ABSTRACT
Reduced anticancer efficacy of cyclophosphamide and platinum salts has been reported in animals treated with anti-Gram-positive antibiotics. These effects were related to translocation of Gram-positive bacteria during mucositis with subsequent induction of cytotoxic oxygen reactive species and tumor invasion by pathogenic Th17 cells. To assess these hypotheses in a clinical setting, we identified patients receiving cyclophosphamide for chronic lymphocytic leukemia (CLL) and cisplatin for relapsed lymphoma. Data originated from the CLL8 trial (NCT00281918) and the Cologne Cohort of Neutropenic Patients (NCT01821456). Relevant antibiotics were defined as compounds with primary activity against Gram-positive bacteria. We evaluated their impact on response, progression-free survival (PFS) and overall survival (OS) by Kaplan–Meier methodology and Cox proportional hazards regression analysis. Among 800 available CLL patients, those receiving anti-Gram-positive antibiotics (n = 45/800) achieved a significantly lower overall response rate (OR 74.3% vs. 90.2%, p = 0.007). Patients with anti-Gram-positive antibiotics progressed significantly earlier, had a reduced OS (median PFS 14.1 vs. 44.1 mo, p < 0.001; median OS 56.1 vs. 91.7 mo, p < 0.001) and multivariate analysis showed that administration of anti-Gram-positive antibiotic treatment was independently associated with reduced PFS (Hazard ratio (HR) 2.090, p = 0.001) and OS (HR 2.966, p < 0.001). Of 122 patients with relapsed lymphoma, those treated with anti-Gram-positive antibiotics (n = 21/122) achieved a significantly lower OR rate (70.3% vs. 42.9%, p = 0.016). Patients with anti-Gram-positive antibiotics progressed significantly earlier than others (median PFS 2.3 vs. 11.5 mo, p = 0.001). As for multivariate analysis, the use of anti-Gram-positive antibiotics was independently associated with reduced PFS (HR 2.237, p = 0.012) and OS (HR 7.831, p < 0.001). Our data supports a potential negative impact of anti-Gram-positive antibiotics on the anticancer activity of cyclophosphamide and cisplatin in a clinical setting.
Introduction
Chemotherapy-induced mucositis is characterized by epithelial barrier inflammation and cell loss. Intestinal mucositis frequently leads to bacterial translocation and subsequent bloodstream infection, dehydration due to diarrhea, and need for parenteral nutrition and intravenous analgesics.Citation1 Neutropenic colitis as its most severe complication has been associated with increased mortality.Citation2 Recent evidence suggests an even more complex role for chemotherapy-induced mucositis. Two independent groups were able to demonstrate in their respective mouse models that disruption of the mucous membranes' barrier function may enable a hitherto unknown mode of action of cytotoxic treatment.Citation3,4
Viaud et al. showed that administration of cyclophosphamide leads to mucositis and successive translocation of Gram-positive bacteria into the mesenteric lymph nodes and spleen, followed by an increase of interferon-γ and a subset of interleukin (IL) 17 secreting splenic T-cells (pathogenic TH17 cells). This finding is dependent on the presence of translocated gut microbiota in the respective histological specimens and could not be reproduced in germ-free mice.Citation4
Furthermore, tumor-bearing mice had reduced regression of tumor load, if pre-treated with antibiotics, whereas injection of pathogenic TH17 cells in antibiotic pre-treated mice restored the antitumor effect of cyclophosphamide. The reduction in the antitumor effect of cyclophosphamide was more pronounced after exposure to an antibiotic with a focus on the Gram-positive spectrum, as opposed to antibiotics targeting the Gram-negative spectrum.
Iida et al. demonstrated that disruption of the microbiota not only impairs the response of subcutaneous tumors to cpg-oligonucleotide and anti-IL-10-receptor antibody immunotherapy, but also to platinum-based chemotherapy agents. The group was able to identify specific Gram-positive bacteria that promoted the antitumor efficacy of the immunotherapeutic combination by priming of tumor-infiltrating myeloid cells. In case of the platinum-based therapeutic approach, antitumor efficacy was improved by bacterial induction of reactive oxygen species through Toll-like receptors.Citation3
Both works demonstrated a link between administration of antibiotics targeted at Gram-positive pathogens, composition of the gut microbiota and efficacy of antineoplastic treatment in mice. Thus far, it remains unclear, whether these findings are transferrable to humans.
To investigate the impact of antibiotic treatment on antineoplastic treatment outcomes in humans, we identified two representative patient populations: first, patients treated with a cyclophosphamide containing first-line therapy for CLL and second, patients treated with a cisplatin containing regimen for relapsed lymphoma. For both populations, we analyzed potential associations between anti-Gram-positive antibiotic treatment and patient outcome.
Results
Out of 817 patients randomized in the CLL 8 trial, 800 (97.9%) patients received chemo(immuno)therapy and had data on concomitant medications available, thus constituting the study population for this analysis.
721 patients (90.1%) received any kind of antibiotic treatment. According to our definition, 45 patients (5.6%) received relevant antibiotics, 676 patients (84.5%) received non-relevant antibiotics and 79 patients (9.9%) received no antibiotics. Of those receiving relevant antibiotic, 24 patients were allocated to receive FC and 21 patients to receive FCR.
Comparison of baseline characteristics of patients receiving relevant antibiotics with those receiving no/irrelevant antibiotics yielded no statistically significant difference with regard to distribution of age, sex, Binet stage, ECOG performance status, genomic aberrations, IGHV and TP53 mutational status as well as s-β2m. Patients receiving relevant antibiotics were, however, more likely to discontinue chemo(immuno)therapy (53.3% [n = 24] vs. 26.6% [n = 201], p < 0.001) and to have elevated levels of s-TK (90.6% [n = 29] vs. 74.4% [n = 416], p = 0.039). There were no significant differences in reasons for early withdrawal between groups ( p = 0.362). Reasons for early withdrawal are specified in Table S1.
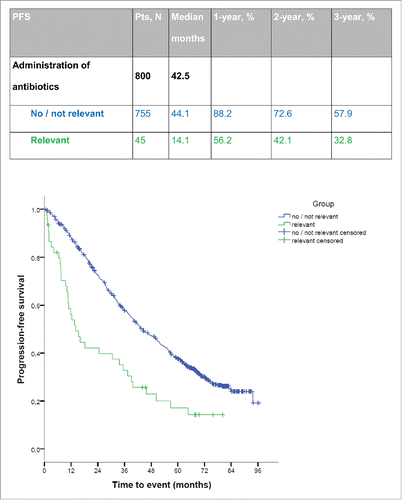
Patient characteristics and outcomes by antibiotic treatment category for each variable are detailed in .
Table 1. Baseline characteristics and outcome CLL patients.
Patients with relevant antibiotics were significantly less likely to achieve an OR compared to patients who received no or irrelevant antibiotics (74.3% [n = 26] vs. 90.2% [n = 651], p = 0.007). There was no significant difference in OR between patients receiving irrelevant and no antibiotics (89.0% [n = 430] vs. 92.1% [n = 70], p = 0.417). See also Fig. S5 for Kaplan–Meier estimates by antibiotic category.
Patients who had received relevant antibiotics progressed significantly earlier (median PFS 14.1 mo) than patients in the no/irrelevant group (median PFS 44.1 mo, p < 0.001) (see ). OS was significantly reduced, as well (median OS 56.1 vs. 91.7 mo, p < 0.001; Fig. S1). PFS and OS were not significantly different between patients receiving irrelevant and no antibiotics (median PFS 43.3 vs. 48.3 mo, p = 0.996 and median OS 90.2 mo vs. not reached, p = 0.528).
Of note, this effect of antibiotic treatment on response, PFS and OS could also be demonstrated analyzing both treatment arms, FC and FCR, separately (Table S2 and Figs. S2 and 3). While most differences observed between groups were statistically significant in this subgroup analysis, only a trend was observed for end of treatment response after treatment with FC (Table S2).
To account for potential bias due to undertreatment resulting from infectious complications, the analysis was repeated in the group of patients having received six cycles (n = 575) of chemotherapy and those having achieved a response at interim staging (n = 666). Patients with six cycles of therapy or response at interim staging respectively, and concomitant relevant antibiotics (n = 21 and n = 30, respectively) progressed significantly earlier (median PFS 24.3/16 mo) than patients who completed therapy without any or only with irrelevant antibiotics (n = 554 and n = 636, respectively; median PFS 52.4/49.9 mo, p < 0.001/0.001). Performance of a dose-intensity approach confirmed our results. To further assess the prognostic role of relevant antibiotics on chemotherapeutic outcome, a multivariate analysis of genetic subgroups was performed. Variables included and results are shown in and Table S3. Administration of relevant antibiotic treatment proved an independent prognostic factor for reduced PFS and OS (HR 2.073, p = 0.002 and HR 2.966, p < 0.001). Substitution of the number of cycles by a dose-intensity variable confirmed our results especially the impact of treatment with relevant antibiotics.
Table 2. Cox regression progression-free survival in CLL patients.
Relapsed lymphoma
Overall, 122 patients with relapsed lymphoma were identified and included in the analysis. There were no missing data. Overall, 98 patients (80.3%) received any kind of antibiotic treatment and among them 21 (21.4%) received relevant and 77 (78.6%) non-relevant antibiotics. There were 24 patients (19.7%) without antibiotic treatment.
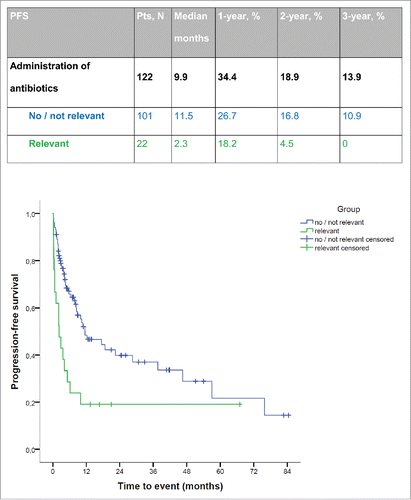
Patient characteristics and outcomes by antibiotic treatment category are detailed in . Concerning baseline characteristics, there were no significant differences in the distribution of age, gender, type of lymphoma, days to relapse and ECOG performance status between patients with and without relevant antibiotic treatment. Patients who received relevant antibiotics were significantly more likely to be categorized as Ann Arbor stage ≥ 3 (85.7% [n = 18] vs. 61.4% [n = 62], p = 0.033) and to have received only one cycle of (R)-DHAP instead of two as planned (42.9% [n = 9] vs. 3% [n = 3], p ≤ 0.001). Overall, nine patients received relevant antibiotics and failed to complete at least two cycles of (R)-DHAP. In none of them, cytotoxic therapy was aborted due to an infectious complication, but due to progression of lymphoma.
Table 3. Baseline characteristics and outcome relapsed lymphoma patients.
Patients who received relevant antibiotics were significantly less likely to achieve OR (42.9% [n = 9] vs. 70.3% [n = 71], p = 0.016), and tended toward failure to achieve CR (9.5% [n = 2] vs. 15.8% [n = 16], p = 0.055).
There was no significant difference in OR between patients receiving irrelevant and no antibiotics (68.4% [n = 54] vs. 77.3% [n = 17], p = 0.418).
Progression was observed significantly earlier in patients who had received relevant antibiotics (median PFS 2.3 mo) compared to patients categorized into the no/irrelevant antibiotics group (median PFS 11.5 mo, p = 0.001) (). OS was significantly reduced, as well (median OS 5.6 vs. 96.8 mo, p < 0.001; Fig. S4).
PFS and OS were not significantly different between patients receiving irrelevant and no antibiotics (median PFS 10.4 vs. 46.6 mo, p = 0.466 and median OS 96.8 mo vs. not reached, p = 0.243).
In the Cox proportional hazards regression analysis administration of relevant antibiotic treatment was an independent prognostic factor for reduced PFS (HR 2.237, p = 0.012) and OS (HR 7.831, p < 0.001; and Table S4).
Table 4. Cox regression progression-free survival in relapsed lymphoma patients.
Discussion
In our analysis, we assessed the influence of antibiotics on the outcome of chemotherapy containing cyclophosphamide and cisplatin for first-line treatment of CLL or relapsed lymphoma, respectively. The populations for this analysis were chosen to best transfer the crucial components in the mouse models of Viaud et al. and Iida et al.Citation3,4 into a clinical setting. In both populations, we identified treatment with antibiotics with an anti-Gram-positive focus as an independent risk factor for significantly reduced PFS and OS.
According to Viaud et al., anticancer efficacy of cyclophosphamide was reduced after administration of anti-Gram-positive antibiotics. Translocation of Gram-positive commensal bacteria into secondary lymphoid organs due to mucositis with subsequent priming and recruitment of pathogenic TH17 cells into the tumor bed was described as the underlying mechanism of action.Citation4 Patients enrolled in the CLL8 study received either FC or FCR, both containing cyclophosphamide, thus, the effect of antibiotics on treatment outcome could be assessed in all study participants. The considerable size of effect of treatment with FC or FCR and the possibility of obtaining long-term follow-up data made this a particularly suitable population for our analysis.
In the mouse model used by Iida et al., antibiotic treatment similarly attenuated the anticancer efficacy of platinum salts. Again, commensal bacteria, in particular from the Gram-positive spectrum, were shown to play a crucial role in the underlying mechanism of action. In their presence, tumor-associated inflammatory cells were induced to produce reactive oxygen species essential for platinum genotoxicity.Citation3 Apart from the treatment of relapsed lymphoma, platinum salts are routinely used in patients with solid tumors. Since standard treatment regimens for these indications do not generally induce neutropenia, antibiotics for infectious complications are rare in these populations. Therefore, we decided to limit the analysis set to patients with relapsed high-grade lymphoma.
The broad variety of factors influencing the prognosis of hematological malignancies, e.g. genetic factors, infections, choice of treatment and general status at baseline represented a major challenge to this study. We addressed this issue with a carefully executed choice of variables and analysis plan. Interaction between the administration of relevant antibiotics and early termination of antineoplastic treatment due to infectious complications remains a potential confounder; this was addressed by evaluating the reasons for early termination of antineoplastic treatment. In patients with CLL, the reasons for withdrawal of study treatment were evenly distributed between the groups and in the relapsed lymphoma population, the only nine patients who received relevant antibiotics and failed to complete at least two cycles of (R)-DHAP did so due to progression of their lymphoma. In light of these data, we feel confident to exclude infectious complications as a confounder.
In patients treated for relapsed lymphoma, median OS in patients with anti-Gram-positive antibiotics (5.6 mo) was significantly lower than in patients receiving no/not relevant antibiotics (median PFS 96.8 mo, p < 0.001). In the CLL population, the difference in median PFS was less pronounced (median PFS 56.1 vs. 91.7 mo, p < 0.001). We assume that the marked difference between groups in the relapsed lymphoma population can be attributed to the lower sample size of this population. Moreover the group of lymphoma patients was hetergenous. Inclusion of data from other cohorts or interventional studies would help to pinpoint the influence of anti-Gram-positive antibiotics in this population.
In the two mouse models that served as a basis for the design of this analysis, Gram-positive bacteria were identified as crucial components in the elucidated pathomechanisms. While we indirectly assessed the presence of these bacteria by focusing on antibiotic treatment, we could not establish a clear cause–effect relationship in the absence of an accompanying microbiome analysis.
Our analyses were limited in size and retrospective in nature. Despite our efforts, the effects of statistical confounders inherent to this kind of setting may not have been fully accounted for. Further efforts, including fecal microbiome analyses, should be made to reproduce our findings and deepen the knowledge on the underlying pathomechanisms, as their implications would be of major concern to physicians and patients alike. Today, bloodstream infections remain a major cause of non-relapse mortality in patients with chemotherapy-induced neutropenia. Empirical use of broad-spectrum antimicrobials continues as a cornerstone of supportive care in hematology. Besides these first-line regimens, linezolid and the glycopeptides, all of which are targeted at Gram-positive pathogens, are frequently used as empirical add on treatment in patients with persistent febrile neutropenia, even though there is no convincing evidence to support any advantages for this strategy.Citation5,6 While the general value of empiric treatment is undisputed, more differentiated strategies are conceivable.
The field of microbiota research is growing quickly. Recent developments highlight the role of specific microbiota in the pathogenesis and therapy of diseases. In 2013, the first-randomized clinical trial on fecal microbiota transplantations highlighted its clinical efficacy in achieving clinical cure for recurrent Clostridium difficile. At the time, a generally reduced diversity of the gut microbiota was proposed as the major predictor for disease recurrence; however, a recent analysis using high-resolution sequencing techniques proposed that a group of only four bacterial species may confer resistance to Clostridium difficile infection, based on their ability to modulate their host's bile acid metabolism.Citation7
In the field of allogeneic stem cell transplantation, antibiotic-induced loss of lower intestinal diversity has been proposed as an independent predictor of adverse outcome, whereas development of graft-versus-host disease seems to be a major contributor to mortality in this setting.Citation8,9 Only recently, commensal bacteria of the genus Blautia were pinpointed as the missing link between changes in microbiota composition, development of graft-versus-host disease and an adverse outcome.Citation10 A second, independent group of researchers has recently come to similar conclusions.Citation11
In line with these top-down research approaches, further specification of those bacterial species hypothesized to modulate the efficacy of cytotoxic treatment may be a first step toward development of a targeted bacteriotherapy, designed to supplement the gut microbiota during indispensable courses of antibiotic treatment.
Before such measures can be considered, further vaIidation of our findings are warranted. Prospective cohorts or analyses conducted in association with randomized controlled trials on the efficacy of cytotoxic treatment are conceivable settings.
In summary, we assessed the impact of anti-Gram-positive antibiotics on the outcome of chemotherapy containing cyclophosphamide and cisplatin for CLL and relapsed lymphoma, respectively. While we identified treatment with these antibiotics as an independent adverse factor for response, PFS and OS, further vaIidation studies should be performed.
Patients and methods
Patient populations
CLL
Data was selected post-hoc from the CLL8 trial (NCT00281918, ClinicalTrials.gov), a prospective multicenter randomized phase III trial evaluating first-line treatment with fludarabine and cyclophosphamide (FC) without or with rituximab (FCR) in 817 CLL patients.Citation12 Patients were eligible for inclusion regardless of age if physically fit and with a low comorbidity burden as defined by a score of ≤ 6 on the cumulative illness rating scale (CIRS). For this analysis, the data of all patients with available information on concomitant medications was used.
Relapsed lymphoma
Data was selected from the Cologne Cohort of Neutropenic Patients (CoCoNut), a non-interventional prospective cohort study assessing risk factors, interventions, and outcome of immunosuppressed patients (NCT01821456, ClinicalTrials.gov). Data from all patients undergoing chemotherapy at the University Hospital Cologne, Germany, are incorporated into the database. For this analysis, the database was searched for patients with relapsed lymphoma undergoing treatment with DHAP (dexamethasone, cytarabine and cisplatin) without or with rituximab (R-DHAP).
Definitions, endpoints and statistical methods
“Relevant” antibiotics were defined as compounds with a primarily Gram-positive spectrum of activity, i.e., vancomycin, teicoplanin, linezolid and daptomycin. Patients receiving other or no antibiotics were grouped into the category “irrelevant or no antibiotics.”
The impact of administration of relevant antibiotics on the outcome of patients treated for CLL and relapsed lymphoma was analyzed. Outcomes assessed were OR, complete response (CR), PFS and OS. The prognostic meaning of administration of relevant antibiotics was evaluated applying the Kaplan–Meier methodology, Log-rank test and Cox proportional-hazards regression analysis. For all analyses, a complete case analysis was performed.
Complete and partial response were pooled as OR. PFS and OS were calculated from randomization (CLL) or start of treatment (relapsed lymphoma), respectively, to disease progression or death. Subjects without documented event for PFS or OS were censored at date of last information. Clinical outcomes were assessed according to NCI-sponsored Working Group criteria and the Revised Response Criteria for Malignant Lymphoma, respectively.Citation13,14 All statistical tests were two-sided and statistical significance was defined by a p value less than 0.05. The analyses were performed with SPSS V21.0 and SAS 9.2.
Variables
The following variables were considered for both, the CLL and relapsed lymphoma patient populations: administration of relevant antibiotics (y/n), age < = 60 y (y/n), ECOG (0–5), female gender (y/n), dose reduction (y/n), OR (y/n), CR (y/n), PFS and OS.
Additionally, variables specific to the underlying condition and associated treatments were analyzed. For the CLL dataset, this included treatment with rituximab (y/n), CIRS score (0–8), Binet stage (A/B/C), genomic aberrations by fluorescent in situ hybridization (FISH; 17p deletion/11q deletion/trisomy 12/normal/13q deletion), immunoglobulin heavy variable chain gene (IGHV) mutated (y/n), serum thymidine kinase (s-TK) > 10 U/L (y/n), serum β2-microglobuline (s-β2m) > 3.5 mg/L (y/n), TP53 mutated (y/n), SF3B1 mutated (y/n) and NOTCH1 mutated (y/n).
For the relapsed lymphoma data set, additional variables included type of lymphoma (Hodgkin's lymphoma/diffuse large cell B-cell lymphoma/T-cell lymphoma/other), Ann Arbor classification at treatment initiation stage ≥ 3 (y/n), time from initiation of last chemotherapy to current relapse treated with (R)-DHAP (days), ECOG ≥ 2 (y/n), carboplatin instead of cisplatin (y/n), administration of < 2 cycles of (R)-DHAP (y/n).
Ethical concerns
For the CLL8 trial and the CoCoNut study, the institutional review board and ethics committee of each institution approved the study protocol. Each patient provided written informed consent before enrolment into the CLL8 trial in accordance with the declaration of Helsinki.
Disclosure of potential conflicts of interest
NP has been a consultant to Novartis and received travel grants from Jazz Pharmaceuticals. JJV has received research grants from Astellas, Gilead Sciences, Infectopharm, Pfizer, and Essex/Schering-Plough; and served on the speakers' bureau of Merck Sharp Dohme/Merck, Gilead Sciences, Pfizer, and Essex/Schering-Plough. DT has served at the speakers' bureau of Astellas Pharma and received honoraria from Merck Sharp and Dohme. LB has received honoraria from MSD and served at the speakers' bureau of Astellas Pharma and MSD. BE has been a consultant to Gilead Sciences, Janssen-Cilag, Mundipharma, GlaxoSmithKline. She has served at the speakers' bureau of Mundipharma, GlaxoSmithKline, Janssen-Cilag, Gilead Sciences, Celgene. She has received research grants from Roche, Mundipharma. She has received travel grants from Roche, GlaxoSmithKline and Mundipharma. PC has served at the speakers' board of Janssen and F. Hoffmann-LaRoche. She has received research funding from Gilead Sciences, F. Hoffmann-LaRoche, GlaxoSmithKline and Janssen-Cilag. She has received travel grants from Astellas Pharma, F. Hoffmann-LaRoche, Gilead Sciences, Janssen-Cilag and Mundipharma. MvBB has received honoraria from Astellas Pharma, Merck Sharp and Dohme and Amgen. He has received received speaker fees from Astellas Pharma and research funding from Astellas Pharma, MSD, Roche, Amgen and Takeda. He has received travel grants from Astellas Pharma, Roche, Amgen, MSD and Mologen. OAC is supported by the German Federal Ministry of Research and Education (BMBF grant 01KN1106) and the European Commission, and has received research grants from, is an advisor to, or received lecture honoraria from 3M, Actelion, Astellas, AstraZeneca, Basilea, Bayer, Celgene, Cidara, Cubist/Optimer, Da Volterra, Daiichi Sankyo, F2G, Genentech, Genzyme, Gilead, GSK, Medpace, Merck Serono, MSD, Miltenyi, NanoMR, Novartis, Parexel, Pfizer, Quintiles, Rempex, Roche, Sanofi Pasteur, Shionogi, Summit, Vical, Vifor, Viropharma. MJGTV has served at the speakers' bureau of Pfizer, Merck/MSD, Gilead Sciences, and Astellas Pharma, received research funding from 3M, DaVolterra, Gilead Sciences and Astellas Pharma and been a consultant to Astellas Pharma, Berlin Chemie, Merck/MSD and DaVolterra. JB, MH, SK, KF, AMF and SS have no conflicts of interest.
KONI_A_1150399_s02.docx
Download MS Word (347.7 KB)Acknowledgments
NP has contributed in conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing and final manuscript approval. JJV has contributed in conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing and final manuscript approval. DT has contributed in collection and assembly of data, manuscript writing and final manuscript approval. LB has contributed in collection and assembly of data, manuscript writing and final manuscript approval. BE has contributed in data analysis and interpretation, manuscript writing and final manuscript approval. PC has contributed in data analysis and interpretation, manuscript writing and final manuscript approval. MvBB has contributed in data analysis and interpretation, manuscript writing and final manuscript approval. MH has contributed in collection and assembly of data, data analysis and interpretation, manuscript writing and final manuscript approval. OAC has contributed in collection and assembly of data, data analysis and interpretation, manuscript writing and final manuscript approval. MJGTV has contributed in conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing and final manuscript approval. JB has contributed in data analysis and interpretation, manuscript writing and final manuscript approval. KF has contributed in conception and design, manuscript writing and final manuscript approval. SK has contributed in data analysis and interpretation, manuscript writing and final manuscript approval. AMF has contributed in collection of data, manuscript writing and final manuscript approval. SS has contributed in data analysis and interpretation, manuscript writing and final manuscript approval.
References
- van der Velden WJ, Herbers AH, Netea MG, Blijlevens NM. Mucosal barrier injury, fever and infection in neutropenic patients with cancer: introducing the paradigm febrile mucositis. Br J Haematol 2014; 167:441-52; PMID:25196917; http://dx.doi.org/10.1111/bjh.13113
- Vehreschild MJ, Meissner AM, Cornely OA, Maschmeyer G, Neumann S, von Lilienfeld-Toal M, Karthaus M, Wattad M, Staib P, Hellmich M et al. Clinically defined chemotherapy-associated bowel syndrome predicts severe complications and death in cancer patients. Haematologica 2011; 96:1855-60; PMID:21859736; http://dx.doi.org/10.3324/haematol.2011.049627
- Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013; 342:967-70; PMID:24264989; http://dx.doi.org/10.1126/science.1240527
- Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013; 342:971-6; PMID:24264990; http://dx.doi.org/10.1126/science.1240537
- Lisboa LF, Miranda BG, Vieira MB, Dulley FL, Fonseca GG, Guimaraes T, Levin AS, Shikanai-Yasuda MA, Costa SF. Empiric use of linezolid in febrile hematology and hematopoietic stem cell transplantation patients colonized with vancomycin-resistant Enterococcus spp. Int J Infect Dis 2015; 33:171-6; PMID:25660090; http://dx.doi.org/10.1016/j.ijid.2015.02.001
- Paul M, Dickstein Y, Borok S, Vidal L, Leibovici L. Empirical antibiotics targeting Gram-positive bacteria for the treatment of febrile neutropenic patients with cancer. Cochrane Database Syst Rev 2014; 1:CD003914; PMID:24425445; http://dx.doi.org/10.1002/14651858.CD003914.pub3
- Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 2015; 517:205-8; PMID:25337874; http://dx.doi.org/10.1038/nature13828
- Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, No D, Gobourne A, Viale A, Dahi PB et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014; 124:1174-82; PMID:24939656; http://dx.doi.org/10.1182/blood-2014-02-554725
- Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, Liu C, West ML, Singer NV, Equinda MJ et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med 2012; 209:903-11; PMID:22547653; http://dx.doi.org/10.1084/jem.20112408
- Jenq R, Taur Y, Devlin S, Ponce D, Goldberg J, Ahr KF, Littmann ER, Ling L, Gobourne AC, Miller LC et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol Blood and marrow Transplant 2015; 21:1373-83; PMID:25977230; http://dx.doi.org/10.1016/j.bbmt.2015.04.016.
- Weber D, Oefner PJ, Hiergeist A, Koestler J, Gessner A, Weber M, Hahn J, Wolff D, Stammler F, Spang R et al. Low urinary indoxyl sulfate levels early after transplantation reflect a disrupted microbiome and are associated with poor outcome. Blood 2015; 126:1723-8; PMID:26209659; http://dx.doi.org/10.1182/blood-2015-04-638858
- Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, Hensel M, Hopfinger G, Hess G, von Grunhagen U et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376:1164-74; PMID:20888994; http://dx.doi.org/10.1016/S0140-6736(10)61381-5
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ; International Workshop on Chronic Lymphocytic Leukemia. Blood. 2008 Jun 15; 111(12):5446-56; http://dx.doi.org/10.1182/blood-2007-06-093906.
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25:579-86; PMID:17242396; http://dx.doi.org/10.1200/JCO.2006.09.2403