ABSTRACT
Glycolytic activity in T cells governs T cell responses by controlling various cellular processes, including proliferation, survival, and effector functions. We recently showed that the tumor microenvironment diminishes T cell antitumor responses by depriving glucose to infiltrating T cells. Moreover, metabolic rewiring tumor-reactive T cells sustain T cell metabolic fitness and antitumor responses.
Abbreviations
| GLUT1 | = | Glucose transporter 1 |
| HIF-1α | = | Hypoxia-inducible factor 1-alpha |
| NFAT | = | Nuclear factor of activated T-cells |
| PCK-1 | = | phosphoenolpyruvate carboxykinase 1 |
| PD-1 | = | Programmed cell death protein 1 |
| PEP | = | phosphoenolpyruvate |
| PKM2 | = | pyruvate kinase M2 isoform |
| SERCA | = | sarcoplasmic or endoplasmic reticulum Ca2+-ATPase |
Malignant transformation is an accumulative process of multiple genomic alterations, including metabolic reprogramming, oncogenic mutations, epigenetic modifications, and cellular signaling alterations.Citation1 Among these traits of tumor cells, deregulated cellular metabolism is an important feature to support unrestricted growth and survival of tumor cells.Citation2 Tumor cells can utilize glucose to generate ATPs in the presence of oxygen, which is well known as Warburg effects (Warburg glycolysis). Although Warburg glycolysis is an inefficient way to consume glucose, this process allows tumor cells to fulfill both bioenergetic and biosynthetic demands. Importantly, oncogenic mutation and signaling cascades have been revealed to initiate metabolic alterations in tumor cells. Therefore, targeting cancer metabolism is considered as a promising strategy for antineoplastic drugs development. However, it remains elusive whether deregulated cancer metabolism promotes tumor progression by other unexplored mechanisms and if targeting cancer metabolism impairs functions of other cells in the tumor microenvironment, such as antitumor immunity.
T cells undergo metabolic reprogramming when they receive activation signal from T cell receptor (TCR) and co-stimulatory receptors.Citation3 Recent studies showed that metabolic reprogramming, including aerobic glycolysis and amino acid metabolism, modulates T cell differentiation and effector functions.Citation4 In addition to influencing the balance between mTOR and AMPK activity, studies suggested that nutrient metabolism modulates T cell function and exhaustion by the activation of metabolic enzymes and the generation of metabolic intermediates.Citation5, 6 Even through studies suggested that TCR signaling cascades are controlled by nutrient metabolism, the underlying mechanisms remain mostly unclear. Moreover, the similarity between cancer cell and T cell metabolism raises two important issues on cancer immunotherapy. First, common metabolic demands in tumor cells and T lymphocytes may result in metabolic competition in the tumor microenvironment, which could lead to T cell dysfunction/exhaustion. Secondly, targeting cancer metabolism may not only suppress tumor growth but also impairs T cell antitumor immune responses. Therefore, understanding the metabolic regulation involved in T cell activation and antitumor responses is a dire need. Also, it is critical to determine whether metabolic regulation can be exploited to improve cancer immunotherapy.
In our recent study, we first found that the tumor microenvironment was glucose deprived and tumor-infiltrating CD4+ T cells displayed gene signature of glucose deprivation in genetically modified murine melanoma model and B16 melanoma model.Citation7 Since hexokinase 2 (HK2) controls the rate-limiting step of glycolysis, we postulated that increased aerobic glycolysis in tumor cells by overexpressing HK2 could lead to immune evasion accompanied with reduced antitumor responses in tumor-infiltrating T cells. Using immune-deficient mice and melanoma cell engraftment model, we found that tumor-infiltrating CD4+ T cells failed to sustain antitumor effector functions and HK2-overexpressing melanoma cells were resistant to CD4+ T-cell-mediated antitumor immunity. In addition to decreased glucose availability in tumors, we also reported that HK2-overexpressing melanoma cells were more potent to suppress glucose uptake ability in CD4+ T cells. Taken together, our results uncover that tumor cells with elevated glycolytic rates are capable to promote T cell dysfunction/exhaustion by restricting glucose to tumor-infiltrating T cells.
We further investigated the underlying metabolic regulation by which glycolysis sustains T cell antitumor responses. Surprisingly, we found that sufficient glycolytic activity in T cells was a critical event to sustain cytoplasmic Ca2+ accumulation and promote nuclear translocation of NFAT1 and IFNγ and CD40L production. Then, we determined that a glycolytic metabolite, phosphoenolpyruvate (PEP), regulated Ca2+-NFAT signaling pathway and the generation of effector molecules by modulating sarcoplasmic or endoplasmic reticulum Ca2+-ATPase (SERCA) activity with genetic and pharmacological approaches. Of note, metabolic adaption can support T cell survival by utilizing oxidative phosphorylation metabolism when T cells experienced glucose starvation. We then investigated if manipulating tumor-reactive T cells to generate PEP from other metabolic intermediates is able to restore T cell antitumor responses in glucose-deprived conditions. To do so, we overexpressed phosphoenolpyruvate carboxykinase 1 (PCK1), which can converts TCA cycle intermediate, oxaloacetate (OAA), into PEP in tumor-specific T cells. Indeed, PCK1-overexpressing T cells sustained Ca2+ flux, NFAT nuclear localization and the effector functions in the glucose-deprived condition. Moreover, PCK1-overexpressing T cells displayed higher antitumor abilities in tumors, but not in other peripheral tissues and spleens. These findings suggest that PCK1 overexpression only boosts effector functions of T cells in the glucose-deprived circumstance. Altogether, the study provided a proof-of-concept evidence that modulating metabolic checkpoints is a promising strategy to manipulate T cell functions in the specialized microenvironment, like tumors. ()
Figure 1. (A) Cancer cells create a glucose deprivation tumor microenvironment by their own glycolytic activity. Deficiency of glucose in the tumor microenvironment promotes exhaustion/dysfunction in tumor infiltrating T cells. (B) PCK-1 overexpression restores the calcium-NFAT signaling pathway and antitumor responses in tumor-reactive T cells infiltrating into the glucose-deprived tumor microenvironment.
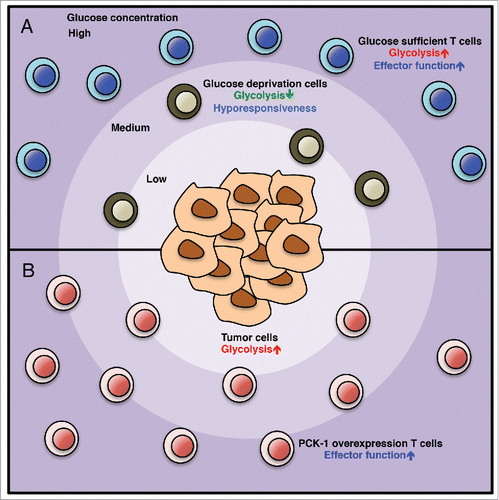
In summary, our findings uncovers that tumor cells cause T cell dysfunction in the tumor microenvironment by depriving glucose in infiltrating T cells. This study further implies that deregulated cancer metabolism could be one of the underlying mechanism which promote immune evasion. Most importantly, it also revealed that metabolic rewiring tumor-reactive T cell is a promising strategy to enhance T cell tumoricidal activity. Of note, PD-1 blockade has also been shown recently to improve metabolic fitness of T cells on fighting against chronic viral infection and tumor cells. Citation8, 9 Taken together, these studies suggest that modulating metabolic activities in immune cells, especially T cells, has therapeutic potential to suppress diseases progression. In addition to aerobic glycolysis, tumor cells also display other metabolic abnormality, such as increased fatty acid synthesis and amino acid metabolism. Therefore, a global understanding of how metabolic state in the tumor microenvironment modulates immune infiltration and the activation status of infiltrating immune cells is an important future direction. Additionally, determining the metabolic regulation mediated by different nutrients in immune cells would be of great interest for future cancer immunotherapy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
P.-C. H. is supported by ISREC research grant (26075483), SNSF Project Grant (31003A_163204), and Ludwig Center for Cancer Research (2607011910).
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer 2011; 11:85-95; PMID:21258394; http://dx.doi.org/10.1038/nrc2981
- MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol 2013; 31:259-83; PMID:23298210; http://dx.doi.org/10.1146/annurev-immunol-032712-095956
- Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity 2013; 38:633-43; PMID:23601682; http://dx.doi.org/10.1016/j.immuni.2013.04.005
- Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O'Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013; 153:1239-51; PMID:23746840; http://dx.doi.org/10.1016/j.cell.2013.05.016
- Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, Hoenger G, Durovic B, Jauch A, Hess C. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol 2013; 14:1064-72; PMID:23955661; http://dx.doi.org/10.1038/ni.2687
- Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, Tsui YC, Cui G, Micevic G, Perales JC et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 2015; 162:1217-28; PMID:26321681; http://dx.doi.org/10.1016/j.cell.2015.08.012
- Staron MM, Gray SM, Marshall HD, Parish IA, Chen JH, Perry CJ, Cui G, Li MO, Kaech SM. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8(+) T cells during chronic infection. Immunity 2014; 41:802-14; PMID:25464856; http://dx.doi.org/10.1016/j.immuni.2014.10.013
- Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 2015; 162:1229-41; PMID:26321679; http://dx.doi.org/10.1016/j.cell.2015.08.016
