ABSTRACT
Histamine receptor 2 (H2) antagonists are widely used clinically for the control of gastrointestinal symptoms, but also impact immune function. They have been reported to reduce tumor growth in established colon and lung cancer models. Histamine has also been reported to modify populations of myeloid-derived suppressor cells (MDSCs). We have examined the impact of the widely used H2 antagonist ranitidine, on both myeloid cell populations and tumor development and spread, in three distinct models of breast cancer that highlight different stages of cancer progression. Oral ranitidine treatment significantly decreased the monocytic MDSC population in the spleen and bone marrow both alone and in the context of an orthotopic breast tumor model. H2 antagonists ranitidine and famotidine, but not H1 or H4 antagonists, significantly inhibited lung metastasis in the 4T1 model. In the E0771 model, ranitidine decreased primary tumor growth while omeprazole treatment had no impact on tumor development. Gemcitabine treatment prevented the tumor growth inhibition associated with ranitidine treatment. In keeping with ranitidine-induced changes in myeloid cell populations in non-tumor-bearing mice, ranitidine also delayed the onset of spontaneous tumor development, and decreased the number of tumors that developed in LKB1−/−/NIC mice. These results indicate that ranitidine alters monocyte populations associated with MDSC activity, and subsequently impacts breast tumor development and outcome. Ranitidine has potential as an adjuvant therapy or preventative agent in breast cancer and provides a novel and safe approach to the long-term reduction of tumor-associated immune suppression.
Abbreviations
| H1 | = | Histamine receptor 1 |
| H2 | = | Histamine receptor 2 |
| H4 | = | Histamine receptor 4 |
| MDSCs | = | myeloid-derived suppressor cells |
| TAMs | = | tumor-associated macrophages |
Introduction
Selective H2 antagonists such as ranitidine and famotidine are some of the most frequently used drugs for the treatment of upper gastrointestinal disorders. In recent years, these drugs have frequently been replaced by proton pump inhibitorsCitation1,2 due to their improved potency and in view of anecdotal reports of myelo-suppressive actions of H2 antagonists.Citation3,4 However, there remains a large population of people, with and without cancer, that regularly take H2 antagonists. Information on how these drugs impact cancer immunity is limited.
H2 expression has been reported on several tumor cell typesCitation5-7 including some types of breast cancer (reviewed in ref. Citation8). Other cells within the tumor microenvironment can also express H2, including immune effector cells, endothelial cells, epithelial cells, and fibroblasts.Citation9,10 H2 signaling has been shown to both enhanceCitation11 and inhibit tumor cell growth in vitro.Citation5,12 H2 antagonists have been reported to be effective in the treatment of certain cancer types, specifically H2-expressing colorectal cancers Citation(13,14, reviewed in ref. Citation15) although the mechanism of these responses remains unclear.
Antitumor immune responses, including tumor immune surveillance, are complex and involve both innate and acquired components. Effective host immune function can reduce the incidence of tumors, limiting their growth and subsequent metastasis. NK cells and CD8+ T cells are recognized as critical effector cells in antitumor immunity and tumor surveillance. Monocytes and macrophage subsets are pivotal in local immune regulation within the tumor microenvironment. Tumor-associated macrophages and a population of cells known as MDSCs that includes both immature neutrophilic and monocytic subsets, have been shown to reduce effective antitumor immune function by inhibiting T cells, NK cells, dendritic cells, and cytotoxic macrophages,Citation16-22 and inducing T regulatory cell development.Citation23 MDSCs have been found to be a barrier in inducing cancer immunity with immunotherapy, and combination treatments that include drugs such as gemcitabine are used to decrease MDSC levels.Citation24-27 In mice, MDSCs can be identified, in part, by the expression of surface markers CD11b, Ly6C and Ly6G, which denote their monocyte and neutrophil lineage.Citation28
Histamine can modulate multiple immune effector cells via H2. It has been implicated in enhanced mobilization of dendritic cells, and decreased IL-12 secretion,Citation29-32 which can then alter subsequent antibody generation, and NK and T cell activity. H2 receptors can also modulate the cytolytic activity of NK cells,Citation33,34 inhibit both Th1 and Th2 cytokine secretionCitation35 and enhance IL-10 secretion.Citation36 Conversely, H2 blockade on T cells alleviates suppression of IFNγ secretionCitation29-31,37 and inhibits IL-10 production.Citation36 H2 signaling on monocytes can block expression of adhesion molecules involved in T cell activation, such as ICAM-1 and CD40. Therefore, in the context of an H2 antagonist, enhanced T cell proliferation and IFNγ secretion can ensue.Citation31 H2 blockade can also alter the cytolytic activity of NK and CD8+ cells, in general reducing NK activityCitation33 and enhancing CD8+ activity.Citation38 Despite these findings, the role of H2 antagonists as regulators of effective immune responses to breast cancer has not been systematically examined.
There are a few studies looking at the impact of histamine on MDSCs. MDSCs express H1 and H2 and immature myeloid cells express histidine decarboxylase (HDC) which is important for MDSC development. HDC expression by myeloid cells was shown to impact tumor growth in a colon cancer model.Citation39 Histamine from mast cells can also modify MDSC activity and symptomatic allergic patients have increased MDSC function although the impact of H2 receptors antagonists was not directly addressed.Citation39-41 A study using cimetidine which has both H2 antagonist and a number of off target effects showed evidence of cimetidine-induced MDSC apoptosis. However, the more selective H2 antagonist famotidine did not have similar effects.Citation40
In the current study, the ability of the widely used selective H2 antagonist ranitidine to modify key myeloid populations in the context of breast tumors was determined. Breast tumor development and metastasis was also studied in three distinct mouse models that highlight different stages of tumor development and spread. Our results demonstrate that ranitidine reduces select populations of monocytic cells consistent with an impact on MDSC, and inhibits initial tumor development, primary tumor growth and metastasis to the lung in breast cancer models.
Results
Ranitidine treatment reduces CD11b+Ly6Chi cells in the spleen and the bone marrow in both naive and tumor-bearing mice
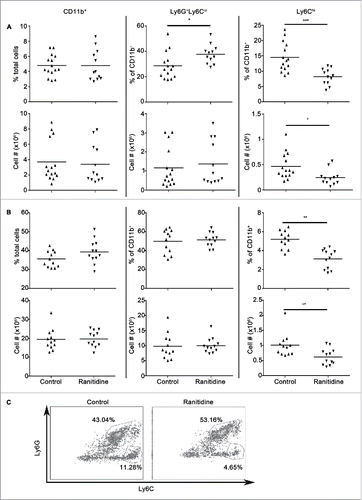
The H2 antagonist ranitidine was orally administered to groups of BALB/c mice at a dose of 8 mg/kg for 8 d and the impact of treatment on splenic and bone marrow myeloid cell populations was determined. Myeloid cells can be identified, in part, by the expression of surface markers CD11b, Ly6C and Ly6G, which denote their monocyte (CD11b+Ly6Chi) and neutrophil (CD11b+Ly6G+Ly6Clo) lineage. Ranitidine treatment was associated with an increased percentage of Ly6G+Ly6Clo cells and decreased percentage of Ly6Chi cells within CD11b+ splenocytes. This was reflected by a decreased frequency of CD11b+Ly6Chi splenocytes (). Total splenocyte numbers were unaltered by ranitidine treatment (7.2 × 107 ± 8.8 × 106, n = 15 vs. 6.4×107 ± 7.5 × 106, n = 12 cells in control and ranitidine-treated, respectively). Bone marrow cells from ranitidine-treated mice also showed a decreased percentage of Ly6Chi cells within the CD11b+ cells and a decreased frequency of CD11b+Ly6Chi cells (). Total bone marrow cellularity was not significantly decreased in ranitidine-treated animals (5.5 × 107 ± 4.0 × 106, n = 12 vs. 5.1 × 107 ± 3.6 × 106, n = 12 cells in control and ranitidine-treated, respectively).
Figure 1. Ranitidine treatment decreases CD11b+Ly6Chi population in the spleen and bone marrow of BALB/c mice. Composition of total CD11b+ cells, Ly6G+Ly6Clo granulocytic cells, and Ly6Chi monocytic cells in spleen (A) and bone marrow (B) of non-tumor-bearing mice with and without 8 d of ranitidine treatment. (C) Representative flow cytometry data showing percentages of Ly6G+Ly6Clo and Ly6Chi in CD11b+ splenocytes. Data points represent individual mice and line represents the mean per group. p < 0.05, p < 0.001, unpaired t-test.
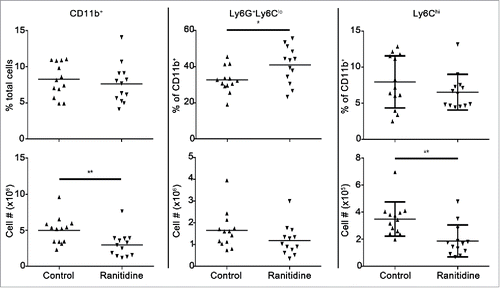
Certain subsets of myeloid cells, such as MDSCs, are primarily upregulated in the context of cancer (for a review see ref. Citation28), therefore the effect of ranitidine on the splenocyte populations in mice bearing 4T1 breast tumors, associated with modulation of MDSC’s,Citation42 was examined. Mice were treated with ranitidine or left untreated for 8 d in the context of breast tumor development. Similar to what was observed in naive mice, there was an increase in the proportion of CD11b+Ly6G+Ly6Clo cells following ranitidine treatment () and a decrease in CD11b+ Ly6Chi monocytic cells in the spleen. There was an overall decrease in the numbers of myeloid cells by approximately 40% in the spleen, which could be attributed to a decrease in monocytes with ranitidine treatment. There was no overall change in lymphoid cell popu-lations.
Figure 2. Ranitidine treatment decreases CD11b+Ly6Chi population in the spleen of 4T1 tumor-bearing BALB/c mice. Composition of total CD11b+ cells, Ly6G+Ly6Clo granulocytic cells, and Ly6Chi monocytic cells in spleen of 4T1 tumor-bearing mice with and without 8 d ranitidine treatment, starting one day prior to tumor cell injection. Data points represent individual mice and line represents the mean per group. p <0.05, p <0.01, unpaired t-test.
Histamine receptor 2 antagonists decrease lung metastasis in the 4T1 breast cancer model
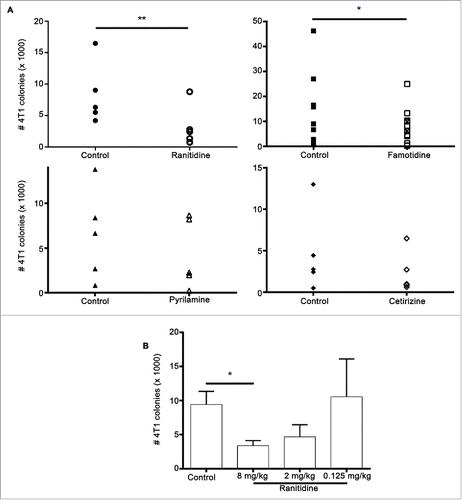
Given that ranitidine altered the populations of myeloid cells in the spleen, the ability of ranitidine treatment to alter tumor outcome was examined. Ranitidine and other selected histamine receptor antagonists, including cetirizine (H1), JNJ7-777120 (H4), cimetidine and pyrilamine (H1 and H2) and famotidine, an alternate H2 antagonist, were examined. None of the drugs showed a significant effect on 4T1 primary tumor endpoint weight (Table S1) or in the growth kinetics of the tumor over 19–21 d (Fig. S1). However, ranitidine had a significant impact on lung tumor metastasis with a mean percent inhibition of 61% compared with control-treated mice (). Animals given oral famotidine also showed a significant decrease (mean percent inhibition of 58%) in lung metastasis. Pyrilamine showed a trend toward metastasis inhibition (mean percent inhibition of 34%) while cetirizine and JNJ7777120 showed no effect on metastasis. The anti-metastatic effect of ranitidine was dose dependent, with the greatest inhibition at an oral dose of 8 mg/kg. Lung tumor burden was similar to control 4T1 tumor bearing mice when a dose of 0.125 mg/kg of ranitidine was administered ().
Figure 3. Histamine receptor antagonists inhibit 4T1 metastasis. (A) Average number of 4T1 colonies derived from lungs of tumor-bearing BALB/c mice treated with ranitidine (8 mg/kg), famotidine (□ 8mg/kg and ⊠ 2mg/kg), pyrilamine (10 mg/kg), and cetirizine (10 mg/kg). (B) Number of 4T1 colonies derived from lungs of tumor-bearing mice treated with decreasing doses of ranitidine. Data points in (A) represent mean of 3–4 mice per group per experiment; data in (B) represent mean ± SEM of 3–42 mice. p < 0.05, p < 0.01, paired t-test (A), ANOVA followed by a Dunnett's test (B).
Evaluation of potential direct effects of histamine receptor antagonists on tumor growth
Some breast cancer cells as well as normal breast tissue can express H2 receptorsCitation43 (for a review see ref. Citation8). Neither H1 nor H2 receptor antagonists had a direct effect on 4T1 cell proliferation or ability to migrate in vitro (Fig. S2). E0771 cells were similarly unaffected by histamine receptor antagonist treatment in vitro (data not shown). In keeping with these findings, neither 4T1 cells nor E0771 cells expressed H1 or H2 receptors (Fig. S3). These findings confirmed that H2 antagonists are not directly affecting the tumor cells via H2 receptors.
CD8+ T cells are not essential for ranitidine effects on metastasis
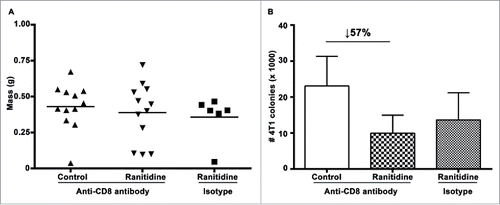
Previous studies have shown that histamine can inhibit CD8+ T cell activation through a decrease in IL-12 productionCitation29 and an increase in IL-10,Citation36 which would lead to a decrease in Th1 cells and IFNγ secretion. Therefore, tumor growth and metastasis in the presence or absence of ranitidine following antibody mediated CD8+ T cell depletion within recipient mice was compared. As in fully immunocompetent mice, there was no alteration in final tumor weight and tumor growth kinetics when mice were treated with ranitidine (). However, there was still a decrease in lung tumor metastasis following ranitidine treatment, even in the absence of CD8+ T cells, with a mean percent inhibition of 57% (three experiments, four mice/group) between control and ranitidine-treated CD8-depleted mice (compared to 61% mean percentage inhibition between control and ranitidine-treated mice without CD8 depletion, ). Therefore, CD8+ T cells are not essential for the ranitidine-induced inhibition of metastasis in the 4T1 model.
Figure 4. Ranitidine does not affect lung metastases by directly affecting CD8+ activity. (A) Final tumor weight of ranitidine treated mice with and without CD8+ depletion. (B) Number of 4T1 cell colonies that developed from lungs of tumor bearing BALB/c mice treated with ranitidine with and without CD8+ depletion. Data from (A) represent average of individual mice and line represents mean per group. Data in (B) represents mean ± SEM of 6–12 mice per group.
Mice treated with ranitidine demonstrate decreased suppression of T cell function compared with control animals
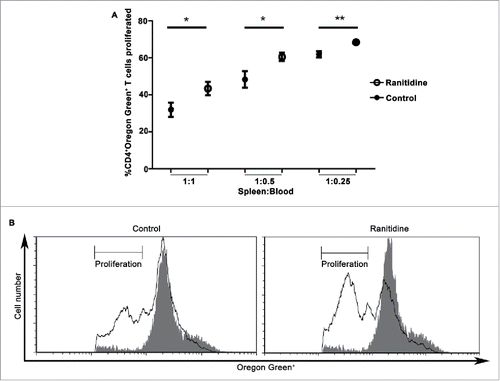
In view of the observed changes in myeloid cells bearing the markers associated with MDSCs, the ability of circulating cells from ranitidine-treated mice to modify T cell proliferation was examined. In view of our results which suggested CD8+ T cells are not responsible for the effect ranitidine has on metastasis, the effect of potential MDSCs were analyzed on CD4+ T cell proliferation. Leukocytes derived from ranitidine-treated mice were significantly less able to suppress the CD4+ T cell proliferation in response to antigen (p < 0.05) when compared with cells from control mice, with the level of proliferation increasing from 32% to 43% (). L-NMMA and nor-NOHA were used, at doses shown to be effective in similar systems, to inhibit NOS2 and Arg1.Citation44,45 However, neither inhibitor alone was able to significantly reduce the inhibitory activity of MDSCs. There was a trend toward decreased suppressive activity with simultaneous inhibition of NOS2 and Arg1 in animals that did not receive ranitidine treatment (mean 13.4% increase in proliferation, with 5/7 mice showing evidence of inhibited MDSC function) which was not observed in the ranitidine treated group that had been shown to have reduced MDSC function (mean 1.6% decrease in proliferation) following NOS2 and Arg1 blockade (n = 7, data not shown). Our results suggest that although there was altered functional activity of MDSCs from mice treated with ranitidine, the main mechanism of suppression of CD4+ T cell responses in this model was not dependent on NOS2 or Arg1.
Figure 5. Peripheral blood leukocytes from ranitidine-treated tumor-bearing mice have decreased suppressive functions. Peripheral blood leukocytes from 4T1 tumor-bearing mice with and without treatment were isolated and plated with Oregon Green-labeled D011.10 splenocytes with ova323-339. After 3 d incubation, proliferation was measured. Data points in (A) represents mean ± SEM of 9 mice. (B) Representative data of one mouse/treatment. Gray histogram represent unstimulated Oregon Green+CD4+ cells. p < 0.05, p < 0.01, unpaired t-test.
Ranitidine decreases primary tumor growth in a second orthotopic model
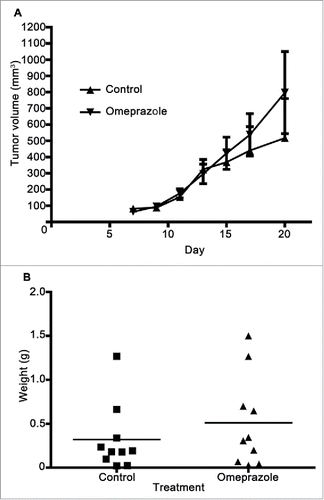
To examine whether the effect of ranitidine was specific to the 4T1 model, a similar experiment using the less metastatic E0771 mouse model of breast cancer in C57BL/6 was performed. Initially, similar tumor growth kinetics was observed with and without ranitidine treatment. However, a decrease in tumor growth occurred in ranitidine-treated animals starting at approximately day 13 post tumor cell injection (). A similar regression was not observed in animals that received control drinking water. At the time of tumor harvest, a significantly decreased final tumor weight was observed in animals that had received ranitidine treatment compared to the control animals (). This simpler, short-term model was employed for additional studies of the mechanism of ranitidine-dependent tumor inhibition. The impact of omeprazole treatment on tumor growth was examined to control for any impact of reduced stomach acid on microbiome or related immune responses. Omeprazole treatment did not alter E0771 tumor growth when compared to control mice (). No significant metastasis to the lung was observed in this model within the experimental time frame.
Figure 6. Ranitidine treatment decreases E0771-GFP tumor growth. (A) E0771 tumors in C57BL/6 mice treated with ranitidine (8 mg/kg) were measured every 2 d starting 7 d post E0771-GFP cell injection. (B) At day 21, the primary tumor was excised and weighed. Data in (A) represents the mean ± SEM tumor volume of 11–12 mice/point. Data points in (B) represent final tumor weight of individual mice. p < 0.05, unpaired t-test.
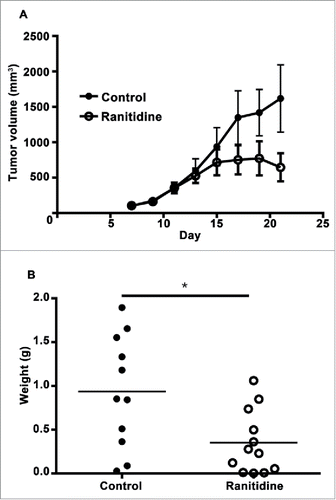
Figure 7. Omeprazole treatment does not alter E0771-GFP tumor development. (A) E0771 tumors in C57BL/6 mice treated with omeprazole (1.275 mg/d in mash) were measured every 2 d starting 7 d post E0771-GFP cell injection. (B) At day 20, the primary tumor was excised and weighed. Data in (A) represents the mean ± SEM tumor volume of 10 mice/point. Data points in (B) represent final tumor weight of individual mice.
Ranitidine does not alter E0771 development if monocytes are depleted by gemcitabine treatment
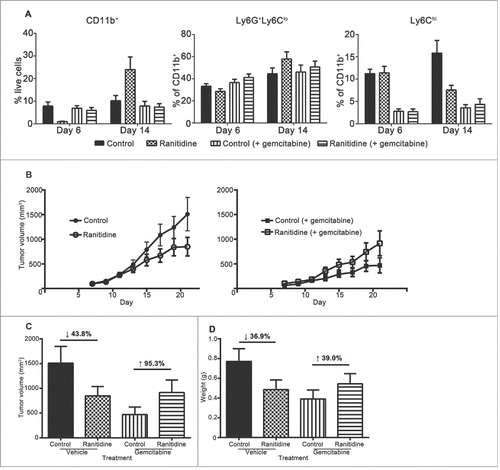
To determine whether the effect ranitidine has on E0771 development was dependent on MDSCs, gemcitabine treatment was used for depletion of MDSCs in vivo.Citation24,25,42,46 Analysis of circulating MDSCs a day after gemcitabine treatment revealed that monocytes were preferentially depleted, with no significant alteration in neutrophils (). Gemcitabine-treated mice had decreased tumor growth compared to untreated mice, and gemcitabine combined with ranitidine-treated mice showed similar tumor development as those treated with gemcitabine alone ().
Figure 8. Gemcitabine treatment prevents ranitidine-induced tumor growth inhibition. (A) Blood samples were taken from each mouse one day after gemcitabine (or vehicle) injection and stained for myeloid cells. (B) E0771 tumors in C57BL/6 mice treated with ranitidine (8 mg/kg) with/without gemcitabine treatment were measured every 2 d starting 7 d post E0771-GFP cell injection. (C–D) At day 20, the primary tumor was measured (C), excised and weighed (D). Data in (A) represents the mean ± SEM of 4–8 mice/group. Data in (B) represents the mean ± SEM tumor volume of 12–24 mice/point. Data points in (C–D) represent the mean ± SEM of 12–24 mice/group.
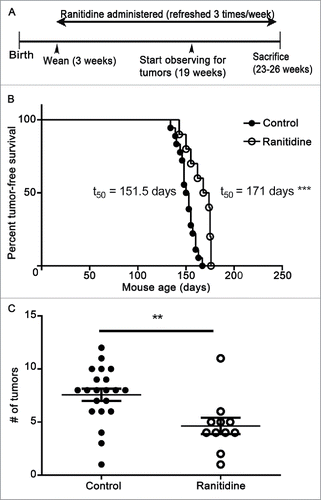
Long-term ranitidine treatment is associated with increased latency in breast tumor onset and a decrease in number of tumors in LKB1−/−/NIC mice
A spontaneous breast tumor model provides a more similar scenario to clinical cancer, and allows assessment of the impact of potential treatments on early tumor development. Therefore, experiments were performed in LKB1−/−/NIC mice, which normally develop mammary tumors within 20–25 weeks of birth.Citation47 LKB1−/−/NIC mice were given ranitidine in the drinking water, initiated at the time of weaning (21 d after birth). 50% of untreated LKB1−/−/NIC mice develop primary mammary tumors by 147 d after birth.Citation47 However, in ranitidine-treated mice there was a significant increase in latency of tumorigenesis by an additional 24 d; t50 of 171 d (). Furthermore, control mice typically have to be euthanized at week 22 due to large tumors and high tumor burden (average of eight tumors/mice) to comply with ethical endpoints. However, mice treated with ranitidine had a reduced tumor burden allowing them to survive until the planned end of the experiment, at week 26. On average, tumor burden at this time point was four tumors/mice, significantly below that observed in the control animals ().
Figure 9. Ranitidine increased breast tumor onset latency and decreases final tumor numbers in LKB1−/−/NIC mice. (A) Representative timeline of experiment. (B) Mice were examined weekly for palpable breast tumors. (C) Endpoint number of tumors was counted. (B) Representative tumor-free survival of control (n=15) and ranitidine-treated (n=10) mice. Data points in (C) represent number of tumors in individual mice. p < 0.001, Log rank test (B); p < 0.01, unpaired t-test (C).
Discussion
New approaches to enhancing effective immune responses to breast cancer are urgently required both as an approach to cancer prevention and to improve the effectiveness of other treatments. Modulation of tumor-associated immune suppression, specifically MDSC populations provides a potential approach to enhancing acquired antitumor immunity which could be effective at multiple stages of tumor development and spread. In the current study, ranitidine treatment in vivo caused a decrease in select monocyte populations in both the spleen and the bone marrow of both naïve and breast tumor-bearing mice. Blood from ranitidine-treated, tumor-bearing mice also showed a reduced ability to suppress T cell proliferation, and depleting MDSCs through gemcitabine treatment ablated the effect of ranitidine on tumor growth. H2 antagonists, when administered in drinking water at doses within the clinical therapeutic range, inhibited lung metastasis in the 4T1 breast tumor model and led to inhibition of initial tumor growth in the E0771 breast cancer model. H2 antagonist treatment also increased the time before tumor development and reduced the number of tumors developing in the LKB1−/−/NIC mouse model of spontaneous breast tumor development. Overall, H2 blockade was beneficial in these breast tumor models. These results are surprising considering that previous studies report that ranitidine decreases the cytotoxic activity of NK cellsCitation33 and that histamine signaling through H2 inhibits reactive oxygen species synthesis by monocytes, thereby enhancing NK cell activity.Citation48-50 These findings demonstrate that ranitidine treatment can function at a variety of stages during tumor development and in multiple breast tumor settings, consistent with an impact on tumor-associated immune suppression.
One previous study has analyzed whether treatment with H2 antagonists can alter breast tumor outcome,Citation51 but in these patients, treatment was provided only at very late stages of disease. In keeping with our findings, another study has suggested a decreased risk of developing lung cancer with long-term H2 antagonist treatment.Citation52 Our results suggest that H2 antagonists could have a potentially beneficial effect if administered either before tumor development or at an early point in tumor development, particularly in those tumor settings where limiting MDSC function would be beneficial. The beneficial effects of ranitidine treatment are not dependent upon H2 expression by the tumor cells and neither of the injectable tumor models we used expressed this receptor.
As H2 antagonists block acid secretion in the stomach, this can cause alterations in the intestinal microbiomeCitation53 which could then alter the immune response.Citation54,55 A recent study revealed that altering the gut microbiome in mice with a predisposition to developing breast tumors increased the number of tumors the mice had, a change that was related to neutrophil activity.Citation56 Omeprazole is a proton pump inhibitor that is capable of inhibiting acid secretion without binding H2 receptors. Other studies have shown that omeprazole can inhibit breast cancer cell proliferation in vitro and experimental metastasis in vivo,Citation57,58 but our results show that, unlike ranitidine, omeprazole treatment does not inhibit E0771 tumor growth.
Eight days of ranitidine treatment were associated with a significant decrease in monocytes in the spleen and bone marrow. This is consistent with the known myelo-suppressive effect of ranitidine.Citation3,4 In humans, it is thought that such myelo-depletion is primarily associated with neutropenia.Citation4 In mice, the ranitidine-induced depletion we observed was monocyte specific. Previous results have shown that the less selective H2 antagonist cimetidine can cause apoptosis of MDSCs,Citation40 but these results were not replicated by the alternate H2 antagonist famotidine, and were not reversed by histamine, suggesting an “off target” mechanism of cimetidine action.
In general, monocytes and macrophages have several pro-tumorigenic effects. Tumor-associated macrophages are important for angiogenesis and microenvironment modulation (for a review, see ref.Citation59). Decreasing the monocyte populations could thereby inhibit tumor growth and development.Citation60,61 The monocyte population of MDSCs is also considered more immunosuppressive than the neutrophil population.Citation62 Ranitidine has previously been demonstrated to have a number of effects on monocytes including modulation of adhesion molecule expression, in keeping with the known functions of H2.Citation31 However, it remains possible that the impact of ranitidine on monocyte populations may be indirect.
MDSCs are a key cell type involved in immune suppression in cancer and can also directly impact tumor cells to induce growth and metastasis.Citation63,64 Increases in circulating MDSCs correlate with disease progression in breast cancer patientsCitation65 and circulating MDSCs in cancer patients are shown to inhibit T cell proliferation.Citation64,65 In our experiments, blood leukocytes from ranitidine-treated 4T1 tumor-bearing mice had less suppressive activity on antigen-driven T cell proliferation than those from control tumor bearing mice. These findings confirm that ranitidine treatment causes an alteration in the functional MDSCs in the blood. Both neutrophilic and monocytic MDSCs could contribute to this response. In keeping with these findings, histamine and histamine-producing cells have previously been demonstrated to have a key role in MDSC regulation in mice, although the most profound effects were observed on granulocytic MDSC's.Citation41,66 This study by Martin et alCitation41 stated that granulocytic MDSCs can be impacted by both H1 and H2 antagonists, but our studies showed no effect by H1 antagonists. This may be in part due to the impact H1 has on other immune cells, which could offset the positive effects H1 blockade has on MDSC inhibition.
NOS2 and Arg1 are often thought of as the predominant mediators in MDSC-mediated suppression but inhibition of these pathways did not significantly modify the suppression of CD4+ T cell responses by peripheral blood cells from 4T1 tumor-bearing mice. However, some populations of MDSCs have been shown to cause suppression via NOS2- and Arg1-independent mechanisms. MDSCs also synthesize reactive oxygen species (ROS), and in some cases have been shown to be the primary mediator in T cell suppression.Citation67-69 In a study by Nagaraj et al., ROS synthesis was the primary mediator of suppression by MDSC, and when gp91 was knocked out, therefore leading to an inability to produce ROS, MDSC function was lost, while MDSCs deficient in NOS2 still had suppressive activity.Citation70 Another study showed that production of TGFβ by MDSCs was very important for hindering tumor immunosurveillance,Citation18,71,72 and that TGFβ in humans is important for immune suppression with no contribution by NOS2 and Arg1 activity.Citation73 Overall, our data may signify that the impact ranitidine had on alterations in immune suppression was dependent on an alteration in the number of MDSCs present in circulation, not due to ranitidine altering expression of NOS2 and Arg1 and also suggests that TGF-β, ROS or a combination of such pathways mediate MDSC function in the blood, in this model.
Gemcitabine is widely used as an inhibitor of MDSC'sCitation24 and was utilized to investigate the role of such MDSCs in the E0771 model of ranitidine-inhibited primary tumor growth. Gemcitabine can selectively cause depletion of MDSCs, while not altering other immune cell numbers.Citation25,46 This depletion was specific to monocytic MDSCs in the E0771 model. Following gemcitabine treatment, ranitidine did not have an effect on tumor development. Notably, other cells from the monocytic lineage, including mature macrophages and tumor-associated macrophages (TAMs) are not impacted by short-term gemcitabine treatment.Citation74 However, it remains possible that long-term ranitidine treatment could alter TAM populations by altering monocytes that would otherwise be recruited and differentiated into TAMs.
H2 blockade has been shown to alleviate inhibition of IFNγ functionCitation35 and modify DC function and migration.Citation29,32 Notably, CD8+ T cell depletion did not alter ranitidine’s effect on 4T1 metastasis. Although CD8+ T cells are important in tumor clearance, they are not the only cell involved.Citation75 MDSCs have been reported to have inhibitory effects on several immune effector cell types including NK cellsCitation18,19 and CD4+ T cellsCitation76,77 directly,Citation21 or via inhibition of DC functionCitation20 or induction of regulatory T cell development.Citation78 In addition, antibody-mediated processes such as complement mediated lysis and antibody-dependent cell-mediated cytotoxicity can also be affected by MDSCs.
In this study, ranitidine had an impact on three distinct models of breast cancer. We have confirmed that this is not due to a direct effect on the tumor cells in the two orthotopic models used. The different degrees and levels of tumor inhibition may be attributed to the differences in mouse genetic backgroundsCitation79,80 and/or the differences in the biology of the tumors themselves as they interact with the immune system. 4T1 cells are not highly immunogenic.Citation81 Modification of monocyte populations, such as MDSCs, has previously been shown to profoundly affect the ability of the 4T1 tumor model to metastasize.Citation42,63,82 The E0771 cell line appeared to be more vulnerable to immune changes mediated by ranitidine at the primary tumor site. In the LKB1−/−/NIC model of spontaneous breast cancer, ranitidine is given over a longer period of time, which allows the ranitidine to modify immune effector cell populations prior to tumor development. The impact of ranitidine-induced MDSC changes on tumor growth and spread will likely vary extensively between tumors and also be related to other effects of ranitidine on immune function of importance in regulating tumor growth or metastasis. Overall, these studies highlight the profound impact that widely used H2 antagonists can have on antitumor immune function and suggest the use of these agents may provide opportunities to reduce tumor-associated immune suppression in breast cancer or reduce breast cancer development in those at high risk.
In conclusion, we have shown that the commonly used H2 antagonist ranitidine can affect monocyte populations in both normal and tumor-bearing animals. Consistent with an inhibition of MDSC populations, ranitidine treatment can also inhibit breast tumor development and spread in three separate breast tumor models including a model of spontaneous breast tumor development. These results suggest that it may be beneficial to consider including a H2 antagonists, as opposed to other regulators of gastric acid secretion, in the context of breast cancer therapy or prevention. Clinical studies are urgently required to address these issues.
Methods
Cell lines
Mouse epithelial breast carcinoma cell line 4T1 and mouse breast adenocarcinoma cell line E0771 (ATCC) transduced with GFP were maintained in a monolayer in Dulbecco's Modified Eagle’s Medium (Hyclone) containing 10% fetal bovine serum, and 1% L-glutamine, HEPES, and penicillin/streptomycin. Selection for GFP-positive E0771 was maintained with 4 μg/mL of puromycin in the media.
Mice
All mouse experiments were pre-approved by the Dalhousie University Committee on Laboratory Animals. Five week old female BALB/c mice and C57BL/6 mice were purchased from Charles River Laboratories and housed in specific pathogen-free conditions at the Carleton Animal Care Facility at Dalhousie University. LKB1−/−/NIC female mice were bred at Dalhousie University.
In vivo orthotopic breast cancer model
Histamine antagonists were added to drinking water one day prior to tumor cell injection and were refreshed every other day. These histamine antagonists remain stable in the drinking water during this time.Citation83,84 The water bottles at this time were weighed before and after the water was changed, to be able to calculate the amount of water drank by each mouse and calculate the amount of drug taken in per mouse. Using this approach, the consumption of drug per mouse per day ranged 6–8 mg/kg. Omeprazole (O104, Sigma Aldrich) was mixed into mash (5 g/mouse/d) at an amount that would give each mouse 70 mg/kg, and given to mice every day, starting one day prior to tumor cell injection.
An adaptation of the protocol of Pulaski and Ostrand-RosenbergCitation85 was employed for orthotopic models. 6–8 week old BALB/c female mice were anesthetized and 100,000 4T1 cells in 50 μL PBS were injected subcutaneously into the mammary fat pad near the fourth nipple. The volume of the tumor was determined by caliper measurements every second day using the equation volume = length × width2/2. At day 7 or day 19–21 post injection, the mice were sacrificed and the primary tumor, spleen, femurs, and lungs were collected. The lungs were digested for one hour at 37°C in the following enzyme cocktail in HBSS: 300 U/mL collagenase VII (C2139, Sigma Aldrich), 6 U/mL elastase (LE425, Elastin Products Company, Inc.), 100 μg/mL DNAse I (11-284-932-001, Roche), 2.5 mM calcium chloride (Fisher), and 2.5 mM magnesium chloride (M2670, Sigma Aldrich), then pushed through a 100 μm nylon filter. Serial dilutions were plated in media containing 60 μM 6-thioguanine (cat. #154-42-7, Alfa Aesar). After 9–12 d of incubation, the 4T1 colonies were fixed and stained with 0.03% methylene blue (M9140, Sigma Aldrich) solution. The plates were scanned using an HP Scanjet G4050 scanner and the colonies were counted using ImageJ software.
For the E0771 model, 6–8 week old female C57BL/6 mice were anesthetized and 200,000 cells in 100 μL of Matrigel® (Corning) were injected subcutaneously into the mammary fat pad near the fourth nipple. Tumor size was tracked as described above with tumors harvested and weighed at day 21.
Spontaneous tumor development model
At the time of weaning (approximately 4 weeks), female LKB1−/−/NIC miceCitation47 were given either control or ranitidine containing water. Drug-treated water was refreshed three times per week. Mice were examined once a week starting at week 19 for tumors and tumor volume was quantified using calipers in a similar method as mentioned above. A mouse was considered tumor bearing when a tumor was palpable and measurable by calipers. At week 23–26, mice were sacrificed and the primary tumors were counted and weighed.
In vivo CD8+ cell depletion
Purified anti-CD8+ antibody (Clone 53–6.7) was generously provided by Dr Thomas Issekutz (Dalhousie University). LEAF™ Purified Rat IgG2a κ isotype control was purchased from Biolegend (Cat. #400533).
Two days prior to 4T1 tumor cell injection, 200 μg of either anti-CD8+ antibody or the isotype control was injected intraperitoneally in BALB/c mice. Eight days post tumor cell injection, 100 μg of antibody was injected intraperitoneally. The efficacy of CD8+ depletion was confirmed by flow cytometry of peripheral blood samples.
In vivo MDSC depletion
The E0771 model was utilized as stated above with ranitidine treatment beginning one day prior to tumor cell injection. Five and 13 d post tumor cell injection, mice were injected intraperitoneally with gemcitabine hydrochloride (60 mg/kg, G6423, Sigma Aldrich) or vehicle. On day 6 and day 14, the efficacy of gemcitabine to deplete monocytes was confirmed by flow cytometry of peripheral blood samples.
Flow cytometry
Antibodies: Rat anti-mouse CD11b-fluorescein isothiocyanate (cat. #11–0112, eBioscience), rat anti-mouse Ly6G-biotin (cat. #12760, Biolegend), rat anti-mouse Ly6C-allophycocyanin (APC) (cat. #17–5932, eBioscience). Appropriate isotype matched control antibodies were used in all experiments.
Splenocytes and bone marrow cells were blocked in FACS buffer containing rat serum. Samples were then mixed with primary antibodies for 15 min on ice, washed, and mixed with streptavidin PerCP for 20 min at 4°C. Following washing, cells were fixed with 1% paraformaldehyde and acquired for analysis using a Becton Dickinson FACSAria II. Results were analyzed using FCS Express software (De Novo Software).
T cell suppression assay
Mice were treated with ranitidine and injected with 4T1 cells as described above. At day 14 post 4T1 injection, peripheral blood was isolated via cardiac puncture. Red blood cells were lysed and leukocytes were resuspended in complete RPMI (Hyclone) media (10% fetal bovine serum, and 1% L-glutamine, HEPES, and penicillin/streptomycin) to a volume equal to the amount originally isolated from each mouse. Samples were serially diluted in 96-well plates in triplicate, before adding D011.10 splenocytes.
Spleens obtained from D011.10 mice were isolated, red blood cells were lysed, and leukocytes were counted. Splenocytes were labeled using 1 μM Oregon Green® 488 dye (O-6149, Thermo Fisher Scientific) in RPMI at 37°C for 15 min. Cells were washed and 200,000 splenocytes were added per well. To each well, 10 μM OVA323–339 peptide was added and the cells were cultured. To determine whether the suppressive effect was dependent on NOS2 and Arg1 activity, L-NMMA and/or nor-NOHA (500 µM) was added.Citation44,45 At day 3, each well was washed and stained with anti-mouse CD4+-PE (cat. #12–004, eBioscience), fixed in 1% paraformaldehyde and acquired using a Becton Dickinson FACS Calibur. Results were analyzed using FCS Express software.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1151591_s02.zip
Download Zip (2.8 MB)Acknowledgments
We would like to thank Bassel Dawod and Yisong Wei for technical assistance, and Cheryl Rafuse and Derek Rowter for their help at the flow cytometry core facilities.
Funding
Ava Vila-Leahey was supported by a trainee award from the Beatrice Hunter Cancer Research Institute (BHCRI) with funds provided by the Canadian Imperial Bank of Commerce as part of The Terry Fox Strategic Health Research Training Program in Cancer Research at the Canadian Institutes for Health Research (CIHR). This work was funded by the Canadian Cancer Society Research Institute, the CIHR (#MOP93517), the BCHRI and the Dept. of Defense Breast Cancer Research Program awards #W81XWH-10-1-0035 and #W81XWH-10-1-0036.
References
- Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. Jama 2013; 310:2435-42; PMID:24327038; http://dx.doi.org/10.1001/jama.2013.280490.
- MacLaren R, Reynolds PM, Allen RR. Histamine-2 receptor antagonists vs proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Intern Med 2014; 174:564-74; PMID:24535015; http://dx.doi.org/10.1001/jamainternmed.2013.14673.
- Agura ED, Vila E, Petersen FB, Shields AF, Thomas ED. The use of ranitidine in bone marrow transplantation. A review of 223 cases. Transplantation 1988; 46:53-6; PMID:3293286; http://dx.doi.org/10.1097/00007890-198807000-00008.
- List AF, Beaird DH, Kummet T. Ranitidine-induced granulocytopenia: recurrence with cimetidine administration. Ann Intern Med 1988; 108:566-7; PMID:3348565; http://dx.doi.org/10.7326/0003-4819-108-4-566.
- Cricco GP, Mohamad NA, Sambuco LA, Genre F, Croci M, Gutierrez AS, Medina VA, Bergoc RM, Rivera ES, Martin GA. Histamine regulates pancreatic carcinoma cell growth through H3 and H4 receptors. Inflamm Res 2008; 57 Suppl 1:S23-4; PMID:18345506; http://dx.doi.org/10.1007/s00011-007-0611-5.
- Boer K, Helinger E, Helinger A, Pocza P, Pos Z, Demeter P, Baranyai Z, Dede K, Darvas Z, Falus A. Decreased expression of histamine H1 and H4 receptors suggests disturbance of local regulation in human colorectal tumours by histamine. Eur J Cell Biol 2008; 87:227-36; PMID:18258331; http://dx.doi.org/10.1016/j.ejcb.2007.12.003.
- Francis H, DeMorrow S, Venter J, Onori P, White M, Gaudio E, Francis T, Greene JF, Jr, Tran S, Meininger CJ et al. Inhibition of histidine decarboxylase ablates the autocrine tumorigenic effects of histamine in human cholangiocarcinoma. Gut 2012; 61:753-64; PMID:21873469; http://dx.doi.org/10.1136/gutjnl-2011-300007.
- Medina VA, Rivera ES. Histamine receptors and cancer pharmacology. Br J Pharmacol 2010; 161:755-67; PMID:20636392; http://dx.doi.org/10.1111/j.1476-5381.2010.00961.x.
- Jutel M, Blaser K, Akdis CA. Histamine receptors in immune regulation and allergen-specific immunotherapy. Immunol Allergy Clin North Am 2006; 26:245-59, vii; PMID:16701143; http://dx.doi.org/10.1016/j.iac.2006.02.006.
- Porretti JC, Mohamad NA, Martin GA, Cricco GP. Fibroblasts induce epithelial to mesenchymal transition in breast tumor cells which is prevented by fibroblasts treatment with histamine in high concentration. Int J Biochem Cell Biol 2014; 51:29-38; PMID:24685678; http://dx.doi.org/10.1016/j.biocel.2014.03.016.
- Cricco GP, Davio CA, Martin G, Engel N, Fitzsimons CP, Bergoc RM, Rivera ES. Histamine as an autocrine growth factor in experimental mammary carcinomas. Agents Actions 1994; 43:17-20; PMID:7741034; http://dx.doi.org/10.1007/BF02005757.
- Medina V, Cricco G, Nunez M, Martin G, Mohamad N, Correa-Fiz F, Sanchez-Jimenez F, Bergoc R, Rivera ES. Histamine-mediated signaling processes in human malignant mammary cells. Cancer Biol Ther 2006; 5:1462-71; PMID:17012845; http://dx.doi.org/10.4161/cbt.5.11.3273.
- Tonnesen H, Knigge U, Bulow S, Damm P, Fischerman K, Hesselfeldt P, Hjortrup A, Pedersen IK, Pedersen VM, Siemssen OJ et al. Effect of cimetidine on survival after gastric cancer. Lancet 1988; 2:990-2; PMID:2902494; http://dx.doi.org/10.1016/S0140-6736(88)90743-X.
- Burtin C, Noirot C, Scheinmann P, Galoppin L, Sabolovic D, Bernard P. Clinical improvement in advanced cancer disease after treatment combining histamine and H2-antihistaminics (ranitidine or cimetidine). Eur J Cancer Clin Oncol 1988; 24:161-7; PMID:3356203; http://dx.doi.org/10.1016/0277-5379(88)90247-7.
- Bolton E, King J, Morris DL. H2-antagonists in the treatment of colon and breast cancer. Semin Cancer Biol 2000; 10:3-10; PMID:10888265; http://dx.doi.org/10.1006/scbi.2000.0301.
- Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, Diaz LA, Jr., Papadopoulos N, Kinzler KW, Vogelstein B et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A 2014; 111:11774-9; PMID:25071169; http://dx.doi.org/10.1073/pnas.1410626111.
- Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol 2005; 174:636-45; http://dx.doi.org/10.4049/jimmunol.174.2.636.
- Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-β 1. J Immunol 2009; 182:240-9; PMID:19109155; http://dx.doi.org/10.4049/jimmunol.182.1.240.
- Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009; 50:799-807; PMID:19551844; http://dx.doi.org/10.1002/hep.23054.
- Poschke I, Mao Y, Adamson L, Salazar-Onfray F, Masucci G, Kiessling R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother 2012; 61:827-38; PMID:22080405; http://dx.doi.org/10.1007/s00262-011-1143-y.
- Watanabe S, Deguchi K, Zheng R, Tamai H, Wang LX, Cohen PA, Shu S. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol 2008; 181:3291-300; PMID:18714001; http://dx.doi.org/10.4049/jimmunol.181.5.3291.
- Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 2008; 111:4233-44; PMID:18272812; http://dx.doi.org/10.1182/blood-2007-07-099226.
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 2006; 66:1123-31; PMID:16424049; http://dx.doi.org/10.1158/0008-5472.CAN-05-1299.
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 2005; 11:6713-21; PMID:16166452; http://dx.doi.org/10.1158/1078-0432.CCR-05-0883.
- Tongu M, Harashima N, Monma H, Inao T, Yamada T, Kawauchi H, Harada M. Metronomic chemotherapy with low-dose cyclophosphamide plus gemcitabine can induce anti-tumor T cell immunity in vivo. Cancer Immunol Immunother 2013; 62:383-91; PMID:22926062; http://dx.doi.org/10.1007/s00262-012-1343-0.
- Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. Five-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 2010; 70:3052-61; PMID:20388795; http://dx.doi.org/10.1158/0008-5472.CAN-09-3690.
- Wesolowski R, Markowitz J, Carson WE, 3rd. Myeloid derived suppressor cells - a new therapeutic target in the treatment of cancer. J Immunother Cancer 2013; 1:10; PMID:24829747; http://dx.doi.org/10.1186/2051-1426-1-10.
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182:4499-506; PMID:19342621; http://dx.doi.org/10.4049/jimmunol.0802740.
- Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J Clin Invest 2001; 108:1865-73; PMID:11748270; http://dx.doi.org/10.1172/JCI200113930.
- van der Pouw Kraan TC, Snijders A, Boeije LC, de Groot ER, Alewijnse AE, Leurs R, Aarden LA. Histamine inhibits the production of interleukin-12 through interaction with H2 receptors. J Clin Invest 1998; 102:1866-73; PMID:9819373; http://dx.doi.org/10.1172/JCI3692.
- Zhang J, Takahashi HK, Liu K, Wake H, Liu R, Sadamori H, Matsuda H, Yagi T, Yoshino T, Mori S et al. Histamine inhibits adhesion molecule expression in human monocytes, induced by advanced glycation end products, during the mixed lymphocyte reaction. Br J Pharmacol 2010; 160:1378-86; PMID:20590628; http://dx.doi.org/10.1111/j.1476-5381.2010.00800.x.
- Dawicki W, Jawdat DW, Xu N, Marshall JS. Mast cells, histamine, and IL-6 regulate the selective influx of dendritic cell subsets into an inflamed lymph node. J Immunol 2010; 184:2116-23; PMID:20083654; http://dx.doi.org/10.4049/jimmunol.0803894.
- Asea A, Hermodsson S, Hellstrand K. Histaminergic regulation of natural killer cell-mediated clearance of tumour cells in mice. Scand J Immunol 1996; 43:9-15; PMID:8560202; http://dx.doi.org/10.1046/j.1365-3083.1996.d01-14.x.
- Hellstrand K, Asea A, Hermodsson S. Role of histamine in natural killer cell-mediated resistance against tumor cells. J Immunol 1990; 145:4365-70; PMID:2147942
- Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, Zak-Nejmark T, Koga R, Kobayashi T, Blaser K et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature 2001; 413:420-5; PMID:11574888; http://dx.doi.org/10.1038/35096564.
- Osna N, Elliott K, Khan MM. Regulation of interleukin-10 secretion by histamine in TH2 cells and splenocytes. Int Immunopharmacol 2001; 1:85-96; PMID:11367520; http://dx.doi.org/10.1016/S0162-3109(00)00268-X.
- Osna N, Elliott K, Khan MM. The effects of histamine on interferon gamma production are dependent on the stimulatory signals. Int Immunopharmacol 2001; 1:135-45; PMID:11367511; http://dx.doi.org/10.1016/S1567-5769(00)00005-9.
- Khan MM, Keaney KM, Melmon KL, Clayberger C, Krensky AM. Histamine regulates the generation of human cytolytic T lymphocytes. Cell Immunol 1989; 121:60-73; PMID:2541932; http://dx.doi.org/10.1016/0008-8749(89)90005-1.
- Yang XD, Ai W, Asfaha S, Bhagat G, Friedman RA, Jin G, Park H, Shykind B, Diacovo TG, Falus A et al. Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+Ly6G+ immature myeloid cells. Nat Med 2011; 17:87-95; PMID:21170045; http://dx.doi.org/10.1038/nm.2278.
- Zheng Y, Xu M, Li X, Jia J, Fan K, Lai G. Cimetidine suppresses lung tumor growth in mice through proapoptosis of myeloid-derived suppressor cells. Mol Immunol 2013; 54:74-83; PMID:23220070; http://dx.doi.org/10.1016/j.molimm.2012.10.035.
- Martin RK, Saleem SJ, Folgosa L, Zellner HB, Damle SR, Nguyen GK, Ryan JJ, Bear HD, Irani AM, Conrad DH. Mast cell histamine promotes the immunoregulatory activity of myeloid-derived suppressor cells. J Leukoc Biol 2014; 96:151-9; PMID:24610880; http://dx.doi.org/10.1189/jlb.5A1213-644R.
- Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol 2009; 9:900-9; PMID:19336265; http://dx.doi.org/10.1016/j.intimp.2009.03.015.
- Lemos B, Davio C, Gass H, Gonzalez P, Cricco G, Martin G, Bergoc R, Rivera E. Histamine receptors in human mammary gland, different benign lesions and mammary carcinomas. Inflamm Res 1995; 44 Suppl 1:S68-9; PMID:8521007; http://dx.doi.org/10.1007/BF01674400.
- Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, Segal DM, Staib C, Lowel M, Sutter G et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol 2003; 170:270-8; PMID:12496409; http://dx.doi.org/10.4049/jimmunol.170.1.270.
- Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res 2007; 67:4507-13; PMID:17483367; http://dx.doi.org/10.1158/0008-5472.CAN-06-4174.
- Gujar SA, Clements D, Dielschneider R, Helson E, Marcato P, Lee PW. Gemcitabine enhances the efficacy of reovirus-based oncotherapy through anti-tumour immunological mechanisms. Br J Cancer 2014; 110:83-93; PMID:24281006; http://dx.doi.org/10.1038/bjc.2013.695.
- Andrade-Vieira R, Xu Z, Colp P, Marignani PA. Loss of LKB1 expression reduces the latency of ErbB2-mediated mammary gland tumorigenesis, promoting changes in metabolic pathways. PloS One 2013; 8:e56567; PMID:23451056; http://dx.doi.org/10.1371/journal.pone.00-56567.
- Hellstrand K, Asea A, Dahlgren C, Hermodsson S. Histaminergic regulation of NK cells. Role of monocyte-derived reactive oxygen metabolites. J Immunol 1994; 153:4940-7; PMID:7963557
- Hansson M, Hermodsson S, Brune M, Mellqvist UH, Naredi P, Betten A, Gehlsen KR, Hellstrand K. Histamine protects T cells and natural killer cells against oxidative stress. J Interferon Cytokine Res 1999; 19:1135-44; PMID:10547153; http://dx.doi.org/10.1089/107999099313073.
- Agarwala SS, Glaspy J, O'Day SJ, Mitchell M, Gutheil J, Whitman E, Gonzalez R, Hersh E, Feun L, Belt R et al. Results from a randomized phase III study comparing combined treatment with histamine dihydrochloride plus interleukin-2 versus interleukin-2 alone in patients with metastatic melanoma. J Clin Oncol 2002; 20:125-33; PMID:11773161; http://dx.doi.org/10.1200/JCO.20.1.125.
- Bowrey PF, King J, Magarey C, Schwartz P, Marr P, Bolton E, Morris DL. Histamine, mast cells and tumour cell proliferation in breast cancer: does preoperative cimetidine administration have an effect? Br J Cancer 2000; 82:167-70; PMID:10638985; http://dx.doi.org/10.1054/bjoc.1999.0895.
- Hsu CL, Chang CH, Lin JW, Wu LC, Chuang LM, Lai MS. Histamine-2 receptor antagonists and risk of lung cancer in diabetic patients - an exploratory analysis. Pharmacoepidemiol Drug Saf 2013; 22:632-40; PMID:23576472; http://dx.doi.org/10.1002/pds.3441.
- Seto CT, Jeraldo P, Orenstein R, Chia N, DiBaise JK. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome 2014; 2:42; PMID:25426290; http://dx.doi.org/10.1186/2049-2618-2-42.
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013; 504:451-5; PMID:24226773; http://dx.doi.org/10.1038/nature12726.
- Wu HJ, Ivanov, II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 2010; 32:815-27; PMID:20620945; http://dx.doi.org/10.1016/j.immuni.2010.06.001.
- Lakritz JR, Poutahidis T, Mirabal S, Varian BJ, Levkovich T, Ibrahim YM, Ward JM, Teng EC, Fisher B, Parry N et al. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget 2015; 6:9387-96; PMID:25831236; http://dx.doi.org/10.18632/oncotarget.3328.
- Goh W, Sleptsova-Freidrich I, Petrovic N. Use of proton pump inhibitors as adjunct treatment for triple-negative breast cancers. An introductory study. J Pharm Pharm Sci 2014; 17:439-46; PMID:25224353.
- Jin UH, Lee SO, Pfent C, Safe S. The aryl hydrocarbon receptor ligand omeprazole inhibits breast cancer cell invasion and metastasis. BMC Cancer 2014; 14:498; PMID:25011475; http://dx.doi.org/10.1186/1471-2407-14-498.
- Panni RZ, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages to combat cancer. Immunotherapy 2013; 5:1075-87; PMID:24088077; http://dx.doi.org/10.2217/imt.13.102.
- Ward R, Sims AH, Lee A, Lo C, Wynne L, Yusuf H, Gregson H, Lisanti MP, Sotgia F, Landberg G et al. Monocytes and macrophages, implications for breast cancer migration and stem cell-like activity and treatment. Oncotarget 2015; 6:14687-99.
- Sharma SK, Chintala NK, Vadrevu SK, Patel J, Karbowniczek M, Markiewski MM. Pulmonary alveolar macrophages contribute to the premetastatic niche by suppressing antitumor T cell responses in the lungs. J Immunol 2015; 194:5529-38; http://dx.doi.org/10.4049/jimmunol.1403215; PMID:26008983; http://dx.doi.org/10.18632/oncotarget.4189
- Haile LA, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J Immunol 2010; 185:203-10; PMID:20525890; http://dx.doi.org/10.4049/jimmunol.0903573.
- Oh K, Lee OY, Shon SY, Nam O, Ryu PM, Seo MW, Lee DS. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res 2013; 15:R79; PMID:24021059; http://dx.doi.org/10.1186/bcr3473.
- OuYang LY, Wu XJ, Ye SB, Zhang RX, Li ZL, Liao W, Pan ZZ, Zheng LM, Zhang XS, Wang Z et al. Tumor-induced myeloid-derived suppressor cells promote tumor progression through oxidative metabolism in human colorectal cancer. J Transl Med 2015; 13:47; PMID:25638150; http://dx.doi.org/10.1186/s12967-015-0410-7.
- Bergenfelz C, Larsson AM, von Stedingk K, Gruvberger-Saal S, Aaltonen K, Jansson S, Jernstrom H, Janols H, Wullt M, Bredberg A et al. Systemic Monocytic-MDSCs Are Generated from Monocytes and Correlate with Disease Progression in Breast Cancer Patients. PloS One 2015; 10:e0127028; PMID:25992611; http://dx.doi.org/10.1371/journal.pone.0127028.
- Saleem SJ, Martin RK, Morales JK, Sturgill JL, Gibb DR, Graham L, Bear HD, Manjili MH, Ryan JJ, Conrad DH. Cutting edge: mast cells critically augment myeloid-derived suppressor cell activity. J Immunol 2012; 189:511-5; PMID:22706087; http://dx.doi.org/10.4049/jimmunol.1200647.
- Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol 2009; 182:5693-701; PMID:19380816; http://dx.doi.org/10.4049/jimmunol.0900092.
- Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res 2001; 61:4756-60; PMID:11406548.
- Zhu J, Huang X, Yang Y. Myeloid-derived suppressor cells regulate natural killer cell response to adenovirus-mediated gene transfer. J Virol 2012; 86:13689-96; PMID:23055553; http://dx.doi.org/10.1128/JVI.01595-12.
- Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med 2007; 13:828-35; PMID:17603493; http://dx.doi.org/10.1038/nm1609.
- Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B et al. Transforming growth factor-β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med 2003; 198:1741-52; PMID:14657224; http://dx.doi.org/10.1084/jem.20022227.
- Fichtner-Feigl S, Terabe M, Kitani A, Young CA, Fuss I, Geissler EK, Schlitt HJ, Berzofsky JA, Strober W. Restoration of tumor immunosurveillance via targeting of interleukin-13 receptor-α 2. Cancer Res 2008; 68:3467-75; PMID:18451175; http://dx.doi.org/10.1158/0008-5472.CAN-07-5301.
- Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 2007; 25:2546-53; PMID:17577033; http://dx.doi.org/10.1200/JCO.2006.08.5829.
- Weizman N, Krelin Y, Shabtay-Orbach A, Amit M, Binenbaum Y, Wong RJ, Gil Z. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene 2014; 33:3812-9; PMID:23995783; http://dx.doi.org/10.1038/onc.2013.357.
- Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 2007; 109:5346-54; PMID:17327412; http://dx.doi.org/10.1182/blood-2006-10-051318.
- Tsukamoto H, Nishikata R, Senju S, Nishimura Y. Myeloid-derived suppressor cells attenuate TH1 development through IL-6 production to promote tumor progression. Cancer Immunol Res 2013; 1:64-76; PMID:24777249; http://dx.doi.org/10.1158/2326-6066.CIR-13-0030.
- Crook KR, Jin M, Weeks MF, Rampersad RR, Baldi RM, Glekas AS, Shen Y, Esserman DA, Little P, Schwartz TA et al. Myeloid-derived suppressor cells regulate T cell and B cell responses during autoimmune disease. J Leukoc Biol 2015; 97:573-82; PMID:25583578; http://dx.doi.org/10.1189/jlb.4A0314-139R.
- Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008; 135:234-43; PMID:18485901; http://dx.doi.org/10.1053/j.gastro.2008.03.020.
- Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock 2004; 22:460-6; PMID:15489639; http://dx.doi.org/10.1097/01.shk.0000142249.08135.e9.
- Chen X, Oppenheim JJ, Howard OM. BALB/c mice have more CD4+CD25+ T regulatory cells and show greater susceptibility to suppression of their CD4+CD25- responder T cells than C57BL/6 mice. J Leukoc Biol 2005; 78:114-21; PMID:15845645; http://dx.doi.org/10.1189/jlb.0604341.
- Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res 1998; 58:1486-93; PMID:9537252.
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011; 475:222-5; PMID:21654748; http://dx.doi.org/10.1038/nature10138.
- Vehabovic M, Hadzovic S, Stambolic F, Hadzic A, Vranjes E, Haracic E. Stability of ranitidine in injectable solutions. Int J Pharm 2003; 256:109-15; PMID:12695016; http://dx.doi.org/10.1016/S0378-5173(03)00067-X.
- Bullock LS, Fitzgerald JF, Mazur HI. Stability of intravenous famotidine stored in polyvinyl chloride syringes. DICP 1989; 23:588-90; PMID:2763581.
- Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol 2001; Chapter 20:Unit 20 2; PMID:18432775; http://dx.doi.org/10.1002/0471142735.im2002s39
