ABSTRACT
Loss of DNA methylation can activate endogenous retroviral expression and dsRNA in cancer cells. This leads to induction of toll-like receptor signaling stimulating an antiviral interferon response. Recent findings provide a therapeutic rationale for combining DNA methylation inhibitors with blockage of immune checkpoint proteins to fight cancer.
In the recent years, exciting therapeutic approaches that activate the host immune system have proven effective toward eliminating diverse solid tumors. These include humanized antibodies targeting various immune checkpoint regulators like CTLA-4, PD-1 and PD-L1.Citation1 Current data has also shown that epigenetic therapies, including the DNA-methylation inhibitors 5-Azacytidine (Aza) and 5-Aza-2′-deoxycytidine (5-Aza-dC) (Decitabine), boost immune signaling of tumor cells.Citation2,3 Therefore, cancer treatments combining inhibition of DNA methylation with blockage of immune checkpoint proteins are a promising new therapeutic direction. Two recent publications shed light on the basic molecular and cellular efficacy regarding the above therapies,Citation3,4 where one common link implicates the innate immune system.
Until the discovery of the toll-like receptors (TLR) in Drosophila melanogaster and subsequent functional translation to humans the innate immune system was thought to be less sophisticated than the adaptive immune system.Citation5 The function of TLRs is to sense “danger” signals, which include nucleic acids or membrane components from exogenous viruses or bacteria. All 10 human TLRs described to date are subdivided by cellular localization (plasma membrane or endosomes) and activation (external membrane lipids or proteins and external nucleic acids).Citation6 Examples of receptors specific for sensing foreign nucleic acids include TLR3 for dsRNA, TLR7/8 for ssRNA and TLR9 for RNA:DNA hybrids (). TLR-nucleic acid binding leads to interferon α/β signaling, downstream activation of interferon stimulated genes (ISGs) and anti-viral and apoptotic functions. Administration of synthetic dsRNA (polyI:C) in humans lead to activation of innate immune pathway members like TLR3, RIG-I, MDA5 and gene expression of ISGs.Citation7 Interestingly, it has been shown that mouse Tlr3, Tlr7 and Tlr9 are essential for control of endogenous retroviruses (ERV).Citation8
Figure 1. Different exogenous and endogenous RNA species induce the innate immune system via TLR, RIG-I and MDA5 resulting in cytokine and interferon signaling. The main ERV RNA species inducing the immune response include dsRNA and ssRNA. RIG-I, retinoic acid inducible gene-1 (or RARRES3); MAVS, mitochondrial antiviral signaling protein (or IPS1); TLR, toll-like receptor; MyD88, myeloid differentiation primary response 88; MDA5, melanoma differentiation-associated 5 (or IFIH1); LGP2, laboratory of genetics and physiology 2 (or DHX58); TRIF, TIR domain-containing adaptor-inducing interferon-β (or TICAM1); NF-kB, nuclear factor kappa-light-chain-enhancer of activated B-cells; IRF, interferon regulatory factor; IFN, interferon.
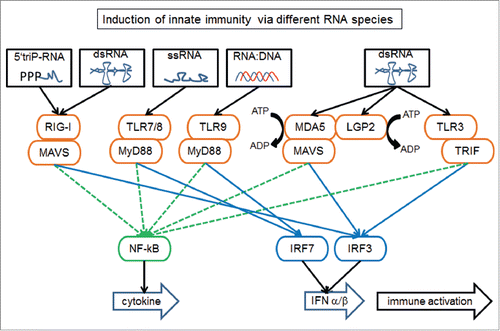
ERVs are derived from past exogenous retroviral infections and constitute approximately 10% and 8% of mouse and human genomes, respectively. In this regard, Tlr3, Tlr7 and Tlr9 deficient mice show no induction of innate immune genes and the type I interferon response and these gene deficiencies result in high expression of ERV RNA leading to viremia and tumorigenesis.Citation8 Like exogenous viruses, activation of TLRs via a variety of endogenous viral nucleic acids represents the initial step for downstream induction of NF-κB and/or IRF signaling pathways and stimulation of the interferon type I response (). Besides RIG-I and MDA5 the interferon promoter-stimulating factor 1 (IPS-1) (MAVS) is also essential for TLR signaling.Citation9 MAVS is the sole adapter for both RIG-I and MDA5 signaling and mediates effective responses against viral RNA (). The Laboratory of Genetics and Physiology 2 (LGP2) gene binds dsRNA, facilitating MDA5 to induce innate immunity via interferon transcription ().Citation10 Following interferon protein secretion and receptor binding ISGs become expressed and lead to immune cell recruitment, cytokine production and cell death to promote viral clearance.
Recently, we and others showed that Aza or 5-Aza-dC treatment of epithelial ovarian and colorectal cancer cell lines led to an induction of ERV dsRNA, which triggered innate type I interferon signaling and apoptosis as if in response to a viral infection.Citation3,4 Critical pathway members in these responses include TLR3, MAVS, MDA5, IRF7, interferon β (IFN-ß) and its receptor. Azaor5-Aza-dC-mediated demethylation and subsequent activation of ERVs led to a cellular viral “infection” alarm, which originated within the tumor cell. Many tumors evolve the ability to mediate a strong suppression of the immune system within the tumor microenvironment. Aza treatment remarkably sensitized melanoma tumor cells in a mouse model to anti-CTLA-4 immune checkpoint therapy demonstrating a significantly reduced tumor burden compared to each compound alone.Citation3 Furthermore, we uncovered that a core group of ISGs, defined as a viral defense signature, divided tumor cell lines upregulated by Aza and primary ovarian, breast, melanoma and colon carcinomas into low and high ISG expressing groups. Impressively, ISG expression of ovarian carcinomas positively correlated with low and high ERV expression.Citation3 We also showed that high expression of the viral defense genes in melanoma patients predicted a lasting clinical response to anti-CTLA-4.Citation3 These results support a link of ERV expression with ISG response in primary tumors, which needs to be investigated further. In light of the sophisticated ways in which tumors suppress the immune systemvia regulation of immune checkpoint proteins, our findings have high translational connotations for considering combinatorial treatments of patients with DNA-methylation inhibitors and other checkpoint inhibitors. These combinatorial treatments could activate the immune response and facilitate tumor clearance so that patients with both low and high ISG and ERV expressing tumors would benefit.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015; 33:1974-82; PMID:25605845; http://dx.doi.org/10.1200/JCO.2014.59.4358
- Li H, Chiappinelli KB, Guzzetta AA, Easwaran H, Yen RW, Vatapalli R, Topper MJ, Luo J, Connolly RM, Azad NS et al. Immune regulation by low doses of the DNA methyltransferase inhibitor 5-azacitidine in common human epithelial cancers. Oncotarget 2014; 5:587-98; PMID:24583822; http://dx.doi.org/10.18632/oncotarget.1782
- Chiappinelli KB, Strissel PL, Desrichard A, Li H, Henke C, Akman B, Hein A, Rote NS, Cope LM, Snyder A et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell 2015; 162:974-86; PMID:26317466; http://dx.doi.org/10.1016/j.cell.2015.07.011
- Roulois D, Loo Yau H, Singhania R, Wang Y, Danesh A, Shen SY, Han H, Liang G, Jones PA, Pugh TJ et al. DNA-Demethylating Agents Target Colorectal Cancer Cells by Inducing Viral Mimicry by Endogenous Transcripts. Cell 2015; 162:961-73; PMID:26317465; http://dx.doi.org/10.1016/j.cell.2015.07.056
- O'Neill LAJ, Golenbock D, Bowie AG. The history of Toll-like receptors — redefining innate immunity. Nat Rev Immunol 2013; 13:453-60; PMID:23681101; http://dx.doi.org/10.1038/nri3446
- De Nardo D. Toll-like receptors: Activation, signalling and transcriptional modulation. Cytokine 2015; 74:181-9; PMID:25846205; http://dx.doi.org/10.1016/j.cyto.2015.02.025
- Caskey M, Lefebvre F, Filali-Mouhim A, Cameron MJ, Goulet JP, Haddad EK, Breton G, Trumpfheller C, Pollak S, Shimeliovich I et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J Exp Med 2011; 208:2357-66; PMID:22065672; http://dx.doi.org/10.1084/jem.20111171
- Yu P, Lübben W, Slomka H, Gebler J, Konert M, Cai C, Neubrandt L, Prazeres da Costa O, Paul S et al. Nucleic acid-sensing Toll-like receptors are essential for the control of endogenous retrovirus viremia and ERV-induced tumors. Immunity 2012; 37:867-79; PMID:23142781; http://dx.doi.org/10.1016/j.immuni.2012.07.018
- Reikine S, Nguyen JB, Modis Y. Pattern Recognition and Signaling Mechanisms of RIG-I and MDA5. Front Immunol 2014; 5:342; PMID:25101084; http:10.3389/fimmu.2014.00342.
- Bruns AM, Leser GP, Lamb RA, Horvath CM. The innate immune sensor LGP2 activates antiviral signaling by regulating MDA5-RNA interaction and filament assembly. Mol Cell 2014; 55:771-81; PMID:25127512; http://dx.doi.org/10.1016/j.molcel.2014.07.003
