ABSTRACT
Tumor immunotherapy based on the use of chimeric antigen receptor modified T cells (CAR T cells) is a promising approach for the treatment of refractory hematological malignancies. However, a robust response mediated by CAR T cells is observed only in a minority of patients and the expansion and persistence of CAR T cells in vivo is mostly unpredictable.Lenalidomide (LEN) is an immunomodulatory drug currently approved for the treatment of multiple myeloma (MM) and mantle cell lymphoma, while it is clinically tested in the therapy of diffuse large B-cell lymphoma of activated B cell immunophenotype. LEN was shown to increase antitumor immune responses at least partially by modulating the activity of E3 ubiquitin ligase Cereblon, which leads to increased ubiquitinylation of Ikaros and Aiolos transcription factors, which in turn results in changed expression of various receptors on the surface of tumor cells. In order to enhance the effectiveness of CAR-based immunotherapy, we assessed the anti-lymphoma efficacy of LEN in combination with CAR19 T cells or CAR20 T cells in vitro and in vivo using various murine models of aggressive B-cell non-Hodgkin lymphomas (B-NHL).Immunodeficient NSG mice were transplanted with various human B-NHL cells followed by treatment with CAR19 or CAR20 T cells with or without LEN. Next, CAR19 T cells were subjected to series of tests in vitro to evaluate their response and signaling capacity following recognition of B cell in the presence or absence of LEN.Our data shows that LEN significantly enhances antitumor functions of CAR19 and CAR20 T cells in vivo. Additionally, it enhances production of interferon gamma by CAR19 T cells and augments cell signaling via CAR19 protein in T cells in vitro. Our data further suggests that LEN works through direct effects on T cells but not on B-NHL cells. The biochemical events underlying this costimulatory effect of LEN are currently being investigated. In summary, our data supports the use of LEN for augmentation of CAR-based immunotherapy in the clinical grounds.
Introduction
The adoptive immunotherapy with autologous T lymphocytes genetically modified ex vivo to express artificial signaling molecule designated CARs represents a novel and promising treatment modality of cancer. So far, the most successful example of CAR-based immunotherapy achievements came from the treatment of patients with B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia (B-ALL, CLL).Citation1 Successfully targeted antigens include CD19 and CD20 which are major B-cell surface antigens and are strongly expressed by malignant B cells. CARs typically encode an extracellular antibody-derived domain that binds to a surface antigen (CD19, CD20, etc.) linked with an intracellular signaling domain that mediates T-cell activation such as TCRζ chain and co-stimulatory domains from CD28 or 4–1BB intracellular chains. The signaling through CAR substitutes for the signaling through endogenous T-cell receptor and leads to a potent and swift cytotoxicity toward target T cells in non-HLA restricted manner.Citation2 In principle, any surface antigen can be targeted with CAR. Up to now, a large number of CARs targeting diverse tumors have been developed and many clinical trials are ongoing.
Despite promising results, resistance to CAR-based immunotherapy is frequently seen.Citation3 The most debated reasons for the observed resistance include a loss of the CAR-specific antigen or a limited proliferation of CAR T cells in vivo as a result of their inefficient activation or even inhibition due to immunosuppressive microenvironment within the tumor stroma.Citation4 Several new approaches that would enhance CAR-based therapy are currently being tested, including an introduction of additional motifs from various co-stimulatory molecules into the intracellular signaling chain of CAR, co-transduction of T cells with genes encoding for essential prosurvival T-cell cytokines, or selective modification of certain T-cell subsets (such as effector memory).Citation2 Another strategy to improve clinical efficacy of CAR-based therapy is based on the targeted reversal of tumor stroma immunosuppressive activity by using different immunomodulatory compounds such as monoclonal antibodies (MAbs) that block particular inhibitory receptors (e.g. CTLA-4, PD-1, LAG-3),Citation5 or small molecules belonging to the class of immunomodulatory agents (IMiDs), namely LEN.
LEN is an IMiD approved for the treatment of MM, mantle cell lymphoma and 5q-syndrome.Citation6 It was demonstrated that LEN binds E3 ubiquitin ligase Cereblon and induces degradation of transcription factors Ikaros and Aiolos.Citation7 It inhibits growth of malignant B cells, inhibits angiogenesis and augments antitumor T-cell responses.Citation8 It has been reported that LEN triggers tyrosine phosphorylation of CD28 on T cells, followed by activation of nuclear factor kappa B.Citation9 In addition, LEN modifies T-cell responses in vivo and leads to increased interleukin (IL)-2 production in both CD4+ and CD8+ T cells, induces the shift of T helper (Th) responses from Th2 to Th1, inhibits expansion of regulatory subset of T cells (Tregs), and improves functioning of immunological synapses in follicular lymphoma and CLL.Citation10,11
In this study, we tested the immunoadjuvant properties of LEN in combination with CAR19 or CAR20 T cells in experimental therapy of aggressive B-cell lymphomas using various mouse xenograft models based on xenotransplantation of both B-NHL cell lines and primary lymphoma cells. Presented data shows that LEN augments activation of CAR19 T cells in vitro and significantly enhances antitumor functions of CAR19 and CAR20 T cells in vivo. Our data additionally demonstrates that LEN works through direct effects on T cells, but not on lymphoma B cell. In general, our data supports the testing of LEN for augmentation of CAR-based immunotherapy in the clinical grounds.
Results
LEN enhances IFNγ secretion and augments signaling in CAR-19 T-cells
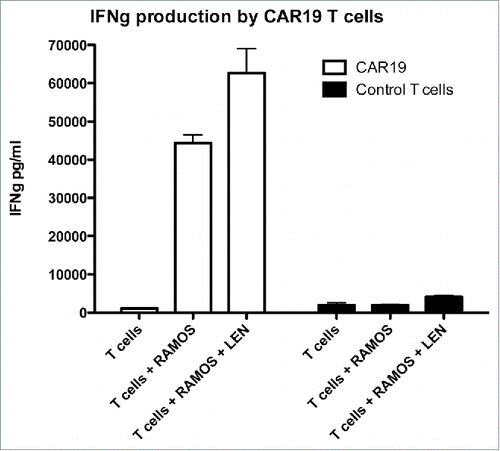
The data in demonstrates that co-incubation of CAR 19 T cells with Ramos cells (Burkitt lymphoma) in the presence of 10 MM LEN results in enhanced production of IFNM. We also show that the response is specific to CD19 since CAR-negative T cells do not produce IFNγ in the presence of B cells.(right panel).
Figure 1. The production of IFNγ by CAR19 T cells following recognition of B-cell lymphoma cell line Ramos is enhanced by Lenalidomide. CAR19 T cells were incubated at 10:3 ratio with Ramos B-cells overnight, in the presence or absence of 10 MM lenalidomide and the production of IFNγ into culture supernatant was determined with ELISA. The experiment was performed twice with similar results.
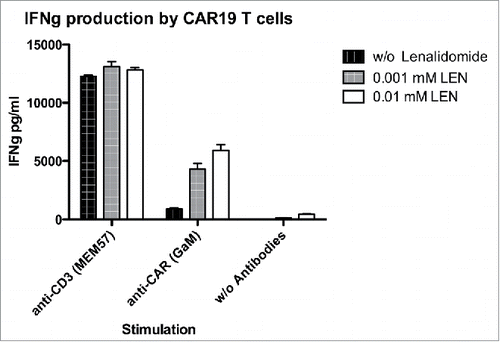
The activation of CAR19 T cells might be influenced by multiple ligands expressed by Ramos B cells. To minimize such interference we activated CAR19 T cells with antibodies to CAR (or TCR) in the presence of LEN (but in the absence of Ramos cells). The data in shows that LEN significantly and dose-dependently enhanced production of IFNγ after stimulation by anti-CAR antibody. Stimulation via endogenous TCR was more efficient than stimulation via CAR19, and in this case LEN did not further augment response of T cells. This finding suggests that stimulation by CAR is suboptimal compared to stimulation via TCR and that such weak stimulation can be markedly augmented by LEN. Further increase of the response in case of strong T cells activation via endogenous TCR cannot be achieved by LEN.
Figure 2. Lenalidomide enhances signaling via CAR19 receptor. To determine the costimulatory effect of lenalidomide, CAR19 T cells were stimulated with immobilized anti-CD3 antibody (clone MEM-57) or, with immobilized anti-CAR serum (polyclonal goat anti-mouse IgG FAB2) in the presence (10 MM, 1 MM) or absence of lenalidomide. The production of IFNγ into culture supernatant was determined with ELISA after overnight co-incubation. The experiment was performed twice with similar results.
The effect of LEN is mediated by direct costimulation of CAR-19 T cells and more effectively augments the response of weakly stimulated CAR-19 T cells
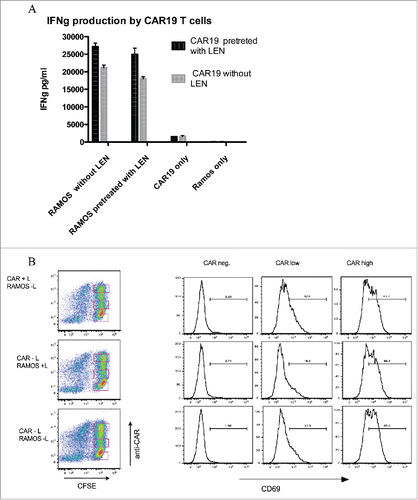
The data in shows that LEN pretreatment of CAR19 T cells enhanced the production of INFγ, while LEN pretreatment of B cell had no effect on IFNγ production. We hypothesized that LEN would enhance the response of weakly or, sub-optimally activated T cells, i.e., those expressing low amounts of CAR19. To answer this question we measured by flow-cytometry the upregulation of activatory molecule CD69 on CAR-19 T cells together with the expression of CAR19 receptor to correlate the level of T cell activation with the amount of CAR19. Similarly as in the previous experiment, CAR19 T cells and Ramos B cells were pre-incubated with LEN. The expression of CAR (y-axis, left panel) was separated into three major subgroups: CAR-negative (C-N), CAR-low (C-L), and CAR-high (C-H) subgroups (the gating is shown in the left panel). The level of CD69 upregulation was then determined in these three subgroups (right panel), the numbers in histograms indicate the percentage of CD69-positive cells. The data shows that LEN pretreatment markedly enhanced upregulation of CD69 (32% vs. 20%) only in the C-L T cells. No upregulation of CD69 was detected in the C-N T cells, while the difference observed in the C-H T cells was insignificant.
Figure 3. Lenalidomide acts directly onto T cells but not B cells to costimulate signaling via CAR19. (A) The production of IFNγ was measured by ELISA following pretreatment of CAR19 T cells or RAMOS cells for 16 h with 10 MM LEN, after wash-out of excess LEN cells were further co-incubated at 10:3 ratio overnight and production of IFNγ was then determined in culture supernatant. (B) The upregulation of activatory marker CD69 following recognition of Ramos B cells was determined on CAR+ T cells by flow cytometry in same setup as in , T cells were labeled with CFSE to easily separate them from B cells, the expression of CAR19 was visualized with Alexa 647 labeled goat anti-mouse serum. Combinations of either pretreated CAR19 T cells (CAR+L, Ramos-L), or Ramos B cells (CAR-L, Ramos+L), or cells without LEN (CAR-L, Ramos-L) are shown. The expression of CD69 shown in histograms was gated on either CAR19 T cells expressing high amounts, low amounts or negative for CAR19. These experiments were performed twice with similar results.
LEN induces Aiolos and Ikaros degradation in T cells
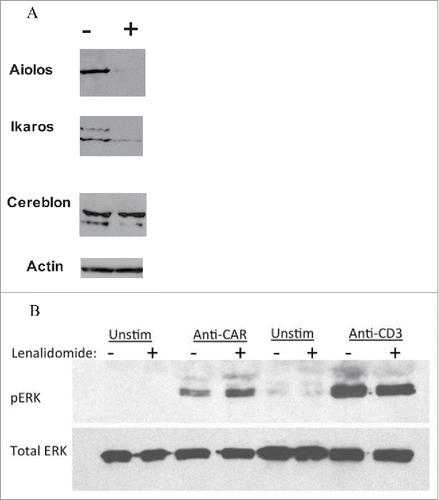
The molecular mechanisms behind the effects of LEN are not exactly known, but it was recently demonstrated that LEN binds to E3 ubiquitin ligase Cereblon and induces ubiquitinylation and degradation of transcription factors Aiolos and Icaros.Citation7 This mechanism was first shown in MM B cells, but was not tested in T cells so far. The data in shows that LEN indeed similarly induces degradation of Aiolos and Ikaros transcription factors in T cells.
LEN increases ERK phosphorylation following CAR activation in T cells
Our data so far demonstrated that LEN augmented CAR signaling resulting in increased production of effector cytokines such as IFNγ, and increased expression of activation markers such as CD69. Additionally, in this experiment we tested the phosphorylation status of ERK kinase following stimulation of T cells with anti-TCR, or anti-CAR antibody. The data in shows that LEN pretreatment augments the phosporylation of ERK following stimulation of CAR. LEN did not further increase the level of pERK after TCR activation suggesting that supra-physiological stimulation such as with anti-TCR antibody is not further enhanced by LEN similarly as in..
LEN enhances antitumor functions of CAR T cells in vivo
To translate the in vitro data into in vivo settings, we tested the immunoadjuvant properties of LEN to antitumor functions of CAR19 or CAR20 T cells using immunodeficient NOD-SCID-gamma chain null mice (NSG mice) transplanted subcutaneously (SC) with human B-NHL cells. In the first series of experiments, we transplanted NSG mice with established B cell lines TMD8 (diffuse large B-cell lymphoma of activated B-cell immunophenotype, ABC-DLBCL) and Ramos (Burkitt lymphoma). Both cell lines grow readily in vitro and in vivo and form large tumors upon SC injection into NSG mice. In the subsequent series of experiments, we used primary lymphoma cell-based murine xenograft models developed in our laboratory designated NEMO (relapsed mantle cell lymphoma MCL), and KTC (refractory ABC-DLBCL).Citation12 It must be emphasized that both NEMO and KTC cells can only be propagated in vivo in NSG mice from primary to secondary recipients.(they do not grow in vitro).
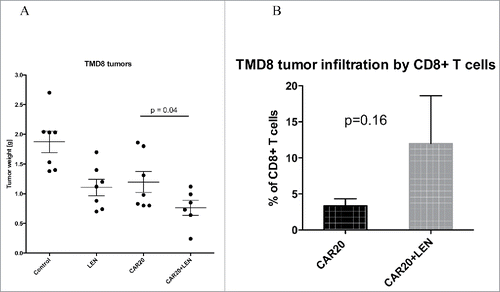
In the experiment shown in , we transplanted NSG mice on day 1 with 5 million TMD8 cells. These cells are highly CD20 positive and only medium positive for CD19, therefore, we used CAR T cells specific to antigen CD20 in this experiment. On days 2 and 3 mice received intravenous (IV) injection of CAR20 T cells (5 million each) followed by daily intraperitoneal (IP) injection of LEN. Fourteen days later mice were euthanized, and tumors excised and analyzed as in the previous experiment. The data in the left panel shows differences in tumor weight between respective groups and similarly to the previous experiment indicates that the tumor progression was slowest in mice treated with the combination of CAR20 T cells and LEN. Interestingly, tumors obtained from these mice also demonstrated highest levels of infiltration with CD8+ T cells.(right panel).
Figure 4. Analysis of changes in protein expression induced by LEN. (A) Downregulation of Ikaros and Aiolos by LEN. CAR19 T cells were pretreated with LEN 10 MM for 24 h (+), or without it (−), cells were then lysed in SDS loading buffer and the levels of Ikaros and Aiolos transcription factors were determined by immunoblotting together with the ubiquitin ligase Cereblon, actin indicates the equal loading. (B) Enhanced ERK activation by ligation of CAR after LEN pre-treatment (10 MM for 24 h). CAR19 T cells were activated by immobilized anti-CAR antibody (goat anti-mouse), or by anti-CD3 antibody (clone MEM-57) for 30 min at 37°C, cells were then lysed in SDS sample buffer and the phosphorylation status of ERK was determined by immunoblotting, the total levels of ERK are shown in the bottom panel. Both experiments were performed twice with similar results.
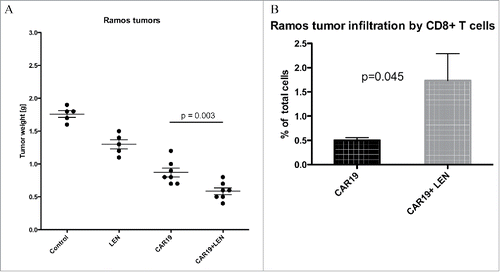
The data in shows results from the experiment, in which NSG mice received SC injection of 10 million Ramos B-cell followed with one dose of 5 million CAR19 T-cells. In previous experiments (not shown) we found out that Ramos cells are highly sensitive to CAR-mediated therapy and therefore mice received only one dose of CAR T cells. One group of CAR19—treated mice received daily injections of LEN similarly as in the previous experiments. After 3 weeks mice were sacrificed and tumors were analyzed. The results showed that the combination of CAR19 and LEN led to the best anti-lymphoma response, and that the tumors in this group contained significantly more CD8+ T cells compared to the tumors obtained from the mice treated with CAR19 T cells only.
Figure 5. LEN enhances antitumor response in vivo to TMD8 DLBCL cells. NSG mice were transplanted SCl with 5 million DLBCL ABC cells TMD8 and then received two doses of 5 million. CAR20 T cells followed with daily IP injection of 10 Mg of LEN, 14 d later mice were sacrificed and the tumors were excised and weighted (A). In panel B we shown the infiltration of tumors by CD8+ T cells which was determined by flow cytometry in cell suspension prepared from excised tumors. This experiment was performed twice with similar results.
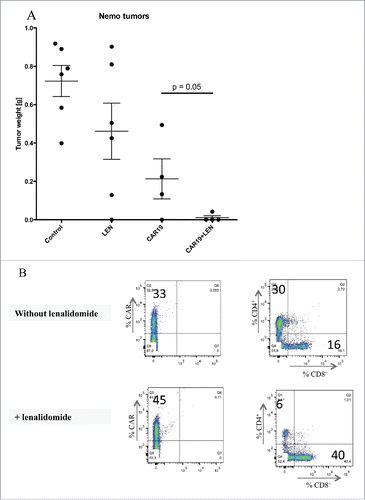
The data in shows results from the experiment, in which NSG mice received SC injection of 5 million NEMO cells followed by injection of 5 million CAR19 T cells 2 and 3 d later, half of these mice received daily LEN. Fourteen days later, mice were euthanized and the tumors were excised and analyzed. Our data showed that LEN markedly augmented CAR-19 mediated suppression of tumor growth in vivo. Interestingly, flow cytometry analysis uncovered that tumors in mice treated with CAR19 T cells plus LEN were infiltrated predominantly with CD8+CAR19 T cells in contrast to the tumors obtained from the mice treated with CAR19 T cells only, which were infiltrated predominantly with CD4+ CAR19 T cells.
Figure 6. LEN enhances antitumor response in vivo to Ramos Burkitt lymphoma cells. NSG mice were transplanted SC with 5 million Ramos cells and then received one dose of 5 million CAR19 T cells followed with daily IP injection of LEN, 21 d later mice were sacrificed, the tumors were excised and weighted (A). In panel B is shown the infiltration of tumors by CD8+ T cells which was determined by flow cytometry in cell suspension prepared from excised tumors. This experiment was performed once.
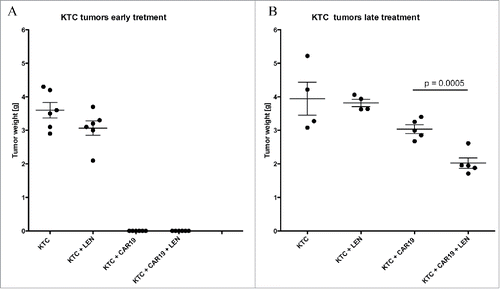
In the last experiment, we tested the efficacy of CAR therapy using a mouse model of primary cell-based ABC-DLBC (i.e., mice xenografted with KTC cells). As the growth of KTC tumors was completely suppressed when treatment with CAR19 T cells was initiated early after tumor inoculation, () we decided to modify the approach and delay the treatment initiation. The treatment consisted of three doses of 5 million. CAR19 T cells at days 10, 12 and 17. Two weeks after first injection of CAR19 T cells the mice were sacrificed, tumors excised and weighted. The data in shows that the tumor growth was significantly suppressed in the group, which received CAR19 T cells plus LEN. In contrast to the previous experiments, we did not find any T cell infiltration in the tumors of any group. The potential explanation for this observation might be the delay in the treatment initiation—the tumors were approximately 0.5 cm in diameter, and the very large tumor mass (compared to numbers of injected CAR T cells) probably led to limited persistence of CAR 19 T cells within tumor stroma.
Figure 8. LEN enhances response antitumor response in vivo to established primary diffuse large B cell lymphoma (A) NSG mice were SC transplanted with 10 million DLBC cell KTC followed with one doses of 5 million CAR19 T cells and daily IP injection LEN, 14 d later mice were sacrificed, the tumors were excised and weighted. (B) NSG mice were SC transplanted with 5 million DLBC cell KTC and left for 10 d to establish cca 0.5 cm tumors, during this period all mice were receiving daily injections LEN. After the tumors were established, mice received three doses of 5 million. CAR19 T cells at days 10, 12 and 17 with, or without LEN. Two weeks after treatment with CAR19 T cells, mice were sacrificed and the size of tumors was determined. The experiment in was performed once.
Discussion
Our data demonstrated that a co-administration of LEN, which is a clinically approved orally available immunomodulatory agent, provides a significant enhancement of CAR-based immunotherapy. LEN leads to stronger signaling via CAR, increased production of IFNγ and upregulation of CD69 by activated CAR T cells. At the subcellular level, we showed that LEN increased phosphorylation of ERK after activation via CAR and the effects of LEN were associated with downregulation of transcription factors Ikaros and Aiolos. We have performed several in vivo experiments using murine models of aggressive human B-NHL (based on xenotransplantation of both cell lines and primary lymphoma cells) and we confirmed that LEN significantly enhanced antitumor functions of CAR T cells in vivo resulting in increased suppression of tumor growth and enhanced infiltration of tumors by cytotoxic CD8+ T cells in most tested models. Our data suggests that that LEN worked through direct effects on CAR T cells by enhancing the cellular response after a weaker stimulus such as after CAR activation.
Figure 7. Lenalidomide enhances antitumor response in vivo to primary mantle cell lymphoma. (A) NSG mice received SC 5 million. NEMO cells followed by IV injection of two doses of 5 million CAR19 T cells and daily IP injection of LEN, 14 d later mice were sacrificed and the tumors were excised weighted. (B) The infiltration of tumors by T cells was analyzed by flow cytometry in a cell suspension prepared from excised tumors using antibodies to CD4+, CD8+ and CAR, one representative mouse is shown. The experiment in was performed once.
CAR-based tumor immunotherapy is currently under intensive clinical investigation, treatment induced long-term remission in patients with B cell acute and B cell chronic leukemia who were resistant to standard therapy including allogeneic bone marrow transplantation.Citation1 Most frequently targeted antigen in these diseases is the CD19 since it is strongly expressed by all malignant B cells, other B cell antigens such as CD20 were also tested.Citation24 CAR-based therapy is not limited only to B cell malignancies, various surface tumor antigens are under pre-clinical and clinical investigation targeting many types of tumors.Citation25
The mechanisms of antitumor activity of LEN and other IMiDs are broad and may affect many cell types in the organism. Direct effects include anti-proliferative and pro-apoptotic effects especially on malignant plasma cells.Citation13 Indirect effects are postulated to be mediated by alterations in tumor microenvironment. IMiDs block interaction of MM cells with bone marrow stromal cells through inhibition of expression of surface adhesion molecules.Citation14 and they inhibit formation of bone lesions in MM by directly inhibiting osteoclast maturation.Citation15 The immunomodulatory effects are associated with non-specific activation of immune system, which then effectively slows tumor progression.Citation16 Unanswered question, however, remains the true nature of immune system activation by IMiDs. Published data suggests that IMiDs (the most frequently studied compound being LEN) might directly modify phenotype of malignant B cell by reducing the expression of inhibitory ligands such as PD-L1 and thus increase the recognition of tumors cells by T cells. Besides effects on malignant B cell,Citation17 IMiDs have direct effects on T cells by providing CD28-like costimulation.Citation9 resulting in enhanced activation and effector cytokine production, additionally, there were observations that IMiDs inhibit the proliferation and function of Tregs.Citation18
Another evidence showing direct effects of IMiDs on T cells was obtained in patients, who underwent allogeneic bone marrow transplantation for MM and were later on LEN treatment. These patients developed severe graft-versus-host-disease (GvHD) and LEN had to be withdrawn from therapy.Citation19 In mouse models, LEN was shown to enhance the response to antitumor vaccination.Citation20 The conclusion from the available data is therefore quite solid in the sense that IMiDs can stimulate antitumor immune reaction by providing activatory signals to tumor-specific T cells and by enhancing the recognition of malignant B cell by reducing expression of their immuneinhibitory receptors. The overall therapeutic effect of IMiDs thus appears to be a combination of direct anti-proliferative effects on tumor cells, indirect effects on tumor stroma, immunomodulatory effects leading to increased recognition of malignant B cells and enhanced response of antitumor Tcells.
Our data shows that LEN increases infiltration of tumors with CD8+ T cells. It is possible that LEN promotes the expansion of CD8+ T cells over CD4+ T cells or reduces the activation-induced cell death (AICD) of activated CD8+ T cells. To investigate this we cultivated CAR T cells in the presence of LEN (up to 7 d) and we did not detect any significant enrichment of CD8+ CAR19 T cells over CD4+ cells. Similarly, the sensitivity of CAR T cells to AICD in vitro was not affected by LEN regardless if they were CD4+ or CD8+ (data not shown). We have also analyzed the tumors for the presence of T-regulatory cells and we did not find any significant infiltration by Tregs in tumors regardless of LEN treatment. However, the behavior of CAR T cells in vivo following recognition of tumor antigen and subsequent activation might be different from what occurs in vitro. Since LEN provides additional costimulatory signals to T cells for example by mimicking CD28 activation,Citation9 it is possible that tumor-specific CD8+ CAR T cells will survive longer within tumor stroma in the presence of LEN.
Our finding that LEN works via direct effects on T cells but not B cells is the result of in vitro observation and can be very hardly proved in vivo. However, this finding does not contradict any so far published data and we indeed agree that LEN has direct antitumor effects, which is demonstrated by inhibition of tumor growth by single-agent LEN (in the absence of CAR treatment, see Results). Our in vivo data suggests that CAR T cell specific and tumor cell specific effects of LEN are synergistic and complementary. The literature shows that direct immunomodulatory effects of LEN on T cells results in enhanced proliferation and stronger production of IL2, IFNγ and TNF-α in vitro.Citation21 Also the exposure of primary CLL cells to LEN in vitro led to induction of costimulatory molecules including CD80, CD86 and FASL on tumor cells, restoring immunological synapse formation and improving autologous tumor cell recognition by T cells.Citation17 We have analyzed the phenotype of B-NHL cell lines Ramos and TMD8 after in vitro treatment with LEN and we did not detect any reproducible changes in the expression of CD19 or CD20, and both cell lines were negative for the expression of PD-L1 or PD-L2 regardless of LEN treatment. In concordance with these findings, the viability and proliferation of B-NHL cell lines in vitro not affected by LEN.
The molecular mechanisms behind LEN actions are connected to the degradation of transcription factors Aiolos and Ikaros via E3 ubiquitin ligase Cereblon.Citation7 Degradation of these substrates, which are negative regulators of IL2 expression in T cells results in enhanced production of IL2 and other cytokines known to regulate T cell function. This mechanism was first shown in malignant plasma cells and later in other B cell types and T cells as well.Citation8 We have confirmed that LEN induced degradation of Ikaros and Aiolos in CAR T cells and besides already discussed effects, we have also found out that LEN leads to increased activation of ERK following CAR stimulation. This finding is another manifestation of increased T cell response after LEN since ERK pathway activity reflects the magnitude of T cell activation.Citation22
Presented experiments were performed with CAR19 T-cells except experiment in , however, we have done several key experiments such as tests for IFNγ production also with CAR20 T cells, the results were similar to the results obtained with CAR19 T cells and therefore are not shown. Next, our results suggest that the costimulatory effects of LEN might be related to the strength of receptor-mediated activation and become significant in sub-optimally stimulated CAR T cells. This is supported by in vitro experiments, which have shown that LEN more effectively enhanced upregulation of CD69 in T cells expressing low amounts of CAR-19 compared to T cells expressing high levels of CAR-19. The lack of enhancement of endogenous TCR signaling by LEN can be the result of the fact that LEN might not further enhance supra-physiological activation of T cells triggered by anti-CD3 antibody. This hypothesis fits with the current model of T cell activation, which integrates signals originating form TCR, costimulatory and cytokine receptors.Citation23
In conclusion, the key message of our paper is that the anti-lymphoma efficacy of CAR-based immunotherapy can be robustly enhanced by concomitant application of immunomodulatory agent LEN. Such combined treatment might lead to a highly effective cancer immunotherapy.
Material and methods
Construction of recombinant scFv fragments
The coding sequences for VL and VH variable domains of anti-CD20 mAb MEM-97 and anti-CD19 mAb B-D3 were obtained by RT-PCRs using total RNA from respective hybridoma cells. PCR products were sequenced and used as templates for reamplification with modified primers allowing assembly of the VL (EcoRV-XhoI) and VH (PstI-BstEII) coding DNA fragments into scFv molecule in the format (VH)-(Gly4Ser)4-(VL). The scFv protein was produced in bacteria and tested for binding to the surface of CD19 and CD20 postive cells by flow-cytometry (data not shown). Subsequently, the scFv segments were in silico assembled with the CAR sequence, codon optimized and the whole sequence was chemically synthesized (Origene) and cloned into pWPXLd vector (Addgene) via BamHI-EcoRI using standard molecular biology techniques.
Cells, media and antibodies
HEK and Ramos cell lines were obtained from ATCC,Citation12 TMD8 was a kind gift of dr. Hernandez-Ilizariturry- it´s origin was authenticated in our laboratory by mutational analysis showing the presence of the specific mutation of CD79B gene (Y196H). The cell lines were grown in IMDM media supplemented with 10% FCS, antibiotics (penicillin, streptomycin) and were mycoplasma negative. Human blood was obtained from volunteers under approval of local ethical committee, samples were processed by Ficoll gradient centrifugation and isolated leukocytes were further used for lentiviral infections. Following fluorochrome labeled antibodies were used: Alexa 647 goat anti-mouse (Jackson Immuno), pacific Blue anti-CD4+, PerCP anti-CD8+, APC anti-CD69, PE anti-CD19 and PE anti-CD20 (all Exbio), for stimulation and western blotting were used unlabeled antibodies specific for: CD3, CD28 (Miltenyi Biotec), TCRzeta (Santa Cruz), Aiolos, Ikaros, pERK, total ERK (all Cell Signaling).
Cell activation, lenalidomide treatment
Goat anti-mouse polyclonal serum (Jackson Immuno), or anti-CD3 antibody MEM-57 were adsorbed onto plastic plates at 37 C for 1 h, washed with PBS and then used for stimulation of CAR19 T cells. In some experiments, T cells and B cells were incubated for 24 h in the presence 10MM LEN in culture media (without IL2), cells were then centrifuged twice to wash out excess of LEN and used for in vitro assay.
Virus production and cell transduction
Lentiviruses (2nd generation vectors) were prepared by transfecting the HEK cells with packaging plasmids psPax and pVSVG (Addgene) and lentiviral vectors pWPXLd containing CAR insert using Metafectene Easy reagent, supernatants were then used directly to infect primary human T cells. For infections, first, tissue culture plastic was coated with antibodies to CD3 and CD28, then ficol-purified leukocytes were resuspended in LV sup. and spin-infected (90 min, 1500 g, RT) in the presence of 5 Mg/uL polybrene, the infection was repeated next day, after 3 d the cells were replated in new flasks without antibodies and were further grown in RPMI media in the presence of 100 U/mL of IL2 (Proleukin).
Interferon gamma assay
The concentration of IFNγ was determined by ELISA assay kit (Biolegend), the absorbances were read with ELISA plate reader at wavelengths specified in the kit. For the assay, we usually incubated 10,000 T cells with 3,000 B cells, in 100 uL of media for 12 h without the presence of IL2, before analysis cells were freeze-thawed. The concentration of IFNγ was calculated in Prism software according to the dilution of IFNγ standard.
Flow cytometry
Cells (1 million) in 50 ML PBS with 1% FCS were incubated with fluorescently labeled antibodies at dilution specified by the manufacturer, usually for 30 min at 4°C, washed twice and analyzed with flow-cytometer (Canto, BD). Single cell suspension was prepared from tumors by cutting them into small pieces with a blade and gentle dissociation by pipetting.
Cytotoxic assay
First, Ramos B cells were labeled with CFSE (5 um for 5 min at RT in PBS), washed and co-incubated with T cells, usually 3 × 103 T cells plus 1 × 104 Ramos cells in 200 uL media for 24 h, the viability of CFSE+ cells was analyzed by flow cytometry after adding the DNA dye Hoechst.
Western blotting
Cells were lysed at concentration 20 million/mL in 1% SDS loading buffer, reduced with 1% DTT (Sigma) at 95°C for 3 min, sonicated and resolved by SDS-PAGE. Proteins were visualized after transfer onto PVDF membrane by immunoblotting.
In vivo experiments and statistical analysis
In vivo studies were approved by the institutional Animal Care and Use Committee. Immunodeficient NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (referred to as NSG mice, Jackson Laboratory) were maintained in individually ventilated cages. Primary DLBCL−based mouse model designated KTC (treatment refractory DLBCL of activated B-cell immunophenotype) was established in the same way as previously described for a primary MCL−based mouse model designated NEMO.Citation5 KTC or NEMO cells were chosen for in vivo experiments, because they represent lymphoma subtypes, for the therapy of which LEN is currently approved (MCL) or under advanced clinical testing (ABC-DLBCL).
NSG mice were kept in individually ventilated cages under SPF conditions, mice 6–12 weeks old of the same sex in a group of 5 to 6 animals were injected SC with indicated number of tumor cells in 0.2 mL of PBS. After specified period, mice received intravenously two doses of 5×10e6 CAR T cells, followed with daily LEN treatment (10 mg/kg in 0.1 mL PBS intraperitoneally). After indicated time, mice were sacrificed, tumors were excised and weighted, then the tumor tissue was minced and dissociated by pipetting and analyzed by flow cytometry. The differences between LEN-treated groups were analyzed by one-tailed unpaired t-test using the Graph Pad Prism software. All animal work was performed under approved ethical guidelines.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
1115940_Supplementary_Material.pdf
Download PDF (286.7 KB)Funding
Financial support—Pavel Otahal: IGA-MZ NT/14030–3, Pavel Klener Jr.: IGA-MZ: NT13201–4/2012, UNCE 204021, PRVOUK P24/LF1/3, PRVOUK-27/LF1/1, Lucie Lateckova: GA-UK 1270214. This research was also partially sponsored and funded by Celgene to PK and MT.
References
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF et al., Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371(16):1507-17; PMID:25317870; http://dx.doi.org/10.1056/NEJMoa1407222
- Maher J. Immunotherapy of malignant disease using chimeric antigen receptor engrafted T cells. ISRN Oncol 2012; 2012:278093; PMID:23304553; http://dx.doi.org/10.5402/2012/278093
- Curran KJ, Seinstra BA, Nikhamin Y, Yeh R, Usachenko Y, van Leeuwen DG, Purdon T, Pegram HJ, Brentjens RJ. Enhancing Antitumor Efficacy of Chimeric Antigen Receptor T Cells Through Constitutive CD40L Expression. Mol Ther 2015; 23(4):769-78; PMID:25582824; http://dx.doi.org/10.1038/mt.2015.4
- Han EQ, Li XL, Wang CR, Li TF, Han SY. Chimeric antigen receptor-engineered T cells for cancer immunotherapy: progress and challenges. J Hematol Oncol 2013; 6:47; PMID:23829929; PMID:24353912; http://dx.doi.org/10.1186/1756-8722-6-47
- John LB, Kershaw MH, Darcy PK. Blockade of PD-1 immunosuppression boosts CAR T-cell therapy. Oncoimmunology 2013; 2(10):e26286; PMID:24353912; http://dx.doi.org/10.4161/onci.26286
- Heise C, Carter T, Schafer P, Chopra R. Pleiotropic mechanisms of action of lenalidomide efficacy in del(5q) myelodysplastic syndromes. Expert Rev Anticancer Ther 2010; 10 (10):1663-72; PMID:20942636; http://dx.doi.org/10.1586/era.10.135
- Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X et al., Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014; 343(6168): 301-5; PMID:24292625; http://dx.doi.org/10.1126/science.1244851
- Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, Ito T, Ando H, Waldman MF, Thakurta A et al., Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN). Br J Haematol 2014; 164(6):811-21; PMID:24328678; http://dx.doi.org/10.1111/bjh.12708
- LeBlanc R, Hideshima T, Catley LP, Shringarpure R, Burger R, Mitsiades N, Mitsiades C, Cheema P, Chauhan D, Richardson PG et al., Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood 2004; 103(5):1787-90; PMID:14512311; http://dx.doi.org/10.1182/blood-2003-02-0361
- Schafer PH, Gandhi AK, Loveland MA, Chen RS, Man HW, Schnetkamp PP, Wolbring G, Govinda S, Corral LG, Payvandi F et al., Enhancement of cytokine production and AP-1 transcriptional activity in T cells by thalidomide-related immunomodulatory drugs. J Pharmacol Exp Ther 2003; 305(3):1222-32; PMID:12649301; http://dx.doi.org/10.1124/jpet.102.048496
- Haslett PA, Corral LG, Albert M, Kaplan G. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med 1998; 187(11):1885-92; PMID:9607928; http://dx.doi.org/10.1084/jem.187.11.1885
- Klanova M, Soukup T, Jaksa R, Molinsky J, Lateckova L, Maswabi BC, Prukova D, Brezinova J, Michalova K, Vockova P et al., Mouse models of mantle cell lymphoma, complex changes in gene expression and phenotype of engrafted MCL cells: implications for preclinical research. Lab Invest 2014; 94(7):806-17; PMID:24862967; http://dx.doi.org/10.1038/labinvest.2014.61
- Verhelle D, Corral LG, Wong K, Mueller JH, Moutouh-de Parseval L, Jensen-Pergakes K, Schafer PH, Chen R, Glezer E, Ferguson GD et al., Lenalidomide and CC-4047 inhibit the proliferation of malignant B cells while expanding normal CD34+ progenitor cells. Cancer Res 2007; 67(2):746-55; PMID:17234786; http://dx.doi.org/10.1158/0008-5472.CAN-06-2317
- Gorgun G, Calabrese E, Soydan E, Hideshima T, Perrone G, Bandi M, Cirstea D, Santo L, Hu Y, Tai YT et al., Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood 2010; 116 (17):3227-37; PMID:20651070; http://dx.doi.org/10.1182/blood-2010-04-279893
- Breitkreutz I, Raab MS, Vallet S, Hideshima T, Raje N, Mitsiades C, Chauhan D, Okawa Y, Munshi NC, Richardson PG et al., Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia 2008; 22(10):1925-32; PMID:18596740; http://dx.doi.org/10.1038/leu.2008.174
- Ramsay AG, Johnson AJ, Lee AM, Gorgün G, Le Dieu R, Blum W, Byrd JC, Gribben JG. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest 2008; 118(7):2427-37; PMID:18551193
- Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood 2012; 120 (7):1412-21; PMID:22547582; http://dx.doi.org/10.1182/blood-2012-02-411678
- Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, Todryk S, Chen R, Muller G, Stirling D et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother 2009; 58(7):1033-45; PMID:19009291; http://dx.doi.org/10.1007/s00262-008-0620-4
- Kneppers E, van der Holt B, Kersten MJ, Zweegman S, Meijer E, Huls G, Cornelissen JJ, Janssen JJ, Huisman C, Cornelisse PB et al., Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: results of the HOVON 76 Trial.Blood 2011; 118(9):2413-9; PMID:21690556; http://dx.doi.org/10.1182/blood-2011-04-348292
- Sakamaki I, Kwak LW, Cha SC, Yi Q, Lerman B, Chen J, Surapaneni S, Bateman S, Qin H. Lenalidomide enhances the protective effect of a therapeutic vaccine and reverses immune suppression in mice bearing established lymphomas. Leukemia 2014; 28(2):329-37; PMID:23765229; http://dx.doi.org/10.1038/leu.2013.177
- Haslett PA, Hanekom WA, Muller G, Kaplan G. Thalidomide and a thalidomide analogue drug costimulate virus-specific CD8+ T cells in vitro. J Infect Dis 2003; 187 (6):946-55; PMID:12660941; http://dx.doi.org/10.1086/368126
- Franklin RA, Tordai A, Patel H, Gardner AM, Johnson GL, Gelfand EW. Ligation of the T cell receptor complex results in activation of the Ras/Raf-1/MEK/MAPK cascade in human T lymphocytes. J Clin Invest 1994; 93(5):2134-40; PMID:8182145; http://dx.doi.org/10.1172/JCI117209
- Marchingo JM, Kan A, Sutherland RM, Duffy KR, Wellard CJ, Belz GT, Lew AM, Dowling MR, Heinzel S, Hodgkin PD. T cell signaling. Antigen affinity, costimulation, and cytokine inputs sum linearly to amplify T cell expansion. Science 2014; 346(6213):1123-7; PMID:25430770; http://dx.doi.org/10.1126/science.1260044
- Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, Lindgren CG, Lin Y et al., CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 2012; 119(17):3940-50; PMID:22308288; http://dx.doi.org/10.1182/blood-2011-10-387969
- Han EQ, Li XL, Wang CR, Li TF, Han SY. Chimeric antigen receptor-engineered T cells for cancer immunotherapy: progress and challenges. J Hematol Oncol 2013; 6:47; PMID:23829929; http://dx.doi.org/10.1186/1756-8722-6-47
