ABSTRACT
Immune-related adverse events (irAEs) induced by checkpoint inhibitors are well known. Since fatal outcomes have been reported early detection and adequate management are crucial. In particular, colitis is frequently observed and can result in intestinal perforation. This is the first report of an autoimmune colitis that was treated according to algorithms but became resistant due to a CMV reactivation. The 32-y-old male patient with metastatic melanoma treated within an anti-PD-1/ipilimumab combination study developed severe immune-mediated colitis (CTCAE grade 3) with up to 18 watery stools per day starting 2 weeks after treatment initiation. After improving upon therapy with immunosuppressive treatment (high dose steroids and infliximab) combined with parenteral nutrition diarrhea again exacerbated. Additionally, the patient had asymptomatic grade 3 CTCAE amylase and lipase elevation. Colitis was monitored by weekly endoscopies and colon biopsies were analyzed histologically with CMV staining, multi-epitope ligand cartography (MELC) and qRT-PCR for inflammatory genes. In the course, CMV reactivation was detected in the colon and treated with antiviral medication in parallel to a reduction of corticosteroids. Subsequently, symptoms improved. The patient showed a complete response for 2 y now including regression of bone metastases. CMV reactivation under checkpoint inhibitor therapy in combination with immunosuppressive treatment for autoimmune side effects has to be considered in these patients and if present treated. Potentially, CMV reactivation is underdiagnosed. Treatment algorithms should include CMV diagnostics.
Abbreviations
| APC | = | antigen-presenting cell |
| CMV | = | cytomegalovirus |
| CTLA-4 | = | cytotoxic T-lymphocyte antigen 4 |
| DC | = | dendritic cell |
| IrAE | = | immune-related adverse events |
| IgG | = | immunoglobulins |
| MELC | = | multi-epitope ligand cartography |
| PD-1 | = | Programmed cell death protein 1. |
Introduction
Ipilimumab, approved for treatment of unresectable metastatic melanoma, binds to CTLA-4 on conventional and regulatory T cells results and leads to an antitumor response via enhanced T cell activation, proliferation and infiltration into the tumor microenvironment.Citation1 It has shown improved overall survival in melanoma patients.Citation2,3 The most common side effects are irAEsCitation4 with colitis in 30% of the patients being the most common side effect.Citation2 Autoimmune colitis can be severe with reported cases of colectomy Citation5-7 and perforations subsequently leading to death.Citation8 A multicenter study documented rare side effects in a large patient cohort documented a high response rate in these patients, even if they had received fewer infusions than recommended.Citation9 Treatment algorithms for managing irAEs recommend symptomatic treatment, therapy with steroids and in more severe cases therapy with infliximab.Citation10 In several publications, a correlation between irAEs and clinical efficacy of the drug has been considered, including a severity-response-effectCitation11-13 however study data did not show this.
More recently, anti-PD-1 antibodies pembrolizumab and nivolumab, that block negative signaling from tumor or stroma cells to T lymphocytes via PD-L1, result in potent antitumor immune responses in different tumor entities including melanoma.Citation14 In a phase 1 trial, safety and efficacy of concurrent or sequenced regimens of ipilimumab and nivolumab were assessed in advanced melanoma.Citation15 Grade 3 and 4 irAEs occurred in 53% of concurrent and in 18% of sequentially treated subjects. The most common treatment related grade 3 or 4 event in all groups was elevation of lipase levels, whereas colitis occurred in 9% of concurrent regimen patients. A phase 2 randomized, double blind study and a phase 3 study with combined ipilimumab/nivolumab treatment of patients with advanced melanoma showed grade 3 or 4 diarrhea in 19%, 7%, and 29% for ipilimumab, nivolumab, and the combination treatment, respectively. Grade 3 or 4 colitis was induced in 27%, 2%, and 24%, in patients treated with ipilimumab, nivolumab, and the combination, respectively.Citation16 To our knowledge, no reports of CMV colitis after checkpoint inhibitor therapy exist in the literature or the companies' data bases (MSD, BMS medical services).
Here, we present a patient with metastatic melanoma who developed severe colitis, therapy-refractory to conventional treatment options under checkpoint inhibitor therapy within an anti PD-1/ipilimumab trial. Reactivation of CMV occurred and further exacerbated symptoms.
Case report
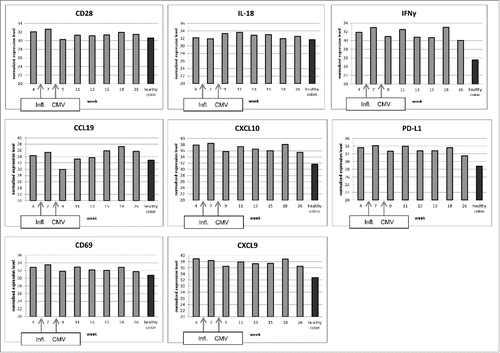
A 32-y-old, male patient with metastatic melanoma stage IV, M1c, of unknown primary tumor presented with a submandibular lymph node metastasis that was excised. Staging revealed osseous metastases in thoracic und lumbar vertebrae and the os ilium. He was included in an ipilimumab/nivolumab combination trial (CA209067; NCT01927419). After the third infusion in week 3 () he was hospitalized because of grade 3 CTCAE diarrhea with up to seven stools per day and a reduced general state of health, vomiting and pyrexia. After infectious work-up was negative including norovirus, Clostridium difficile, salmonella, shigella, yersinia, and campylobacter, he was diagnosed with immune-related colitis and treated with prednisolone (2 mg/kg body weight per day) which did not improve symptoms. Diarrhea frequency was up to 14 stools per day. Colonoscopy showed significant signs of inflammation in the colon and the sigmoid with thickening of the enteric walls and hypervascularization (). Histology revealed a florid colitis with mucosal destruction, hemorrhages, ulcerations, crypt abscesses and impairment of architecture (). Thus, infliximab (5 mg/kg body weight i.v.) was administered in accordance with current algorithmsCitation17,18 which quickly reduced diarrhea frequency to nine stools per day. In addition, grade 3 elevation of lipase (715 U/L; ULN < 60 U/L) and α amylase (253 U/L; ULN < 110 U/L) occurred without any clinical signs of pancreatitis. He was dismissed with 80 mg prednisone daily but relapsed with grade 3 diarrhea upon slowly tapering the steroid and did not respond when steroids were increased again to 2 mg/kg. Thus, in week 7 after start of immunotherapy, the patient was hospitalized again with persistent hemorrhagic watery stools 10–12 times a day and weakness. Endoscopy showed microhemorrhagic colitis with inflammation in rectum and sigmoid, without signs of CMV colitis () and serology showed CMV IgG. Stool cultures again excluded an infectious origin (C. difficile, salmonella, shigella, yersinia, and campylobacter) and the patient was retreated with infliximab however this time without improvement. Radiologic exams because of sustained cough showed no signs for pneumonia, pneumonitis and the pancreatic enzymes declined. The state of health of the patient was stagnant, so infliximab treatment was repeated. Additionally, prophylactic antibiotics (metronidazole, ciprofloxacin) were administered to remove potential antigenic stimuli of bacteria and parenteral nutrition was initiated. A follow-up sigmoidoscopy due to higher frequency of bowel movements up to 18 times a day showed a clear deterioration with crypt abscesses, erosions and hemorrhagic lesions, whereas a haustration of colon and sigmoid was still present on the abdominal CT. Surgery with ileostomy was considered. Since the abdomen remained soft on palpation and radiologic imaging excluded a perforation no surgical intervention was performed despite persisting watery, but less hemorrhagic stools. After interdisciplinary consultation with gastroenterologists, surgeons and dermatologists a fourth dose of infliximab was administered. In the further course, testing for CMV DNA in EDTA blood by real-time PCR was positive with 140,000 copies of CMV DNA per mL, and immunohistochemistry showed positive staining for CMV now indicating CMV colitis (; week 9). Furthermore, colon biopsies in week 9 were positive for CMV DNA with 20,000 copies per 100,000 cells, whereas in the biopsies taken 2 and 5 weeks earlier (week 4 and 7) no CMV-DNA could be detected (detection limit approximately 100 copies/100,000 cells). Hence, ganciclovir 5 mg/kg body weight twice daily i.v. was started and upon dismissal switched to valganciclovir 900 mg twice daily p.o. and symptoms improved. Due to low total IgG values, immunoglobulins 0.5 mg/kg body weight were administered once daily on three consecutive days intravenously. Pre-therapy IgG had been normal (1360 mg/dL; normal range 751–1560 mg/dL). Staging carried out in week 11 revealed a complete response with sclerosing of the osseous metastases, no additional metastases and a non-measurable target lesion. In week 13, the patient presented again with persisting watery and hemorrhagic stools and was retreated with immunoglobulins. Despite antiviral therapy CMV staining in the colon biopsy was still positive in weeks 11, 13 and 15, so antiviral treatment was escalated to the i.v. route again. Therapy with prednisolone was continued at 55 mg daily p.o. and endoscopies with CMV staining in biopsy specimen, gene expression and MELC analysesCitation19 were performed three times in the following 2 months. The number of CMV-positive cells in the colon biopsies declined continuously under therapy with ganciclovir but persisted. Corresponding results of expression of selected immunologically relevant genes are shown (). Especially CD28, a co-stimulatory molecule on the surface of naive T cells, and CCL19, a chemokine that attracts dendritic cells (DCs) and activated B cells, decreased significantly at the time of reactivation of CMV. In the following weeks, both markers rose in parallel to the reduction of CMV in the colon. A difference between the first two time points without CMV and the following ones can be seen with respect to expression of CD69, IL-18 and IFNγ, CXCL9 and CXCL10, and of PD-L1. This could possibly imply the involvement of these immune-mediators in CMV reactivation in our patient. However, these results have to be viewed in light of multiple interfering mechanisms (immune checkpoint blockade, melanoma, immune-suppressive drugs). In comparison to a healthy colon, activation markers were expressed at much higher concentrations at almost all time points. By MELC, an increase in the number of immune cells and a shift in the allocation within the tissue could be shown (). Natural killer cells (CD56+) and CTLA-4 positive cells increased over time, and B cells (CD20+), CD8+ T cells, memory T cells (CD45RO+) and CD45+ T cells additionally formed ectopic follicles (i.e., tertiary lymphoid structures). The analyses were performed in two independent MELC experiments and healthy colon was stained for comparison. Since colitis persisted systemic antiviral therapy with foscarnet 90 mg/kg body weight twice daily i.v. was started in week 16 due to suspected resistance against ganciclovir, which was however subsequently not confirmed, so that ganciclovir 5 mg/kg body weight twice daily i.v. could be reinitiated. Reduction of prednisolone to 12.5 mg at dismissal did not worsen the frequency of bowel movements. Histopathological analysis showed persisting but declining signs of inflammation and CMV positivity until the last assessment in week 26, and endoscopy pointed to an improvement of edema, hemorrhages and ulcerations in the colon. Upon discontinuation of ganciclovir stool frequency increased again up to 12 times daily. Treatment with loperamid was not effective at any point. A final colonoscopy in week 33 still indicated a discontinued ulcerative and hemorrhagic colitis, yet watery and hemorrhagic stools persisted to some extent, so that administration of high-dose mesalazine in combination with the steroid was started. A year later, the patient is now doing better with only three to four bowel movements per day and without signs of tumor recurrence.
Figure 1. Clinical course of the patient. Dark gray bars mark the symptoms the patient developed with the frequency of bowel movements represented by corresponding columns, whereas white boxes indicate the applied therapies over time. In weeks labeled with x, endoscopy has been performed, index 1 indicating sigmoidoscopy and index 2 indicating colonoscopy.
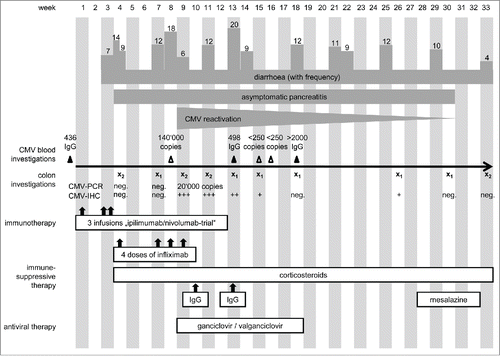
Figure 2. (A) High-definition endoscopy. Pictures 1–4 of weeks 7, 11, 13, and 15 describe both in high definition white-light (3, 4) and in virtual chromoendoscopy (1, 2) an erythematous, granulomatous and oedematous mucosal inflammation of the rectosigmoid colon with a high mucosal vulnerability (spontaneous diffuse mucosal bleeding). Images 2 and 4 show a particular grainy white pattern suggesting an overlapped infectious origin confirmed by immunohistochemistry and microbiology as a CMV colitis which superposed and complicated the initial autoimmune T cell driven ipilimumab colitis. As a comparison, picture 5 taken 3 mo after week 33 shows an almost complete mucosal restitution with reminiscent erythema, hypervascularity and healed scars in the colon. (B) Immunohistochemistry of colon biopsies with CMV staining. [A]: pre-CMV initial biopsy showed prominent plasma cell-rich inflammatory infiltrates in the lamina propria and notable crypt abscess formation. [B]: CMV-stage: the crypt epithelium showed regenerative changes with large intranuclear inclusions, the lamina propria contained mixed infiltrates with prominent dilated capillaries. Inset: CMV was strongly positive in four nuclei in this field (arrows). [C]: post-CMV biopsy showed still prominent mononuclear cell infiltration but no neutrophilic crypt abscesses, note follicular hyperplasia. (C) Multi-epitope ligand cartography (MELC) of cryo-conserved colon tissue. Samples taken at two different time points and a control sample from healthy colon have been stained for different antibodies using the MELC-technique.Citation19 Natural killer cells (CD56+) and CTLA-4 positive cells increased over time, and B cells (CD20+), CD8+ T-cells, memory T cells (CD45RO+) and CD45+ T cells additionally formed follicles. Depicted is an overlay (20X magnification) of NK cells (red), CD8+ T cells (green) and B cells (blue).
![Figure 2. (A) High-definition endoscopy. Pictures 1–4 of weeks 7, 11, 13, and 15 describe both in high definition white-light (3, 4) and in virtual chromoendoscopy (1, 2) an erythematous, granulomatous and oedematous mucosal inflammation of the rectosigmoid colon with a high mucosal vulnerability (spontaneous diffuse mucosal bleeding). Images 2 and 4 show a particular grainy white pattern suggesting an overlapped infectious origin confirmed by immunohistochemistry and microbiology as a CMV colitis which superposed and complicated the initial autoimmune T cell driven ipilimumab colitis. As a comparison, picture 5 taken 3 mo after week 33 shows an almost complete mucosal restitution with reminiscent erythema, hypervascularity and healed scars in the colon. (B) Immunohistochemistry of colon biopsies with CMV staining. [A]: pre-CMV initial biopsy showed prominent plasma cell-rich inflammatory infiltrates in the lamina propria and notable crypt abscess formation. [B]: CMV-stage: the crypt epithelium showed regenerative changes with large intranuclear inclusions, the lamina propria contained mixed infiltrates with prominent dilated capillaries. Inset: CMV was strongly positive in four nuclei in this field (arrows). [C]: post-CMV biopsy showed still prominent mononuclear cell infiltration but no neutrophilic crypt abscesses, note follicular hyperplasia. (C) Multi-epitope ligand cartography (MELC) of cryo-conserved colon tissue. Samples taken at two different time points and a control sample from healthy colon have been stained for different antibodies using the MELC-technique.Citation19 Natural killer cells (CD56+) and CTLA-4 positive cells increased over time, and B cells (CD20+), CD8+ T-cells, memory T cells (CD45RO+) and CD45+ T cells additionally formed follicles. Depicted is an overlay (20X magnification) of NK cells (red), CD8+ T cells (green) and B cells (blue).](/cms/asset/bfcd2039-d918-41ab-8182-7e4c7ec1d6eb/koni_a_1128611_f0002_oc.gif)
Figure 3. Semiquantitative gene expression in colon tissue. Probes have been taken at indicated time points. The RT-PCR results have been normalized on RPL37a. All of the selected cytokines, chemokines and surface molecules play an important role in the regulation of immune reactions. Arrows indicate the time points of infliximab administration and of CMV reactivation. As control, we used healthy colon mRNA from other individuals. The depicted values for healthy colon are mean values of five different samples (mean standard deviation 0.61).
Discussion
We present here the first case of an autoimmune colitis under checkpoint therapy which improved in response to treatment according to current algorithms but became resistant because a CMV reactivation occurred in the colon. Such a complication might be more often recognized if CMV diagnostics would be included in the current algorithms.
Colitis is a well-known side effect of ipilimumab and perforations, even fatal ones have been described.Citation5-8 About 5–20% of grade 3 or 4 colitis and several cases in which a colectomy had to be performed due to the severity are reported in the literature.Citation2,6-9 The onset of ipilimumab-induced colitis is usually from week 5 onwards after the first infusion.Citation17 In anti-PD1 therapy with nivolumab or pembrolizumab, frequency and severity of colitis seems to be lower.Citation33 For pembrolizumab, the frequency of severe diarrhea was only 1%.Citation20 Nivolumab induced grade 3–4 diarrhea in 1% of patients and in 8% for the combination of ipilimumab + nivolumab.Citation16
Histological findings in colitis under immunotherapy show various phenotypes including neutrophil rich enterocolitis-like, autoimmune colitis-like patterns with intraepithelial lymphocytes and increased apoptosis, and even overlapping features.Citation6 Furthermore, chronic pictures with infiltrating lymphocytes and granulomas have been reported.Citation8 Another study observed lymphocytes, plasma cells, eosinophils and neutrophils with crypt microabscesses and occasional apoptotic epithelial cells.Citation21 The presented case initially featured pathohistological findings like plasma cell-rich infiltrates in the tunica propria, granulocyte infiltration, apoptotic bodies, regenerating epithelium, crypt abscesses, confluent erosions and hemorrhages, all in accordance with a severe autoimmune colitis. Intestinal follicular hyperplasia as seen in this patient has been described in diversion colitis patientsCitation22, 23 and in inflammatory bowel diseases (IBD).Citation24,25 In intestinal viral infections, no follicular hyperplasia in the gut has been reported. Therefore, the pseudofollicles featured in this case most likely correspond to the autoimmune colitis induced by checkpoint inhibitor therapy yet further fueled by CMV reactivation, resembling IBD.
In perforating colitis under immunotherapy, a contribution of C. difficile has been suggested in previous reports, but this factor was excluded in our patient.Citation6 Recently, it has been published that in mice commensal bacteria play a significant role in the antitumor response following different immune-modulating therapies.Citation26, 27 Here, damage to the microbiota in the gut was suggested to counteract antitumor effects mediated by the modulation of myeloid-derived cell functions in the tumor microenvironment. These findings depict a supporting role for microorganisms in immunotherapy. Reactivated viruses, on the other hand, may have a negative impact on the course of disease. In the presented case, reactivation of CMV may have occurred due to immunosuppression, which was necessary to treat the autoimmune colitis induced by checkpoint inhibitor therapy or it might have been triggered by the immune activation resulting from checkpoint blockade. Hepatitis reactivation has been described in patients under treatment with ipilimumab (personal communication). Nevertheless, ipilimumab treatment of HCV infected patients has been considered safe so far.Citation28 In a patient with malignant melanoma, even suppression of HCV has been described under therapy. Minter et al. reported on a case with a decrease of the viral load under ipilimumab from initially almost 400,000 IU/mL to 12 IU/mL in the blood shortly after therapy.Citation29 In contrast to these human findings, a study in simian immunodeficiency virus (SIV)-infected anti-CTLA-4 antibody treated macaques revealed increased T cell activation in rectal mucosa, which resulted in higher loss of CD4+ T cells and correlated with enhanced viral replication in the mucosa.Citation30 In human immunodeficiency virus (HIV), it seems that the immune modulating therapy with ipilimumab counteracts viral replication.Citation31 CMV reactivation has so far been described after therapy with ipilimumab only in a single case report published by our group that showed autoimmune colitis and simultaneous CMV hepatitis but not colitis.Citation32 It is however well known that prolonged immunosuppression leads to CMV reactivation. Despite these facts, so far CMV diagnostic is not included into the treatment algorithms for any of the checkpoint inhibitors. This case illustrates for the first time that CMV reactivation can further complicate autoimmune colitis under checkpoint inhibitor therapy, and must be treated intensely to avoid a fatal outcome. Early diagnosis is obviously critical and a CMV diagnostic workup should in our view be incorporated into the current algorithms.
Conclusion
This case of persistent and anti-TNF-refractory colitis was induced by (i) checkpoint inhibitor therapy and (ii) reactivation of CMV. In all irAEs that are treated with immunosuppression infections including reactivations of viral infections have to be considered and when detected treated while—if possible—reducing immunosuppression.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We especially thank Waltraud Leisgang and Elisabeth Thurau who performed immunohistochemical stainings and PCR analyses as well as Klaus Korn and Antje Knöll for their cooperation on the virologic diagnostic. We thank David Feltquate, Arvin Yang and his team for continuous support and very constructive input for the management of this patient. Additionally, we thank Stephen Hodi, Jeffrey Weber and Jedd Wolchok for their advice.
Funding
We thank the Verein zur Förderung des Tumorzentrums der Universität Erlangen-Nürnberg e.V. for financial support of the translational research. BMS funded the clinical study.
References
- Salama AK, Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res 2011; 17(14):4622-8; PMID:21467163; http://dx.doi.org/10.1158/1078-0432.CCR-10-2232
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363(8):711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364(26):2517-26; PMID:21639810; http://dx.doi.org/10.1056/NEJMoa1104621
- Hanaizi Z, van Zwieten-Boot B, Calvo G, Lopez AS, van Dartel M, Camarero J, Abadie E, Pignatti F. The European Medicines Agency review of ipilimumab (Yervoy) for the treatment of advanced (unresectable or metastatic) melanoma in adults who have received prior therapy: summary of the scientific assessment of the Committee for Medicinal Products for Human Use. Eur J Cancer 2012; 48(2):237-42; PMID:22030452; http://dx.doi.org/10.1016/j.ejca.2011.09.018
- Berman D, Parker SM, Siegel J, Chasalow SD, Weber J, Galbraith S, Targan SR, Wang HL. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun 2010; 10:11; PMID:21090563
- Mitchell KA, Kluger H, Sznol M, Hartman DJ. Ipilimumab-induced perforating colitis. J Clin Gastroenterol 2013; 47(9):781-5; PMID:23632354; http://dx.doi.org/10.1097/MCG.0b013e31828f1d51
- Slingerland M, Nortier JW, Veenendaal RA, Kapiteijn E. Severe colitis while responding to ipilimumab in metastatic melanoma. Acta Oncol 2012; 51(6):805-7; PMID:22551308; http://dx.doi.org/10.3109/0284186X.2012.682630
- Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 2006; 24(15):2283-9; PMID:16710025; http://dx.doi.org/10.1200/JCO.2005.04.5716
- Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, Bergmann T, Bockmeyer CL, Eigentler T, Fluck M et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One 2013; 8(1): e53745; PMID:23341990; http://dx.doi.org/10.1371/journal.pone.0053745
- Johnston RL, Lutzky J, Chodhry A, Barkin JS. Cytotoxic T-lymphocyte-associated antigen 4 antibody-induced colitis and its management with infliximab. Dig Dis Sci 2009; 54(11):2538-40; PMID:19104936; http://dx.doi.org/10.1007/s10620-008-0641-z
- Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol 2005; 23(25):6043-53; PMID:16087944; http://dx.doi.org/10.1200/JCO.2005.06.205
- Lutzky J, Wolchok J, Hamid O, Lebbe C, Pehamberger H, Linette G, de Pril V, Ibrahim R, Hoos A, O'Dayet S et al. Association between immune-related adverse events (irAEs) and disease control or overall survival in patients (pts) with advanced melanoma treated with 10 mg/kg ipilimumab in three phase II clinical trials. J Clin Oncol 27:15s, 2009 (suppl; abstr 9034); http://meetinglibrary.asco.org/content/34815-65
- Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res 2007; 13(22 Pt 1):6681-8; PMID:17982122; http://dx.doi.org/10.1158/1078-0432.CCR-07-0187
- Hamid O, Carvajal RD. Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Expert Opin Biol Ther 2013; 13(6):847-61; PMID:23421934; http://dx.doi.org/10.1517/14712598.2013.770836
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369(2):122-33; PMID:23724867; http://dx.doi.org/10.1056/NEJMoa1302369
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373:23-34; PMID:26027431; http://dx.doi.org/10.1056/NEJMoa1504030
- Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012; 30(21):2691-7; PMID:22614989; http://dx.doi.org/10.1200/JCO.2012.41.6750
- Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD. Toxicities of the Anti-PD-1 and Anti-PD-L1 Immune Checkpoint Antibodies. Ann Oncol pii: mdv383. [Epub ahead of print] Review 2015; 26(12):2375-91; http://dx.doi.org/10.1093/annonc/mdv383
- Ostalecki C, Konrad A, Thurau E, Schuler G, Croner RS, Pommer AJ, ael Stürzl M. Combined multi-gene analysis at the RNA and protein levels in single FFPE tissue sections. Exp Mol Pathol 2013; 95(1):1-6; PMID:23583336; http://dx.doi.org/10.1016/j.yexmp.2013.03.008
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369(2):134-44; PMID:23724846; http://dx.doi.org/10.1056/NEJMoa1305133
- Lord JD, Hackman RC, Moklebust A, Thompson JA, Higano CS, Chielens D, Steinbach G, McDonald GB. Refractory colitis following anti-CTLA4 antibody therapy: analysis of mucosal FOXP3+ T cells. Dig Dis Sci 2010; 55(5):1396-405; PMID:19507029; http://dx.doi.org/10.1007/s10620-009-0839-8
- Yeong ML, Bethwaite PB, Prasad J, Isbister WH. Lymphoid follicular hyperplasia–a distinctive feature of diversion colitis. Histopathology 1991; 19(1):55-61; PMID:1916687; http://dx.doi.org/10.1111/j.1365-2559.1991.tb00894.x
- Edwards CM, George B, Warren BF. Diversion colitis: new light through old windows. Histopathology 1999; 35(1):86-7; PMID:10383813; http://dx.doi.org/10.1046/j.1365-2559.1999.0728b.x
- Ell SR, Frank PH. Spectrum of lymphoid hyperplasia: colonic manifestations of sarcoidosis, infectious mononucleosis, and Crohn's disease. Gastrointest Radiol 1981; 6(4):329-32; PMID:7308713; http://dx.doi.org/10.1007/BF01890279
- Yeung MM, Melgar S, Baranov V, Oberg A, Danielsson A, Hammarström S, Hammarström ML. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TcR-gammadelta expression. Gut 2000; 47(2):215-27; PMID:10896913; http://dx.doi.org/10.1136/gut.47.2.215
- Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013; 342(6161):967-70; PMID:24264989; http://dx.doi.org/10.1126/science.1240527
- Zitvogel L, Galluzzi L, Viaud S, Vétizou M, Daillère R, Merad M, Kroemer G. Cancer and the gut microbiota: an unexpected link. Sci Transl Med 2015; 7(271):271ps1; PMID:25609166; http://dx.doi.org/10.1126/scitranslmed.3010473
- Yazici O, Sendur MA, Aksoy S. Hepatitis C virus reactivation in cancer patients in the era of targeted therapies. World J Gastroenterol 2014; 20(22):6716-6724; PMID:24944464; http://dx.doi.org/10.3748/wjg.v20.i22.6716
- Minter S, Willner I, Shirai K. Ipilimumab-induced hepatitis C viral suppression. J Clin Oncol 2013; 31(19):e307-8; PMID:23690418; http://dx.doi.org/10.1200/JCO.2012.46.5831
- Cecchinato V, Tryniszewska E, Ma ZM, Vaccari M, Boasso A, Tsai WP, Petrovas C, Fuchs D, Heraud JM, Venzon D et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol 2008; 180(8):5439-47; PMID:18390726; http://dx.doi.org/10.4049/jimmunol.180.8.5439
- Wightman F, Solomon A, Kumar SS, Urriola N, Gallagher K, Hiener B, Palmer S, Mcneil C, Garsia R, Lewin SR. Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. AIDS 2015; 29(4):504-6; PMID:25628259; http://dx.doi.org/10.1097/QAD.0000000000000562
- Uslu U, Agaimy A, Hundorfean G, Harrer T, Schuler G, Heinzerling L. Autoimmune colitis and subsequent CMV-induced hepatitis after treatment with ipilimumab. J Immunother 2015; 38(5):212-5; PMID:25962110; http://dx.doi.org/10.1097/CJI.0000000000000081
- L Hofmann, A Forschner, C Loquai, SM Goldinger, L Zimmer, S Ugurel, MI Schmidgen, R Gutzmer, JS Utikal, D Göppner, JC Hassel, F Meier, J Tietze, I Thomas, C Weishaupt, M Leverkus, R Wahl, U Dietrich, C Garbe, MC Kirchberger, T Eigentler, C Berking, A Gesierich, AM Krackhardt, D Schadendorf, G Schuler, R Dummer, LM Heinzerling. Cutaneous, gastrointestinal, hepatic, endocrine and renal side effects of anti-PD-1 therapy. Eur J Cancer (in press); http://dx.doi.org/10.1016/j.ejca.2016.02.025
