ABSTRACT
Tumor-infiltrated macrophages were potential targets of the immune therapy for patients with colon cancer. Colony stimulating factor 1 (CSF1) is a primary chemoattractant and functional regulator for macrophages, and therefore would be a feasible intervention for the macrophage-targeting therapeutics. However, the expression of CSF1 in colon cancer microenvironment and its roles in cancer development is largely unknown. In the present study, we found that CSF1 was over-expressed exclusively in colon cancer cells and was correlated with macrophages infiltration. The high CSF1 expression and macrophages infiltration were related to the tumor–node-metastasis (TNM) stage of colon cancer, and suggested to be positively associated with survival of colon cancer patients. In the in vitro studies based on an indirect Transwell system, we found that co-culture with macrophage promoted CSF1 production in colon cancer cells. Further investigation on regulatory mechanisms suggested that CSF1 production in colon cancer cells was dependent on PKC pathway, which was activated by IL-8, mainly produced by macrophages. Moreover, colon cancer cell-derived CSF1 drove the recruitment of macrophages and re-educated their secretion profile, including the augment of IL-8 production. The mice tumor xenografts study also found that over-expression of CSF1 in colon cancer cells promoted intratumoral infiltration of macrophages, and partially suppressed tumor growth. In all, our results demonstrated that CSF1 was an important factor in the colon cancer microenvironment, involving in the interactions between colon cancer cells and tumor-infiltrated macrophages.
Abbreviations
| CCL | = | chemokine (C-C motif) ligand |
| CM | = | conditioned medium |
| CSF1 | = | colony stimulating factor 1 |
| IL | = | interkeukin |
| mAb | = | monoclonal antibody |
| NAb | = | neutralizing antibody |
| PKC | = | protein Kinase C |
| shRNA | = | short hairpin RNA |
| TMA | = | tissue microarray |
| TNM | = | tumor-node-metastasis. |
Introduction
Colorectal cancer ranks top three of the most commonly diagnosed cancer and cancer-related death worldwide.Citation1 The incidence rate of colorectal cancer increases rapidly in eastern Asia over the recent years.Citation2 In spite of the improved therapeutic strategies based upon the classical TNM system, with resection of primary tumor as the first choice, there are still 10% of patients with stage I/II disease, and up to 30% of patients with stage III disease develop recurrence after curative resection and die of it.Citation3 The high recurrence rate in early stage indicates that the conventional TNM staging has limitations. One possible explanation is that tumor progression is more than an autonomous process concerning only cancer cells, but a dynamic process incorporating the interactions between cancer cells and microenvironment.Citation4 There are emerging interests to investigate the reciprocal interactions occur in tumor tissue, trying to develop innovative prognostic predictors and therapeutic strategies.
Immune cells are highly represented in the colorectal cancer microenvironment. The in situ immunocytes infiltration profoundly modulates the outcome of patients,Citation5 underlining the importance of local immune contexture in therapeutic interventions. The most abundant immune cells infiltrating in solid tumor are macrophages, representing up to 50% of all tumor mass.Citation6 In colorectal cancer, about 70% of all patients exhibited high macrophages infiltration in tumor tissue.Citation7 However, despite the high infiltration rate, the functions of macrophages in colorectal cancer are still controversial. Although some reports indicate that tumor-infiltrating macrophages induce progression and metastasis,Citation8,9 others argue by demonstrating a high survival advantage of intratumoral macrophages to colorectal cancer patients,Citation7,10 Such inconsistency is comprehensible, considering that tumor-infiltrated macrophages are heterogeneous, composed of both tumor-killing and tumor-promoting subpopulations.Citation11,12 Based on the above observations, a macrophage-targeting immune therapy would be effective for colorectal cancer, if their antitumor activities are enhanced to overwhelm the tumor-promoting potential. Great efforts have been made to explore feasible strategies for such functional modulation.
CSF1 is a major lineage modulator for the survival, differentiation and chemotaxis of macrophages. The overexpression of CSF1 is found in various types of cancer, and associated with higher macrophages infiltration.Citation13-15 A recent study also reported CSF1 expression in more than half (56.7%) of patients with colorectal cancer.Citation16 The above observations suggest that CSF1 intervention would be a feasible strategy for macrophage-related immune therapy against colon cancer. However, despite of the well-acknowledged correlations between CSF1 level and number of macrophages, there are still debates about the roles of CSF1 in cancer development.Citation17 Based on in vitro observations, opposite CSF1-targeting strategies, such as recombinant CSF1 and CSF1 inhibitor, has been applied in a range of mice models and some cases of patients.Citation18-22 These therapeutics exhibit clinical benefits depending on types of cancer, adjuvant interventions and patient status, which bring more confusion to clinical applications of CSF1. The above observations suggest that a throughout investigation about CSF1 in tumor microenvironment is necessary, before CSF1-relevant strategies are applied in colon cancer. In this study, we investigated the expression pattern and clinical value of CSF1 in patients with colon cancer, and the regulatory mechanisms by which the high CSF1 microenvironment was formed. We also preliminarily explored the effects of colon cancer-derived CSF1 on biological functions of intratumoral macrophages.
Results
CD68 and CSF1 expression in colon cancer and their correlation
In order to analyze macrophages infiltration in colon cancer, tumor specimens from 46 patients diagnosed with colon cancer were stained for CD68, a marker of human macrophages. As shown in , CD68+ cells were present throughout the stroma of tumor tissue, and predominantly clustered in peritumoral stroma along the invasive front. Most CD68+ cells were closely adjacent to tumor cells. Significantly higher numbers of CD68+ cells were observed in both intratumoral and peritumoral stroma compared with normal colon mucosa. The number of CD68+ cells in tumor was correlated with the number in peritumoral stroma (r = 0.665, p < 0.001), but not in normal tissue ().
Figure 1. The CD68 and CSF1 expression in colon cancer tissue. (A) Numbers of CD68+ cells in normal mucosa, peritumoral or intratumoral stoma of 46 cases of primary colon cancer. Each symbol represented one tissue sample. (B) The correlation of infiltrated CD68+ cell number between normal tissue and intratumoral stroma (upper) and peritumoral and intratumoral stroma (down). (C) (Upper) A typical view of CSF1 expression in peritumoral and tumor tissue in colon cancer (small: 100×; large: 400×). (Down) The percentage of CSF1-high cases in normal and tumor samples. (D) A typical CD68 and CSF1 expression in two consecutive sections (small: 100×; large: 400×). (E) The correlation between CD68+ cell numbers and CSF1 expression score in each tumor sample. **p < 0.01
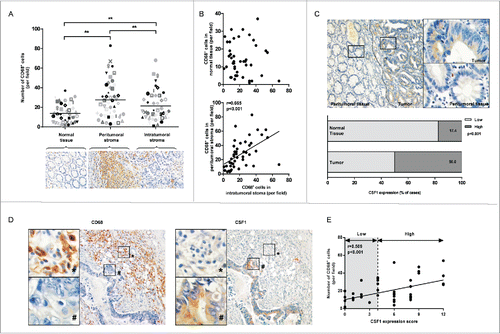
We next explored CSF1 expression in colon cancer patients. CSF1 was observed to distribute homogenously and exclusively in colon cancer cells. The staining intensity was significantly higher compared with peritumoral tissue () or normal tissue (data not shown). We further semiquantitatively scored the immunoreactivity of CSF1 in normal colon mucosa and tumor by a well-established system described in the Materials and Methods, and all the cases were classified into low and high expression groups according to the score. The results demonstrated that 50.0% (23 cases) of tumor specimens was considered CSF1 highly expressed, while only 17.4% (8 cases) of normal tissue was in high group (p < 0.001, ).
The correlation of CD68+ cells and CSF1 expression in colon cancer were also investigated. As shown in , little CD68+ cells were positive for CSF1 staining, and CSF1-expressing tumor cells were also negative for CD68. However, there was a correlation between number of tumor-infiltrated CD68+ cells and CSF1 immunoscore in colon cancer (r = 0.509, p < 0.001. ).
CSF1 expression and macrophages infiltration are associated with prognosis of colon cancer patients
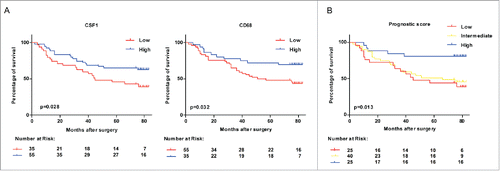
We went on to investigate the relationship of CSF1 expression and macrophages infiltration with prognosis by a tissue microarray (TMA) analysis consisting of 90 colon cancer cases that underwent primary surgery. The 5-y overall survival (OS) was 45.6% for the total population. Univariate analysis revealed that number of involved lymph nodes, TNM stage, grade and growth pattern of tumor were significant prognostic factors for survival (p < 0.05, ).
Table 1. 5-y overall survival according to clinicopathological parameters.
The total study population was divided into CSF1 low/high and CD68 low/high subgroups, based on the model described in the Materials and Methods. Here, we found that CSF1 high group had a significant survival advantage compared with CSF1 low group (p = 0.028, ). This survival advantage was also shown in CD68 high group (p = 0.032, ). Finally, we generated a prognostic score based on CSF1 and CD68 expression. This prognostic score categorized colon cancer patients into low (CSF1loCD68lo), intermediate (CSFloCD68hi/CSFhiCD68lo) and high (CSF1hiCD68hi) groups. As shown in , the prognostic score composed of CSF1 and CD68 had more significant value in predicting the prognosis than one single factor (p = 0.013).
Figure 2. Survival of colon cancer patients categorized by CSF1 and CD68 expression. (A) All colon cancer cases in tissue microarray with follow-up data (n=90) were categorized according to expression of CSF1 (left) and CD68 (right) respectively, and survival analysis between two subgroups was performed. (B) Survival analysis was performed between subgroups based on a prognostic score combined with CSF1 and CD68. Low: CSF1loCD68lo; Intermediate: CSFloCD68hi/CSFhiCD68lo; High: CSF1hiCD68hi. Survival curves were plotted by Kaplan–Meier method and the log-rank test was used to determine statistical significance.
Next, a multivariate analysis was adopted to explore the importance of CSF1 and CD68 expression in prognostic prediction compared with other clinicopathologic parameters. Significant factors in univariate analysis, including tumor stage, grade and tumor growth pattern were taken into multivariate analysis, along with the CSF1-CD68 prognostic score. The result suggested that the CSF1-CD68 prognostic score remained independently associated with OS, with relative risks for the low and intermediate groups of 3.603 (95% CI: 1.289–10.071) and 3.114 (95% CI: 1.153–8.412), respectively ().
Table 2. Cox proportional hazard model in 90 colon cancer cases.
CSF1 expression and macrophages infiltration correlate to clinicopathologic parameters of colon cancer patients
showed the correlation between CSF1/CD68 expression and clinicopathologic parameters. When combined the cases in TMA and primary tumor specimens, CSF1 was found to correlate with tumor stage and number of involved lymph nodes (N stage), while CD68 showed correlation with tumor stage and tumor infiltration degree (T stage). No correlation was observed in gender, age, tumor location, metastasis, grade, tumor type and growth pattern of tumor.
Table 3. Intratumoral CSF1 and CD68 expression in relation to clinicopathological parameters of colon cancer patients.
CSF1 expressed by colon cancer cells is promoted by indirect contact with macrophages
In the above study, we found the over-expression of CSF1 in colon cancer cells and its positive correlation with macrophages infiltration. Both CSF1 expression and macrophages infiltration were predictors for prognosis of colon cancer patients. In order to explore how the high-CSF1 microenvironment is modulated and what its pathophysiological activities are, an indirect co-culture model was established based on Transwell chamber (). THP-1-derived macrophages were co-cultured with colon cancer cell line HT-29 for 12 h or 24 h, then the supernatant was collected and CSF1 concentration was measured by ELISA. The results showed that THP-1-derived macrophages produced relatively high level of CSF1 (about 2 ng/1 × 106 cells), while basic CSF1 expression in HT-29 cells was negligible (). Interestingly, CSF1 in the supernatant was significantly increased after 24 h of co-culture (). To further identify the sources of elevated CSF1, we separately measured CSF1 transcription in THP-1-derived macrophages and HT-29 cells over intended period of co-culture. We found that CSF1 transcription in HT-29 cells was greatly upregulated after the co-culture, while it was not significantly altered in THP-1-derived macrophages (). CSF1 upregulation was also found in HT-29 conditioned medium (CM) after co-culture (). The same co-culture model was also applied to two other colon cancer cell lines, SW480 and SW620. However, after the co-culture only increase in CSF1 transcription was found, while the CSF1 secretion of these two cell lines was not upregulated (Fig. S2).
Figure 3. Co-culture with THP-1-derived macrophages promoted CSF1 production in HT-29 colon cancer cells. (A) A diagrammatic illustration for the co-culture procedure. Upper: To obtain the co-culture medium, 2×105 of HT-29 cells were co-cultured with intended numbers of THP-1-derived macrophages for 24 h, then the co-culture medium and cells were collected and measured separately. Down: To obtain conditioned medium (CM), HT-29 cells and THP-1-derived macrophages were cultured alone in new medium for another 24 h after co-culture, then the CM were collected. (B) CSF1 concentration in THP-1-derived macrophages, HT-29 cells and co-culture medium for 12 h and 24 h. Mφ, the CM of THP-1-derived macrophages alone; HT-29, the CM of HT-29 cells alone; Mφ+HT-29, the co-culture medium of THP-1-derived macrophages and HT-29 cells. (C) CSF1 transcription in THP-1-derived macrophages and HT-29 cells after intended period of co-culture. (D) CSF1 concentration in CM of THP-1-derived macrophages and HT-29 cells, alone or 24 h after the co-culture. (E) CSF1 transcription in THP-1-derived macrophages and HT-29 cells co-cultured in different cell ratio. All subgroups were compared to the control. (F) A typical immunofluorescent image for CSF1 expression pattern in HT-29 cells, alone or after 24 h of co-culture with macrophages. Mφ represented THP-1-derived macrophages. The RT-PCR data were normalized to the control and shown as fold change. Each bar represents the mean±SD (n = 3, *p < 0.05, **p < 0.01).
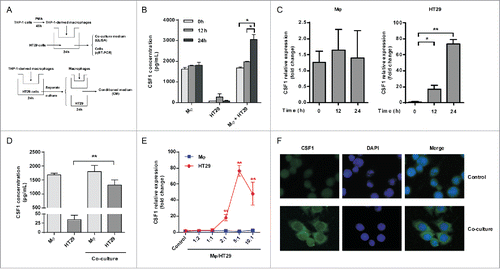
To determine whether CSF1 upregulation was associated with numbers of macrophages and cancer cells, HT-29 cells were co-cultured with increasing amounts of THP-1-derived macrophages. Here, we found that CSF1 transcription became increasing at a 2:1 ratio of macrophages to cancer cells. This upregulation was more significant at 5:1 ratio and started to decrease at 10:1 ratio ().
We further explored CSF1 distribution pattern in HT-29 cells by immunofluorescence. As shown in , CSF1 protein was restrained to nucleus when cultured alone. However, the primary expression site of CSF1 changed to cytoplasm after the co-culture.
Macrophages promoted CSF1 expression in colon cancer cells via IL-8-PKC pathway
We went on to investigate the mechanisms by which THP-1-derived macrophages modulated CSF1 expression in HT-29 cells. Interleukin-8 (IL-8 or CXCL8), a proinflammatory chemokine highly produced by tumor-associated macrophages, was regarded as an important regulator in the tumor microenvironment.Citation23,24 Here, we found that THP-1-derived macrophages produced high level of IL-8 compared with HT-29 cells, and its production was even increased after co-culture (). In order to verify whether IL-8 modulated CSF1 expression in HT-29 cells, IL-8-neutralizing antibody (IL-8 NAb, 2 μg/mL) was added into the co-culture system. CSF1 transcription and secretion of HT-29 cells in the co-culture system was significantly decreased in the presence of IL-8 NAb ( and ).
Figure 4. IL-8 promoted CSF1 in HT-29 cells via PKC pathway. (A and B) IL-8 transcription (A) and concentration in CM (B) of THP-1-derived macrophages and HT-29 cells, alone or after co-culture. (C) THP-1-derived macrophages and HT-29 cells were cultured alone or co-cultured with/without IL-8 NAb (2 μg/mL) for 24 h. Then cells were collected respectively and CSF1 transcription was measured. (D) HT-29 cells were co-cultured with THP-1-derived macrophages with/without IL-8 NAb (2 μg/mL) for 24 h, then were cultured alone in new medium for another 24 h. CSF1 concentration in HT-29 CM was measured. (E) HT-29 cells were treated by phorbol 12-myristate 13-acetate (PMA, 100 ng/mL) for 24 h, and CSF1 transcription was measured. (F) HT-29 cells co-cultured with THP-1-derived macrophages with/without IL-8 NAb were measured for phosphorylation of PKC isoforms. Mφ represented THP-1-derived macrophages. The qRT-PCR data were normalized to the control and shown as fold change. Each bar represents the mean ± SD (n = 3, *p < 0.05, **p< 0.01).
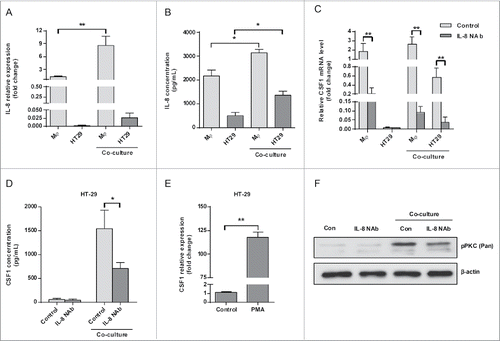
During the research, we accidentally found that PMA significantly increased the transcription of CSF1 in HT-29 cells (). As PMA is a potent activator of PKC pathway, we tried to explore whether PKC pathway was involved in the CSF1 upregulation process. As shown in , co-culture with THP-1-derived macrophages significantly increased the phosphorylation of PKC pathway. The phosphorylation was significantly decreased when IL-8 NAb was added into the co-culture system.
Colon cancer cell-derived CSF1 is responsible for macrophages recruitment and secretion profile redirection
The function of elevated CSF1 expression in colon cancer cells was further investigated. As CSF1 was reported as a chemotactic factor for monocytes/macrophages, we firstly explored the recruitment effect of HT-29-derived CSF1 on THP-1-derived macrophages. First, HT-29 conditioned medium (CM) alone or co-cultured was analyzed for recruiting potential for THP-1-derived macrophages by transwell assay. Because SW480 cells had similar malignancy with HT-29 cells (Duke's B) and was negative for CSF1 production, the SW480 CM was used as a negative control. The number of macrophages recruited by co-cultured HT-29 CM was significantly higher than HT-29 CM alone, and also higher than the co-cultured SW480 CM. (). In order to verify whether CSF1 was involved in the recruitment of THP-1-derived macrophages, CSF1-neutralizing antibody (CSF1 NAb, 100 ng/mL) was added into the HT-29 CM before Transwell assay. The results demonstrated that CSF1 NAb decreased number of macrophages recruited by the co-cultured HT-29 CM, while did not significantly alter the attracting effect of HT-29 CM alone (). The role of HT-29-derived CSF1 in macrophages recruitment was further verified by RNA interference. CSF1 shRNA was transfected into HT-29 cells before co-culture. As shown in , CSF1 interfering in HT-29 cells significantly attenuated the attracting effect of the co-cultured HT-29 CM, when compared with scramble shRNA interfering.
Figure 5. HT-29-derived CSF1 recruited THP-1-derived macrophages and directed their cytokines/chemokines production. (A) The CM of THP-1-derived macrophages and HT-29 cells were applied to Transwell assay, and numbers of THP-1-derived macrophages recruited by CM were counted. The SW480 CM was used as a negative control for cancer cell-derived CSF1. (B) The HT-29 CM, alone or co-cultured, were applied for Transwell assay, with/without CSF1 NAb (100 ng/mL), and recruited THP-1-derived macrophages were counted. (C) The CM of HT-29 transfected by control or CSF-shRNA were used for Transwell assay. The recruited THP-1-derived macrophages were counted. (D) THP-1-derived macrophages were co-cultured with HT-29 cells transfected by control or CSF1-shRNA for 24 h, and the transcription of typical pro- or anti-inflammatory cytokines/chemokines was measured. The cytokines/chemokines with significant change were exhibited. Mφ-HT-29: HT-29 CM after 24 h of co-culture; Mφ-SW480: SW480 CM after 24 h of co-culture. The qRT-PCR data were normalized to the control and shown as fold change. Each bar represents the mean ± SD (n = 3, *p < 0.05, **p< 0.01).
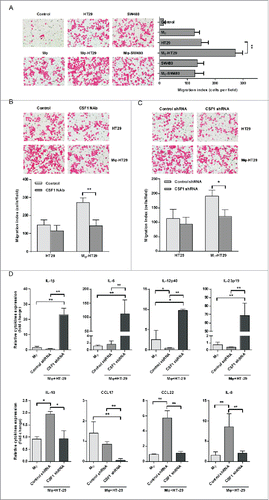
The above results have shown that CSF1 produced by HT-29 cells promoted the recruitment of THP-1-derived macrophages. We went on to explore the effects of HT-29-derived CSF1 on secretion activities of macrophages. THP-1-derived macrophages were cultured alone or co-cultured with CSF1 shRNA-interfered HT-29 cells, and transcriptions of various cytokines and chemokines were measured. Here, we found that pro-inflammatory cytokines transcription, such as IL-1β, IL-6 and IL-23, was significantly increased in macrophages, when co-cultured with CSF1 shRNA-interfered HT-29 cells. However, the transcription of anti-inflammatory cytokines and chemokines, including IL-10, CCL17 and CCL22, was downregulated (). In addition, the transcription of IL-8 in THP-1-derived macrophages was also decreased when co-cultured with CSF1 shRNA-interfered HT-29 cells ().
CSF1 overexpression in colon cancer cells promotes macrophages infiltration in xenograft and partially suppresses tumor growth
The effects of CSF1 expressed by cancer cells on properties of tumor-infiltrated macrophages were further validated in vivo. A CSF1 over-expression xenograft model was formed by injecting recombinant adenovirus vector expressing human CSF1 (Ad-hCSF1) into the right tumor of nude mice, which were bearing HT-29 colon cancer xenografts on bilateral legs (). Overexpression of human CSF1 in tumor tissue was confirmed by qRT-PCR (). The injection significantly increased the number of tumor-infiltrated macrophages not only in the right tumor, but also in the non-injected left tumor (). In addition, injection of Ad-hCSF1 also promoted mouse IL-8 mRNA level in the right tumor compared with control groups ().
Figure 6. Adenovirus-mediated intratumoral CSF1 over-expression affected macrophages infiltration and tumor growth in vivo. (A) A diagrammatic illustration for the tumor model study procedure. 1 × 107 HT-29 cells in 100 μL RPMI 1640 medium was subcutaneously injected into bilateral legs of BALB/c nude mice. After 12 d of tumor inoculation, recombinant adenovirus vector expressing human CSF1 (Ad-hCSF1) or control vector (both 5 × 1010 vp/100 μL) was injected into the right tumor. Mice were sacrificed and tumors were isolated on day 27. (B) (Left) Typical views of CD68+ cells in tumor xenografts treated with control vector or Ad-hCSF1 (400×). (Right) The mean number of CD68+ cells infiltrated in tumor. (C) mRNA levels of human CSF1 (upper) and mouse IL-8 (down) in right tumors of two groups were analyzed after 15 d of adenovirus vector injection. (D) Tumor volume in two groups was measured every 3 d and statistical analysis was performed. Tumor volumes were calculated as (length × width2)/2. (E) Mean colon cancer xenograft weights on day 27, treated with control vector or Ad-hCSF1. Data were represented as mean ± SD (n = 5, *p < 0.05, ** p < 0.01).
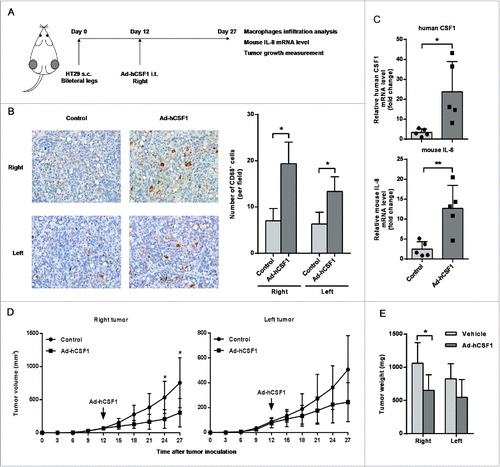
We further investigated the role of cancer cell-derived CSF1 in tumor growth using the same xenograft model. The right tumor volume in Ad-hCSF1-treated group became significantly reduced compared with that in control group after 12 d of injection. The volume of non-injected left tumor in experimental group was also slightly smaller than that of control mice, but only marginal significance (p = 0.099) was reached at the end of observation (). The tumor weight analysis was consistent with tumor volume. Significant difference was only observed in the right tumor between Ad-hCSF1-injected and control mice ().
Discussion
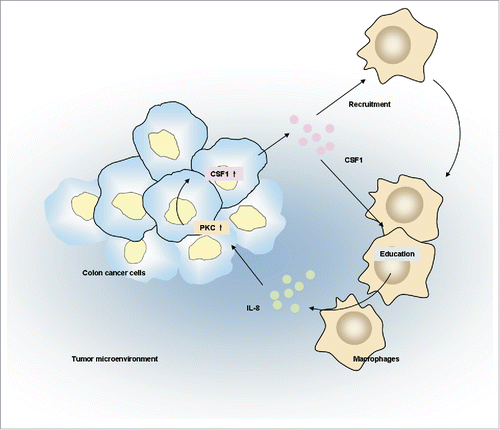
Macrophages are potential therapeutic targets for colon cancer. CSF1, a major macrophage lineage regulator, would be a feasible strategy for macrophage-related immune therapy. However, the roles of CSF1 in colon cancer development are largely unknown. In this study, we found that CSF1 was overexpressed in colon cancer cells and its expression was correlated with numbers of tumor-infiltrated macrophages. Tumor CSF1 expression and macrophages infiltration was associated with prognosis of colon cancer patients. The in vitro studies suggested that macrophages promoted CSF1 expression in colon cancer cells through IL-8. Subsequently, colon cancer-derived CSF1 enhanced macrophages recruitment and regulated their secretion profile. Taken together, these studies revealed reciprocal interactions between tumor-infiltrated macrophages and colon cancer cells through CSF1. A schematic diagram was made to depict the above mechanisms ().
Figure 7. The schematic diagram depicting interactions between colon cancer cells and tumor infiltrated macrophages depending on CSF1.
There are growing evidences implicating that both tumor-infiltrated macrophages and macrophage-educating microenvironment are important players in the process of tumor development. In the first part of this study, we found high macrophages infiltration and CSF1 over-expression in colon cancer microenvironment, which were consistent with previous studies.Citation7,10,16 Increased macrophages infiltration and CSF1 expression were associated with improved survival of patients. In addition, a high prognostic score, which contained cases with both high CSF1 and CD68 expression, was shown to be an independent prognostic marker by multivariate analysis. Our in vivo study, showing that overexpression of human CSF1 in colon cancer xenografts reduced tumor growth, partially supported these observations in patients. The above results were somewhat unexpected, because CSF1 showed dominantly protumorigenic role in other types of malignancies.Citation13,25,26 However, it should be noticed that the roles of CSF1 in cancer development is largely reflected by the function of tumor-infiltrated macrophages, because it plays not only a potent chemoattractant for macrophages, but also functional regulator.Citation27 Previous studies suggested that CSF1 promoted the antitumor activity of macrophages by enhancing their phagocytosis and cytotoxicity to cancer cells,Citation28-30 but we had not known whether tumor-derived CSF1 helped to educate such antitumor potential. In the in vitro study, we found that colon cancer-derived CSF1 significantly decreased the transcription of some pro-inflammatory cytokines in macrophages, which was shown as negative factors for development of colon cancer.Citation31-34 Such immuno-regulatory effects could be more complicated in vivo, as we found that CSF1 over-expression in xenografts mediated by adenovirus showed a mixed pro-/anti-inflammatory profile in host (data not shown). Nevertheless, the above results suggested that tumor-derived CSF1 was involved in the recruitment and re-education of tumor-infiltrated macrophages. These regulations might support to establish an antitumorigenic microenvironment in colon cancer.
In the second part of our study, we explored the mechanisms by which the high CSF1 microenvironment in colon cancer was formed. Surprisingly, we found that colon cancer cells HT-29 was negative for CSF1, but the transcription and secretion of CSF1 was significantly promoted after co-culture with THP-1-derived macrophages. This result showed reciprocal interactions between macrophages and colon cancer cells, that is, CSF1-responding macrophages recruited into the tumor tissue supported the overexpression of CSF1 in cancer cells, creating a unique high-CSF1 tumor landscape. Meanwhile, it should be noticed that the upregulation of CSF1 in HT-29 cells required higher density of THP-1-derived macrophages (). The recruitment of macrophages by CSF1 might result in increased macrophage to cancer cell ratio, which further promoted CSF1 expression of cancer cells. In addition, the promoting effects of macrophages on CSF1 production was supposed to be general, as transcriptional upregulation was also observed in other colon cancer cell lines, SW480 and SW620 (Fig. S2), and several breast and lung cancer cell lines (data not shown). However, although co-culture with macrophages significantly promoted CSF1 transcription in SW480 and SW620 cells, it did not alter CSF1 secretion. One explanation was the relatively lower level of basic transcription of CSF1 in SW480 and SW620 cells. In fact, quantitative RT-PCR showed that CSF1 transcription in HT-29 cells was about 10 times higher than SW480 and SW620 cells. In addition, it should not be excluded that post-transcriptional or post-translational modifications occurred in SW480 and SW620 cells, which need further investigation.
During the studies exploring the mechanisms regulating CSF1 production, we found that IL-8, a pro-inflammatory cytokines and chemokines largely produced by macrophages, was involved in the upregulation of CSF1 in HT-29 cells. Moreover, secreted IL-8 in co-culture medium activated PKC pathway, which was responsible for CSF1 transcription in HT-29 cells. There were elegant studies showing that IL-8 was one of the most significant upregulated chemokines in both serum and tumor site in patients with colorectal cancer, and associated with poorer outcomes.Citation23,24 Our results provided additional information about the roles of IL-8 in colon cancer by regulating CSF1 production. In addition, we noticed that cancer cell-derived CSF1 amplified IL-8 production of macrophages, which was consistent with previous studies.Citation35,36 The interactions between cancer cell-derived CSF1 and macrophage-derived IL-8 represented another positive feedback loop in colon cancer tissue. As both CSF1 and IL-8 were both important factors with immune-regulating capacity, this feedback loop might provide a synergetic microenvironment for the functions and migration potential of tumor-infiltrated immune cells.
The specific mechanisms by which IL-8 promoted CSF1 expression need further investigation when considering the complexity of the PKC pathway. Fifteen isoforms existed in the PKCs family, which were divided into three groups (classical, atypical and novel) according to calcium dependency and activators.Citation37 As a member of phorbol esters, PMA was well-acknowledged activator of classical PKCs, including the isoforms α, βI, βII, and γ, and novel PKCs such as isoforms δ, ε, η, and θ. In the present study, we only explored the total phosphorylation level of all members of classical and novel PKC isoforms, and found that IL-8 in the co-culture medium enhanced their phosphorylation. The specific roles of single PKC isoform in the whole system were still not able to distinguish in the present study, which would be explored in future by isoform-specific inhibitors and antibodies.
In all, here we tried to explore the clinical value of high-CSF1 microenvironment in colon cancer and its regulatory mechanisms. Our results showed that CSF1 was a key mediator in the reciprocal interactions between colon cancer cells and tumor-infiltrated macrophages, and two positive feedback loops involving CSF1 were identified in the present study: First, tumor-infiltrated macrophages promoted CSF1 production of colon cancer cells, which subsequently recruited more macrophages into the tumor microenvironment; Secondly, CSF1 secreted by cancer cells promoted macrophage-derived IL-8, which in turn supported the formation of a high-CSF1 microenvironment. Therefore, strategies targeting CSF1 might synchronously affect two primary components in tumor tissue: cancer cells and tumor-infiltrated macrophages. Further investigations were still needed to obtain the full view of the biological functions of high-CSF1 microenvironment in colon cancer before any clinical attempt was performed.
Materials and methods
Antibodies, reagents and recombinant adenovirus vectors
Antibodies to CD68 (ab955) and CSF1 (ab52864) for immunohistochemistry were purchased from Abcam. Phorbol 12-myristate 13-acetate (PMA, #79346) was purchased from Sigma. Monoclonal anti-human CSF1 (AF216) and IL-8 (MAB208) neutralizing antibodies (NAb) were purchased from R&D Systems. Phosphorylated PKC (pan) (#9739) and β-actin (#4967) for western blot were purchased from Cell Signaling Technology. The recombinant adenovirus vector expressing human CSF1 (Ad-hCSF1) was purchased from ViGene Biosciences.
Patients and clinical samples
Tumor specimens of 46 patients diagnosed with primary colon cancer were obtained during the surgical tumor resection at Qilu Hospital of Shandong University, during the period from 2006 to 2014. None of the patients had received preoperative radiotherapy. The study was approved by the Ethics Committee of Qilu Hospital of Shandong University, and informed consent was obtained from each patient. The histopathologic characteristics including the TNM stage, grade, tumor type and growth pattern were determined by established histological criteria.
The TMA-obtained 90 colon cancer cases were purchased from Shanghai Outdo Biotech Company. The survival data was collected from 2008 to 2014.
Immunohistochemistry and immunofluorescence
Paraffin-embedded samples were cut into 5-μm sections and then processed for immunohistochemistry. After the incubation with CD68 and CSF1 antibodies (1:200 of CD68, 1:100 of CSF1) at 4°C overnight, the specimens were stained with 3′-diaminobenzidine and the nuclei were counterstained with hematoxylin. Samples were viewed under the Olympus IX81 microscope and images were produced using DP Controller.
For immunofluorescent staining of CSF1, HT-29 cells were grown to 80% confluence in six-well plates. Cells were fixed in cold acetone/methanol (1:1) and permeabilized with 0.1% Triton X-100 in PBS. The fixed cells were incubated with primary CSF1 antibodies followed by incubation with FITC-conjugated anti-rabbit IgG (AP132F, Millipore). 1 μg/mL DAPI (D212, Dojindo) was used for nuclear counterstaining. The fluorescence was monitored on a Nikon Eclipse E600 microscope. Images were acquired using a SPOT camera and adjusted using Adobe Photoshop software.
CD68 and CSF1 evaluation
We evaluated CD68 and CSF1 expression in tumor specimens according to the previous protocols with minor modification.Citation38 The specimens were scored by two independent observers not knowing any prognosis or clinicopathologic variables. Disagreements were evaluated a third time by another conclusive judgment. For CD68 evaluation in primary tumor samples, we counted the numbers of CD68+ cells in 10 random fields over the whole section and obtained the mean number per field. For CD68 evaluation in TMA, the numbers of CD68+ cells in each tissue core were directly counted. Then the mean number of CD68+ cells infiltrated in primary samples and tissue cores was calculated (19.7 per field). All cases with the number < 20 per field were considered low, the number ≥ 20 considered high.
The immunoreactivity of CSF1 was semiquantitatively scored by a well-established system which incorporated both the percentage of positive cells and the stain intensity, with minor modifications.Citation39 In short, a mean percentage of positive cells was determined among five fields at ×400 and then assigned to one of five categories: < 5% (0), 5–25% (1), 25–50% (2), 50–75% (3), and ≥75% (4). The mean intensity of staining was also determined in five fields and scored as: weak (1), moderate (2), and strong (3). Then the percentage of positive cells and staining intensity were multiplied to generate a weighted score for each tumor specimen. We subgrouped the tumor specimens according to the intensity scores into low (which included scores 0 to +4) and high (which included scores +6 to +12) groups. Some typical images of tumor samples with different intensity scores were provided as Fig. S1.
Cell culture and generation of THP-1-derived macrophages
Human colon cancer cell line HT-29, SW480, SW620 and acute monocytic leukemia cell line THP-1 was obtained from ATCC. The cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in incubator with 95% air, 5% CO2.
To generate THP-1-derived macrophages, 1 × 106 THP-1 cells were treated with 100 ng/mL PMA for 48 h Citation40 and adherent cells were collected for further studies. For Transwell chamber co-culture, 2 × 105 HT-29, SW480 or SW620 cells were seeded in 2 mL RPMI 1640 medium/10% FBS into each well of a six-well plate. After 24 h, medium was replaced by 3 mL pre-warmed fresh medium, and THP-1-derived macrophages were seeded into the upper well (0.4-μm pores; Millipore) at different density. After the co-culture for specific period of time, the co-culture medium and two types of cells were collected respectively. In other studies, colon cancer cells and THP-1-derived macrophages were separated and cultured alone in 2 mL fresh medium for another 24 h. Then conditioned medium were collected respectively. A diagrammatic illustration for the co-culture procedure was provided as .
Enzymes linked immunosorbent assay (ELISA)
CSF1 (#274967) and IL-8 (#281427) concentration in culture supernatants were determined by ELISA kits (R&D Systems) according to the manufacturer's instructions.
Quantitative RT-PCR
RNA from cell lines and mice tumor xenografts was extracted by TRIzol Reagent (Invitrogen). cDNA was synthesized by reverse transcription. Quantitative RT-PCR (qRT-PCR) was performed on the LightCycler 2.0 Instrument (Roche). GAPDH was used as an internal control. The primer sequences used in this study were listed in Table S1.
Migration assay
1 × 105 THP-1-derived macrophages were added into the upper compartment of the inserts (24-well plate, 8-μm pores, BD Biosciences) in 100 μL of serum-free medium. 600 μL RPMI 1640 medium containing 10% FBS, or corresponding conditioned medium was added into the lower chamber of the Transwell plate. In some cases, IL-8 NAb (2 μg/mL) was added into the conditioned medium in lower chamber 2 h before incubation. After incubating at 37°C in 5% CO2 for 12 h, the migrated cells on the lower surface of the filter were fixed by 10% formalin and stained with eosin. Five random fields of each well were photographed and cell numbers were counted.
shRNA transient transfection
CSF1-targeting shRNA sequences inserted into the GV248 vector with an eGFP reporter were purchased from Shanghai GeneChem Company. A nonspecific shRNA was used as a negative control. HT-29 cells were plated in six-well plates and 60% confluence was reached before transfection. Then cells were transfected with CSF1-shRNA (2 µg plasmid) by 5 µL Lipofectamine™ 2000 (Invitrogen) according to the manufacturer's instructions. The transfection efficiency at 48 h was more than 90% determined by flow cytometric analysis.
Western blot
Cell lysates containing equal amounts of total proteins were electrophoresed in 10% SDS-PAGE and then transferred onto a nitrocellulose membrane. After blocking with TBST (Tris-buffered saline, 0.1% Tween 20) containing 5% nonfat dried milk, the membranes were incubated with primary antibodies against pPKC and β-actin overnight at 4°C. Then the membranes were washed with TBST and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h. The protein bands were detected by enhanced chemiluminescence.
Tumor model study
The study procedures were approved by the Animal Care and Use Committee of Shandong University and conformed to the legal mandates and national guidelines for the care and maintenance of laboratory animals. Five-week-old male BALB/c nude mice were purchased from Beijing HFK Bioscience Co. Ltd and maintained under sterilized conditions. HT-29 cells (1 × 107/100 μL RPMI 1640 medium) were injected subcutaneously into bilateral legs of BALB/c nude mice. Tumor length and width were measured every 3 d by a caliper. Tumor volumes were calculated by (length × width2)/2. After 12 d, when the volume of subcutaneous tumors reached 50–100 mm3, mice were randomly divided into two groups (n = 5) receiving injection of the control vector or Ad-hCSF1 (both 5 × 1010 vp) into the right tumor. On day 27, mice were sacrificed, and tumors were isolated and weighted. One portion of tumor was processed for paraffin embedding and stained by CD68 antibody to evaluate the density of macrophages. The remainder was processed for RNA extraction and qRT-PCR. A diagrammatic illustration for the tumor model study was provided as .
Statistical analysis
Data were presented as the mean ± SD, based on triplet experiments. Student's t test and ANOVA were used for statistical comparisons. To determine the correlation between two variables, nonparametric Spearman correlation was performed. For the determination of the differences of clinicopathological parameters between different groups, cross-tabulations were analyzed with χ2-test or Fisher's exact test. To estimate the cancer-specific survival, Kaplan–Meier analysis was used and comparisons between groups were done with the log-rank test. Factors associated with cancer-specific survival with a p value lower than 0.05 were further tested in multivariate analysis by Cox model. All statistical analysis was performed using SPSS software version 13.0. p value less than 0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
KONI_A_1122157_supplementary_material.zip
Download Zip (1.6 MB)Funding
This work was supported by the National Natural Science Foundation of China (grant number 31300752, 31470885, 31270971, 81072406 and 81300510).
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69-90; PMID:21296855; http://dx.doi.org/10.3322/caac.20107
- Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin 2009; 59:366-78; PMID:19897840; http://dx.doi.org/10.3322/caac.20038
- Kobayashi H, Mochizuki H, Sugihara K, Morita T, Kotake K, Teramoto T, Kameoka S, Saito Y, Takahashi K, Hase K et al. Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study. Surgery 2007; 141:67-75; PMID:17188169; http://dx.doi.org/10.1016/j.surg.2006.07.020
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298-306; PMID:22419253; http://dx.doi.org/10.1038/nrc3245
- Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 2009; 86:1065-73; PMID:19741157; http://dx.doi.org/10.1189/jlb.0609385
- Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res 2007; 13:1472-9; PMID:17332291; http://dx.doi.org/10.1158/1078-0432.CCR-06-2073
- Etoh T, Shibuta K, Barnard GF, Kitano S, Mori M. Angiogenin expression in human colorectal cancer: the role of focal macrophage infiltration. Clin Cancer Res 2000; 6:3545-51; PMID:10999742
- Hong F, Wu BX, Li Z. Molecular regulation of macrophages in unleashing cancer-related inflammation. Oncoimmunology 2014; 3:e27659; PMID:24778928; http://dx.doi.org/10.4161/onci.27659
- Chaput N, Svrcek M, Auperin A, Locher C, Drusch F, Malka D, Taieb J, Goere D, Ducreux M, Boige V. Tumour-infiltrating CD68+ and CD57+ cells predict patient outcome in stage II-III colorectal cancer. Br J Cancer 2013; 109:1013-22; PMID:23868006; http://dx.doi.org/10.1038/bjc.2013.362
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141:39-51; PMID:20371344; http://dx.doi.org/10.1016/j.cell.2010.03.014
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014; 41:49-61; PMID:25035953; http://dx.doi.org/10.1016/j.immuni.2014.06.010
- Smith HO, Anderson PS, Kuo DY, Goldberg GL, DeVictoria CL, Boocock CA, Jones JG, Runowicz CD, Stanley ER, Pollard JW. The role of colony-stimulating factor 1 and its receptor in the etiopathogenesis of endometrial adenocarcinoma. Clin Cancer Res 1995; 1:313-25; PMID:9815987
- Behnes CL, Bremmer F, Hemmerlein B, Strauss A, Strobel P, Radzun HJ. Tumor-associated macrophages are involved in tumor progression in papillary renal cell carcinoma. Virchows Arch 2014; 464:191-6; PMID:24327306; http://dx.doi.org/10.1007/s00428-013-1523-0
- Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res 2007; 67:2649-56; PMID:17363585; http://dx.doi.org/10.1158/0008-5472.CAN-06-1823
- Liu H, Zhang Z, Tabuchi T, Wang S, Wang J. The role of pro-inflammatory cytokines and immune cells in colorectal carcinoma progression. Oncol Lett 2013; 5:1177-82; PMID:23599759
- Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 2012; 119:1810-20; PMID:22186992; http://dx.doi.org/10.1182/blood-2011-09-379214
- Jakubowski AA, Bajorin DF, Templeton MA, Chapman PB, Cody BV, Thaler H, Tao Y, Filippa DA, Williams L, Sherman ML et al. Phase I study of continuous-infusion recombinant macrophage colony-stimulating factor in patients with metastatic melanoma. Clin Cancer Res 1996; 2:295-302; PMID:9816172
- Mizutani K, Takeuchi S, Ohashi Y, Yakushiji M, Nishimura H, Takahashi T, Maruhashi T, Ueda K, Noda K, Watanabe Y et al. Clinical usefulness of macrophage colony-stimulating factor for ovarian cancers: Long-term prognosis after five years. Oncol Rep 2003; 10:127-31; PMID:12469157
- Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med 2013; 19:1264-72; PMID:24056773; http://dx.doi.org/10.1038/nm.3337
- Strachan DC, Ruffell B, Oei Y, Bissell MJ, Coussens LM, Pryer N, Daniel D. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8 T cells. Oncoimmunology 2013; 2:e26968; PMID:24498562; http://dx.doi.org/10.4161/onci.26968
- Casbon AJ, Lohela M, Werb Z. Delineating CSF-1-dependent regulation of myeloid cell diversity in tumors. Oncoimmunology 2015; 4:e1008871; PMID:26155427; http://dx.doi.org/10.1080/2162402X.2015.1008871
- Ning Y, Lenz HJ. Targeting IL-8 in colorectal cancer. Expert Opin Ther Targets 2012; 16:491-7; PMID:22494524; http://dx.doi.org/10.1517/14728222.2012.677440
- Lee YS, Choi I, Ning Y, Kim NY, Khatchadourian V, Yang D, Chung HK, Choi D, LaBonte MJ, Ladner RD et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer 2012; 106:1833-41; PMID:22617157; http://dx.doi.org/10.1038/bjc.2012.177
- Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 2001; 193:727-40; PMID:11257139; http://dx.doi.org/10.1084/jem.193.6.727
- Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia 2002; 7:147-62; PMID:12465600; http://dx.doi.org/10.1023/A:1020399802795
- Chockalingam S, Ghosh SS. Macrophage colony-stimulating factor and cancer: a review. Tumour Biol 2014; 35:10635-44; PMID:25238879; http://dx.doi.org/10.1007/s13277-014-2627-0
- Grugan KD, McCabe FL, Kinder M, Greenplate AR, Harman BC, Ekert JE, van Rooijen N, Anderson GM, Nemeth JA, Strohl WR et al. Tumor-associated macrophages promote invasion while retaining Fc-dependent anti-tumor function. J Immunol 2012; 189:5457-66; PMID:23105143; http://dx.doi.org/10.4049/jimmunol.1201889
- Sanda MG, Bolton E, Mule JJ, Rosenberg SA. In vivo administration of recombinant macrophage colony-stimulating factor induces macrophage-mediated antibody-dependent cytotoxicity of tumor cells. J Immunother 1992; 12:132-7; PMID:1504054; http://dx.doi.org/10.1097/00002371-199208000-00008
- Munn DH, Garnick MB, Cheung NK. Effects of parenteral recombinant human macrophage colony-stimulating factor on monocyte number, phenotype, and antitumor cytotoxicity in nonhuman primates. Blood 1990; 75:2042-8; PMID:2186820
- Belluco C, Nitti D, Frantz M, Toppan P, Basso D, Plebani M, Lise M, Jessup JM. Interleukin-6 blood level is associated with circulating carcinoembryonic antigen and prognosis in patients with colorectal cancer. Ann Surg Oncol 2000; 7:133-8; PMID:10761792; http://dx.doi.org/10.1007/s10434-000-0133-7
- Jedinak A, Dudhgaonkar S, Sliva D. Activated macrophages induce metastatic behavior of colon cancer cells. Immunobiology 2010; 215:242-9; PMID:19457576; http://dx.doi.org/10.1016/j.imbio.2009.03.004
- De Simone V, Pallone F, Monteleone G, Stolfi C. Role of T17 cytokines in the control of colorectal cancer. Oncoimmunology 2013; 2:e26617; PMID:24498548; http://dx.doi.org/10.4161/onci.26617
- Rakhesh M, Cate M, Vijay R, Shrikant A, Shanjana A. A TLR4-interacting peptide inhibits lipopolysaccharide-stimulated inflammatory responses, migration and invasion of colon cancer SW480 cells. Oncoimmunology 2012; 1:1495-506; PMID:23264896; http://dx.doi.org/10.4161/onci.22089
- Varney ML, Olsen KJ, Mosley RL, Bucana CD, Talmadge JE, Singh RK. Monocyte/macrophage recruitment, activation and differentiation modulate interleukin-8 production: a paracrine role of tumor-associated macrophages in tumor angiogenesis. In Vivo 2002; 16:471-7; PMID:12494891
- Hashimoto S, Yoda M, Yamada M, Yanai N, Kawashima T, Motoyoshi K. Macrophage colony-stimulating factor induces interleukin-8 production in human monocytes. Exp Hematol 1996; 24:123-8; PMID:8641333
- Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J 1998; 332 (Pt 2):281-92; PMID:9601053; http://dx.doi.org/10.1042/bj3320281
- Wen Z, Liu H, Li M, Li B, Gao W, Shao Q, Fan B, Zhao F, Wang Q, Xie Q et al. Increased metabolites of 5-lipoxygenase from hypoxic ovarian cancer cells promote tumor-associated macrophage infiltration. Oncogene 2015; 34:1241-52; PMID:24662827; http://dx.doi.org/10.1038/onc.2014.85
- Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res 1998; 58:5071-4; PMID:9823313
- Zhao P, Gao D, Wang Q, Song B, Shao Q, Sun J, Ji C, Li X, Li P, Qu X. Response gene to complement 32 (RGC-32) expression on M2-polarized and tumor-associated macrophages is M-CSF-dependent and enhanced by tumor-derived IL-4. Cell Mol Immunol 2015; 12:692-9; PMID:24662827; http://dx.doi.org/10.1038/cmi.2014.108.
