lead to development of GvHD, Shah et al reported on severe GvHD in 5/9 patients after treatment with donor-derived IL-15/4–1BBL-activated NK cells following HLA-matched, T-cell-depleted SCT. This gives rise for several questions such as to the safety in using manipulated feeder cells.Citation80,81 The key to future success is to optimize NK cell processing methods in order to achieve sufficient numbers of NK-DLI with the most efficient tumor cytotoxicity and clinical responses.
The majority of NK-DLI were generated through utilization of peripheral blood mononuclear cells (PBMC) either by apheresis or ficoll separation under GMP conditions. Although PBMC consists of 5–20 % NK cells, it is not possible to achieve always sufficient numbers of potent NK cells for multiple applications. Thus, various techniques to expand NK cells ex vivo have been developed (). For example, Alici has designed a system where they can expand NK-DLI in a clinically compatible manner without feeders reaching highly cytotoxic NK cells.Citation16 They completed a clinical trial using donor-derived ex vivo-expanded NK cells in terminal cancer patients that had CLL, kidney cancer, colorectal cancer, and hepatocellular carcinoma with promising results.Citation76 Having optimized the procedure for NK cell expansion in a closed-automated bioreactor a first-in-man phase I/II clinical trial was initiated (EudraCT: 2010–022330–83).Citation17,82
Another approach in expanding NK cells ex vivo makes use of feeder cells that provide essential stimulatory signals for NK cells proliferation. Monocytes, irradiated PBMC, feeder cell lines and engineered feeder cell lines are the most commonly used sources for stimulation of NK-cell expansion through humoral signals and cell-to-cell contact. On the other hand, UCB is also an essential source for achieving clinically relevant doses of NK cells when autologous cells are not optimal or readily available. Recently, expansion of active, tumor cytotoxic and pure NK cells under GMP conditions was demonstrated through use of UCB as a source.Citation21
Moreover, the cell line NK92 which is a human NK cell line and is cytotoxic to a wide range of malignant cells,Citation21 has also been used as a source of NK cells for GMP-grade cellular therapy products.Citation39 After irradiation (to prevent proliferation), these cells can be used effectively in immunotherapeutic approaches without compromising their cytotoxic function. For example, clinical-grade NK92 cells have been manufactured and were recently safely used as antitumor therapy for end-stage patients with different tumors.Citation37 One of the advantages of using such master cell bank is an appealing opportunity in the manufacture of cellular therapy products since it is possible to establish a comprehensive standardization and characterization of the cell source.
Many of the NK expansion protocols are based on initial enrichment of NK cells either through cell selection or sorting in order to achieve pure cell therapy product and avoid unwanted side effects especially stemming from T cells. One of the methods of enriching initial NK cell numbers and final NK cell purity is the clinical grade immunomagnetic depletion of T cells and/or B cells and myeloid cells as shown by Miller et al.Citation2 Additionally, direct immunomagnetic selection of NK cells for production of NK cell therapy products, is another way to enrich initial content of NK cells. Nevertheless, in order to get activated, NK cells might require physical and cytokine-dependent communication with other cells such as monocytes;Citation83 thus, it is essential to fine-tune the enrichment of NK cells or make use of feeder cells or optimize the cytokine cocktail used in ex vivo NK cell expansion protocols.
Most GMP-grade NK cell therapy protocols include IL-2 as a main cytokine to stimulate NK cell activation and proliferation. Recent advances in production of GMP quality cytokines enabled further optimization of cytokine supplementation during NK cell expansion. For example, use of IL-15 in combination with IL-2 improved product viability and NK cell proliferationCitation14 that highlights necessity of other cytokines to achieve NK-cell product potency especially when it comes to the NK cell expansion protocols that are not using feeder support. In addition, it could be shown, that cytokine (IL-12/IL-15)-activated NK cells transferred into naïve hosts can be detected up to 3 weeks later when they are phenotypically similar to naive cells.Citation84 Moreover, IL-12/15/18-preactivated NK cells led to sustained effector function against cancer,Citation85 which contributed to a recently opened trial of Todd Fehninger (ClinicalTrials.gov: NCT01898793) at St Louis Washington University using IL-12/15/18 stimulated NK cells in patients with AML. Besides NK cell source, feeder support and cytokine stimulation, other parameters such as expansion platform, cell culture media and serum supplementation are also very important in achieving clinically relevant cell numbers, viability and tumor cytotoxicity. There are very few GMP quality media optimally working for ex vivo NK cell expansion protocols. Most commonly preferred media are X-VIVO serum-free media (BioWhittaker, Verviers, Belgium), AIM V (Life Technologies, Grand Island, NY), or stem cell growth medium (SCGM; CellGenix, Freiburg, Germany). Generally, medium is supplemented by human AB serum or fetal bovine serum from certified sources.
Finally, there are numerous variables that may impact quality and quantity of NK cell products. Future trials will evaluate the contribution of each factor to the product purity, potency, and safety as well as identify NK cell products that can be reproducibly manufactured with the optimal safety and antitumor responses.
Quality control for NK cell phenotype and functionality
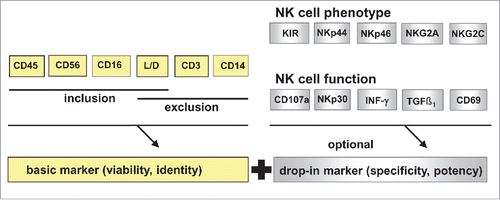
Multi-color flow cytometric quality control (QC) provides the platform for both quantification of NK cell phenotypes and NK cell functionality. While the ISHAGE (International Society for Hematotherapy and Graft Engineering)Citation86 protocol for CD34+ cells was successfully established >15 y ago leading to a broad international use, harmonization for enumeration of NK cell products is still a major challenge. The lack of accredited QC methods makes comparison of technical and clinical results from different NK studies tedious. Ideally, a QC panel for NK cells should consist of (i) a stable validated backbone to determine cell viability, cell number and to confirm the identity/purity of the CD56+CD3− NK cells and (ii) optional, variable “drop-in” markers to further specify the cells regarding subpopulations, functionality or potency. The development of new dyes and the multitude of biomarkers available today allow the design of such a modular assay.Citation87
Flow cytometric quantification should be performed in single platform, no-wash-preparation technique including live/dead staining in order to enumerate the absolute number of viable CD56+CD3− NK cells. Ideally, for NK cells the backbone constitutes the antibodies CD45, CD56, CD3, CD16, CD14 or CD14/DRAQ7, live/dead staining such as 7-AAD or PI and counting beadsCitation67 as presented in . The gating strategy is based on viable lymphocyte subtyping, using low scatter, positive expression of CD56 and CD45 antigens, CD3 as a negative discrimination marker, CD14 as a dump channel and CD16 to differentiate between immune regulatory and cytotoxic NK cells. Additionally, in the same panel various “drop-in” antibodies can be included for staining with different other markers of interest such as KIRs, NCRs, NKG2A, NKG2C, NKG2D, CD62L, CD57, CD244 and activating markers (CD69, HLA-DR, CD25). Finally, enumeration of functionality is possible as a “drop in” system as well, but this does not allow the use of the single platform no wash system. For example, CD107a can be adopted for quantification of degranulationCitation35,36 and intracellular cytokine staining (such as INFγ or TGF-β) for estimation of NK cell functionality.
Figure 2. Modular quality control for NK cells. For NK cell phenotyping on accredited flow cytometer a backbone including the antibodies CD45, CD56, CD3, CD16, CD14 or CD14/DRAQ7 and live/dead staining such as 7-AAD or PI for NK cell identity should be accompanied by different variable drop in markers like NCRs or KIRs for specificity and potency. For NK cell functionality, the same backbone might be used in combination to intracellular staining or specific target labeling in case of effector: target cytotoxicity. NCR = natural cytotoxicity receptors; KIR = Killer immunglobuline like receptors.
Advanced flow cytometric analyses in single platform no wash technique is also possible to use for a proper quantification of NK cell cytotoxicity against the MHC class I-negative cell line K562 or against the patient's individual leukemicCitation88 or tumor cells.Citation89 Whole blood NK cells, purified or cytokine activated NK cells and malignant cells are co-cultured in different effector: target ratios. Absolute cell counts are determined using counting beads and cytotoxicity is defined as the loss of vital target cells relative to a control.
In order to harmonize QC panels for NK cells, several world-wide exchange programs have been initiated for improved harmonization and compatibility between NK-cell based immunotherapies in the future.
Conclusion and future direction
Considerable progress has been made in clinical NK cell studies during the past decade. Improved strategies in KIR typing in order to select an auspicious NK cell donor, optimization of large scale NK cell manufacturing protocols and harmonization in QC for cell release enables a platform for future clinical studies. In addition, establishment of fully automated clinical-scale protocols gives rise for cost-effective NK cell production. Although these advances generates hope, there is also a need for caution regarding GvHD development if donor-derived IL-15/4–1BBL-activated NK cells are used. This refers to well-established application of purified activated CD56+CD3− NK-DLI, which led to high safety for the patients, even if haploidentical or third party NK cells were transfused. Highly purified NK cells may also open a new field of immunotherapy by using genetic engineered CAR expressing NK cells for redirecting against various malignancies. In contrast to CAR T cells, which have entered clinical trials successfully, but which are still in discussion for insertion of suicide genes to improve safety, primary mature NK cells have a limited lifespan only and therefore seemed to be excellent effector cells for genetic manipulation with CARs.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgements
We thank P. Kottaridis, C. Marden, J. North, S. Grace in UK and Dr Schaap, Dr Dolstra from Radboud UMC, Nijmegen, NL for their support to the respective study.
Funding
This work was supported in part by the European Union's Seventh-Framework-Program FP7/2007–2013/, under REA grant agreement both no 317013 (NATURIMMUN) and no 602366 (AGORA), by the St Baldrick's Foundation in USA, by the UK Leukemia Lymphoma Research and the German Ministry of Education (IFB-Tx), Ref No 01E00802.
References
- Stern M, Passweg JR, Meyer-Monard S, Esser R, Tonn T, Soerensen J, Paulussen M, Gratwohl A, Klingebiel T, Bader P et al. Pre-emptive immunotherapy with purified natural killer cells after haploidentical SCT: a prospective phase II study in two centers. Bone Marrow Transplant 2013; 48:433-8; PMID:22941380; http://dx.doi.org/10.1038/bmt.2012.162
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005; 105:3051-7; PMID:15632206; http://dx.doi.org/10.1182/blood-2004-07-2974
- Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295:2097-100; PMID:11896281; http://dx.doi.org/10.1126/science.1068440
- Locatelli F, Pende D, Mingari MC, Bertaina A, Falco M, Moretta A, Moretta L. Cellular and molecular basis of haploidentical hematopoietic stem cell transplantation in the successful treatment of high-risk leukemias: role of alloreactive NK cells. Front Immunol 2013; 4:15; PMID:23378843; http://dx.doi.org/10.3389/fimmu.2013.00015
- Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology 2014; 3:e28147; PMID:25340009; http://dx.doi.org/10.4161/onci.28147
- Glienke W, Esser R, Priesner C, Suerth JD, Schambach A, Wels WS, Grez M, Kloess S, Arseniev L, Koehl U. Advantages and applications of CAR-expressing natural killer cells. Front Pharmacol 2015; 6:21; PMID:25729364; http://dx.doi.org/10.3389/fphar.2015.00021
- Huenecke S, Zimmermann SY, Kloess S, Esser R, Brinkmann A, Tramsen L, Koenig M, Erben S, Seidl C, Tonn T et al. IL-2-driven regulation of NK cell receptors with regard to the distribution of CD16+ and CD16- subpopulations and in vivo influence after haploidentical NK cell infusion. J Immunother 2010; 33:200-10; PMID:20145545; http://dx.doi.org/10.1097/CJI.0b013e3181bb46f7
- Brehm C, Huenecke S, Quaiser A, Esser R, Bremm M, Kloess S, Soerensen J, Kreyenberg H, Seidl C, Becker PS et al. IL-2 stimulated but not unstimulated NK cells induce selective disappearance of peripheral blood cells: concomitant results to a phase I/II study. PloS One 2011; 6:e27351; PMID:22096557; http://dx.doi.org/10.1371/journal.pone.0027351
- Kloess S, Huenecke S, Piechulek D, Esser R, Koch J, Brehm C, Soerensen J, Gardlowski T, Brinkmann A, Bader P et al. IL-2-activated haploidentical NK cells restore NKG2D-mediated NK-cell cytotoxicity in neuroblastoma patients by scavenging of plasma MICA. Eur J Immunol 2010; 40:3255-67; PMID:21061445; http://dx.doi.org/10.1002/eji.201040568
- Ullrich E, Koch J, Cerwenka A, Steinle A. New prospects on the NKG2D/NKG2DL system for oncology. Oncoimmunology 2013; 2:e26097; PMID:24353908; http://dx.doi.org/10.4161/onci.26097
- Brehm C, Huenecke S, Esser R, Kloess S, Quaiser A, Betz S, Zimmermann O, Soerensen J, Passweg JR, Klingebiel T et al. Interleukin-2-stimulated natural killer cells are less susceptible to mycophenolate mofetil than non-activated NK cells: possible consequences for immunotherapy. Cancer Immunol Immunother 2014; 63:821-33; PMID:24806448; http://dx.doi.org/10.1007/s002-62-014-1556-5
- Esser R, Muller T, Stefes D, Kloess S, Seidel D, Gillies SD, Aperlo-Iffland C, Huston JS, Uherek C, Schonfeld K et al. NK cells engineered to express a GD2 -specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med 2012; 16:569-81; PMID:21595822; http://dx.doi.org/10.1111/j.1582-4934.2011.01343.x
- Schonfeld K, Sahm C, Zhang C, Naundorf S, Brendel C, Odendahl M, Nowakowska P, Bonig H, Kohl U, Kloess S et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol Ther 2015; 23:330-8; PMID:25373520; http://dx.doi.org/10.1038/mt.2014.219
- Siegler U, Meyer-Monard S, Jorger S, Stern M, Tichelli A, Gratwohl A, Wodnar-Filipowicz A, Kalberer CP. Good manufacturing practice-compliant cell sorting and large-scale expansion of single KIR-positive alloreactive human natural killer cells for multiple infusions to leukemia patients. Cytotherapy 2010; 12:750-63; PMID:20491532; http://dx.doi.org/10.3109/14653241003786155
- Meyer-Monard S, Passweg J, Siegler U, Kalberer C, Koehl U, Rovo A, Halter J, Stern M, Heim D, Alois Gratwohl JR et al. Clinical-grade purification of natural killer cells in haploidentical hematopoietic stem cell transplantation. Transfusion 2009; 49:362-71; PMID:19389215; http://dx.doi.org/10.1111/j.1537-2995.2008.01969.x
- Alici E, Sutlu T, Bjorkstrand B, Gilljam M, Stellan B, Nahi H, Quezada HC, Gahrton G, Ljunggren HG, Dilber MS. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood 2008; 111:3155-62; PMID:18192509; http://dx.doi.org/10.1182/blood-2007-09-110312
- Sutlu T, Stellan B, Gilljam M, Quezada HC, Nahi H, Gahrton G, Alici E. Clinical-grade, large-scale, feeder-free expansion of highly active human natural killer cells for adoptive immunotherapy using an automated bioreactor. Cytotherapy 2010; 12:1044-55; PMID:20795758; http://dx.doi.org/10.3109/14653249.2010.504770
- Cany J, Dolstra H, Shah N. Umbilical cord blood-derived cellular products for cancer immunotherapy. Cytotherapy. 2015 Jun; 17(6):739-48; http://dx.doi.org/10.1016/j.jcyt.2015.03.005
- Spanholtz J, Tordoir M, Eissens D, Preijers F, van der Meer A, Joosten I, Schaap N, de Witte TM, Dolstra H. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PloS One 2010; 5:e9221; PMID:20169160; http://dx.doi.org/10.1371/journal.pone.0009221
- Cany J, van der Waart AB, Spanholtz J, Tordoir M, Jansen JH, van der Voort R, Schaap NM, Dolstra H. Combined IL-15 and IL-12 drives the generation of CD34-derived natural killer cells with superior maturation and alloreactivity potential following adoptive transfer. Oncoimmunology 2015; 4:e1017701; PMID:26140247; http://dx.doi.org/10.1080/2162402X.2015.1017701
- Spanholtz J, Preijers F, Tordoir M, Trilsbeek C, Paardekooper J, de Witte T, Schaap N, Dolstra H. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PloS One 2011; 6:e20740; PMID:21698239; http://dx.doi.org/10.1371/journal.pone.00-20740
- Chouaib S, Pittari G, Nanbakhsh A, El Ayoubi H, Amsellem S, Bourhis JH, Spanholtz J. Improving the outcome of leukemia by natural killer cell-based immunotherapeutic strategies. Front Immunol 2014; 5:95; PMID:24672522; http://dx.doi.org/10.3389/fimmu.2014.00095
- Lee DA, Verneris MR, Campana D. Acquisition, preparation, and functional assessment of human NK cells for adoptive immunotherapy. Methods Mol Biol 2010; 651:61-77; PMID:20686960; http://dx.doi.org/10.1007/978-1-60761-786-0_4
- Shah N, Martin-Antonio B, Yang H, Ku S, Lee DA, Cooper LJ, Decker WK, Li S, Robinson SN, Sekine T et al. Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PloS One 2013; 8:e76781; PMID:24204673; http://dx.doi.org/10.1371/journal.pone.0076781
- Zhu S, Phatarpekar PV, Denman CJ, Senyukov VV, Somanchi SS, Nguyen-Jackson HT, Mace EM, Freeman AF, Watowich SS, Orange JS et al. Transcription of the activating receptor NKG2D in natural killer cells is regulated by STAT3 tyrosine phosphorylation. Blood 2014; 124:403-11; PMID:24891320; http://dx.doi.org/10.1182/blood-2013-05-499707
- Tarek N, Lee DA. Natural killer cells for osteosarcoma. Adv Exp Med Biol 2014; 804:341-53; PMID:24924184; http://dx.doi.org/10.1007/978-3-319-04843-7_19
- Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A, Curtsinger J, McKenna D et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy 2011; 13:98-107; PMID:20849361; http://dx.doi.org/10.3109/14653249.2010.515582
- Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, Cooley S, Weisdorf D, Miller JS. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother 2010; 59:1739-44; PMID:20680271; http://dx.doi.org/10.1007/s00262-010-0896-z
- Wiernik A, Foley B, Zhang B, Verneris MR, Warlick E, Gleason MK, Ross JA, Luo X, Weisdorf DJ, Walcheck B et al. Targeting Natural Killer Cells to Acute Myeloid Leukemia In Vitro with a CD16´33 Bispecific Killer Cell Engager and ADAM17 Inhibition. Clin Cancer Res 2013; Jul 15; 19(14):3844-55; PMID:23690482; http://dx.doi.org/10.1158/1078-0432.CCR-13-0505
- Miller JS. Therapeutic applications: natural killer cells in the clinic. Hematology Am Soc Hematol Educ Program 2013; 2013:247-53; PMID:24319187; http://dx.doi.org/10.1182/asheducation-2013.1.247
- Kottaridis PD, North J, Tsirogianni M, Marden C, Samuel ER, Jide-Banwo S, Grace S, Lowdell MW. Two-Stage Priming of Allogeneic Natural Killer Cells for the Treatment of Patients with Acute Myeloid Leukemia: A Phase I Trial. PloS One 2015; 10:e0123416; PMID:26062124; http://dx.doi.org/10.1371/journal.pone.0123416
- Katodritou E, Terpos E, North J, Kottaridis P, Verrou E, Gastari V, Chadjiaggelidou C, Sivakumaran S, Jide-Banwo S, Tsirogianni M et al. Tumor-primed natural killer cells from patients with multiple myeloma lyse autologous, NK-resistant, bone marrow-derived malignant plasma cells. Am J Hematol 2011; 86:967-73; PMID:21919039; http://dx.doi.org/10.1002/ajh.22163
- Sabry M, Tsirogianni M, Bakhsh IA, North J, Sivakumaran J, Giannopoulos K, Anderson R, Mackinnon S, Lowdell MW. Leukemic priming of resting NK cells is killer Ig-like receptor independent but requires CD15-mediated CD2 ligation and natural cytotoxicity receptors. J Immunol 2011; 187:6227-34; PMID:22084431; http://dx.doi.org/10.4049/jimmunol.1101640
- Sabry M, Lowdell MW. Tumor-Primed NK Cells: Waiting for the Green Light. Front Immunol 2013; 4:408; PMID:24324471; http://dx.doi.org/10.3389/fimmu.2013.00408
- Fischer L, Penack O, Gentilini C, Nogai A, Muessig A, Thiel E, Uharek L. The anti-lymphoma effect of antibody-mediated immunotherapy is based on an increased degranulation of peripheral blood natural killer (NK) cells. Exp Hematol 2006; 34:753-9; PMID:16728280; http://dx.doi.org/10.1016/j.exphem.2006.02.015
- Penack O, Gentilini C, Fischer L, Asemissen AM, Scheibenbogen C, Thiel E, Uharek L. CD56dimCD16neg cells are responsible for natural cytotoxicity against tumor targets. Leukemia 2005; 19:835-40; PMID:15744340; http://dx.doi.org/10.1038/sj.leu.2403704
- Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, Suttorp M, Seifried E, Ottmann OG, Bug G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013; 15:1563-70; PMID:24094496; http://dx.doi.org/10.1016/j.jcyt.2013.06.017
- Klingemann H. Challenges of cancer therapy with natural killer cells. Cytotherapy 2015; 17:245-9; PMID:25533934; http://dx.doi.org/10.1016/j.jcyt.2014.09.007
- Tam YK, Martinson JA, Doligosa K, Klingemann HG. Ex vivo expansion of the highly cytotoxic human natural killer-92 cell-line under current good manufacturing practice conditions for clinical adoptive cellular immunotherapy. Cytotherapy 2003; 5:259-72; PMID:12850795; http://dx.doi.org/10.1080/14653240310001523
- Klingemann HG. Cellular therapy of cancer with natural killer cells-where do we stand? Cytotherapy 2013; 15:1185-94; PMID:23768925; http://dx.doi.org/10.1016/j.jcyt.2013.03.011
- Koepsell SA, Kadidlo DM, Fautsch S, McCullough J, Klingemann H, Wagner JE, Miller JS, McKenna DH, Jr. Successful “in-flight” activation of natural killer cells during long-distance shipping. Transfusion 2013; 53:398-403; PMID:22574659; http://dx.doi.org/10.1111/j.1537-2995.2012.03695.x
- Klingemann H, Grodman C, Cutler E, Duque M, Kadidlo D, Klein AK, Sprague KA, Miller KB, Comenzo RL, Kewalramani T et al. Autologous stem cell transplant recipients tolerate haploidentical related-donor natural killer cell-enriched infusions. Transfusion 2013; 53:412-8; quiz 1; PMID:22738379; http://dx.doi.org/10.1111/j.1537-2995.2012.03764.x
- Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 2012; 1:1223-5; PMID:23243584; http://dx.doi.org/10.4161/onci.21335
- Arpinati M, Curti A. Immunotherapy in acute myeloid leukemia. Immunotherapy 2014; 6:95-106; PMID:24341888; http://dx.doi.org/10.2217/imt.13.152
- Curti A, Ruggeri L, D'Addio A, Bontadini A, Dan E, Motta MR, Trabanelli S, Giudice V, Urbani E, Martinelli G et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood 2011; 118:3273-9; PMID:21791425; http://dx.doi.org/10.1182/blood-2011-01-329508
- Leung W. Infusions of allogeneic natural killer cells as cancer therapy. Clin Cancer Res 2014; 20:3390-400; PMID:24987108; http://dx.doi.org/10.1158/1078-0432.CCR-13-1766
- Leung W. Use of NK cell activity in cure by transplant. Br J Haematol 2011; 155:14-29; PMID:21812770; http://dx.doi.org/10.1111/j.1365-2141.2011.08823.x
- Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P, Niethammer D, Handgretinger R. Determinants of antileukemia effects of allogeneic NK cells. J Immunol 2004; 172:644-50; PMID:14688377; http://dx.doi.org/10.4049/jimmunol.172.1.644
- Beziat V, Traherne J, Malmberg JA, Ivarsson MA, Bjorkstrom NK, Retiere C, Ljunggren HG, Michaelsson J, Trowsdale J, Malmberg KJ. Tracing dynamic expansion of human NK-cell subsets by high-resolution analysis of KIR repertoires and cellular differentiation. Eur J Immunol 2014; 44:2192-6; PMID:24723455; http://dx.doi.org/10.1002/eji.201444464
- Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, Marsh SG, Geraghty D, Spellman S, Haagenson MD et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 2010; 116:2411-9; PMID:20581313; http://dx.doi.org/10.1182/blood-2010-05-283051
- Bari R, Rujkijyanont P, Sullivan E, Kang G, Turner V, Gan K, Leung W. Effect of donor KIR2DL1 allelic polymorphism on the outcome of pediatric allogeneic hematopoietic stem-cell transplantation. J Clin Oncol 2013; 31:3782-90; PMID:24043749; http://dx.doi.org/10.1200/JCO.2012.47.4007
- Sullivan EM, Jeha S, Kang G, Cheng C, Rooney B, Holladay M, Bari R, Schell S, Tuggle M, Pui CH et al. NK cell genotype and phenotype at diagnosis of acute lymphoblastic leukemia correlate with postinduction residual disease. Clin Cancer Res 2014; 20:5986-94; PMID:25281696; http://dx.doi.org/10.1158/1078-0432.CCR-14-0479
- Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Marsh SG, Spellman S, Haagenson MD, Saeturn K, Ladner M et al. Donor killer cell Ig-like receptor B haplotypes, recipient HLA-C1, and HLA-C mismatch enhance the clinical benefit of unrelated transplantation for acute myelogenous leukemia. J Immunol 2014; 192:4592-600; PMID:24748496; http://dx.doi.org/10.4049/jimmunol.1302517
- Foley B, Felices M, Cichocki F, Cooley S, Verneris MR, Miller JS. The biology of NK cells and their receptors affects clinical outcomes after hematopoietic cell transplantation (HCT). Immunol Rev 2014; 258:45-63; PMID:24517425; http://dx.doi.org/10.1111/imr.12157
- Leung W, Iyengar R, Triplett B, Turner V, Behm FG, Holladay MS, Houston J, Handgretinger R. Comparison of killer Ig-like receptor genotyping and phenotyping for selection of allogeneic blood stem cell donors. J Immunol 2005; 174:6540-5; PMID:15879158; http://dx.doi.org/10.4049/jimmunol.174.10.6540
- Bari R, Bell T, Leung WH, Vong QP, Chan WK, Das Gupta N, Holladay M, Rooney B, Leung W. Significant functional heterogeneity among KIR2DL1 alleles and a pivotal role of arginine 245. Blood 2009; 114:5182-90; PMID:19828694; http://dx.doi.org/10.1182/blood-2009-07-231977
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002; 99:754-8; PMID:11806974; http://dx.doi.org/10.1182/blood.V99.3.754
- Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res 2011; 17:6287-97; PMID:21844012; http://dx.doi.org/10.1158/1078-0432.CCR-11-1347
- Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 2009; 69:4010-7; PMID:19383914; http://dx.doi.org/10.1158/0008-5472.CAN-08-3712
- Berg M, Lundqvist A, McCoy P, Jr., Samsel L, Fan Y, Tawab A, Childs R. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy 2009; 11:341-55; PMID:19308771; http://dx.doi.org/10.1080/14653240902807034
- Lundqvist A, Berg M, Smith A, Childs RW. Bortezomib treatment to potentiate the anti-tumor immunity of ex-vivo expanded adoptively infused autologous natural killer cells. J Cancer 2011; 2:383-5; PMID:21750690; http://dx.doi.org/10.7150/jca.2.383
- Luhm J, Brand JM, Koritke P, Hoppner M, Kirchner H, Frohn C. Large-scale generation of natural killer lymphocytes for clinical application. J Hematother Stem Cell Res 2002; 11:651-7; PMID:12201953; http://dx.doi.org/10.1089/15258160260194794
- Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, Klingemann H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy 2008; 10:625-32; PMID:18836917; http://dx.doi.org/10.1080/14653240802301872
- Klingemann HG, Martinson J. Ex vivo expansion of natural killer cells for clinical applications. Cytotherapy 2004; 6:15-22; PMID:14985163; http://dx.doi.org/10.1080/14653240310004548
- Iyengar R, Handgretinger R, Babarin-Dorner A, Leimig T, Otto M, Geiger TL, Holladay MS, Houston J, Leung W. Purification of human natural killer cells using a clinical-scale immunomagnetic method. Cytotherapy 2003; 5:479-84; PMID:14660043; http://dx.doi.org/10.1080/14653240310003558
- Lim O, Lee Y, Chung H, Her JH, Kang SM, Jung MY, Min B, Shin H, Kim TM, Heo DS et al. GMP-compliant, large-scale expanded allogeneic natural killer cells have potent cytolytic activity against cancer cells in vitro and in vivo. PloS One 2013; 8:e53611; PMID:23326467; http://dx.doi.org/10.1371/journal.pone.0053611
- Koehl U, Brehm C, Huenecke S, Zimmermann SY, Kloess S, Bremm M, Ullrich E, Soerensen J, Quaiser A, Erben S et al. Clinical grade purification and expansion of NK cell products for an optimized manufacturing protocol. Front Oncol 2013; 3:118; PMID:23730623; http://dx.doi.org/10.3389/fonc.2013.00118
- Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J, Dandekar V, Mei Z, Jackson K, Vera J et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy 2012; 14:1131-43; PMID:22900959; http://dx.doi.org/10.3109/14653249.2012.700767
- Tanaka J, Sugita J, Shiratori S, Shigematu A, Asanuma S, Fujimoto K, Nishio M, Kondo T, Imamura M. Expansion of NK cells from cord blood with antileukemic activity using GMP-compliant substances without feeder cells. Leukemia 2012; 26:1149-52; PMID:22143670; http://dx.doi.org/10.1038/leu.2011.345
- Granzin M, Soltenborn S, Muller S, Kollet J, Berg M, Cerwenka A, Childs RW, Huppert V. Fully automated expansion and activation of clinical-grade natural killer cells for adoptive immunotherapy. Cytotherapy 2015; 17:621-32; PMID:25881519; http://dx.doi.org/10.1016/j.jcyt.2015.03.611
- Passweg JR, Tichelli A, Meyer-Monard S, Heim D, Stern M, Kuhne T, Favre G, Gratwohl A. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia 2004; 18:1835-8; PMID:15457184; http://dx.doi.org/10.1038/sj.leu.2403524
- Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui CH, Leung W. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol 2010; 28:955-9; PMID:20085940; http://dx.doi.org/10.1200/JCO.2009.24.4590
- Motohashi S, Ishikawa A, Ishikawa E, Otsuji M, Iizasa T, Hanaoka H, Shimizu N, Horiguchi S, Okamoto Y, Fujii S et al. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res 2006; 12:6079-86; PMID:17028247; http://dx.doi.org/10.1158/1078-0432.CCR-06-0114
- Krause SW, Gastpar R, Andreesen R, Gross C, Ullrich H, Thonigs G, Pfister K, Multhoff G. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: a clinical phase i trial. Clin Cancer Res 2004; 10:3699-707; PMID:15173076; http://dx.doi.org/10.1158/1078-0432.CCR-03-0683
- Yoon SR, Lee YS, Yang SH, Ahn KH, Lee JH, Kim DY, Kang YA, Jeon M, Seol M, Ryu SG et al. Generation of donor natural killer cells from CD34(+) progenitor cells and subsequent infusion after HLA-mismatched allogeneic hematopoietic cell transplantation: a feasibility study. Bone Marrow Transplant 2010; 45:1038-46; PMID:19881555; http://dx.doi.org/10.1038/bmt.2009.304
- Barkholt L, Alici E, Conrad R, Sutlu T, Gilljam M, Stellan B, Christensson B, Guven H, Bjorkstrom NK, Soderdahl G et al. Safety analysis of ex vivo-expanded NK and NK-like T cells administered to cancer patients: a phase I clinical study. Immunotherapy 2009; 1:753-64; PMID:20636021; http://dx.doi.org/10.2217/imt.09.47
- Koehl U, Sorensen J, Esser R, Zimmermann S, Gruttner HP, Tonn T, Seidl C, Seifried E, Klingebiel T, Schwabe D. IL-2 activated NK cell immunotherapy of three children after haploidentical stem cell transplantation. Blood Cells Mol Dis 2004; 33:261-6; PMID:15528141; http://dx.doi.org/10.1016/j.bcmd.2004.08.013
- Szmania S, Lapteva N, Garg T, Greenway A, Lingo J, Nair B, Stone K, Woods E, Khan J, Stivers J et al. Ex vivo-expanded natural killer cells demonstrate robust proliferation in vivo in high-risk relapsed multiple myeloma patients. J Immunother 2015; 38:24-36; PMID:25415285; http://dx.doi.org/10.1097/CJI.0000000000000059
- Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, Rigatos G, Papamichail M, Perez SA. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother 2010; 59:1781-9; PMID:20703455; http://dx.doi.org/10.1007/s00262-010-0904-3
- Shah NN, Baird K, Delbrook CP, Fleisher TA, Kohler ME, Rampertaap S, Lemberg K, Hurley CK, Kleiner DE, Merchant MS et al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell-depleted stem cell transplantation. Blood 2015; 125:784-92; PMID:25452614; http://dx.doi.org/10.1182/blood-2014-07-592881
- Lee DA. The off-target effects of nonspecific NK cells. Blood 2015; 125:744-5; PMID:25634612; http://dx.doi.org/10.1182/blood-2014-12-616359
- Sutlu T, Alici E. Ex vivo expansion of natural killer cells: a question of function. Cytotherapy 2011; 13(6):767-8; PMID:21375422; http://dx.doi.org/10.3109/14653249.2011.563295.
- Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood 1992; 80:2221-9; PMID:1421393
- Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A 2009; 106:1915-9; PMID:19181844; http://dx.doi.org/10.1073/pnas.0813192106
- Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med 2012; 209:2351-65; http://dx.doi.org/10.1084/jem.20120944
- Keeney M, Chin-Yee I, Weir K, Popma J, Nayar R, Sutherland DR. Single platform flow cytometric absolute CD34+ cell counts based on the ISHAGE guidelines. International Society of Hematotherapy and Graft Engineering. Cytometry 1998; 34:61-70; PMID:9579602; http://dx.doi.org/10.1002/(SICI)1097-0320(19980415)34:2%3c61::AID-CYTO1%3e3.0.CO;2-F
- Sack U, Barnett D, Demirel GY, Fossat C, Fricke S, Kafassi N, Nebe T, Psarra K, Steinmann J, Lambert C. Accreditation of flow cytometry in Europe. Cytometry B Clin Cytom 2013; 84:135-42; PMID:23554222; http://dx.doi.org/10.1002/cyto.b.21079
- Zimmermann SY, Esser R, Rohrbach E, Klingebiel T, Koehl U. A novel four-colour flow cytometric assay to determine natural killer cell or T-cell-mediated cellular cytotoxicity against leukaemic cells in peripheral or bone marrow specimens containing greater than 20% of normal cells. J Immunol Methods 2005; 296:63-76; PMID:15680151; http://dx.doi.org/10.1016/j.jim.2004.10.014
- Kloss S, Bochennek K, Huenecke S, Zimmermann SY, Kuci S, Muller T, Wels WS, Klingebiel T, Esser R, Koehl U. A novel five-colour flow cytometric assay to determine NK cell cytotoxicity against neuroblastoma and other adherent tumour cells. J Immunol Methods 2007; 325:140-7; PMID:17663991; http://dx.doi.org/10.1016/j.jim.2007.06.013

