ABSTRACT
We recently reported that therapeutic vaccination with live tumor antigen-producing Salmonella typhimurium rescues dysfunctional endogenous T cell responses and eradicates long-established tumors refractory to αCTLA-4 and αPD-L1 checkpoint inhibitor blockade. Here, we show that live intravenously injected or heat-killed (HK) intratumorally injected Salmonella typhimurium, even when not producing tumor antigen, synergize with adoptive T cell therapy to eradicate tumors. These data demonstrate that the combination of adoptive T cell transfer with the injection of live or dead Salmonella typhimurium is a promising approach for cancer treatment.
Introduction
Despite adoptive T cell therapy demonstrating promise for the treatment of long-established preclinical and human tumors,Citation1-3 many malignancies appear resistant to transferred T cells. This holds true even for metastatic melanoma, recognized as the most immunogenic tumor, whereby complete durable regression is restricted to 20% of patients treated with adoptive transfer of autologous tumor-infiltrating lymphocytes.Citation4 Tumors become resistant to T cell therapy by evading immune recognition and/or suppressing the effector response.Citation5-9
We previously studied the requirements for T cells to reject preclinical tumors and discovered that adoptively transferred T cells eradicate tumors expressing high- but not low-levels of antigen.Citation1,10 High antigen expression by cancer cells is required for tumor stromal cells to cross-present cancer cell antigen.Citation1 Cross-presentation stimulates T cells to secrete TNF-α and IFNγ that are required for destruction of the tumor stroma, which is a requisite step for tumor eradication.Citation11-13 In addition to high antigen expression by cancer cells, we later discovered that cross-presentation also requires tumor peptides to possess high affinity for MHC-I.Citation13 If these two requirements for cross-presentation are not met, T cell therapy is ineffective as tumors relapse with outgrowth of antigen-loss variants.Citation1,13 This is not only evident preclinically, but also applies to clinical melanoma in which responses to adoptive transfer or ipilimumab correlate with tumors expressing mutant peptides that possess high peptide-MHC (p-MHC) affinity.Citation14,15
Although adoptive T cell therapy can cause regression of clinical tumors when targeting mutant peptides with high p-MHC affinity, these tumors may eventually relapse.Citation14 The percentage of tumors that relapse, despite having this favorable T cell-peptide targeting, remains unknown. However, in metastatic melanoma, this likely occurs with high frequency given its 80% relapse rateCitation4 despite often being immunogenic. Relapse in these patients likely occurs due to tumors expressing low levels of antigen that are below the threshold needed for cross-presentation. To overcome this constraint, we hypothesized that loading the tumor stroma with exogenous antigen would sensitize tumors to adoptive T cell therapy. In the present study, we used live attenuated Salmonella typhimurium A1-R bacteriaCitation16 that were engineered to produce tumor antigen for loading stroma. This was based on our previous experience demonstrating that these antigen-expressing bacteria can (i) deliver exogenous tumor peptide into APCs for efficient MHC-I loading and (ii) preferentially replicate in tumors following intravenous injection.Citation17 Here, we show that the combination of adoptive T cell transfer with intravenous administration of antigen-producing bacteria prevents tumor relapse against long-established tumors that express low levels of antigen. Unexpectedly, however, prevention of relapse was even more effective when adoptive T cell transfer was combined with live bacteria not expressing tumor antigen. Since live bacteria may not be tolerated by cancer patients that become neutropenic through lymphodepletive conditioning which is a requirement for many adoptive T cell protocols,Citation18 we also investigated intratumoral heat killed (HK) bacteria combined with T cell transfer and discovered that this approach also synergized with T cells to eradicate tumors.
Results and discussion
Live intravenous bacteria combined with adoptive T cell therapy leads to tumor eradication
We first evaluated whether tumor antigen-producing Salmonella typhimurium A1-R could prevent relapse of low antigen-expressing tumors following T cell therapy. To investigate this question, we used the MC57 fibrosarcoma cell line that expresses a fusion protein consisting of a trimer of the SIINFEKL (SIINF) peptide from chicken ovalbumin protein and EGFP (). SIINF was used as a model tumor-specific peptide due to its inherent high affinity for MHC-I,Citation13 which resembles natural tumor-specific peptidesCitation19 that are being targeted clinically.Citation14,15 Consistent with the high affinity of SIINF for MHC-I, MC57 tumors expressing high levels of SIINF are consistently eradicated by adoptively transferred SIINF-specific OT-1 T cells.Citation13 This effect is dependent on exclusive targeting of the SIINF antigen because MC57-SIINF tumors were established in TCR-transgenic mice (2C) that lack tumor-reactive endogenous T cells.Citation13 Since the natural expression level of mutant tumor antigens is likely to be lower than the engineered high expression level of SIINF that we previously characterized in the MC57 cell line, we generated a low SIINF-expressing MC57 cell line (MC57 SIINF-LO) to utilize for the purpose of studying tumor relapse (). In order to load tumor stroma with exogenous antigen, we utilized intravenous Salmonella typhimurium A1-R based on its ability to produce high levels of SIINF (A1-R SIINF) and preferentially replicate in tumors.Citation17 Notably, this bacterium simultaneously stimulates systemic and intratumoral SIINF-specific CD8+ T cell responses while maintaining preferential tumor colonization.Citation17 Consistent with these findings, we show here that these recombinant bacteria stimulate the robust proliferation of adoptively transferred OT-1 T cells in mice ().
Figure 1. Combining intravenous delivery of live bacteria with adoptive T cell therapy prevents tumor relapse. (A) Diagram of the SIINFEKL-AAY repeat fused to EGFP. (B) MC57 parental, MC57-SIINFEKL high antigen-expressing cell line (MC57-SIINF-HI), and MC57-SIINFEKL low antigen-expressing cell line (MC57-SIINF-LO) were analyzed for SIINF expression by EGFP fluorescence using flow cytometry. (C) C57BL/6 mice were injected intravenously with CFSE-labeled OT-1 splenocytes. On the next day, A1-R SIINF or A1-R control bacteria were injected intravenously. Five days later, lymph nodes were harvested and proliferation of transferred OT-1 T cells was assessed by CFSE dilution using flow cytometry. (D) 2C mice were injected s.c. with MC57-SIINF-LO cells. After tumors were established for ≥ 2 weeks, mice were treated i.v. with monotherapeutic OT-1 splenocytes, A1-R control + OT-1, or A1-R SIINF + OT-1. Treatment with bacteria was 1 d prior to OT-1 T cell transfer. Each line represents an individual tumor-bearing mouse. Mice were compiled from four independent experiments, with three experiments containing all three treatment groups. Tumor relapse rate following combined treatment with OT-1 T cells and A1-R control was significantly lower (p < 0.05) compared to the relapse rate with OT-1 T cells alone. As a monotherapeutic control, 2C mice with long-established MC57 tumors were treated with live intravenous A1-R. Blue arrows represent the initial time of treatment. Green crosses represent mice that died during the experiment. (E) A relapsed MC57-SIINFEKL-LO tumor was re-isolated and analyzed for SIINFEKL expression by EGFP fluorescence. Data shown represent re-isolated tumors from two mice treated with OT-1 T cell monotherapy.
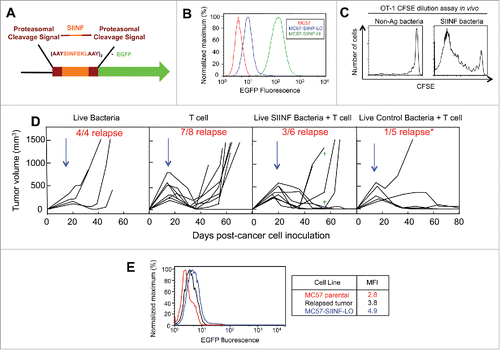
Given that long-established preclinical tumors provide an accurate modeling system for clinical tumors,Citation20 MC57-SIINF-LO tumors were established ≥2 weeks in 2C mice and reached 402 ± 170 mm3 prior to treatment with adoptively transferred OT-1 T cells and/or live bacteria. Tumors were established in 2C transgenic mice in order to assess how to best target a single MHC-I-binding peptide by adoptive transfer without interference from murine endogenous T cells that may not accurately resemble endogenous T cells found in elderly heavily pretreated cancer patients.Citation21 Treatment with OT-1 T cells, alone, led to initial tumor regression but tumors subsequently relapsed in 88% of mice (). When relapsed MC57-SIINF-LO tumors were harvested and analyzed, they expressed a reduced level of EGFP compared to the original cell line suggesting partial antigen loss due to T cell targeting (). The combination treatment of OT-1 T cells and intravenously injected A1-R SIINF improved tumor control with the tumor relapse rate decreasing to 50%. However, 2/7 mice died approximately 40 d post-treatment. Surprisingly, the combination of OT-1 T cells with A1-R control (non-SIINF expressing) bacteria provided maximum tumor control with a relapse rate of 20% (p < 0.05 when compared to monotherapy OT-1 treatment) and was safer compared to the combination of SIINF-expressing bacteria and OT-1 T cells (). Consistent with our previous findings,Citation17 treatment with A1-R bacteria as a monotherapy did not prevent MC57 tumors from growing progressively (), despite having the capacity to colonize tumors between 2–6 × 107 cfu per g tumor tissue.
Collectively, these data demonstrate that live-attenuated bacteria can synergize with T cells to eradicate tumors. Interestingly, the best tumor control and safety was achieved using bacteria that did not produce tumor antigen (). The reduced efficacy when using tumor antigen-producing bacteria may be explained by our previous observation that these bacteria colonize normal organs—although at a ≈1000x-fold reduced level compared to the tumor—where they stimulate peripheral tumor-specific CD8+ T cell responses.Citation17 This may recruit transferred T cells to the periphery and thereby limit the number of T cells infiltrating the tumor. Further, T cells stimulated in the periphery may damage normal organs which could account for deaths that we observed following this treatment combination (). In contrast, live control SIINF-negative bacteria do not induce peripheral tumor-specific T cell responses (), but, importantly, still colonize tumors where they seem to enhance T cell efficacy.
Intratumoral heat-killed bacteria combined with adoptive T cell therapy leads to tumor eradication
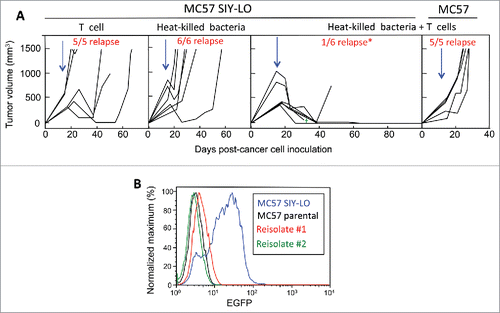
To enhance the effectiveness of adoptively transferred T cells, cancer patients undergo lymphodepletive regimens that also cause neutropenia prior to T cell infusion.Citation18 These lymphodepletive regimens are unlikely to be compatible with the use of live bacteria, as demonstrated by neutrophil depletion resulting in quick death of tumor-bearing mice treated with live attenuated Salmonella typhimurium.Citation22 To address this limitation, we next evaluated whether adoptive T cell transfer is enhanced when using sterile bacterial products by testing the use of intratumorally-injected HK bacteria. This approach was investigated in the MC57-SIYRYYGL (SIY) modelCitation23 in which cancer cells express varying levels of antigen and, therefore, better resemble the heterogeneity of human tumors,Citation24 when compared to the MC57-SIINF-LO cell line that expresses a homogenously low level of antigen (). Treatment of MC57-SIY tumors, established for ≥2 weeks in TCR-transgenic mice of irrelevant specificity (OT-1) and 439 ± 146 mm3, with adoptively transferred SIY-specific 2C T cells achieved initial tumor regression that was followed by relapse in 100% of mice (). Analysis of tumor cells from relapsed tumors revealed that relapse occurred with loss of the targeted SIY antigen (). Similarly, treatment with intratumoral HK bacteria caused initial tumor regression that was followed by tumor relapse in 100% of mice (). In contrast, treatment with the combination of T cells and parental non-SIY HK bacteria consistently eradicated tumors with relapse in the 3 mo post-treatment follow-up period restricted to 17% of mice (p < 0.05 when compared to each monotherapeutic treatment, ). This durable tumor rejection may have been associated with mice developing a memory T cell phenotype, but this effect was not analyzed in this study. However, in this combination treatment group, 1 out of 7 treated mice died 16 d post-treatment at which time the mouse possessed minimal residual disease (). In this tumor model, the synergy observed between T cells and HK bacteria was antigen-specific since the treatment of MC57 tumors absent for SIY expression was completely ineffective ().
Figure 2. Combining intratumorally injected heat-killed bacteria with adoptive T cell therapy prevents tumor relapse. (A) MC57 SIY-LO cancer cells were injected s.c. onto the backs of OT-1 mice. Tumors were established ≥2 weeks prior to being treated with intravenously-injected adoptively-transferred activated 2C T cells, intratumoral HK bacteria, or combined 2C T cells + HK bacteria. Data represent mice compiled from three independent experiments, with each experiment consisting of each treatment group. The tumor relapse rate following combined treatment with HK bacteria and T cells was significantly lower (p < 0.05) compared to either monotherapy. Similarly, MC57 EGFP tumors were treated with the combination of HK bacteria and 2C T cells in two independent experiments. Blue arrows represent the initial time of treatment and the green cross represents one mouse death. (B) Re-isolated cancer cells from two relapsed MC57 SIY-LO tumors following monotherapy 2C T cell treatment were analyzed for SIY expression by EGFP fluorescence using flow cytometry. The MC57 parental and MC57 SIY-LO cell line, that was originally injected into mice, were used in this analysis. Data represent three relapsed tumors from two independent experiments.
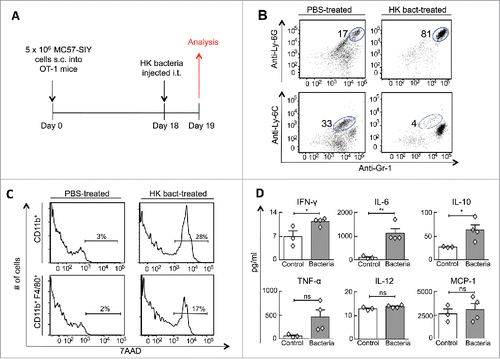
We next investigated the role of HK bacteria in T cell-mediated tumor rejection. Because our previous work demonstrated that T cells eradicate tumors by secreting TNF-α and IFNγ that subsequently mediate the destruction of tumor stroma,Citation11 we hypothesized that HK bacteria synergize with T cells by inducing changes in the tumor stroma that cannot be generated by T cells alone. To examine this notion, long-established MC57-SIY tumors were intratumorally injected with HK bacteria and analyzed for tumor stroma cellular composition and cytokine profile the following day (). Treatment with HK bacteria, compared to the lack of or PBS-treatment, markedly increased the percentage of neutrophils in the stroma while reducing the percentage of monocytes. Gr-1+ Ly6C+ monocytes comprised 11–33% of intratumoral CD11b+ cells in control mice, but only 0.5–7.0% in HK bacteria-treated mice (). Similarly, the neutrophil to monocyte ratio increased from 0.5:1–7:1 in controls to 10:1–200:1 in HK bacteria-treated mice (). Further analysis also revealed that HK bacteria induced the quick death of intratumoral myeloid cells since HK bacteria-treated tumors, when compared to controls, contained 15–20% more non-viable CD11b+ cells that included 10% more non-viable F4/80+ macrophages (). A cytokine array of tumor lysates revealed that HK bacteria-treated tumors possessed higher IFNγ, IL-6 and IL-10 levels (p < 0.05), with a strong trend for TNF-α (p = 0.08, ).
Figure 3. Intratumorally injected heat-killed bacteria transform the tumor microenvironment's cellular composition and cytokine profile. (A) MC57-SIY cancer cells were injected s.c. into OT-1 mice. Tumors were established for 18 d before receiving an intratumoral-injection with HK bacteria or PBS. One day later, mice were sacrificed and tumors were analyzed. (B) Tumor cell suspensions were analyzed for expression of Gr1, Ly6C, and Ly6G. The percentage of live CD11b+ cells identified as neutrophils (Gr1+ Ly6G+) and monocytes (Gr1+Ly6C+) are shown. Data are representative of four HK bacteria-treated mice and three control mice (untreated or PBS-treated) from two independent experiments. (C) Tumors from (B) were also analyzed for expression of CD11b, F4/80 and the 7AAD non-viability probe. 7AAD staining of gated myeloid cells (CD11b+) and gated macrophages (CD11b+, F4/80+) are shown. (D) Cytokine concentrations from tumor homogenates were quantified from tumors using a cytokine bead array. Data consist of four HK bacteria-treated mice and three control mice (untreated or PBS-treated) compiled from two independent experiments.
Because tumor rejection using HK bacteria and T cells requires cancer cells to express cognate antigen (), signaling derived from both cancer cell antigen and HK bacteria are required for T cells to reject tumors. As T cell-targeting against cancer cell antigen, alone, induces tumor regression but failure to completely reject tumors, HK bacteria likely provide an antigen-independent boost to T cells. First, HK bacteria-induced high levels of the pleotropic cytokine IL-10 (), which has been shown to increase both CD8+ T cell numbers and effector function in tumors.Citation25 Second, HK bacteria shifted the neutrophil:monocyte ratio toward a neutrophil-predominated response that has been associated with enhanced T cell efficacy.Citation26-28 Notably, it's important to note that HK bacteria also have a direct antitumor impact as demonstrated by monotherapeutic administration of HK bacteria inducing tumor regression (). Therefore, in the process of generating a pro-inflammatory tumor microenvironment, HK bacteria may provide both T cell-dependent and -independent antitumor effects.
While HK bacteria synergize with adoptive T cell transfer to reject tumors, it is important to note that this dual therapy may possess increased toxicity based on the single mouse that died 16 d post-treatment (). Mortality, if related to treatment, may have been due to cytokine release syndrome since this syndrome is mediated by IL-6 and other pro-inflammatory cytokines upregulated by HK bacteria () or adoptive T cell therapy.Citation29,30 Notably, our preclinical study did not monitor or treat for cytokine release syndrome which can be managed clinically with aggressive supportive fluid hydration and IL-6 neutralizing antibody.Citation29,30
Concluding remarks
Our previous study utilizing bacteria for therapeutic vaccination demonstrated that live bacteria must express tumor antigen in order to rescue endogenous dysfunctional tumor-infiltrating T cells.Citation17 In contrast, our current findings demonstrate that in the absence of tumor antigens expressed/shed by live bacteria/bacterial products, respectively, adoptive T cell therapy can still be enhanced by bacteria to reject tumors. These findings collectively suggest that there are different requirements for rescuing or enhancing endogenous T cell- versus adoptively transferred T cell-effector function(s). However, while unable to be assessed in our TCR transgenic model system, adoptive transfer combined with bacteria may also secondarily prime endogenous tumor-specific T cell responses in the context of simultaneous cancer cell killing and administration of bacterial adjuvant. Future studies should investigate this possibility in immunocompetent old mice rather than young mice because their endogenous T cell repertoire likely better represents the repertoire of elderly cancer patients.Citation21
To the best of our knowledge, these findings demonstrate for the first time that intratumorally injected HK bacteria synergize with T cells to eradicate long-established tumors. Translating the use of locally injected HK bacteria to the clinic is a realistic goal based on the current success treating early non-muscle-invasive bladder cancer with attenuated Bacille Calmette Guerin (BCG) bacteria.Citation31 Furthermore, newly available image-guided injection technologiesCitation32 allow for a highly precise intratumoral delivery. Thus, further optimization of our approach combining local injection of sterile bacterial products with adoptive transfer will be important for treating cancer patients with T-cell resistant locally advanced tumors not amenable to surgical resection.Citation33
Materials and methods
Mice, cell lines, and reagents
SIINFEKL-Kb-specific TCR-transgenic OT-I mice were provided by M. Mescher (University of Minnesota, Twin Cities, MN) and the SIYRYYGL-Kb-specific TCR-transgenic 2C mice were provided by J. Chen (Massachusetts Institute of Technology, Cambridge, MA). C57BL/6 mice were purchased from the Jackson Laboratory. Mice were maintained in a specific pathogen-free barrier facility at the University of Chicago according to the Institutional Animal Care and Use Committee guidelines.
The methylcholanthrene-induced C57BL/6-derived fibrosarcoma MC57 cell line was provided by P. Ohashi (University of Toronto, Toronto, Ontario, Canada) with permission of H. Hengartner (University Hospital Zurich, Zurich, Switzerland). MC57-SIY and MC57-SIINF were previously generated by transduction with MFG-SIY-EGFP and MFG-SIINF-EGFP respectively.Citation13,23 The original MC57-SIINF line was bulk-sorted for low antigen expression to generate the cell line used in this study. Cancer cells were cultured in DMEM and 5% FCS (Gemini Bio-Products, West Sacramento, CA) at 37˚C in a 5 or 10% CO2 dry incubator. Salmonella typhimurium A1-R was provided by AntiCancer Inc. (San Diego, California, United States).
Antibodies used for flow cytometry were the following: anti-Ly6C (AL-21) from BD PharMingen; anti-CD11b (M1/70), anti-Ly6G (1A8) from Biolegend; anti-Gr1 (RB6-8C5) and anti-F4/80 (BM8) from eBioscience. To detect SIINFEKL reactive T cells, a staining solution referred to as SIINF-dX was used that consisted of SIINFEKL peptide-loaded Kb-DimerX [(Kb)2-IgG] from BD PharMingen, anti-mouse IgG1–phycoerythrin, and mouse immunoglobulin G1 (IgG1) isotype control.
Bacterial preparations
Live Salmonella typhimurium A1-R SIINF and A1-R control that expressed EGFP were prepared as previously described.Citation17 HK bacteria were prepared by growing A1-R control (not expressing tumor antigen) logarithmically until the OD600=0.5–0.7. Bacteria were then washed twice in 1X PBS, resuspended in 50–100 μl PBS, and subsequently HK at 65˚C for 1 h. Bacterial killing was confirmed by plating on LB agar.
CFSE dilution study
OT-1/Thy1.1 mice were sacrificed. Splenocytes were incubated in Tris NH4Cl for 2 min to lyse red blood cells. Lymphocytes at 5 × 107 cells per mL were labeled with 10 µM CFSE in PBS at room temperature for 15 min. Cells were then washed twice in 10% FCS-containing DMEM prior to being intravenously injected into C57BL/6 mice. On the next day, mice were injected with A1-R SIINF or A1-R control. Five days later, lymph nodes were isolated and single-cell suspensions were prepared. Flow cytometric analysis of CFSE dilution, a marker of T cell proliferation, was performed by gating on SIINF-dX+CD8+ cells.
Colony formation unit assay
OT-1 mice bearing long-established MC57 tumors were intravenously injected with 5 × 107 cfu of Salmonella typhimurium A1-R. 2 or 4 d later, a colony formation unit assay was conducted as previously described.Citation17
T cell activation
2C mice were sacrificed, spleens harvested, and splenocytes treated with NH4Cl for red blood cell lysis. The splenocytes were then cultured at 5 × 106 cells per mL, with 3 mL of cells per well in a 6-well plate. Cells were cultured in RPMI containing 10% FCS, 2 mM glutamine, 50 µM ß-mercaptoethanol, 1 mM Hepes, 1 mM sodium pyruvate, 1X non-essential amino acids, 100 U/mL penicillin, 100 µg/mL streptomycin, and 50 µg/mL gentamicin (all reagents from Gibco, Carlsbad, CA). To this culture, SIY peptide (gift from S. Meredith, University of Chicago, Chicago, IL) was added for a final concentration of 1 μM. T cells were activated for 3 d prior to adoptive transfer.
Tumor treatment
Cancer cells (5 × 106/200 μL) were injected subcutaneously onto the backs of mice: MC57-SIY into OT-1 mice and MC57-SIINF into 2C mice. We calculated tumor volumes using 3 orthogonal axes (a, b, and c) with volume calculated as abc/2. Once tumors had grown for at least 2 weeks, mice were treated with either naive OT-1 splenocytes (one spleen per recipient) or activated 2C T cells (derived from 0.5–1 spleen per recipient) by intravenous injection. For bacterial treatments, 1–2 × 107 live bacteria were injected intravenously or 4 × 108–2 × 109 HK bacteria were injected intratumorally into multiple regions of each tumor. To minimize leakage during intratumoral injection, mice were anesthetized using isoflurane and needles were suspended at the injection site for at least 5–10 sec during each injection.
Preparing tumor cell suspensions and readapting cancer cells to culture
Tumor cell suspensions were prepared by (i) excising and cutting the tumor into 2–3 mm fragments, (ii) incubating fragments with collagenase D and DNase I, followed by the addition of trypsin, (iii) pipetting fragment-containing solution for 2 min, and (iv) filtering solution through a 70–μm nylon mesh to generate a single cell suspension. Cells were subsequently used for flow cytometric analysis or readaptation to cell culture. Readapted cancer cells were cultured for at least 2 weeks prior to evaluating antigen levels by flow cytometry.
Cytometric bead array
Cytokine measurement was performed with the mouse inflammation CBA kit from BD Biosciences, according to the manufacturer's guidelines. Tumor cell lysates were centrifuged at 5000 rpm, and supernatants separated. Supernatants were diluted in PBS buffer prior to analysis.
Statistics
Relapse was defined as tumor progression within the first 60 d post-cancer cell inoculation. Fisher's exact 2-tailed test was used to determine if there was a difference in relapse rates between treatment groups. For analysis of cytokine level differences between groups, a 2-tailed unpaired t-test was used.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by the NIH grants R01-CA37516, R01-CA22677, and P01-CA97296, to H. Schreiber and the Graduate Training in Growth and Development grant T32 HD009007 to DC. Binder. AntiCancer Inc. provided the Salmonella typhimurium A1-R strain.
References
- Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med 2004; 10:294-8; PMID:14981514; http://dx.doi.org/10.1038/nm999
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008; 26:5233-9; PMID:18809613; http://dx.doi.org/10.1200/JCO.2008.16.5449
- Thomas DL, Kim M, Bowerman NA, Narayanan S, Kranz DM, Schreiber H, Roy EJ. Recurrence of intracranial tumors following adoptive T cell therapy can be prevented by direct and indirect killing aided by high levels of tumor antigen cross-presented on stromal cells. J Immunol 2009; 183:1828-37; PMID:19592642; http://dx.doi.org/10.4049/jimmunol.0802322
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011; 17:4550-7; PMID:21498393; http://dx.doi.org/10.1158/1078-0432.CCR-11-0116
- Singh S, Ross SR, Acena M, Rowley DA, Schreiber H. Stroma is critical for preventing or permitting immunological destruction of antigenic cancer cells. J Exp Med 1992; 175:139-46; PMID:1309851; http://dx.doi.org/10.1084/jem.175.1.139
- Van Waes C, Monach PA, Urban JL, Wortzel RD, Schreiber H. Immunodominance deters the response to other tumor antigens thereby favoring escape: prevention by vaccination with tumor variants selected with cloned cytolytic T cells in vitro. Tissue Antigens 1996; 47:399-407; PMID:8795140; http://dx.doi.org/10.1111/j.1399-0039.1996.tb02575.x
- Wu TH, Schreiber K, Arina A, Khodarev NN, Efimova EV, Rowley DA, Weichselbaum RR, Schreiber H. Progression of cancer from indolent to aggressive despite antigen retention and increased expression of interferon-gamma inducible genes. Cancer Immun 2011; 11:2; PMID:21714479
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37; PMID:22461641; http://dx.doi.org/10.1126/scitranslmed.3003689
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515:568-71; PMID:25428505; http://dx.doi.org/10.1038/nature13954
- Spiotto MT, Schreiber H. Rapid destruction of the tumor microenvironment by CTLs recognizing cancer-specific antigens cross-presented by stromal cells. Cancer Immun 2005; 5:8; PMID:15934727
- Zhang B, Karrison T, Rowley DA, Schreiber H. IFN-gamma- and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers. J Clin Invest 2008; 118:1398-404; PMID:18317595; http://dx.doi.org/10.1172/JCI33522
- Briesemeister D, Sommermeyer D, Loddenkemper C, Loew R, Uckert W, Blankenstein T, Kammertoens T. Tumor rejection by local interferon gamma induction in established tumors is associated with blood vessel destruction and necrosis. Int J Cancer 2011; 128:371-8; PMID:20333679; http://dx.doi.org/10.1002/ijc.25350
- Engels B, Engelhard VH, Sidney J, Sette A, Binder DC, Liu RB, Kranz DM, Meredith SC, Rowley DA, Schreiber H. Relapse or eradication of cancer is predicted by Peptide-major histocompatibility complex affinity. Cancer Cell 2013; 23:516-26; PMID:23597565; http://dx.doi.org/10.1016/j.ccr.2013.03.018
- Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med 2013; 19:747-52; PMID:23644516; http://dx.doi.org/10.1038/nm.3161
- van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, van Dijk LJ, Behjati S, Hilkmann H, El Atmioui D et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol 2013; 31:e439-42; PMID:24043743; http://dx.doi.org/10.1200/JCO.2012.47.7521
- Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, Xu M, Penman S, Hoffman RM. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci U S A 2005; 102:755-60; PMID:15644448; http://dx.doi.org/10.1073/pnas.0408422102
- Binder DC, Engels B, Arina A, Yu P, Slauch JM, Fu YX, Karrison T, Burnette B, Idel C, Zhao M et al. Antigen-specific bacterial vaccine combined with anti-PD-L1 rescues dysfunctional endogenous T cells to reject long-established cancer. Cancer Immunol Res 2013; 1:123-33; PMID:24455752; http://dx.doi.org/10.1158/2326-6066.CIR-13-0058
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015; 348:62-8; PMID:25838374; http://dx.doi.org/10.1126/science.aaa4967
- Dubey P, Hendrickson RC, Meredith SC, Siegel CT, Shabanowitz J, Skipper JC, Engelhard VH, Hunt DF, Schreiber H. The immunodominant antigen of an ultraviolet-induced regressor tumor is generated by a somatic point mutation in the DEAD box helicase p68. J Exp Med 1997; 185:695-705; PMID:9034148; http://dx.doi.org/10.1084/jem.185.4.695
- Wen FT, Thisted RA, Rowley DA, Schreiber H. A systematic analysis of experimental immunotherapies on tumors differing in size and duration of growth. Oncoimmunology 2012; 1:172-8; PMID:22720238; http://dx.doi.org/10.4161/onci.1.2.18311
- Schreiber K, Arina A, Engels B, Spiotto MT, Sidney J, Sette A, Karrison TG, Weichselbaum RR, Rowley DA, Schreiber H. Spleen cells from young but not old immunized mice eradicate large established cancers. Clin Cancer Res 2012; 18:2526-33; PMID:22415314; http://dx.doi.org/10.1158/1078-0432.CCR-12-0127
- Westphal K, Leschner S, Jablonska J, Loessner H, Weiss S. Containment of tumor-colonizing bacteria by host neutrophils. Cancer Res 2008; 68:2952-60; PMID:18413765; http://dx.doi.org/10.1158/0008-5472.CAN-07-2984
- Liu RB, Engels B, Schreiber K, Ciszewski C, Schietinger A, Schreiber H, Jabri B. IL-15 in tumor microenvironment causes rejection of large established tumors by T cells in a noncognate T cell receptor-dependent manner. Proc Natl Acad Sci U S A 2013; 110:8158-63; PMID:23637340; http://dx.doi.org/10.1073/pnas.1301022110
- Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature 2013; 501:328-37; PMID:24048065; http://dx.doi.org/10.1038/nature12624
- Emmerich J, Mumm JB, Chan IH, LaFace D, Truong H, McClanahan T, Gorman DM, Oft M. IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res 2012; 72:3570-81; PMID:22581824; http://dx.doi.org/10.1158/0008-5472.CAN-12-0721
- Garcia-Hernandez Mde L, Hamada H, Reome JB, Misra SK, Tighe MP, Dutton RW. Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J Immunol 2010; 184:4215-27; PMID:20237297; http://dx.doi.org/10.4049/jimmunol.0902995
- Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest 2011; 121:4746-57; PMID:22056381; http://dx.doi.org/10.1172/JCI58814
- Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S, Bronte V. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep 2012; 2:628-39; PMID:22959433; http://dx.doi.org/10.1016/j.celrep.2012.08.006
- Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014; 124:188-95; PMID:24876563; http://dx.doi.org/10.1182/blood-2014-05-552729
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371:1507-17; PMID:25317870; http://dx.doi.org/10.1056/NEJMoa1407222
- Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer–a current perspective. Nat Rev Urol 2014; 11:153-62; PMID:24492433; http://dx.doi.org/10.1038/nrurol.2014.15
- Ojha T, Rizzo L, Storm G, Kiessling F, Lammers T. Image-guided drug delivery: preclinical applications and clinical translation. Expert Opin Drug Deliv 2015; 12:1203-7; PMID:26083469
- Hoffman RM editor. Bacterial Therapy of Cancer: Methods and Protocols. Methods in Molecular Biology 1409. Walker, John M., series ed. Humana Press (Springer Science+ Business Media New York), 2015.
