ABSTRACT
Insulin-like growth factor II mRNA-binding protein 3 (IMP-3), an oncofetal antigen identified using genome-wide cDNA microarray analyses, is overexpressed in several malignancies. IMP-3-derived cytotoxic T lymphocyte (CTL) epitopes have been used for peptide-based immunotherapies against various cancers. In addition to CTLs, induction of tumor-associated antigen (TAA)-specific helper T (Th) cells is crucial for establishment of effective antitumor immunity. In this study, we aimed to identify IMP-3-derived long peptides (IMP-3-LPs) carrying CTL and promiscuous Th-cell epitopes for use in cancer immunotherapy. IMP-3-derived Th-cell epitopes that bind to multiple HLA-class II molecules were predicted by in silico analysis, and their immunogenicity was determined by utilizing human T cells. We identified two highly immunogenic IMP-3-LPs presented by multiple HLA-class II molecules. One of the IMP-3-LPs encompassed two CTL epitopes that have been used for peptide-vaccine immunotherapy in ongoing clinical trials. IMP-3-LPs-specific Th cells responded to autologous dendritic cells (DCs) loaded with the recombinant IMP-3 proteins, suggesting that these s (LPs) can be naturally processed and presented. The IMP-3-LPs and specific Th cells augmented the expansion of IMP-3-specific CTLs, which was further enhanced by programmed cell death-1 (PD-1) blockade. In addition, IMP-3-LP encapsulated in liposomes was efficiently cross-presented in vitro, and this LP successfully cross-primed CTLs in HLA-A2 transgenic mice (Tgm) in vivo. Furthermore, one of the IMP-3-LPs induced IMP-3-specific Th cells from peripheral blood mononuclear cells (PBMCs) of head-and-neck malignant tumor (HNMT) patients. These findings suggest the potential usefulness of IMP-3-LPs in propagating both Th cells and CTLs and may have implications for IMP-3-LPs-based cancer immunotherapy.
Abbreviations
| APC | = | antigen-presenting cell |
| CTL | = | cytotoxic T lymphocyte |
| DC | = | dendritic cell |
| ELISPOT | = | enzyme-linked immunospot |
| GM-CSF | = | granulocyte macrophage colony-stimulating factor |
| HD | = | healthy donor |
| HLA | = | human histocompatibility leukocyte antigen |
| HNMT | = | head-and-neck malignant tumor |
| IL | = | interleukin |
| IMP-3 | = | insulin-like growth factor II mRNA-binding protein 3 |
| LP | = | long peptide |
| mAb | = | monoclonal antibody |
| OS | = | overall survival |
| PBMC | = | peripheral blood mononuclear cell |
| PD-1 | = | programmed cell death-1 |
| SP | = | short peptide |
| TAA | = | tumor-associated antigen |
| Tgm | = | transgenic mice |
| Th | = | helper-T |
| Th1 | = | T-helper type 1 |
Introduction
In the past, many cancer immunotherapies have focused primarily on tumor-reactive CTLs, which have the capacity to directly engage and kill malignant cells. Vaccination with TAAs-derived CTL epitope peptides (short peptides; SPs) is one of the therapeutic modalities used to elicit tumor-specific CTL responses in cancer patients. Therefore, to achieve effective and safe T-cell-mediated antitumor immunity in cancer patients, it is important to identify cancer-specific, oncogenic, and immunogenic TAAs as targets for cancer immunotherapy.Citation1 Recent identification of new TAAs by genome-wide cDNA microarray analyses coupled with isolation of cancer cells by laser-microbeam microdissection can be useful in selecting novel promising targets.Citation1
IMP-3 is one of oncofetal TAAs, which is frequently overexpressed in HNMT, lung cancer, esophageal cancer and various other malignancies, but rarely in normal adult organs.Citation2-4 Because the expression of IMP-3 is also detectable in normal adult testis,Citation5,6 IMP-3 is also considered as a cancer/testis antigenCitation7 as well as an oncofetal antigen, indicating the clinical significance of IMP-3 as a target for antigen-specific cancer immunotherapy. IMP-3 is a translational activator of insulin-like growth factor II (IGF-II) mRNA, promoting cancer cell proliferation through the IGF-II-dependent pathway,Citation8 and also contributes to tumor cell invasion.Citation9 Indeed, higher expression of IMP-3 has been known as one of the biomarkers for poor prognosis of various cancers including HNMT.Citation10-12 We identified highly immunogenic IMP-3-derived SPs (IMP-3-SPs) that can induce HLA-A2 (A*02:01) and HLA-A24 (A*24:02)-restricted CTLs from PBMCs of cancer patients.Citation6,13 Phase I/II clinical trials of peptide-based cancer immunotherapy using these IMP-3-SPs are underway.Citation14-16 Initial results from these trials indicate that the peptide vaccines are well tolerated, induce vaccinated SP-specific CTL responses, and in some patients have resulted in inhibition of tumor enlargement or reduction of tumor size, which has contributed to prolonged overall survival (OS).Citation16
However, it has previously been shown that vaccination with HLA-class I-restricted CTL epitope alone does not always elicit a sufficient immune response to induce effective antitumor immunity.Citation17 One likely cause for the ineffectiveness of SP-based immunotherapy is the induction of immunological tolerance in CD8+ T cells. SPs, which generally consist of 9 or 10 amino acids, can induce tolerance or anergy of CD8+ T cells when presented by HLA-class I molecules expressed on non-professional antigen-presenting cells (APCs) because of the lack of signaling from co-stimulatory molecules.Citation17,18 Synthetic long peptides (LPs) encompassing not only CD4+ Th-cell epitopes but also CTL epitopes have the potential to overcome this weakness because they do not bind directly to HLA-class I molecules expressed on non-APCs because of their long amino-acid sequence. Professional APCs such as DCs engulf and process LPs, then present CTL and Th-cell epitopes in the context of HLA class I and class II molecules, respectively.Citation17 Consequently, LPs can elicit TAA-specific CD4+ and CD8+ T-cell responses without inducing immunological tolerance.
Generation of efficient CTL-mediated antitumor responses generally depends on immunological help provided by TAA-specific CD4+ T cells.Citation19-23 Recent studies have reported the functional roles of TAA-specific Th1 cells within the tumor milieu. TAA-specific Th cells can induce senescence of tumor cells through combined stimulus with CD4+ T cell-derived IFNγ and TNF-α.Citation22 Moreover, TAA-specific Th1 cells enable CTLs to infiltrate the tumor site.Citation23 These Th1 cells also mediate anti-angiogenic effectsCitation24 or exhibit direct cytotoxic activity for tumor cells.Citation25,26 Furthermore, Hoyer et al. reported that simultaneous encounter of Th cells and CTLs with the same DC significantly enhanced antigen-specific CTL expansion.Citation27 Thus, an ideal peptide-based cancer immunotherapy might be a single polypeptide containing multiple epitopes for both Th1 cells and CTLs to induce robust antitumor CD4+ T cell and CD8+ T-cell responses.
In this study, we identified two IMP-3-LPs that induced antigen-specific Th cells with Th1 polarization characteristics in healthy donors (HDs) and HNMT patients. Interestingly, one of IMP-3-LPs encompassed multiple CTL and Th-cell epitopes. This peptide activated IMP-3-specific CTLs both in vitro and in vivo through cross-presentation. Our findings may have important implications for future clinical trials of LP-based cancer immunotherapy.
Results
Prolonged OS correlated with IMP-3-specific CTL responses in HNMT patients vaccinated with IMP-3-SP
Recently, in the phase II clinical trial of the immunotherapy utilizing vaccination with HLA-A24-restricted multiple TAA-derived SPs including IMP-3-SP for treatment of patients with metastatic/refractory squamous cell carcinoma of head-and-neck, we observed that the OS of vaccinated patients was significantly longer than non-vaccinated patients who received the best supportive care.Citation16 Herein, we have re-evaluated updated survival data of vaccinated HNMT patients. Based on their IMP-3-SP reactivity, CTL responses specific to the HLA-A24-resticted IMP3-SP after vaccination were observed in 55.6% of the patients, and these patients showed a significantly longer OS than those without any IMP-3-specific CTL response ().
Figure 1. Prediction of IMP-3-derived and promiscuous HLA-class II binding peptides encompassing CTL epitopes by using a computer algorithm. (A) Prolonged overall survival (OS) correlated with IMP-3-specific CTL responses in HNMT patients vaccinated with IMP-3-SP. The OS was compared between patients with an IMP-3-specific CTL response and those without an IMP-3-specific CTL response. (B) Immunohistochemical analyses of the IMP-3 protein in tumor tissues (original magnification ×200). The upper panel shows immunohistochemical staining with anti-IMP-3 antibody (Ab) in normal human placental tissue (positive control) and normal human oral tissue (negative control). The middle panel shows immunohistochemical staining with anti-IMP-3 Ab in tissue sections of squamous cell carcinoma in HNMT20, 26, and 29. Positive staining for IMP-3 was defined as dark brown cytoplasmic staining in malignant cells. The lower panel shows immunohistochemical staining with isotype-matched control Ab in each tumor tissues. (C) The amino-acid sequence of human IMP-3 protein was analyzed using an algorithm (IEDB analysis resource, consensus method). Numbers on the horizontal axis indicate amino-acid positions at the N-terminus of IMP-3-derived 15-mer peptides. A lower consensus percentile rank indicates stronger binding affinity to HLA-class II molecules. Predicted amino-acid sequences of LPs, IMP-3-LP1 (IMP-3192–212-LP, 21-mer), IMP-3-LP2 (IMP-3402–423, 22-mer), and IMP-3-LP3 (IMP-3507–527, 21-mer) with high consensus percentile ranks for multiple HLA-class II allelic products (DRB1*09:01; blue, DRB1*04:05; red, DRB15:02: green) are indicated with black bars. (D) Amino-acid sequences of three predicted IMP-3-LPs are shown. Nonamer SPs (A2-IMP-3199–207, A24-IMP-3508–516, and A2-IMP-3515–523) that are recognized by HLA-A2 or HLA-A24-restricted CTLs are indicated with underlined bold letters.
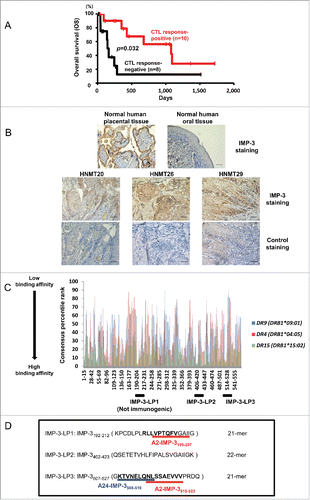
We examined the expression of IMP-3 by immunohistochemistry using tumor tissues obtained from five vaccinated HNMT patients (HNMT20, 24, 26, 29, and 35), and confirmed IMP-3 expression in four out of five patients' tumor tissues (Table S1). Importantly, IMP-3 expression was detected in all three patients tested who had IMP-3-specific CTL responses after vaccinations (HNMT20, 26, and 29) (), whereas in two patients without IMP-3-specific CTL responses (HNMT24 and 35), IMP-3 expression was detected in tumor tissue from one patient (HNMT35) but not from another patient (HNMT24) (Fig. S1). These observations may support the hypothesis that the IMP-3-specific immune responses induced by SP vaccination contribute to the improved prognosis of the patients, and suggest that IMP-3 is a potential target for cancer immunotherapy of HNMT.
Prediction and selection of potentially promiscuous HLA-class II-binding IMP-3-LPs
To predict immunogenic IMP-3-derived Th-cell epitopes which can bind to several common HLA-class II molecules with high affinity, we first checked the amino-acid sequence of IMP-3 using an immune epitope database (IEDB) computer algorithm, as previously described ( and Table S2).Citation28,29 As shown in , the three IMP-3-LPs (LP1; IMP-3192–212-LP, LP2; IMP-3402–423-LP and LP3; IMP-3507–527-LP) were predicted to have strong binding affinity with multiple common HLA-class II molecules (e.g., HLA-DR9, DR4 or DR15). IMP-3-LP1 contained the CTL epitope that was recognized by HLA-A2-restricted CTLs (A2-IMP-3199–2076), and IMP3-LP3 also contained two known CTL epitopes that were recognized by HLA-A24 or A2-restricted CTLs (A24-IMP-3508–51613; A24-IMP-3-SP and A2-IMP-3515–5236; A2-IMP-3-SP) (). Another peptide, IMP-3-LP2, did not contain any known CTL epitopes.
Identification of IMP-3-LPs encompassing Th-cell epitopes
To determine the actual immunogenicity of the three candidate IMP-3-LPs, we examined whether IMP-3-LP-specific CD4+ T cells could be induced from PBMCs of HDs by in vitro stimulation with IMP-3-LPs. CD4+ T cells isolated from PBMCs of five HDs were stimulated at weekly intervals with autologous DCs and PBMCs pulsed with synthesized IMP-3-LPs. After at least two rounds of stimulation, expanded CD4+ T cells were harvested and their responses to the IMP-3-LPs were examined using IFNγ enzyme-linked immunospot (ELISPOT) assays. HLA genotypes of the HDs are shown in and Table S3. The Th cells generated from HLA-DR53-positive HD1 produced a significant amount of IFNγ in response to IMP-LP2-pulsed PBMCs in an HLA-DR-dependent manner (). The bulk Th cells were also specifically activated by IMP-3-LP2-pulsed mouse fibroblast L-cell line transduced with HLA-DR53 genes (L-DR53), but not unpulsed or IMP-3-LP2-pulsed L-DR4 cells, indicating that IMP-3-LP2 was presented by HLA-DR53.
Figure 2. Induction of IMP-3-LPs-specific Th cells from HDs. (A, B) IMP-3-LP-specific Th cells were generated from a DR53+ HD (HD1, A) or DR9+ HD (HD4, B). IMP-3-LP-specific Th cells were generated from HDs by stimulation with the indicated IMP-3-LPs (LP2; A, LP3; B). The generated Th cells were re-stimulated with autologous PBMCs or HLA-class II-expressing L cells pulsed with IMP-3-LP. The number of IFNγ-producing Th cells was analyzed by ELISPOT assay. Representative data from at least three independent experiments with similar results are shown. The HLA-class II genotype of the donor is indicated at the top of the panels. The underlined HLA-class II alleles encode HLA-class II molecules presenting the cognate peptides to Th cells. (C, D) An HLA-DR53-restricted and IMP-3-LP2-specific Th clone (HD1, C) and an HLA-DR8 or DR14-restricted and IMP-3-LP3-specific Th clone (HD3, D) were stimulated with autologous DCs loaded with indicated proteins with or without HLA-DR blocking Ab. Representative data from three independent duplicate experiments with similar results are shown.
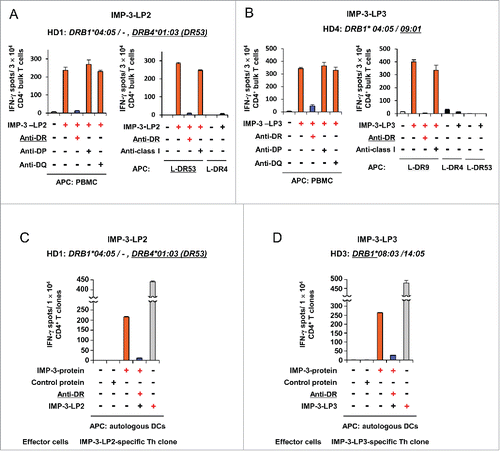
Table 1 Identification of IMP-3-derived and promiscuous HLA-class II-restricted CD4+ T cell epitopes encompassing cytotoxic T lymphocyte (CTL) epitopes
We also examined whether IMP-3-LP2 was presented by other HLA-class II molecules, and consequently stimulated CD4+ T cells from other HDs. We found that IMP-3-LP2 induced HLA-DR8 (DRB1*08:03) (HD2, Fig. S2A), HLA-DR (DRB1*08:03 or 14:05) (HD3, Fig. S2B), HLA-DR4 (DRB1*04:05) (HD5, Fig. S2C), and HLA-DQ-restricted Th cells (HD5, Fig. S2D). These findings indicate that IMP-3-LP2 has the potential to bind to multiple HLA-class II molecules and induce TAA-specific Th cells in several different donors.
Next, we assessed whether IMP-3-LP3 (IMP-3507–527-LP)-specific Th cells were primed by LP stimulation. In an HLA-DR9-positive donor (HD4), the generated Th cells produced a significant amount of IFNγ in response to IMP-3-LP3-pulsed PBMCs in an HLA-DR-dependent manner (). The bulk Th cells specifically recognized L-DR9 cells pulsed with IMP-3-LP3. IMP-LP3 also induced HLA-DR9 (DRB1*09:01)-restricted Th cells from the other HLA-DR9-positive donor, HD5 (Fig. S2E). Furthermore, HLA-DR8 (DRB1*08:03) or DR14 (DRB1*14:05)-restricted Th cells were also generated by IMP-3-LP3 stimulation (HD3, Fig. S2F), indicating that IMP-3-LP3 encompasses multiple Th-cell epitopes, similar to the multi-epitope characteristic of IMP-3-LP2.
IMP-3-LP1 (IMP-3192–212-LP) was predicted to have strong binding affinity with multiple HLA-class II molecules. However, stimulation with LP1 did not prime LP-specific Th cells in any of our HDs (data not shown), and thus we concluded that IMP-3-LP1 was not immunogenic.
IMP-3-LPs encompass naturally processed Th-cell epitopes
We next tested whether IMP-3-LPs-derived Th-cell epitopes were produced by intracellular processing of IMP-3 protein and presented by HLA-class II molecules in DCs. The HLA-DR53-restricted IMP-3-LP2-reactive Th clone generated from HD1 and autologous DCs loaded with recombinant IMP-3 protein were used as responders and APCs, respectively. As shown in , the IMP-3-LP2-specific Th clone was activated upon stimulation with IMP-3 protein-loaded DCs but not with control protein-loaded DCs, indicating that IMP-3-LP2 encompasses naturally processed and HLA-DR53-restricted Th-cell epitope. The IMP-3-LP3-specific Th clone generated from HD3 was also activated by IMP-3 protein-loaded DCs (). These results suggest that both IMP-3-LP2 and IMP-3-LP3 contain Th-cell epitopes that are naturally processed from the IMP-3 protein.
IMP-3-LPs induce Th1-type CD4+ T cells
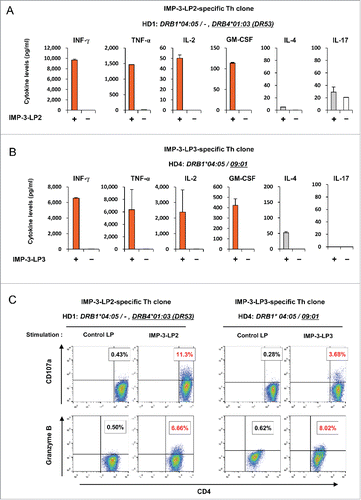
To examine the characteristics of IMP-3-LP-specific Th cells, we measured the levels of various cytokines secreted from IMP-3-LP-specific Th cells in response to stimulation with autologous PBMCs pulsed with cognate peptide. Both IMP-3-LP2- and LP3-specific Th clones produced large amounts of Th1 cytokines, such as IFNγ, TNF-α, IL-2, and GM-CSF, but less IL-4 and IL-17 after re-stimulation with cognate peptides (), suggesting that IMP-3-LP-specific Th cells have Th1-polarized characteristics. Bulk IMP-3-LP-specific Th cells also displayed Th1-like cytokine production profiles (Fig. S3A and S3B). In addition, CD107a and granzyme B were detected in the IMP-3-LP2-specific (HD1) and LP3-specific (HD4) Th clone when stimulated with cognate peptide (), suggesting that IMP-3-LP-reactive Th cells may also possess cytotoxic activity.
Figure 3. Pattern of cytokine production by IMP-3-LP-specific Th clones. (A, B) IMP-3-LP2-specific Th clone (A) or LP3-specific Th clone (B) were stimulated with or without cognate peptides. Concentrations of indicated cytokines in the culture supernatant were measured. Data are presented as the mean ± SD of triplicate assays. (C ) IMP-3-LP-specific Th clones were re-stimulated with cognate peptide or control LP. The numbers in the dot plots indicate the frequencies of CD107a+ or granzyme B+ cells in CD4+ Th clones.
IMP-3-specific Th cells promote the expansion of IMP-3-specific CTLs
Next, we investigated whether IMP-3-specific Th clones (Th clone) promote the expansion of IMP-3-specific CTLs. For this purpose, HLA-A2-restricted and IMP-3515–523-SP-specific CTLs (A2-IMP-3-SP-specific CTLs) were generated from HD4 as described previously,Citation6 and these CTLs were co-cultured with autologous DCs pulsed with A2-IMP-3-SP (SP) in the presence of IMP-3-LP3 (LP), Th clone, or LP plus Th clone(). After 1 week of in vitro culture, the frequencies of CD8+HLA-A2/IMP-3515–523 tetramer+ cells were analyzed. As compared with SP stimulation alone, SP combined with LP or Th clone did not significantly promote the expansion of CTLs (5.38% vs. 6.77% vs. 8.65%) (). Although this LP encompasses the HLA-A2-restricted and IMP-3-derived CTL epitope, the frequencies of CTLs were not increased by the stimulation with SP plus LP, suggesting that efficient cross-presentation of IMP-3-LP3 did not occur under these culture conditions. However, simultaneous stimulation of CTLs/ Th clone with both SP and LP resulted in a significant increase of IMP-3-specific CD8+ T cells. A similar trend was observed in terms of mean fluorescent intensity of tetramer staining (). These results suggest that IMP-3-LP3-specific Th cells activated with IMP-3-LP3 stimulation augment the expansion of IMP-3-SP-specific CTLs. IMP-3-LP2-specific Th clone also enhanced the frequencies of IMP-3-SP-specific CTLs (Fig. S4A).
Figure 4. IMP-3-specific Th cells promote the expansion of A2-IMP-3-SP-specific CTLs. (A) HLA-A2-restricted and IMP-3515–523-SP-specific CTLs (A2-IMP-3-SP-specific CTLs) were generated from a HLA-A2+ HD (HD4), and they were cultured for 7 d with A2-IMP-3-SP (SP) in the presence or absence of IMP-3-LP3 (LP) and LP3-specific Th clone (Th clone). (B) At the end of culture, the frequencies of A2-IMP-3-SP-specific CTLs were measured by HLA-A2/IMP-3515–523 tetramer staining. Representative plots of CD8+ T cells from three independent experiments with similar results are shown. The numbers inside the plots indicate the percentage of CD8+ HLA-A2/ IMP-3515–523 tetramer+T cells. (C) Mean fluorescence intensity of HLA-A2/IMP-3515–523 tetramer-reactive CD8+ T cells. (D) A2-IMP-3-SP-specific CTLs were stimulated with SP in the presence or absence of LP, Th clone, control monoclonal Ab (mAb), and anti-PD-1 mAb. On day 7, CD8+ T cells were analyzed by staining the HLA-A2/IMP-3515–523 tetramer. Representative data are shown from four independent experiments.*p <0.05, **p <0.01. N.S., not significant. (E) Immature DCs were cultured in the presence or absence of autologous IMP-3-LP3-specific Th clones and the cognate peptide. After 48 h of co-culture, the expression of CD40 and CD86 on gated DCs was analyzed. The gray-filled histograms show isotype-matched control staining.
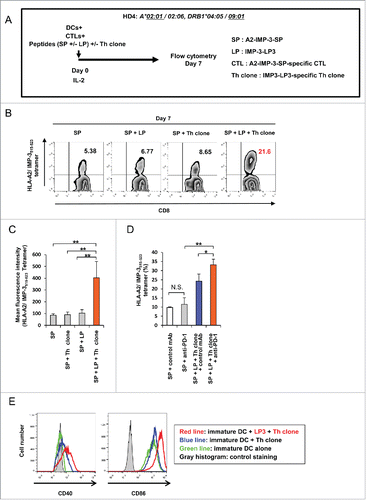
Interestingly, when both Th clone and CTLs were activated in the presence of antibody (Ab) against PD-1, the frequencies of CD8+ tetramer+ cells were further increased compared with identical full stimulation in the absence of anti-PD-1 Ab. This result suggests that PD-1 blockade has a strongly synergistic effect on CTL activation when both CTLs and Th cells are activated ().
IMP-3-specific Th cells induce DC activation in an antigen-specific manner
To investigate the mechanism by which activated CD4+ T cells augment the expansion of IMP-3-specific CTLs, we examined the expression levels of DC activation markers, CD40 and CD86, on DCs after co-culture with IMP-3-LP-specific Th cells stimulated with cognate LPs (). Expression of both CD40 and CD86 on co-cultured DCs was upregulated when IMP-3-LP3-specific Th clones were stimulated with LP-pulsed DCs, indicating that activated IMP-3-LP3-specific Th clones enable DCs to upregulate co-stimulatory molecules induced by cognate interaction. Similar results were obtained using the IMP-3-LP2-specific Th clone established from HD1 (Fig. S4B). From these observations, we speculate that IMP-3-LP-specific Th cells contribute to enhanced CTL expansion through at least in part DC activation.
IMP-3-LP activates IMP-3-SP-specific CTLs through cross-presentation in vitro
Although we could not observe cross-presentation of soluble IMP-3-LP3 to activate cognate CTLs in our initial in vitro experiments (), we chose to re-evaluate this because we thought that the lack of cross-presentation of soluble IMP-3-LP in an in vitro setting may be due to inefficient delivery of the LPs into the HLA-class I-mediated antigen presentation pathway in DCs. We therefore utilized pH-sensitive liposomes, which are taken up efficiently by DCs and reportedly deliver entrapped antigens from the endosome to the cytosol to enhance cross-presentation.Citation30 As shown in , A2-IMP-3-SP-specific bulk CTLs specifically produced IFNγ in response to stimulation with DCs loaded with IMP-3-LP3 encapsulated in liposomes but not DCs loaded with soluble IMP-3-LP3, liposome-encapsulating control LP, or liposome alone. Moreover, specific IFNγ production was inhibited by addition of an anti-HLA-class I mAb. These results indicated that A2-IMP-3-SP-specific CTLs were indeed stimulated through the cross-presentation of IMP-3-LP3 by DCs in vitro.
Figure 5. Efficient cross-presentation of IMP-3-LP3 encapsulated in liposomes in vitro and cross-priming with IMP-3-LP3 in HLA-A2 Tgm in vivo. (A) A2-IMP-3-SP-specific CTLs derived from HD4 (HLA-A2+) were stimulated in vitro with autologous DCs pulsed with IMP-3-LP3 encapsulated in liposomes (Lip-IMP-3-LP3), IMP-3-LP2 encapsulated in liposomes (Lip-control LP), or liposomes alone (Lip alone). CTL responses were analyzed by IFNγ ELISPOT assays. Representative data from three independent experiments with similar results are shown. (B) HLA-A2 Tgm were immunized with IMP-3-LP3 emulsified in IFA. Seven days after the second immunization, CD8+ T cells were isolated from the pooled inguinal lymph nodes and were stimulated ex vivo with bone marrow-derived DCs pulsed with A2-IMP-3-SP or A2-HIV-SP. The numbers of IFNγ-producing CD8+ T cells were assessed using an ex vivo ELISPOT. Representative data from three independent experiments (two or three mice in each group) that were performed in duplicate or triplicate (all yielded similar results) are shown.**p <0.01.
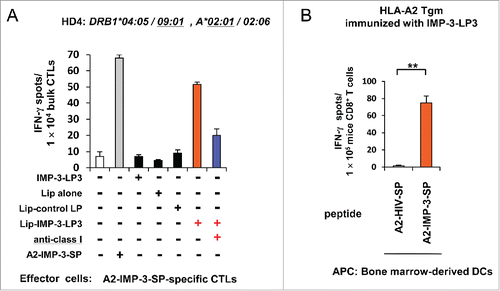
IMP-3-LP encompassing CTL-epitopes can prime IMP-3-SP-specific CTLs through cross-presentation in vivo
We further confirmed the ability of IMP-3-LP3 to cross-prime A2-IMP-3-SP-specific CTLs in vivo using HLA-A2 Tgm. HLA-A2 Tgms were immunized twice with IMP-3-LP3. The CD8+ T cells from HLA-A2 Tgm vaccinated with IMP-3-LP3 specifically produced IFNγ in response to stimulation with bone marrow-derived DCs pulsed with A2-IMP-3-SP (), suggesting that IMP-3-LP3 can prime A2-IMP-3-SP-specific CTLs by cross-presentation in vivo.
IMP-3-LP induces peptide-specific Th cells in HNMT patients
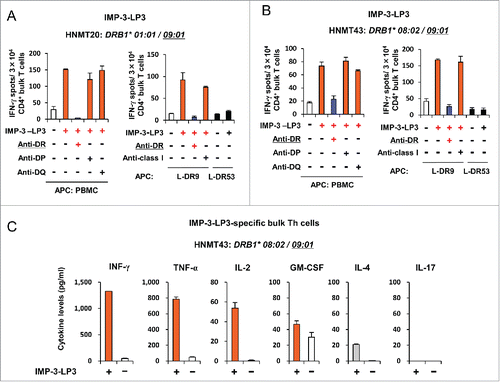
We attempted to induce IMP-3-LP-specific Th cells from three patients with advanced stage HNMT (HNMT20, 29, and 43), who have been administrated long-term vaccinations with IMP-3-SP activating HLA-A24-restricted CTLs. Patient characteristics are summarized in Table S1. From patients HNMT20 and HNMT43, we generated DCs differentiated from autologous CD14+ cell-derived myeloid cell lines, which were transduced with cMYC plus BMI1 to provide unlimited expansion of DCs and enabled us to use them as a source of APCs (Imamura et al., manuscript submitted).Citation31 As observed in the HDs, isolated CD4+ T cells from HNMT patients were stimulated with autologous DCs firstly, followed by stimulation with autologous PBMCs pulsed with IMP-3-LPs. After at least two rounds of stimulation, the antigen-specific responses of patient CD4+ T cells were examined using INFγ ELISPOT assays. As shown in , IMP-3-LP3 induced peptide-specific Th cells from two patients, HNMT20 and HNMT43. The CD4+ T cells generated from HNMT43 displayed a Th1-polarized cytokine secretion pattern including relatively higher IFNγ and TNF-α production (). These results suggest that IMP-3-LP may induce antigen-specific Th cells with Th1-like characteristics not only from HDs but also from HNMT patients, highlighting the potential for the applicability of LP-vaccination in cancer patients.
Figure 6. Induction of IMP-3-LP-specific Th cells from HNMT patients. (A, B) CD4+ T cells isolated from HNMT20 and HNMT43 were stimulated with autologous DCs and PBMCs pulsed with IMP-3-LPs. After two rounds of stimulation, the generated Th cells were re-stimulated with autologous PBMCs or HLA-class II-expressing L cells pulsed with IMP-3-LP3. The number of IFNγ-producing Th cells was analyzed by ELISPOT assay. Representative data from at least three independent experiments with similar results are shown. The HLA-class II genotype of the donor is indicated at the top of the panels. The underlined HLA-class II alleles encode HLA-class II molecules presenting cognate peptides to Th cells. (C) Profiles of cytokine produced upon LP stimulation in IMP-3-LP3-specific Th clones generated from HNMT43 patient. Data are presented as the mean ±SD of triplicate assays.
Discussion
In this study, we identified two immunogenic IMP-3-LPs that were naturally processed from the IMP-3 protein and elicited Th1-like CD4+ T-cell responses. IMP-3-LP-specific Th clones significantly augemented the expansion of IMP-3-SP-specific CTLs, at least through DC activation. In addition, IMP-3-LP3, which also encompasses both HLA-A2 and -A24-restricted CTL epitopes, induced A2-IMP-3-SP-specific CTLs via the cross-presentation pathway both in vitro and in vivo. We also demonstrated that IMP-3-LP boosted peptide-specific Th cells with Th1-like characteristic from PBMCs of HNMT patients. Our findings suggest that vaccination with IMP-3-LPs, either LPs alone or in combination with TAA-specific SPs, is useful to elicit stronger antitumor CD4+ and CD8+ T-cell immunity and may improve the clinical outcome of patients.
CD4+ T cells can provide the help in priming and activating CTLs through DC activation via CD40–CD40L interaction.Citation32,33 Aarntzen and colleagues attempted to investigate the immunological and clinical responses to vaccination with DCs pulsed with both HLA-class I and II-restricted epitopes compared with HLA-class I-restricted epitope alone to evaluate the clinical relevance of TAA-specific Th cells.Citation34 They showed that activation of TAA-specific Th cells with DCs pulsed with both HLA-class I and II-restricted epitopes effectively enhanced TAA-specific CTL frequencies and immune responses, contributing to improved clinical outcome. Likewise, in this study, we demonstrated that the addition of IMP-3-LP and LP-specific Th clone into the culture augmented the expansion of A2-IMP-3-SP-specific CTLs, possibly through DC activation (, S4A and S4B). These data suggest that IMP-3-LPs administered in combination with A2-IMP-3-SP immunotherapy may augment the elicitation of tumor-specific CTLs in cancer patients.
Disis et al. demonstrated that vaccination with HER-2/neu-derived LP which encompassed an HLA-A2-binding CTL epitope could elicit both HER-2/neu-specific T-cell responses and the embedded epitope-specific CTL responses in metastatic breast cancer patients.Citation35 In the present study, we demonstrated that IMP-3-LP3 could prime A2-IMP-3-SP-specific CTLs in vivo and activate these CTLs in vitro through cross-presentation by DCs. Therefore, vaccination with IMP-3-LP3 may potentially elicit both tumor-specific Th cells and CTL responses in clinical applications. Melief et al. showed that LPs encompassing CTL epitopes can induce superior T cell immune responses compared to a vaccine containing minimal CTL epitopes because of the increased duration of antigen presentation by APCs.Citation17,18 However, in the present study, vaccination with IMP-3-LP3 was not superior to A2-IMP-3-SP in induction of IMP-3-specific CTLs in the HLA-A2 Tgm model (data not shown). Thus, further work is necessary to elucidate whether IMP-3-LP alone or in combination with IMP-3-SP would elicit a stronger IMP-3-specific CTL response in vivo.
In the present study, we did not observe the cross-presentation of soluble IMP-3-LP3 in our in vitro experiments. However, cross-presentation of IMP-3-LP3 encapsulated in liposome was successful probably because of the efficient delivery of LP to the cytoplasm of DC. Furthermore, sufficient cross-priming could be observed in vivo using soluble LP, suggesting that liposomes may not be necessary for cross-presentation in our in vivo experiments. One possible reason for this apparent discrepancy is that IFA was used as an adjuvant in our in vivo experiments. IFA promotes antigen persistence at the injection site and prolongs cross-presentation by DCs.Citation36 Thus, IFA-embedded IMP-3-LP could be presented by DCs and cross-prime CTLs in vivo in HLA-A2 Tgm.
The subset of CD4+ T cells induced after vaccinations with HLA-class II-restricted antigens is of particular interest when induction of tumor-reactive CD4+ T cells is desired. A number of previous reports demonstrated that not only cytokine milieu, but also the strength of antigen stimulation resulting from the affinity/avidity between TCR and peptide-MHC ligands determined the fate of Th1/Th2 polarization in vitro and in vivo.Citation37-40 A high dose of peptide or a strongly agonistic ligand favors development of Th1-type CD4+ T cells whereas a low dose of peptide or a weakly agonistic ligand favors Th2 cells. In this study, both IMP-3-LP-specific bulk CD4+ T cells and Th clones generated from HDs produced preferentially Th1-type cytokines in response to IMP-3-LPs. Based on these observations, the two identified IMP-3-LPs might provide high-avidity TCR signals that preferentially polarize toward a Th1 phenotype at least in HDs.
Using PBMCs of HNMT patients, we also successfully induced IMP-3-LP-specific Th-1 like CD4+ T cells by in vitro stimulations with IMP-3-LP in this study. However, Tatsumi et al. previously demonstrated that CD4+ T cells derived from cancer patients tended to display Th2-polarized cytokine secretion patterns in response to in vitro stimulation with TAA-derived LPs.Citation41 Several previous reports also showed that the frequencies of CD4+ CD25high Foxp3+ regulatory T cells (Tregs) were increased in patients with several types of cancers,Citation42,43 and vaccination with TAA-derived LP sometimes induced LP-specific Tregs.Citation44 In the present study, IMP-3-LP-specific Th clones generated from HNMT patients in vitro culture did not express FoxP3 (data not shown), suggesting that they were not Tregs. However, at the current moment, we cannot conclude that vaccination of IMP-3-LP can always elicit LP-specific and Th1-polarized immune responses in many cancer patients because we could not distinguish whether IMP-3-LP-specific Th-cell responses detected in HNMT patients were elicited by boosting in vivo LP-specific Th-cell responses or by priming naive CD4+ T cells in vitro. Further studies using a larger cohort of cancer patients will be required for clarification of physiological relevance of IMP-3-LP for Th1-cell induction.
Previous reports have demonstrated that not only CTLs, but also CD4+ T cells can demonstrate cytotoxicity against both virus-infected cells and tumor cells.Citation25,26 As shown in , IMP-3-LP-specific Th cells expressed CD107a and granzyme B, molecules associated with cytotoxic activities, in response to stimulations with cognate peptide. Although we did not investigate cytotoxic activity directed against cancer cells, our data suggest that IMP-3-LP-specific Th cells may provide not only helper function but also cytotoxic activity, which can be an additional benefit for cancer immunotherapy.
There have been several reports regarding HLA-class II-restricted helper peptides which are capable of binding to more than one HLA-class II moleculeand eliciting polyclonal Th-cell responses.Citation45-47 Using the cell lines engineered to express HLA-class II molecules, we have demonstrated herein that IMP-3-LPs were presented by HLA-DR9, HLA-DR4, HLA-DR8, HLA-DR53, HLA-DR8/14, and HLA-DQ3/4, suggesting that vaccinations with a combination of IMP-3-LP2 and LP3 could cover the majority of the Japanese population (Table S4).Citation48 It has been demonstrated that Th-cell or CTL responses to other epitopes of the same proteins or other antigens not included in the vaccinations (epitope spreading or antigen spreading) were often observed in cancer patients with positve clinical responses to the TAA-derived peptide vaccinations.Citation49,50 These accumulating evidences suggest that Th-cell and CTL responses against broader epitope repertoires may be one of the key points to achieve better clinical responses to cancer vaccinations. In the present study, we identified two immunogenic IMP-3-LPs (IMP-3-LP2 and LP3) encompassing several Th-cell epitopes, and IMP-3-LP3 also contains two IMP-3-derived CTL epitopes. Thus, in terms of not only broad patient coverage but also simultaneous induction of broader IMP-3-specific Th-cell and CTL responses, the cancer vaccine formulation containing a combination of IMP-3-LP2 and LP3 may have an advantage compared to vaccine formulation containing either IMP-3-LP2 or LP3.
Recent clinical trials have validated that antibodies interrupting immune checkpoints, such as CTL associated protein-4, PD-1, and PD-1 ligand, can elicit antitumor immunity and mediate durable tumor regressions. In animal models, combination therapy with immune checkpoint blockade and various types of tumor vaccine has demonstrated enhanced activation of vaccine-induced tumor-specific effector cells, especially CTLs, and synergistic antitumor effect.Citation51-53 Consistent with these studies, we demonstrated that anti-PD-1 Ab further augmented the expansion of IMP-3-SP-specific CTLs when both CTLs and Th clone were activated, although we did not observe this effect when CTLs were stimulated with SP plus anti-PD-1 Ab as compared with SP alone (). We hypothesized that the ineffectiveness of PD-1 blockade when given in combination with SP stimulation alone might be due to limited activation of CTLs, resulting in insufficient numbers of PD-1-positive cells to show the effect of PD-1 blockade. In fact, the expression levels of PD-1 on CTLs stimulated with SP alone were lower than that stimulated with SP plus LP plus Th clone (Fig. S4C). Therefore, these observations suggest that a combination therapy with IMP-3 peptide-based immunotherapy and immune checkpoint blockades may be a good candidate for future cancer immunotherapy and this combination therapy may be more effective when CTLs and Th cells can be sufficiently activated.
In conclusion, we identified two immunogenic IMP-3-LPs that can induce multiple HLA-class II-restricted Th cells. One of the IMP-3-LPs encompassed IMP-3-CTL epitopes and induced both IMP-3-specific Th cells and CTLs. Thus, our results suggest that IMP-3-LPs provide a useful tool for propagation of both IMP-3-specific Th cells and CTLs, leading to induction of stronger antitumor responses. These findings may support clinical trials of IMP-3-LPs-based immunotherapy for various types of cancers.
Materials and methods
Cell lines
Mouse fibroblast cell lines (L cells), genetically engineered to express DR4 (DRB1*04:05, L-DR4), DR8 (DRB1*08:03, L-DR8), DR9 (DRB1*09:01, L-DR9), DR15 (DRB1*15:02, L-DR15), or DR53 (DRB4*01:03, L-DR53) were used as APCs. These L cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS).
Patients
The phase II clinical trial of cancer immunotherapy using three HLA-A24-binding TAA-derived SPs for patients with advanced or metastatic HNMT was approved by the Institutional Review Board of Kumamoto University, Kumamoto, Japan. This trial was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) number 000008379 (CTR-8379). The design of the clinical trial, patient eligibility, and treatment protocol were described previously.Citation16 Patients were enrolled after providing written informed consent. Blood samples were collected from three HNMT patients enrolled in this trial to analyze the immune responses of Th cells reactive to IMP-3-LPs.
Prediction of HLA-class II binding peptides
To predict possible promiscuous HLA-class II binding human IMP-3-derived LPs, the amino-acid sequence of the human IMP-3 protein was analyzed using a computer algorithm (IEDB analysis resource, consensus method, http://tools.immuneepitope.org/analyze/html/mhc_II_binding.html).Citation28,29 The software analyzed 15 amino-acid-long sequences offset to encompass the entire IMP-3 protein. The 21 and 22 amino-acid-LPs, IMP-3192–212-LP (LP1) (KPCDLPLRLLVPTQFVGA-IIG), IMP-3402–423-LP (LP2) (QSETETVHLFIPALSVGAIIGK) and IMP-3507–527-LP (LP3)(GKTVNELQNLSSAEVVVPRDQ), with overlapping high consensus percentile ranks for multiple HLA-class II molecules encoded for by DRB1*09:01, DRB1*04:05 or DRB1*15:02 alleles, were selected and synthesized ( and ). IMP-3-LP1 and IMP-3-LP3 included IMP-3-derived 9-mer CTL epitopes that were recognized by HLA-A2 or A24-restricted CTLs ().
Synthetic peptides, recombinant proteins and LPs encapsulated in liposomes
Two human IMP-3-derived SPs (A2-IMP-3-SP and A24-IMP-3-SP) and three IMP-3-LPs (LP1, LP2, and LP3) were synthesized (MBL; purity >95%; ). An HIV-related SP (A2-HIV-SP) and a CDCA1-derived SP (A2-CDCA1-SP) that binds to HLA-A2 were used as negative control SPs.Citation54 Peptides were dissolved in dimethylsulfoxide at 10 mg/mL, and stored at −80°C. The recombinant whole IMP-3 and CDCA1 protein were generated in Escherichia coli BL21 transformed with a pET28a vector encoding IMP-3 (Novagen, 69864–3). Both IMP-3 protein and CDCA1 protein were assessed by SDS-PAGE and purified. CDCA1 protein was used as a control. The liposomes loaded with IMP-3-LP3 and IMP-3-LP2 (as a control) were produced as previously described.Citation30
Generation of antigen-specific CD4+ T cells from healthy donors and HNMT patients
The protocol for isolation and use of PBMCs from HDs and HNMT patients was approved by the Institutional Review Board of Kumamoto University. We obtained PBMCs from five HDs and three HNMT patients, all with written informed consents. Genotyping of HLA-A, DRB1, and DPB1 alleles was performed at the HLA Laboratory (Kyoto, Japan) (Table S1 and S3). Induction of antigen-specific CD4+ T cells was performed as described previously.Citation55-58 Monocyte-derived DCs that were used as APCs were generated from CD14+ cells as described previously.Citation6 We generated CD14+ cell-derived myeloid cell lines from purified CD14+ cells from the HNMT patients, followed by lentivirus vectors transduction with cMYC plus BMI1 genes to promote proliferation.Citation31 When we stimulated IMP-3-LP-specific Th cells derived from HNMT patients with cognate peptide, we sometimes used autologous CD14+ cell-derived myeloid cell line-DCs as APCs, which were differentiated from CD14+ cell-derived myeloid cell lines by the addition of IL-4 (20 ng/mL) to the culture.Citation31 In some instances, T cells were cloned by limiting dilution for further studies as described previously.Citation59
Assessment of T cell responses to peptides and proteins
The immune response of Th cells to APCs pulsed with peptides or proteins was assessed by IFNγ ELISPOT assays (BD Biosciences, 551849) as described previously.Citation6,55-58 To determine the allelic restriction of HLA molecules, mAbs against HLA-DR (BioLegend, 307612), HLA-DP (Abcam, ab106312), HLA-DQ (Abcam, ab23632), or HLA-class I (Abcam, ab23755) were added into the culture. All mAbs were used at 5 μg/mL.
Cytokine analysis
IMP-3-LP-specific bulk CD4+ T cells (3 × 104/well) or Th clones (1 × 104/well) were cultured with autologous PBMCs (3 × 104/well) in the presence of cognate peptide in 96-well plates. After 24 h, cytokine (IFNγ, TNF-α, IL-2, GM-CSF, IL-4, IL-17) levels in the culture supernatants were measured using the Bio-Plex system (Bio-Rad) according to the manufacturer's instructions.
Detection of CD107a and granzyme B expression by flow cytometry
The expression of CD107a in IMP-3-LP-specific Th cells upon stimulation with cognate peptides was analyzed with an immunocyto CD107a detection kit (MBL, 4844) according to the manufacturer's instructions as described previously.Citation6,55 To analyze the expression of intracellular granzyme B, Th cells were stimulated with cognate LP or control peptide (10 µg/mL) in the presence of brefeldin A (Sigma-Aldrich, B5936) for 5 h. After stimulation, the cells were fixed for 15 min in 4% paraformaldehyde and incubated with PE-conjugated anti-human CD4 Ab (eBioscience, 12-0049-42) and FITC-labeled anti-human granzyme B Ab (BD Biosciences, 561998) or FITC-labeled isotype control mouse IgG1 in 0.25% saponin (Sigma-Aldrich, S7900) for 20 min and then analyzed by flow cytometry.
The synergistic effect of IMP-3-LP on activation of A2-IMP-3-SP-specific CTLs
Induction of A2-IMP-3-SP-reactive CTLs from an HLA-A2+ donor (HD1 and HD4) by stimulation with A2-IMP-3-SP was performed as described previously.Citation6 A2-IMP-3-SP-specific CTLs (1 × 105) were co-cultured with autologous immature DCs (1 × 105) in 24-well plates, followed by addition of SP alone (A2-IMP-3-SP, 10 μg/mL), SP plus LP (IMP-3-LP, 20 μg/mL), SP plus LP-specific Th clone (2 × 104), or SP plus LP plus Th clone in a final volume of 2 mL. IL-2 (20 U/mL) was added on day 0. After culture for 7 d, the cells were stained with PE-labeled tetramer of the HLA-A*02:01/IMP-3-A2515–523-complex (MBL) together with a FITC-labeled anti-human CD8 mAb (Beckman Coulter, 6602385). In some experiments, anti-PD-1 mAb (10 µg/mL, BioLegend, 329912) or control mice IgG mAb (10 µg/mL) was added in the culture.
Analysis of surface marker expressed on DCs after co-culture with IMP-3-specific Th cells
Immature CD14+ monocyte-derived DCs were generated as described previously.Citation55-58 One hundred thousand DCs were loaded with IMP-3-LP (20 μg/mL) for 3 h and then co-cultured with LP-specific Th cells (1 × 105) for a further 48 h. After co-culture, the cells were stained with PE-labeled anti-CD40, FITC-labeled anti-CD86 mAbs (BD Biosciences, 560963 and 555657, respectively), and PerCP-labeled anti-CD4 mAb (eBioscience, 45-0049-42) and marker expression on the CD4− fraction (DC) was analyzed by flow cytometry.
In vitro and in vivo cross-presentation assay
In some experiments, to assess the cross-presentation of IMP-3-LP3, we used DCs loaded with LP encapsulated in liposomes. Liposomes were prepared as previously described.Citation30 Immature DCs were prepared and pulsed with LP encapsulated in liposome (equivalent to 20 µg/mL of LP) for 4 h. The number of IFNγ producing-A2-IMP-3-SP-specific bulk CTLs in response to DCs loaded with liposome-encapsulated IMP-3-LP3 was counted by ELISPOT assay. Stimulation with SP-pulsed DCs was used as a positive control; unpulsed DCs, DCs pulsed with liposomes alone, and DCs pulsed with IMP3-LP2 encapsulated in liposomes served as negative controls. The in vivo cross-priming assay was done as described previously.Citation55-58
Immunohistochemical examination
Immunohistochemical staining for IMP-3 using a mouse anti-human IMP-3 mAb (DAKO, M3626) was conducted as des-cribed previously.Citation2,12 Normal human placental tissue (Abcam, ab4360) that is known to express IMP-3Citation60 and normal oral tissue was used as a positive and negative control, respectively. Isotype-matched mAb (DAKO, X0943) was used as a control for IMP-3 mAb.
Statistical analysis
Statistical analyses were performed using Prism 4.0 software (GraphPad). Multiple group comparisons were performed by one-way analysis of variance (ANOVA) followed by Tukey's post hoc tests. In some cases, data were also analyzed using an unpaired Student's t-test when comparing two experimental groups. p values less than 0.05 were considered significant. The OS was measured from the date treatment started to the date of death or last follow-up. The progression-free survival (PFS) was measured from the date treatment started to the date of documented progression or death. Patients who were alive and not known to have progressed were censored. The OS and PFS were analyzed according to the Kaplan–Meier method, and statistical differences were assessed using the log-rank test.
Disclosure of potential conflicts of interest
Yasuharu Nishimura is supported by funding from OncoTherapy Science, Inc. Koji Yoshida was an employee of OncoTherapy Science, Inc. and is a current employee of AstraZeneca K.K. Takuya Tsunoda was an employee of OncoTherapy Science, Inc. and is a current employee of Merck-Living Innovation.
KONI_A_1123368_supplementary_material.zip
Download Zip (2.6 MB)Acknowledgments
We thank Yusuke Nakamura of Tokyo University for his important contributions to the clinical trial and Tokunori Ikeda of Kumamoto University for helpful technical advices. We also thank Wataru Kumamaru and Mitsuho Onimaru of Kyusyu University for their valuable cooperation in our experiments.
Funding
Grant Support: this research was supported by MEXT Grant-in-Aid for Scientific Research on Innovative Areas, Grant Number 22133005; JSPS KAKENHI, Grant Number 23650609, 24300334 and 15H04311; a research grant from the Princess Takamatsu Cancer Research Fund, No. 10-24215; funding from OncoTherapy Science; and in part by the Scholarship of the Graduate School of Medical Sciences, Kumamoto University, Japan.
References
- Nishimura Y, Tomita Y, Yuno A, Yoshitake Y, Shinohara M. Cancer immunotherapy using novel tumor-associated antigenic peptides identified by genome-wide cDNA microarray analyses. Cancer Sci 2015; 106:505-11; PMID:25726868; http://dx.doi.org/10.1111/cas.12650
- Clauditz TS, Wang CJ, Gontarewicz A, Blessmann M, Tennstedt P, Borgmann K, Tribius S, Sauter G, Dalchow C, Knecht R et al. Expression of insulin-like growth factor II mRNA-binding protein 3 in squamous cell carcinomas of the head and neck. J Oral Pathol Med 2013; 42:125-32; PMID:22643116; http://dx.doi.org/10.1111/j.1600-0714.2012.01178.x
- Findeis-Hosey JJ, Yang Q, Spaulding BO, Wang HL, Xu H. IMP3 expression is correlated with histologic grade of lung adenocarcinoma. Hum Pathol 2010; 41:477-84; PMID:20004948; http://dx.doi.org/10.1016/j.humpath.2009.10.004
- Zheng W, Yi X, Fadare O, Liang SX, Martel M, Schwartz PE, Jiang Z. The oncofetal protein IMP3: a novel biomarker for endometrial serous carcinoma. Am J Surg Pathol 2008; 32:304-15; PMID:18223334; http://dx.doi.org/10.1097/PAS.0b013e3181483ff8
- Hammer NA, Hansen T, Byskov AG, Rajpert-De Meyts E, Grondahl ML, Bredkjaer HE, Wewer UM, Christiansen J, Nielsen FC. Expression of IGF-II mRNA-binding proteins (IMPs) in gonads and testicular cancer. Reproduction 2005; 130:203-12; PMID:16049158; http://dx.doi.org/10.1530/rep.1.00664
- Tomita Y, Harao M, Senju S, Imai K, Hirata S, Irie A, Inoue M, Hayashida Y, Yoshimoto K, Shiraishi K et al. Peptides derived from human insulin-like growth factor-II mRNA binding protein 3 can induce human leukocyte antigen-A2-restricted cytotoxic T lymphocytes reactive to cancer cells. Cancer Sci 2011; 102:71-8; PMID:21087352; http://dx.doi.org/10.1111/j.1349-7006.2010.01780.x
- Mizukami Y, Kono K, Daigo Y, Takano A, Tsunoda T, Kawaguchi Y, Nakamura Y, Fujii H. Detection of novel cancer-testis antigen-specific T-cell responses in TIL, regional lymph nodes, and PBL in patients with esophageal squamous cell carcinoma. Cancer Sci 2008; 99:1448-54; PMID:18452554; http://dx.doi.org/10.1111/j.1349-7006.2008.00-844.x
- Liao B, Hu Y, Herrick DJ, Brewer G. The RNA-binding protein IMP-3 is a translational activator of insulin-like growth factor II leader-3 mRNA during proliferation of human K562 leukemia cells. J Biol Chem 2005; 280:18517-24; PMID:15753088; http://dx.doi.org/10.1074/jbc.M500270200
- Vikesaa J, Hansen TV, Jonson L, Borup R, Wewer UM, Christiansen J, Nielsen FC. RNA-binding IMPs promote cell adhesion and invadopodia formation. EMBO J 2006; 25:1456-68; PMID:16541107; http://dx.doi.org/10.1038/sj.emboj.7601039
- Sitnikova L, Mendese G, Liu Q, Woda BA, Lu D, Dresser K, Mohanty S, Rock KL, Jiang Z. IMP3 predicts aggressive superficial urothelial carcinoma of the bladder. Clin Cancer Res 2008; 14:1701-6; PMID:18347170; http://dx.doi.org/10.1158/1078-0432.CCR-07-2039
- Schaeffer DF, Owen DR, Lim HJ, Buczkowski AK, Chung SW, Scudamore CH, Huntsman DG, Ng SS, Owen DA. Insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3) overexpression in pancreatic ductal adenocarcinoma correlates with poor survival. BMC Cancer 2010; 10:59; PMID:20178612; http://dx.doi.org/10.1186/1471-2407-10-59
- Li S, Cha J, Kim J, Kim KY, Kim HJ, Nam W, Cha IH. Insulin-like growth factor II mRNA-binding protein 3: a novel prognostic biomarker for oral squamous cell carcinoma. Head Neck 2011; 33:368-74; PMID:20652886; http://dx.doi.org/10.1002/hed.21609
- Suda T, Tsunoda T, Daigo Y, Nakamura Y, Tahara H. Identification of human leukocyte antigen-A24-restricted epitope peptides derived from gene products upregulated in lung and esophageal cancers as novel targets for immunotherapy. Cancer Sci 2007; 98:1803-8; PMID:17784873; http://dx.doi.org/10.1111/j.1349-7006.2007.00603.x
- Kono K, Mizukami Y, Daigo Y, Takano A, Masuda K, Yoshida K, Tsunoda T, Kawaguchi Y, Nakamura Y, Fujii H. Vaccination with multiple peptides derived from novel cancer-testis antigens can induce specific T-cell responses and clinical responses in advanced esophageal cancer. Cancer Sci 2009; 100:1502-9; PMID:19459850; http://dx.doi.org/10.1111/j.1349-7006.2009.01200.x
- Kono K, Iinuma H, Akutsu Y, Tanaka H, Hayashi N, Uchikado Y, Noguchi T, Fujii H, Okinaka K, Fukushima R et al. Multicenter, phase II clinical trial of cancer vaccination for advanced esophageal cancer with three peptides derived from novel cancer-testis antigens. J Transl Med 2012; 10:141; PMID:22776426; http://dx.doi.org/10.1186/1479-5876-10-141
- Yoshitake Y, Fukuma D, Yuno A, Hirayama M, Nakayama H, Tanaka T, Nagata M, Takamune Y, Kawahara K, Nakagawa Y et al. Phase II clinical trial of multiple peptide vaccination for advanced head and neck cancer patients revealed induction of immune responses and improved OS. Clin Cancer Res 2015; 21:312-21; PMID:25391695; http://dx.doi.org/10.1158/1078-0432.CCR-14-0202
- Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer 2008; 8:351-60; PMID:18418403; http://dx.doi.org/10.1038/nrc2373
- Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, van der Burg SH, Offringa R. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur J Immunol 2008; 38:1033-42; PMID:18350546; http://dx.doi.org/10.1002/eji.200737995
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 2003; 421:852-6; PMID:12594515; http://dx.doi.org/10.1038/nature01441
- Kennedy R, Celis E. T helper lymphocytes rescue CTL from activation-induced cell death. J Immunol 2006; 177:2862-72; PMID:16920921; http://dx.doi.org/10.4049/jimmunol.177.5.2862
- Chamoto K, Tsuji T, Funamoto H, Kosaka A, Matsuzaki J, Sato T, Abe H, Fujio K, Yamamoto K, Kitamura T et al. Potentiation of tumor eradication by adoptive immunotherapy with T-cell receptor gene-transduced T-helper type 1 cells. Cancer Res 2004; 64:386-90; PMID:14729649; http://dx.doi.org/10.1158/0008-5472.CAN-03-2596
- Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013; 494:361-5; PMID:23376950; http://dx.doi.org/10.1038/nature11824
- Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res 2010; 70:8368-77; PMID:20940398; http://dx.doi.org/10.1158/0008-5472.CAN-10-1322
- Teramoto K, Kontani K, Fujita T, Ozaki Y, Sawai S, Tezuka N, Fujino S, Itoh Y, Taguchi O, Kannagi R et al. Successful tumor eradication was achieved by collaboration of augmented cytotoxic activity and anti-angiogenic effects following therapeutic vaccines containing helper-activating analog-loaded dendritic cells and tumor antigen DNA. Cancer Immunol Immunother 2007; 56:331-42; PMID:16896967; http://dx.doi.org/10.1007/s00262-006-0192-0
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010; 207:637-50; PMID:20156971; http://dx.doi.org/10.1084/jem.20091918
- Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4+ T cells differentiated in vivo eradicate established melanoma. J Exp Med 2010; 207:651-67; PMID:20156973; http://dx.doi.org/10.1084/jem.20091921
- Hoyer S, Prommersberger S, Pfeiffer IA, Schuler-Thurner B, Schuler G, Dorrie J, Schaft N. Concurrent interaction of DCs with CD4+ and CD8+ T cells improves secondary CTL expansion: It takes three to tango. Eur J Immunol 2014; 44:3543-59; PMID:25211552; http://dx.doi.org/10.1002/eji.201444477
- Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol 2008; 4:e1000048; PMID:18389056; http://dx.doi.org/10.1371/journal.pcbi.1000048
- Wang P, Sidney J, Kim Y, Sette A, Lund O, Nielsen M, Peters B. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinformatics 2010; 11:568; PMID:21092157; http://dx.doi.org/10.1186/1471-2105-11-568
- Yuba E, Kono Y, Harada A, Yokoyama S, Arai M, Kubo K, Kono K. The application of pH-sensitive polymer-lipids to antigen delivery for cancer immunotherapy. Biomaterials 2013; 34:5711-21; PMID:23639528; http://dx.doi.org/10.1016/j.biomaterials.2013.04.007
- Haruta M, Tomita Y, Imamura Y, Matsumura K, Ikeda T, Takamatsu K, Nishimura Y, Senju S. Generation of a large number of functional dendritic cells from human monocytes expanded by forced expression of cMYC plus BMI1. Hum Immunol 2013; 74:1400-8; PMID:23811433; http://dx.doi.org/10.1016/j.humimm.2013.05.017
- Bevan MJ. Helping the CD8+ T-cell response. Nat Rev Immunol 2004; 4:595-602; PMID:15286726; http://dx.doi.org/10.1038/nri1413
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 1998; 393:480-3; PMID:9624005; http://dx.doi.org/10.1038/31002
- Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, Schreibelt G, Mus R, De Wilt JH, Haanen JB et al. Targeting CD4+ T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res 2013; 73:19-29; PMID:23087058; http://dx.doi.org/10.1158/0008-5472.CAN-12-1127
- Disis ML, Wallace DR, Gooley TA, Dang Y, Slota M, Lu H, Coveler AL, Childs JS, Higgins DM, Fintak PA et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol 2009; 27:4685-92; PMID:19720923; http://dx.doi.org/10.1200/JCO.2008.20.6789
- Nelson D, Bundell C, Robinson B. In vivo cross-presentation of a soluble protein antigen: kinetics, distribution, and generation of effector CTL recognizing dominant and subdominant epitopes. J Immunol 2000; 165:6123-32; PMID:11086045; http://dx.doi.org/10.4049/jimmunol.165.11.6123
- Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med 1995; 182:1579-84; PMID:7595228; http://dx.doi.org/10.1084/jem.182.5.1579
- Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med 1995; 182:1591-6; PMID:7595230; http://dx.doi.org/10.1084/jem.182.5.1591
- Yamane H, Zhu J, Paul WE. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. J Exp Med 2005; 202:793-804; PMID:16172258; http://dx.doi.org/10.1084/jem.20051304
- van Panhuys N, Klauschen F, Germain RN. T-cell-receptor-dependent signal intensity dominantly controls CD4+ T cell polarization In Vivo. Immunity 2014; 41:63-74; PMID:24981853; http://dx.doi.org/10.1016/j.immuni.2014.06.003
- Tatsumi T, Kierstead LS, Ranieri E, Gesualdo L, Schena FP, Finke JH, Bukowski RM, Mueller-Berghaus J, Kirkwood JM, Kwok WW et al. Disease-associated Bias in T Helper Type 1 (Th1)/Th2 CD4+ T Cell Responses Against MAGE-6 in HLA-DRB10401+ Patients With Renal Cell Carcinoma or Melanoma. J Exp Med 2002; 196:619-28; PMID:12208877; http://dx.doi.org/10.1084/jem.20012142
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942-9; PMID:15322536; http://dx.doi.org/10.1038/nm1093
- Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res 2007; 13:6301-11; PMID:17975141; http://dx.doi.org/10.1158/1078-0432.CCR-07-1403
- Francois V, Ottaviani S, Renkvist N, Stockis J, Schuler G, Thielemans K, Colau D, Marchand M, Boon T, Lucas S et al. The CD4+ T-cell response of melanoma patients to a MAGE-A3 peptide vaccine involves potential regulatory T cells. Cancer Res 2009; 69:4335-45; PMID:19435913; http://dx.doi.org/10.1158/0008-5472.CAN-08-3726
- Kobayashi H, Song Y, Hoon DS, Appella E, Celis E. Tumor-reactive T helper lymphocytes recognize a promiscuous MAGE-A3 epitope presented by various major histocompatibility complex class II alleles. Cancer Res 2001; 61:4773-8; PMID:11406551
- Zarour HM, Maillere B, Brusic V, Coval K, Williams E, Pouvelle-Moratille S, Castelli F, Land S, Bennouna J, Logan T et al. NY-ESO-1 119-143 is a promiscuous major histocompatibility complex class II T-helper epitope recognized by Th1- and Th2-type tumor-reactive CD4+ T cells. Cancer Res 2002; 62:213-8; PMID:11782380
- Grabowska AK, Kaufmann AM, Riemer AB. Identification of promiscuous HPV16-derived T helper cell epitopes for therapeutic HPV vaccine design. Int J Cancer 2015; 136:212-24; PMID:24824905; http://dx.doi.org/10.1002/ijc.28968
- Nakajima FNJ, Yokota T. Analysis of HLA haplotypes in Japanese, using high resolution allele typing. MHC 2001; 8:1-32
- Inderberg-Suso EM, Trachsel S, Lislerud K, Rasmussen AM, Gaudernack G. Widespread CD4+ T-cell reactivity to novel hTERT epitopes following vaccination of cancer patients with a single hTERT peptide GV1001. Oncoimmunology 2012; 1:670-86; PMID:22934259; http://dx.doi.org/10.4161/onci.20426
- Corbiere V, Chapiro J, Stroobant V, Ma W, Lurquin C, Lethe B, van Baren N, Van den Eynde BJ, Boon T, Coulie PG. Antigen spreading contributes to MAGE vaccination-induced regression of melanoma metastases. Cancer Res 2011; 71:1253-62; PMID:21216894; http://dx.doi.org/10.1158/0008-5472.CAN-10-2693
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:22437870; http://dx.doi.org/10.1038/nrc3239
- Sawada Y, Yoshikawa T, Shimomura M, Iwama T, Endo I, Nakatsura T. Programmed death-1 blockade enhances the antitumor effects of peptide vaccine-induced peptide-specific cytotoxic T lymphocytes. Int J Oncol 2015; 46:28-36; PMID:25354479; http://dx.doi.org/10.3892/ijo.2014.2737
- Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res 2013; 73:3591-603; PMID:23633484; http://dx.doi.org/10.1158/0008-5472.CAN-12-4100
- Harao M, Hirata S, Irie A, Senju S, Nakatsura T, Komori H, Ikuta Y, Yokomine K, Imai K, Inoue M et al. HLA-A2-restricted CTL epitopes of a novel lung cancer-associated cancer testis antigen, cell division cycle associated 1, can induce tumor-reactive CTL. Int J Cancer 2008; 123:2616-25; PMID:18770861; http://dx.doi.org/10.1002/ijc.23823
- Tomita Y, Yuno A, Tsukamoto H, Senju S, Kuroda Y, Hirayama M, Irie A, Kawahara K, Yatsuda J, Hamada A et al. Identification of promiscuous KIF20A long peptides bearing both CD4+ and CD8+ T-cell epitopes: KIF20A-specific CD4+ T-cell immunity in patients with malignant tumor. Clin Cancer Res 2013; 19:4508-20; PMID:23714729; http://dx.doi.org/10.1158/1078-0432.CCR-13-0197
- Tomita Y, Yuno A, Tsukamoto H, Senju S, Yoshimura S, Osawa R, Kuroda Y, Hirayama M, Irie A, Hamada A et al. Identification of CDCA1-derived long peptides bearing both CD4+ and CD8+ T-cell epitopes: CDCA1-specific CD4+ T-cell immunity in cancer patients. Int J Cancer 2014; 134:352-66; PMID:24734272; http://dx.doi.org/10.1002/ijc.28376
- Tomita Y, Yuno A, Tsukamoto H, Senju S, Kuroda Y, Hirayama M, Imamura Y, Yatsuda J, Sayem MA, Irie A et al. Identification of immunogenic LY6K long peptide encompassing both CD4+ and CD8+ T-cell epitopes and eliciting CD4+ T-cell immunity in patients with malignant disease. Oncoimmunology 2014; 3:e28100; PMID:25340007; http://dx.doi.org/10.4161/onci.28100
- Sayem MA, Tomita Y, Yuno A, Hirayama M, Irie A, Tsukamoto H, Senju S, Yuba E, Yoshikawa T, Kono K et al. Identification of glypican-3-derived long peptides activating both CD8+ and CD4+ T-cells; prolonged overall survival in cancer patients with Th cell response. OncoImmunology 2016; 5:e1062209; PMID:26942076; http://dx.doi.org/10.1080/2162402X.2015.1062209
- Tabata H, Kanai T, Yoshizumi H, Nishiyama S, Fujimoto S, Matsuda I, Yasukawa M, Matsushita S, Nishimura Y. Characterization of self-glutamic acid decarboxylase 65-reactive CD4+ T-cell clones established from Japanese patients with insulin-dependent diabetes mellitus. Hum Immunol 1998; 59:549-60; PMID:9757911; http://dx.doi.org/10.1016/S0198-8859(98)00050-0
- Radfar F, Achak F, Rajaei F. The relationship between IMP3 expression in colorectal adenocarcinoma and clinicopathologic findings. Biotech Health Sci 2015; 2:e27414; http://dx.doi.org/10.17795/bhs27414
