ABSTRACT
Interleukin-2 (IL-2) is one of the key cytokines with pleiotropic effects on immune system. It has been approved for the treatment of metastatic renal cell carcinoma and metastatic melanoma. Recent progress has been made in our understanding of IL-2 in regulating lymphocytes that has led to exciting new directions for cancer immunotherapy. While improved IL-2 formulations might be used as monotherapies, their combination with other anticancer immunotherapies, such as adoptive cell transfer regimens, antigen-specific vaccination, and blockade of immune checkpoint inhibitory molecules, for example cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) mono-antibodies, would held the promise of treating metastatic cancer. Despite the comprehensive studies of IL-2 on immune system have established the application of IL-2 for cancer immunotherapy, a number of poignant obstacles remain for future research. In the present review, we will focus on the key biological features of IL-2, current applications, limitations, and future directions of IL-2 in cancer immunotherapy.
Abbreviations
| ACT | = | adoptive T cell therapy |
| CTLA-4 | = | cytotoxic T lymphocyte-associated antigen 4 |
| DC | = | dendritic cells |
| IL-2 | = | interleukin-2 |
| JAK | = | Janus family tyrosine kinases |
| LAK | = | lymphokine activated killer |
| MAPK | = | mitogen-activated protein kinase |
| MMPs | = | matrix metalloproteases |
| NK | = | nature killer cell |
| PD-1 | = | programmed death 1 |
| PD-L1 | = | programmed death ligand 1 |
| PI3K | = | phosphoinositide 3-kinase |
| PSA | = | prostate specific antigen |
| STAT | = | signal transducer and activator of transcription |
| TAAs | = | tumor-associated antigens |
| TCR | = | T cell receptor |
| Th1 | = | T helper-1 |
| Th2 | = | T helper-2 |
| Th17 | = | T helper-17 |
| TILs | = | tumor-infiltrating lymphocytes |
| TNF-α | = | tumor necrosis factor α |
| Treg | = | T regulatory cell |
Introduction
Cancer is one of the most common lethal diseases in the world, with 14 million new cases diagnosed annually and is also the leading cause of deaths worldwide, causing 8.2 million deaths annually as World Health Organization (WHO) reported in the World Cancer Report 2014. Although the identification of a large amount of driver oncogenes and subsequent targeted therapy have resulted in a prolongation of overall survival in these with driver mutations, survival remains dismal as a whole and novel therapeutic approaches are still urgently needed. Recently there has been a breakthrough in harnessing the immune system to treat malignant tumors. Cytokines are small glycoproteins that bind to cell surface receptors and regulate the development, survival, and function of immune cell. Thus, cytokines have been extensively studied as potential therapeutic agents to manipulate the immune response to tumor cells.
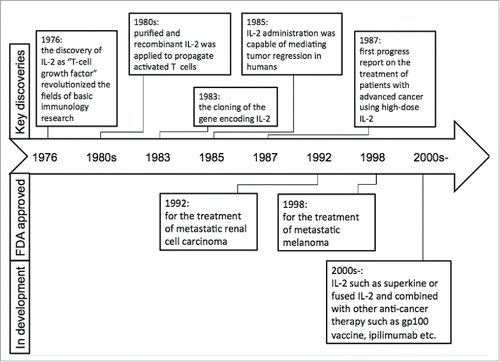
IL-2 is one of the key cytokines with pleiotropic effects on the immune system. The discovery of IL-2 as “T-cell growth factor” (TCGF) in 1976 quickly revolutionized the fields of basic immunology research and immunotherapy for human cancers.Citation1 IL-2 was an early candidate for cancer immunotherapy and was approved for the treatment of metastatic renal cell carcinoma (1992) and later for metastatic melanoma (1998) by FDA. Much progress has been made recently in our understanding of IL-2 in regulating lymphocytes that has led to exciting new directions in cancer immunotherapy (). There are several excellent reviews on IL-2, which examine the molecular biology of its expression, its role in immune cell signaling and immune development, as well as the structural biology of cytokines and their receptors.Citation2-5 In the present review, we will focus on the key biological features of IL-2, current applications, limitations, and future directions of IL-2 in cancer immunotherapy.
The biology of IL-2 and its receptors
IL-2 is a small 15.5-kDa four α-helical bundle cytokine, which has been one of the most studied cytokines since its discovery about 38 y ago. It is produced predominately by antigen-simulated CD4+ T cells, while it can also be produced by CD8+ cells, natural killer (NK) cells, and activated dendritic cells (DC).Citation6-8 IL-2 is an important factor for the maintenance of CD4+ regulatory T cells and plays a critical role in the differentiation of CD4+ T cells into a variety of subsets. It can promote CD8+ T-cell and NK cell cytotoxicity activity, and modulate T-cell differentiation programs in response to antigen, promoting naive CD4+ T-cell differentiation into T helper-1 (Th1) and T helper-2 (Th2) cells while inhibiting T helper-17 (Th17) differentiation.Citation9-11
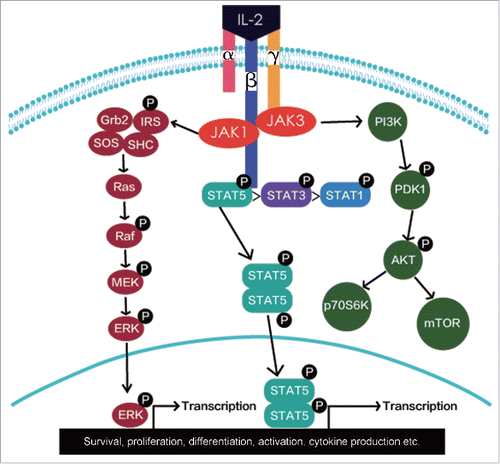
IL-2 receptor is composed of the three subunits IL-2Rα (CD25), IL-2Rβ (CD122), and IL-2Rγ (CD132) (). The αβγ trimeric complex forms the highest affinity receptor. IL-2Rα is unique to IL-2 and is expressed by a number of immune cells including T regulatory cells (Treg), activated CD4+ and CD8+T cells, B cells, mature DCs, endothelial cells, and so on.Citation12-16 The α chain is overexpressed (8–10-fold) compared with the βγ chains. It is believed that the α chain functions to bind IL-2 initially, localizing it to the cell surface, effectively increasing its concentration and also inducing a conformational change in IL-2 which then subsequently binds to the βγ chains on the cell surface.Citation3,4 The expression of IL-2Rα in naive T cells can be triggered rapidly by T cell receptor (TCR) and co-stimulatory signals followed by a positive IL-2/IL-2Rα feedback loop.Citation2 Unlike naive T cells, NK cells, and memory phenotype CD8+ cells express high levels of βγ and some NK cells can also express α chain after the stimulation by IL-2. Of note, Tregs, which act to dampen the immune response, constitutively express high levels of α chain.Citation17 This enables them to consume IL-2 more efficiently than effector CD4+, CD8+, and NK cells, even at a low level.Citation18
The β chain is shared with the IL-15 receptor and the γ chain can be partnered with several other cytokines (e.g., IL-15 and IL-21) receptor chains and is critically involved in signaling. Both of them belong to hematopoietin receptor family.Citation19 IL-2Rβ is mainly expressed by multiple lymphoid populations such as Treg, memory CD8+ T cells, NK cells, and NKT cells. Similar to the IL-2Rβ subunit, the IL-2Rγ subunit is expressed mostly by haematopoietic cells.Citation20,21 Interestingly, the IL-2Rγ subunit is stored intracellularly and its expression by CD4+ is triggered only upon activation.Citation22
The signaling pathways of IL-2
IL-2 binds to its receptors at different affinities.Citation23 The isolated IL-2Rα binds IL-2 with low-affinity (Kd-10−8 M) without transducing a signal and the heterodimeric IL-2Rβγ binds IL-2 with intermediate-affinity (Kd-10−9 M) and transduces intracellular signals. When all three IL-2 receptor subunits form an IL-2Rαβγ trimeric complex, its binding affinity to IL-2 is greater than binding to either a single IL-2 receptor subunit or the IL-2Rβγ heterodimer.Citation24-26 Binding of IL-2 to the IL-2Rβγ or IL-2Rαβγ complex leads to the activation of multiple signaling pathways with initial signal transduction involving the recruitment of Janus family tyrosine kinases (JAK1 and JAK3) to the cytoplasmic domains of IL-2Rβγ or IL-2Rαβγ. The activation of JAK kinases results in the recruitment and phosphorylation of signal transducer and activator of transcription 1 (STAT1), STAT3, STAT5A, and STAT5B. Then, three major downstream signaling pathways including the STAT signaling pathway, the phosphoinositide 3-kinase (PI3K-AKT) signaling pathway, and the mitogen-activated protein kinase (MAPK) signaling pathway are activated ().Citation2 These pathways have mediated the survival, proliferation, differentiation, activation, cytokine production etc. in different types of immune cells.Citation2,18
The application of IL-2 in cancer immunotherapy
IL-2 as monotherapy
In 1985, 25 previously treated patients with metastatic cancer were treated with increasing high dose (HD) IL-2 at an escalated dose of 60,000–600,000 IU/kg until intolerable toxicity. In this first series of 25 patients, 4 of 7 patients with metastatic melanoma and 3 of 3 patients with metastatic renal cancer showed regression of metastatic tumor.Citation27 The study first demonstrated that IL-2 was capable of mediating tumor regression in humans, and thus it was further evaluated in the subsequent studies in these two kinds of cancer types. In a phase II trial, multiple cycles of HD IL-2 at a dose of 600,000–720,000 IU/kg with up to 15 bolus infusions were administered every 8 h or as many as the patient could tolerate in 255 patients with metastatic renal cell carcinoma, which showed a complete response of 7% and an overall response rate of 15%.Citation28 Hence, IL-2 was approved for metastatic renal cell carcinoma in 1992 and later it was approved for metastatic melanoma in 1998 by FDA. Although IL-2 has been demonstrated capable of mediating tumor regression, it is insufficient to improve patients' survival due to its dual functional properties on T cells and severe adverse effect in high dose. Nowadays, IL-2 monotherapy is not the optimal and standard treatment in both metastatic renal cell carcinoma and metastatic melanoma. Efforts to further improve the efficacy of IL-2 therapy are focused on its combination with other anticancer immunotherapies.
IL-2 combined with other cytokines
Though HD IL-2 monotherapy showed promising results in metastatic renal cell carcinoma and melanoma, the toxicity and cost limited its application in a large population. Thus, some investigators evaluated the efficacy of regimens containing low-dose IL-2 combined with other cytokines, such as interferon α (IFN-α). Several phase II trials evaluated HD bolus IL-2 alone, intravenous (IV) IL-2 and IFN, and subcutaneous IL-2 and IFN in patients with metastatic renal cell carcinoma and showed a similar response rates and median overall survivals.Citation29-31 The addition of IV IFN to HD IL-2 did not seem to improve efficacy with an increasing toxicity. Furthermore, in a randomized phase III trial, patients with advanced renal cancer were assigned to receive either low-dose IL-2 and IFN every 6 weeks or HD IL-2 every 12 weeks. The results showed that HD IL-2 produced a statistically significant improvement in response rate (23.2% vs. 9.9% p = 0.018) and response duration (median 24 vs. 15 mo) compared with low-dose IL-2 and IFN-α.Citation32 Other two randomized studies also demonstrated that there were no significant differences in overall survival between HD IL-2 and IL-2 combined with IFN.Citation33,34 Taken together, these results indicated that HD IL-2 is superior to both lower doses of IL-2 or IL-2 and IFN in terms of response rates and duration of response.
IL-2 combined with other cell-based immunotherapy
As mentioned above, IL-2 can promote the activation and cell growth of T and NK cells. Thus, early combination strategies were initiated to investigate IL-2 incorporating immune cells such as lymphokine activated killer (LAK) cells and T cells. Compared with HD IL-2 monotherapy, co-administration of LAK cells with IL-2 yielded a clinical response rate of 20–35%, however, mostly with a transient response in solid tumors.Citation35-37 Another study focused on utilizing an adoptive T cell therapy (ACT) that combines the infusion of ex vivo expanded tumor-infiltrating T cells (TILs) with HD IL-2 regimen in patients with metastatic melanoma.Citation38 In this approach, HD IL-2 is used to expand TILs from tumor fragments to large numbers for a period of 5–6 weeks. Then, these TILs undergo further rapid expansion in the presence of HD IL-2, feeder cells, and anti-CD3 for an additional 2 weeks to reach billions of cells for later infusion.Citation39 The promising results were reported in numerous phase II clinical trials, with an approximately 50% clinical response rate and 13% of durable complete regression in patients with metastatic melanoma.Citation40,41 Although IL-2-based TIL therapy is very promising, TILs expanded in the presence of IL-2 exhibit a more differentiated phenotype that can shorten their long-term persistence and survival in vivo. These drawbacks may compromise clinical benefits of this treatment. To improve the quality of TILs, some researchers attempted to use other cytokines such as IL-7, IL-15, and IL-21 to grow TILs. These studies have shown that these cytokines could maintain the expression of CD28 by CD8+ TILs more efficiently than IL-2 during the rapid expansion of TILs.Citation42
IL-2 combined with chemotherapeutic agents
IL-2 combined with chemotherapeutic agents (so-called biochemotherapy [BCT]) including cisplatin and dacarbazine has been extensively investigated in patients with metastatic melanoma over the past two decades.Citation43 Composite results from a variety of inpatient regimens show a response rate about 50%, with 10% to 20% complete responses and a median survival of 11 to 12 mo. Despite promising antitumor activity reported in initial studies, BCT regimens have consistently failed to produce statistically significant benefits in overall survival in randomized phase III trials. Of seven previously reported phase III trials involving a spectrum of BCT combinations,Citation44-50 only a single-institution trial comparing sequential administration of cisplatin, vinblastine, and dacarbazine (CVD) followed by IL-2 and IFN with CVD reported an increase in overall survival with a statistically marginal significance. Moreover, two meta-analyses of the literature encompassing 18 trials and more than 2,600 patients in which BCT (including IFN, IL-2, or IL-2 plus IFN regimens) was compared with chemotherapy alone showed higher response rates, but no survival advantage, for the BCT regimens.Citation51,52 Although BCT produced slightly higher response rates and longer median progression-free survival than CVD alone, this was not associated with either improved overall survival or durable responses. Considering the extra toxicity and complexity, this concurrent BCT regimen cannot be recommended for patients with metastatic melanoma. New combinations of IL-2 with other chemotherapeutic agents should be investigated.
IL-2 combined with targeted therapy
Targeted therapy has deeply revolutionized the current strategy for cancer treatments, especially after the discovery of BCR-ABL in leukemia and EGFR mutation in non-small-cell lung cancer (NSCLC). Unfortunately, not all of patients would benefit from targeted therapy and nearly all patients who initially respond to targeted inhibitors inevitably develop acquired resistance to the treatment.Citation53-55 In advanced NSCLC, the imbalance of the IL-2/IL-2R system, with the decline in IL-2 levels and the significantly high-soluble IL-2 receptor (sIL-2R) concentrations, has been observed and associated with poor prognosis.Citation56 On the other hand, the role of IL-2 activation in the restoration of the immunocompetence of lymphocytes against lung cancer has been demonstrated.Citation57 Other authors found that EGFR-TKI affects the cancer-related networks of pro-inflammatory cytokines and activates the lymphocytic responses, which suggested a possible synergism between the EGFR molecular pathway inhibition and immune system modulation in tumor shrinkage.Citation58,59 In a phase II study,Citation60 70 consecutive patients with advanced NSCLC were divided into gefitinib (G) and gefitinib + IL-2 (GIL-2) group. The author observed a significant higher overall response rate (16.1% vs. 5.1%, p < 0.001) and a similar disease control rate (41.9% vs. 41%, p > 0.05). The median time to progression was similar (3.5 vs. 4.1 mo, p > 0.05) while the median OS was significantly prolonged in the GIL-2 group (20.1 vs. 6.9 mo, p = 0.002), which showed that IL-2 might improve the outcome of EGFR-TKI. A recent retrospective analysis examined the safety and efficacy of HD-IL2 following TKI therapy in patients with metastatic renal cell carcinoma,Citation61 which showed that prior TKI did not affect the effect of subsequent HD IL-2 therapy. These results suggested the combination of IL-2 could increase the efficacy of targeted inhibitors. However, there is still lack the randomized compared study in patients with driver mutations. Thus, whether other targeted inhibitors combined IL-2 have this effect remains unknown and requires further investigation.
IL-2 combined with peptide vaccines
Theoretically, IL-2 has a synergistic effect with cancer vaccines in the treatment of human malignancies.Citation62 When IL-2 is administered in conjunction with cancer vaccines such as recombinant viruses, naked DNA, or peptide antigens, it can dramatically enhance antitumor effects. A previous phase II study demonstrated that patients with metastatic melanoma receiving HD IL-2 plus the gp100 peptide vaccine had a higher response rate than expected among patients who are treated with IL-2 alone.Citation63 A recent phase III trial further confirmed this result.Citation64 In this trial, patients with advanced melanoma were randomly assigned to receive HD IL-2 alone or gp100 plus incomplete Freund's adjuvant (Montanide ISA-51) once per cycle, followed by IL-2. The vaccine plus IL-2 group had a significant improvement in centrally verified overall clinical response (16% vs. 6%), longer progression-free survival (median 2.2 vs. 1.6 mo; p = 0.008) and overall survival (median 17.8 vs. 11.1 mo; p = 0.06) compared with the IL-2 group. These studies illustrated that the addition of cytokines could enhance the effect of vaccine therapy in patients with melanoma and highlighted the potential of using rational combinations of immune agents in treating patients with metastatic cancer.
IL-2 combined with immune checkpoint inhibitors
Tumor cells can escape from the immune system via several mechanisms. One important way is by adapting immune inhibitory pathways called immune checkpoints. Some checkpoints are co-stimulatory, which are required for T-cell activation such as CD28 and its ligands B7.1 (CD80) and B7.2 (CD86). Other checkpoints inhibit T-cell activation such as CTLA-4 and PD-1 immune checkpoints.Citation65-67 CTLA-4 is capable of suppressing effector immune responses on T cells and multiple animal models have suggested enhanced antitumor immunity with CTLA-4 blockade.Citation68-70 IL-2 administration may also mediate antitumor effects. In addition, IL-2 also stimulates T-regulatory cells that constitutively express CTLA-4 and can suppress immune reactions. Hence, IL-2 might enhance antitumor reactivity in the presence of CTLA-4 blockade. In fact, a phase I/II study had assessed the antitumor activity and autoimmune toxicity of CTLA-4 blockade in combination with IL-2.Citation71 Disappointingly, the objective response rate is not superior to single administration and there is no evidence to support a synergistic effect of CTLA-4 blockade plus IL-2 although durable cancer regressions were seen in patients treated with this combination. Interestingly, a recent study also suggested that the efficacy of CTLA-4 blockade was significantly improved by recombinant IL-2 in mouse and elevated serum IL-2Rα predicted resistance to CTLA-4 blockade in patients with advanced melanoma.Citation72 Hence, we suppose that only patients presenting a high baseline sIL-2Ra concentration might benefit from CTLA-4 blockade in combination with IL-2. To date, the combination of IL-2 with CTLA-4 inhibitors seems have no extra benefit for cancer immunotherapy. However, whether IL-2 has a synergistic antitumor effect with other immune checkpoint inhibitors (such as PD-1/PD-L1 antibody, nivolumab, or pembrolizumab) need more basic and clinic research.
The limitations of IL-2 immunotherapy against cancer
Undoubtedly, IL-2 showed great potential in treating metastatic cancers. However, its application in the clinic remains relatively restricted due to several shortcomings. First, IL-2 has the dual functional properties that it can act on both Tregs as well as effector T cells.Citation5 As a result, some studies have used IL-2 to enhance antitumor immune responses and other studies have used IL-2 to dampen autoimmune responses. Furthermore, both HD and low-dose IL-2 therapy preferentially induce the expansion of CD4+CD25+Foxp3+ Treg and the Treg level remains elevated after each cycle of HD IL-2 therapy.Citation73-75 A study by Sim et al. has shown that HD IL-2 induces a large expansion of a specific CD4+CD25+Foxp3+ Treg subset that expresses ICOS. These ICOS+ Tregs had a higher proliferative capacity in response to IL-2 and displayed a more immunosuppressive phenotype. Patients who showed no response to HD IL-2 had significantly greater expansion of ICOS+ Treg after the first cycle of therapy compared with these who responded,Citation75 which suggested an inhibitory role of these cells that could be a crucial limiting factor in preventing antitumor lymphocyte activity and tumor eradication during HD IL-2 therapy. Tregs would undergo a rapid reconstitution during HD IL-2 and TIL therapy, which was found to be associated with poor clinical response. Meanwhile, the reconstitution of endogenous Tregs was correlated with the dose of IL-2 doses during TIL therapy.Citation76
Another major drawback is the severe toxicities of HD IL-2 therapy. Due to rapid elimination and metabolism via the kidney, IL-2 has a short serum half-life of several minutes. Thus, to achieve an optimal immune-modulatory effect, IL-2 should be given in a high dose, which will inevitably result in severe toxicities. HD IL-2-induced severe toxicities including vascular leak syndrome (VLS), pulmonary edema, hypotension, and heart toxicities.Citation77-79 Several mechanisms have been proposed but still not clearly clarified. It is believed that the induction of pro-inflammatory cytokines such as IL-1, IL-6, tumor necrosis factor α (TNF-α), and IFNγ were potential contributors to IL-2-induced VLS. In addition, Krieg et al. reported that binding of IL-2 to the high-affinity IL-2Rα-expressing endothelial cells induced an acute vasodilation effect and VLS.Citation16 Other studies have also suggested that elevated levels of eNOS, angiopoietin 2, or a protein fragment of the IL-2 molecule designated as permeability-enhancing peptide may lead to VLS.Citation80-82
Strategies to improve efficacy of IL-2 immunotherapy
IL-2 mutants
Ideally, we hope the IL-2 could efficiently activate NK cells and T effector cells without Treg expansion. To achieve this goal, IL-2 mutants were created, which had different binding properties for the IL-2 receptor components. Initial mutational approaches to improve IL-2 efficacy for effector T cells focused on enhancing binding to the α chain of the IL-2 receptor.Citation83-86 Disappointingly, this was not as successful as envisioned because these mutants might actually downregulate immune responses in vivo when delivered systemically due to the global stimulation of Tregs that express the α chain component of the high affinity IL-2 receptor. Recently, two novel IL-2 mutants, namely F42K and R38A, have been described and characterized.Citation87,88 These IL-2 mutants have changed IL-2Rα binding domains that greatly decrease their binding affinity to IL-2Rα while having an affinity similar to that of native IL-2 to the IL-2Rβγ complex. Moreover, these mutants can activate LAK cells without the production of high levels of pro-inflammatory cytokine (IFNγ, IL-1β, TNF-α) and prevent VLS.Citation88 Furthermore, some other studies have suggested that IL-2 mutants have less effect in stimulating a large expansion of Treg when compared with native IL-2. These findings are crucial and encouraging since expansion of Treg by wild-type IL-2 is another main limitation of HD IL-2 therapy. A more recent paper identified IL-2 mutants using yeast displaying a higher affinity to the β chain.Citation89 One of these mutants, termed as “superkine” or “super-2” reflecting its enhanced agonist properties, also showed improved antitumor activity and exhibited less VLS compared to native IL-2. The higher affinity of the mutated IL-2 for the β chain of the IL-2 receptor may be important to explain the reason that modified IL-2 might function in vivo. Others have explored an alternative strategy in which IL-2 mutants were engineered to carry four point mutations that limit their interaction with IL-2Rα to avoid the expansion of Tregs. Taken together, these studies clearly showed that it was feasible to structurally alter IL-2 to accentuate or reduce particular biologic properties to modify the function of IL-2, which might be an important breakthrough in the use of IL-2 for cancer immunotherapy.
Antibody–cytokine fusions or immunocytokines
Another novel approach attempts to deliver IL-2 to tumor sites by genetically fusing cytokines with antibodies (also called immunocytokine), or antibody components such as a single-chain variable fragment (scFv), which could bind tumor-associated antigens (TAAs).Citation90,91 The major advantage of this approach is that it could improve the half-life of cytokine and enhance the immune-modulatory effect of cytokines with less toxicity. For example, IL-2 was conjugated to an antibody reactive with ganglioside 2 (GD2), which would eventually accumulate at the tumor site due to the binding of the antibody to the GD2 antigen on the tumor. As a consequence, the local concentration of IL-2 is increased at the tumor site. Currently, two immunocytokines, Hu14.18-IL2 and L19-IL2 (Darleukin), are in phase II clinical studies. Hu14.18-IL2 consists of an IgG antibody that recognizes GD2, and L19-IL2 is a diabody with two human IL-2 molecules that are genetically fused to the C-terminus of each scFv domain. Pre-clinical studies have demonstrated encouraging therapeutic outcome in vivo.Citation92-95 In the phase I clinical trials, Hu14.18-IL2 and L19-IL2 have shown a mild and reversible toxicity profiles.Citation96,97 In addition Hu14.18-IL12 showed that 58% of melanoma patients achieved stable disease after the first cycle of treatment in the phase I clinical study. Currently, L19-IL2 is in phase II trials to validate its efficacy in patients with metastatic melanoma in combination with dacarbazine (NCT02076646 and NCT01253096).
Protease activated cytokines
Severe toxicities limit the wide application of HD IL-2 in clinical practice. Recently, a new strategy was developed to reduce its adverse effects. This strategy employs a fusion protein (FP) in which IL-2 is joined covalently to a specific inhibitory binding component separated by a protease cleavage site. The local concentration of the specific binding inhibitor is extraordinarily high and specially binds to IL-2. However, after cleavage by proteases that are over-expressed locally at the tumor site [such as matrix metalloproteases (MMPs) or prostate-specific antigen (PSA)], IL-2 will be available to interact with high-affinity receptors on immune cells. These receptors are 10- to 1,000-fold more avid than the specific binding of the isolated α chain. Moreover, immune cells can produce additional cytokines after stimulation. This approach successfully demonstrated that the FP could reduce tumor growth in a mouse colon cancer model in vivo.Citation98 In this study, antibody/IL-2 complexes might be able to bind preferentially to cells that express particular combinations of receptors, which is meaningful since it might preferentially stimulate effector cells such as CD8+ cells and NK cells via eliminating inhibitory components. As a result, specificity will increase, which might further increase its efficacy. This method could not only increase the efficacy of immunotherapy by preferentially altering the tumor microenvironment and enhancing particular subsets of immune cells but also reduce toxicity since it could specially identify tumor cells.
Future directions of IL-2-based immunotherapy
Despite the comprehensive studies of IL-2 on immune system have established the application of IL-2 for tumor immunotherapy, a number of poignant obstacles remain for future research. First, the dose and timing of these new IL-2-based reagents, the immunogenicity of the novel molecules, and their effective combination remain unclear. Secondly, although a lot of studies have focused on the role of IL-2 on T-cells and NK cells, the IL-2R can be expressed by other haematopoietic cells, in particular B cells that can express IL-2Rα along with the β and γ subunits. The role of intermediate and high-affinity IL-2R signaling in B cells and different B cell subsets needs to be clarified. Thirdly, the role of IL-2 in regulating CD4+ T cell lineage commitment into different effector types, especially the switch between Treg and Th17 differentiation still remain controversial.Citation99 Lastly, successful immunotherapies might be a combination, which includes not only one of effective cytokines, but also other immunologic approaches. In the combination, which one could bring survival benefit and will be transformed into clinical application need further research in the future.
Conclusions
IL-2 plays a critical role in the activation of immune system that could be a useful way to eradicate cancer. As monotherapy, IL-2 has been demonstrated capable of mediating tumor regression and was approved for metastatic renal cell carcinoma and metastatic melanoma by FDA. Nevertheless, IL-2 as monotherapy is insufficient to improve patients' survival due to its dual functional properties on T cells and severe adverse effect in high dose. The complexity of IL-2 or IL-2 mutants with one or more of these other common γ chain cytokine family members, named as “superkines” may stimulate unique and more potent signaling effects on lymphocytes through the simultaneously triggering of multiple signaling complexes. Their alone or combinations with other anticancer immunotherapies, such as adoptive cell transfer regimens, antigen-specific vaccination, and blockade of immune checkpoint inhibitory molecules (e.g., CTLA-4 and PD-1/PD-L1 antibodies), have shown to overcome these drawbacks and bring some survival benefit in patients with advanced cancer. These strategies might hold the promise of treating metastatic cancer in the future.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Tao Jiang, Caicun Zhou, and Shengxiang Ren designed this study. Tao Jiang drafted the manuscript; Caicun Zhou and Shengxiang Ren revised the paper; all authors approved the final version of the manuscript.
Funding
This study was supported in part by grants from the National Natural Science Foundation of China (No. 81372392), key project of Shanghai Municipal Commission of Health and Family Planning (No. 2013zyjb0401) and Outstanding Yong Doctor Program of Shanghai Municipal Commission of Health and Family Planning (No. XYQ2013097). This study was also supported by the National Natural Science Foundation of China (No. 81201707) and Science and Technology Commission of Shanghai Municipality (No. 12ZR1426000).
References
- Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 1976; 193:1007-8; PMID:181845; http://dx.doi.org/10.1126/science.181845
- Malek TR. The biology of interleukin-2. Annu Rev Immunol 2008; 26:453-79; PMID:18062768; http://dx.doi.org/10.1146/annurev.immunol.26.021607.090357
- Wang X, Lupardus P, Laporte SL, Garcia KC. Structural biology of shared cytokine receptors. Annu Rev Immunol 2009; 27:29-60; PMID:18817510; http://dx.doi.org/10.1146/annurev.immunol.24.021605.090616
- Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013; 38:13-25; PMID:23352221; http://dx.doi.org/10.1016/j.immuni.2013.01.004
- Skrombolas D, Frelinger JG. Challenges and developing solutions for increasing the benefits of IL-2 treatment in tumor therapy. Expert Rev Clin Immunol 2014; 10:207-17; PMID:24410537; http://dx.doi.org/10.1586/1744666X.2014.875856
- Paliard X, de Waal Malefijt R, Yssel H, Blanchard D, Chretien I, Abrams J, de Vries J, Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol 1988; 141:849-55; PMID:2969394; http://dx.doi.org/10.0022-l767/88/1413-0849$02.00
- Leonard WJ. Cytokines and immunodeficiency diseases. Nat Rev Immunol 2001; 1:200-8; PMID:11905829; http://dx.doi.org/10.1038/35105066
- Yui MA, Sharp LL, Havran WL, Rothenberg EV. Preferential activation of an IL-2 regulatory sequence transgene in TCR gamma delta and NKT cells: subset-specific differences in IL-2 regulation. J Immunol 2004; 172:4691-9; PMID:15067044; http://dx.doi.org/10.4049/jimmunol.172.8.4691
- Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol 2010; 10:225-35; PMID:20336151; http://dx.doi.org/10.1038/nri2735
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol 2003; 21:713-58; PMID:12500979; http://dx.doi.org/10.1146/annurev.immunol.21.120601.140942
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 2010; 140:845-58; PMID:20303875; http://dx.doi.org/10.1016/j.cell.2010.02.021
- Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev 2011; 241:260-8; PMID:21488902; http://dx.doi.org/10.1111/j.1600-065X.2011.01018.x
- Leonard WJ, Kronke M, Peffer NJ, Depper JM, Greene WC. Interleukin 2 receptor gene expression in normal human T lymphocytes. Proc Natl Acad Sci U S A 1985; 82:6281-5; PMID:3929255; http://dx.doi.org/10.1073/pnas.82.18.6281
- Brisslert M, Bokarewa M, Larsson P, Wing K, Collins LV, Tarkowski A. Phenotypic and functional characterization of human CD25+ B cells. Immunology 2006; 117:548-57; PMID:16556269; http://dx.doi.org/10.1111/j.1365-2567.2006.02331.x
- Kronin V, Vremec D, Shortman K. Does the IL-2 receptor alpha chain induced on dendritic cells have a biological function? Int Immunol 1998; 10:237-40; PMID:9533452; http://dx.doi.org/10.1093/intimm/10.2.237
- Krieg C, Letourneau S, Pantaleo G, Boyman O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci U S A 2010; 107:11906-11; PMID:20547866; http://dx.doi.org/10.1073/pnas.1002569107
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009; 30:636-45; PMID:19464986; http://dx.doi.org/10.1016/j.immuni.2009.04.010
- Sim GC, Radvanyi L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev 2014; 25:377-90; PMID:25200249; http://dx.doi.org/10.1016/j.cytogfr.2014.07.018
- Cosman D. The hematopoietin receptor superfamily. Cytokine 1993; 5:95-106; PMID:8392875; http://dx.doi.org/10.1016/1043-4666(93)90047-9
- Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev 2006; 17:349-66; PMID:16911870; http://dx.doi.org/10.1016/j.cytogfr.2006.07.003
- Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol 1998; 70:1-81; PMID:9755337; http://dx.doi.org/10.1016/S0065-2776(08)60386-7
- Bani L, David D, Moreau JL, Cayota A, Nakarai T, Ritz J, Theze J. Expression of the IL-2 receptor gamma subunit in resting human CD4 T lymphocytes: mRNA is constitutively transcribed and the protein stored as an intracellular component. Int Immunol 1997; 9:573-80; PMID:9138018; http://dx.doi.org/10.1093/intimm/9.4.573
- Rickert M, Wang X, Boulanger MJ, Goriatcheva N, Garcia KC. The structure of interleukin-2 complexed with its alpha receptor. Science. 2005; 308:1477-80; PMID:15933202; http://dx.doi.org/10.1126/science.1109745
- Minami Y, Kono T, Miyazaki T, Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol 1993; 11:245-68; PMID:8476561; http://dx.doi.org/10.1146/annurev.iy.11.040193.001333
- Smith KA. Interleukin-2: inception, impact, and implications. Science 1988; 240:1169-76; PMID:3131876; http://dx.doi.org/10.1126/science.3131876
- Waldmann T, Tagaya Y, Bamford R. Interleukin-2, interleukin-15, and their receptors. Int Rev Immunol 1998; 16:205-26; PMID:9505189; http://dx.doi.org/10.3109/08830189809042995
- Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 1985; 313:1485-92; PMID:3903508; http://dx.doi.org/10.1056/NEJM198512053132327
- Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014; 192:5451-8; PMID:24907378; http://dx.doi.org/10.4049/jimmunol.1490019
- Atkins MB, Sparano J, Fisher RI, Weiss GR, Margolin KA, Fink KI, Rubinstein L, Louie A, Mier JW, Gucalp R et al. Randomized phase II trial of high-dose interleukin-2 either alone or in combination with interferon alfa-2b in advanced renal cell carcinoma. J Clin Oncol. 1993; 11:661-70; PMID:8478661; http://dx.doi.org/10.1146/annurev.iy.11.040193.001333
- Clark JI, Kuzel TM, Lestingi TM, Fisher SG, Sorokin P, Martone B, Viola M, Sosman JA. A multi-institutional phase II trial of a novel inpatient schedule of continuous interleukin-2 with interferon alpha-2b in advanced renal cell carcinoma: major durable responses in a less highly selected patient population. Ann Oncol 2002; 13:606-13; PMID:12056712; http://dx.doi.org/10.1093/annonc/mdf105
- Dutcher JP, Fisher RI, Weiss G, Aronson F, Margolin K, Louie A, Mier J, Caliendo G, Sosman JA, Eckardt JR et al. Outpatient subcutaneous interleukin-2 and interferon-alpha for metastatic renal cell cancer: five-year follow-up of the Cytokine Working Group Study. Cancer J Sci Am 1997; 3:157-62; PMID:9161781
- McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff MS et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 2005; 23:133-41; PMID:15625368; http://dx.doi.org/10.1200/JCO.2005.03.206
- Negrier S, Escudier B, Lasset C, Douillard JY, Savary J, Chevreau C, Ravaud A, Mercatello A, Peny J, Mousseau M et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d'Immunotherapie. N Engl J Med 1998; 338:1272-8; PMID:9562581; http://dx.doi.org/10.1056/NEJM199804303381805
- Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 2003; 21:3127-32; PMID:12915604; http://dx.doi.org/10.1200/JCO.2003.02.122
- Mazumder A, Grimm EA, Zhang HZ, Rosenberg SA. Lysis of fresh human solid tumors by autologous lymphocytes activated in vitro with lectins. Cancer Res 1982; 42:913-8; PMID:7059990; http://dx.doi.org/10.0008-5472/82/0042-0000$02.00
- Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med 1982; 155:1823-41; PMID:6176669; http://dx.doi.org/10.1084/jem.155.6.1823
- Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, White DE. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg 1989; 210:474-84; discussion 484-5; PMID:2679456; http://dx.doi.org/10.1097/00000658-198910000-00008
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 2005; 23:2346-57; PMID:15800326; http://dx.doi.org/10.1200/JCO.2005.00.240
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008; 26:5233-9; PMID:18809613; http://dx.doi.org/10.1200/JCO.2008.16.5449
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 2012; 12:269-81; PMID:22437939; http://dx.doi.org/10.1038/nri3191
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008; 8:299-308; PMID:18354418; http://dx.doi.org/10.1038/nrc2355
- Li Y, Liu S, Hernandez J, Vence L, Hwu P, Radvanyi L. MART-1-specific melanoma tumor-infiltrating lymphocytes maintaining CD28 expression have improved survival and expansion capability following antigenic restimulation in vitro. J Immunol 2010; 184:452-65; PMID:19949105; http://dx.doi.org/10.4049/jimmunol.0901101
- Atkins MB. Cytokine-based therapy and biochemotherapy for advanced melanoma. Clin Cancer Res 2006; 12:2353s-8s; PMID:16609058; http://dx.doi.org/10.1158/1078-0432.CCR-05-2503
- Keilholz U, Goey SH, Punt CJ, Proebstle TM, Salzmann R, Scheibenbogen C, Schadendorf D, Lienard D, Enk A, Dummer R et al. Interferon alfa-2a and interleukin-2 with or without cisplatin in metastatic melanoma: a randomized trial of the European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Clin Oncol 1997; 15:2579-88; PMID:9215828; http://dx.doi.org/10.0732-183X/97/1507-0003$3.00/0
- Eton O, Legha SS, Bedikian AY, Lee JJ, Buzaid AC, Hodges C, Ring SE, Papadopoulos NE, Plager C, East MJ et al. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: results from a phase III randomized trial. J Clin Oncol 2002; 20:2045-52; PMID:11956264; http://dx.doi.org/10.1200/JCO.2002.07.044
- Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Seipp CA, Einhorn JH, White DE, Steinberg SM. Prospective randomized trial of the treatment of patients with metastatic melanoma using chemotherapy with cisplatin, dacarbazine, and tamoxifen alone or in combination with interleukin-2 and interferon alfa-2b. J Clin Oncol 1999; 17:968-75; PMID:10071291
- Keilholz U, Punt CJ, Gore M, Kruit W, Patel P, Lienard D, Thomas J, Proebstle TM, Schmittel A, Schadendorf D et al. Dacarbazine, cisplatin, and interferon-alfa-2b with or without interleukin-2 in metastatic melanoma: a randomized phase III trial (18951) of the European Organisation for Research and Treatment of Cancer Melanoma Group. J Clin Oncol 2005; 23:6747-55; PMID:16170182; http://dx.doi.org/10.1200/JCO.2005.03.202
- Ridolfi R, Chiarion-Sileni V, Guida M, Romanini A, Labianca R, Freschi A, Lo Re G, Nortilli R, Brugnara S, Vitali P et al. Cisplatin, dacarbazine with or without subcutaneous interleukin-2, and interferon alpha-2b in advanced melanoma outpatients: results from an Italian multicenter phase III randomized clinical trial. J Clin Oncol 2002; 20:1600-7; PMID:11896110; http://dx.doi.org/10.1200/JCO.20.6.1600
- Bajetta E, Del Vecchio M, Nova P, Fusi A, Daponte A, Sertoli MR, Queirolo P, Taveggia P, Bernengo MG, Legha SS et al. Multicenter phase III randomized trial of polychemotherapy (CVD regimen) versus the same chemotherapy (CT) plus subcutaneous interleukin-2 and interferon-alpha2b in metastatic melanoma. Ann Oncol 2006; 17:571-7; PMID:16469753; http://dx.doi.org/10.1093/annonc/mdl007
- Atkins MB, Hsu J, Lee S, Cohen GI, Flaherty LE, Sosman JA, Sondak VK, Kirkwood JM, Eastern Cooperative Oncology G. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol 2008; 26:5748-54; PMID:19001327; http://dx.doi.org/10.1200/JCO.2008.17.5448
- Sasse AD, Sasse EC, Clark LG, Ulloa L, Clark OA. Chemoimmunotherapy versus chemotherapy for metastatic malignant melanoma. Cochrane Database Syst Rev 2007; CD005413; PMID:17253556; http://dx.doi.org/10.1002/14651858.CD005413.pub2
- Ives NJ, Stowe RL, Lorigan P, Wheatley K. Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: a meta-analysis of 18 trials involving 2,621 patients. J Clin Oncol 2007; 25:5426-34; PMID:18048825; http://dx.doi.org/10.1200/JCO.2007.12.0253
- Redmond KL, Papafili A, Lawler M, Van Schaeybroeck S. Overcoming Resistance to Targeted Therapies in Cancer. Semin Oncol. 2015; 42:896-908; PMID:26615134; http://dx.doi.org/10.1053/j.seminoncol.2015.09.028
- Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015; 372:1689-99; PMID:25923549; http://dx.doi.org/10.1056/NEJMoa1411817
- Tan CS, Gilligan D, Pacey S. Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer. Lancet Oncol 2015; 16:e447-59; PMID:26370354; http://dx.doi.org/10.1016/S1470-2045(15)00246-6
- De Vita F, Turitto G, di Grazia M, Frattolillo A, Catalano G. Analysis of interleukin-2/interleukin-2 receptor system in advanced non-small-cell lung cancer. Tumori 1998; 84:33-8; PMID:9619711
- Chen YM, Yang WK, Whang-Peng J, Tsai WY, Hung YM, Yang DM, Lin WC, Perng RP, Ting CC. Restoration of the immunocompetence by IL-2 activation and TCR-CD3 engagement of the in vivo anergized tumor-specific CTL from lung cancer patients. J Immunother 1997; 20:354-64; PMID:9336742; http://dx.doi.org/10.1097/00002371-199709000-00004
- Umekawa K, Kimura T, Kudoh S, Suzumura T, Oka T, Nagata M, Mitsuoka S, Matsuura K, Nakai T, Yoshimura N et al. Plasma RANTES, IL-10, and IL-8 levels in non-small-cell lung cancer patients treated with EGFR-TKIs. BMC Res Notes 2013; 6:139; PMID:23566546; http://dx.doi.org/10.1186/1756-0500-6-139
- Yamamoto N, Honma M, Suzuki H. Off-target serine/threonine kinase 10 inhibition by erlotinib enhances lymphocytic activity leading to severe skin disorders. Mol Pharmacol 2011; 80:466-75; PMID:21606217; http://dx.doi.org/10.1124/mol.110.070862
- Bersanelli M, Buti S, Camisa R, Brighenti M, Lazzarelli S, Mazza G, Passalacqua R. Gefitinib plus interleukin-2 in advanced non-small cell lung cancer patients previously treated with chemotherapy. Cancers (Basel) 2014; 6:2035-48; PMID:25271833; http://dx.doi.org/10.3390/cancers6042035
- Lam ET, Wong MK, Agarwal N, Redman BG, Logan T, Gao D, Flaig TW, Lewis K, Poust J, Monk P et al. Retrospective analysis of the safety and efficacy of high-dose interleukin-2 after prior tyrosine kinase inhibitor therapy in patients with advanced renal cell carcinoma. J Immunother 2014; 37:360-5; PMID:25075565; http://dx.doi.org/10.1097/CJI.0000000000000044
- Overwijk WW, Theoret MR, Restifo NP. The future of interleukin-2: enhancing therapeutic anticancer vaccines. Cancer J Sci Am 2000; 6(Suppl 1):S76-80; PMID:10685664
- Smith FO, Downey SG, Klapper JA, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Restifo NP, Levy CL et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res 2008; 14:5610-8; PMID:18765555; http://dx.doi.org/10.1158/1078-0432.CCR-08-0116
- Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011; 364:2119-27; PMID:21631324; http://dx.doi.org/10.1056/NEJMoa1012863
- Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily–CTLA-4. Nature 1987; 328:267-70; PMID:3496540; http://dx.doi.org/10.1038/328267a0
- Adachi K, Tamada K. Immune checkpoint blockade opens an avenue of cancer immunotherapy with a potent clinical efficacy. Cancer Sci 2015; 106:945-50; PMID:25981182; http://dx.doi.org/10.1111/cas.12695
- Brahmer JR. Immune checkpoint blockade: the hope for immunotherapy as a treatment of lung cancer? Semin Oncol 2014; 41:126-32; PMID:24565586; http://dx.doi.org/10.1053/j.seminoncol.2013.12.014
- van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med 1999; 190:355-66; PMID:10430624; http://dx.doi.org/10.1084/jem.190.3.355
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996; 271:1734-6; PMID:8596936; http://dx.doi.org/10.1126/science.271.5256.1734
- Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res 2000; 60:2444-8; PMID:10811122
- Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Haworth LR, Levy C et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol 2005; 12:1005-16; PMID:16283570; http://dx.doi.org/10.1245/ASO.2005.03.536
- Hannani D, Vetizou M, Enot D, Rusakiewicz S, Chaput N, Klatzmann D, Desbois M, Jacquelot N, Vimond N, Chouaib S et al. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res 2015; 25:208-24; PMID:25582080; http://dx.doi.org/10.1038/cr.2015.3
- Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood 2006; 107:2409-14; PMID:16304057; http://dx.doi.org/10.1182/blood-2005-06-2399
- Berntsen A, Brimnes MK, thor Straten P, Svane IM. Increase of circulating CD4+CD25highFoxp3+ regulatory T cells in patients with metastatic renal cell carcinoma during treatment with dendritic cell vaccination and low-dose interleukin-2. J Immunother 2010; 33:425-34; PMID:20386464; http://dx.doi.org/10.1097/CJI.0b013e3181cd870f
- Sim GC, Martin-Orozco N, Jin L, Yang Y, Wu S, Washington E, Sanders D, Lacey C, Wang Y, Vence L, Hwu P, Radvanyi L. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Invest 2014; 124:99-110; PMID:24292706; http://dx.doi.org/10.1172/JCI46266
- Yao X, Ahmadzadeh M, Lu YC, Liewehr DJ, Dudley ME, Liu F, Schrump DS, Steinberg SM, Rosenberg SA, Robbins PF. Levels of peripheral CD4(+)FoxP3(+) regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood 2012; 119:5688-96; PMID:22555974; http://dx.doi.org/10.1182/blood-2011-10-386482
- Gately MK, Anderson TD, Hayes TJ. Role of asialo-GM1-positive lymphoid cells in mediating the toxic effects of recombinant IL-2 in mice. J Immunol 1988; 141:189-200; PMID:3259967; http://dx.doi.org/10.0022-l767/88/1411-0189$02.0
- Peace DJ, Cheever MA. Toxicity and therapeutic efficacy of high-dose interleukin 2. In vivo infusion of antibody to NK-1.1 attenuates toxicity without compromising efficacy against murine leukemia. J Exp Med 1989; 169:161-73; PMID:2783332; http://dx.doi.org/10.1084/jem.169.1.161
- Assier E, Jullien V, Lefort J, Moreau JL, Di Santo JP, Vargaftig BB, Lapa e Silva JR, Theze J. NK cells and polymorphonuclear neutrophils are both critical for IL-2-induced pulmonary vascular leak syndrome. J Immunol 2004; 172:7661-8; PMID:15187148; http://dx.doi.org/10.4049/jimmunol.172.12.7661
- Epstein AL, Chen FM, Taylor CR. A novel method for the detection of necrotic lesions in human cancers. Cancer Res 1988; 48:5842-8; PMID:3048650
- Gallagher DC, Bhatt RS, Parikh SM, Patel P, Seery V, McDermott DF, Atkins MB, Sukhatme VP. Angiopoietin 2 is a potential mediator of high-dose interleukin 2-induced vascular leak. Clin Cancer Res 2007; 13:2115-20; PMID:17404094; http://dx.doi.org/10.1158/1078-0432.CCR-06-2509
- Samlowski WE, Kondapaneni M, Tharkar S, McGregor JR, Laubach VE, Salvemini D. Endothelial nitric oxide synthase is a key mediator of interleukin-2-induced hypotension and vascular leak syndrome. J Immunother 2011; 34:419-27; PMID:21577143; http://dx.doi.org/10.1097/CJI.0b013e31821dcb50
- Rao BM, Girvin AT, Ciardelli T, Lauffenburger DA, Wittrup KD. Interleukin-2 mutants with enhanced alpha-receptor subunit binding affinity. Protein Eng 2003; 16:1081-7; PMID:14983090; http://dx.doi.org/10.1093/protein/gzg111
- Rao BM, Driver I, Lauffenburger DA, Wittrup KD. Interleukin 2 (IL-2) variants engineered for increased IL-2 receptor alpha-subunit affinity exhibit increased potency arising from a cell surface ligand reservoir effect. Mol Pharmacol 2004; 66:864-9; PMID:15385640; http://dx.doi.org/10.1124/mol.66.4
- Shanafelt AB, Lin Y, Shanafelt MC, Forte CP, Dubois-Stringfellow N, Carter C, Gibbons JA, Cheng SL, Delaria KA, Fleischer R et al. A T-cell-selective interleukin 2 mutein exhibits potent antitumor activity and is well tolerated in vivo. Nat Biotechnol. 2000; 18:1197-202; PMID:11062441; http://dx.doi.org/10.1038/81199
- Margolin K, Atkins MB, Dutcher JP, Ernstoff MS, Smith JW, 2nd, Clark JI, Baar J, Sosman J, Weber J, Lathia C et al. Phase I trial of BAY 50–4798, an interleukin-2-specific agonist in advanced melanoma and renal cancer. Clin Cancer Res 2007; 13:3312-9; PMID:17545537; http://dx.doi.org/10.1158/1078-0432.CCR-06-1341
- Heaton KM, Ju G, Grimm EA. Human interleukin 2 analogues that preferentially bind the intermediate-affinity interleukin 2 receptor lead to reduced secondary cytokine secretion: implications for the use of these interleukin 2 analogues in cancer immunotherapy. Cancer Res 1993; 53:2597-602; PMID:8495422
- Heaton KM, Grimm EA. Differential inhibition of lymphokine-activated killing, proliferation, and cytokine secretion by humanized antibodies against the low- and intermediate-affinity interleukin-2 receptors. A novel model for activation of human peripheral blood mononuclear cells by interleukin 2. Hum Immunol 1995; 42:274-80; PMID:7759316; http://dx.doi.org/10.1016/0198-8859(94)00106-Z
- Levin AM, Bates DL, Ring AM, Krieg C, Lin JT, Su L, Moraga I, Raeber ME, Bowman GR, Novick P et al. Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkine’. Nature 2012; 484:529-33; PMID:22446627; http://dx.doi.org/10.1038/nature10975
- Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov 2006; 5:147-59; PMID:16424916; http://dx.doi.org/10.1038/nrd1957
- List T, Neri D. Immunocytokines: a review of molecules in clinical development for cancer therapy. Clin Pharmacol 2013; 5:29-45; PMID:23990735; http://dx.doi.org/10.2147/CPAA.S49231
- Sabzevari H, Gillies SD, Mueller BM, Pancook JD, Reisfeld RA. A recombinant antibody-interleukin 2 fusion protein suppresses growth of hepatic human neuroblastoma metastases in severe combined immunodeficiency mice. Proc Natl Acad Sci U S A 1994; 91:9626-30; PMID:7937818; http://dx.doi.org/10.1073/pnas.91.20.9626
- Carnemolla B, Borsi L, Balza E, Castellani P, Meazza R, Berndt A, Ferrini S, Kosmehl H, Neri D, Zardi L. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood 2002; 99:1659-65; PMID:11861281; http://dx.doi.org/10.1182/blood.V99.5.1659
- Schliemann C, Palumbo A, Zuberbuhler K, Villa A, Kaspar M, Trachsel E, Klapper W, Menssen HD, Neri D. Complete eradication of human B-cell lymphoma xenografts using rituximab in combination with the immunocytokine L19-IL2. Blood 2009; 113:2275-83; PMID:19005180; http://dx.doi.org/10.1182/blood-2008-05-160747
- `Wagner K, Schulz P, Scholz A, Wiedenmann B, Menrad A. The targeted immunocytokine L19-IL2 efficiently inhibits the growth of orthotopic pancreatic cancer. Clin Cancer Res 2008; 14:4951-60; PMID:18676770; http://dx.doi.org/10.1158/1078-0432.CCR-08-0157
- Osenga KL, Hank JA, Albertini MR, Gan J, Sternberg AG, Eickhoff J, Seeger RC, Matthay KK, Reynolds CP, Twist C et al. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children's Oncology Group. Clin Cancer Res 2006; 12:1750-9; PMID:16551859; http://dx.doi.org/10.1158/1078-0432.CCR-05-2000
- Ko YJ, Bubley GJ, Weber R, Redfern C, Gold DP, Finke L, Kovar A, Dahl T, Gillies SD. Safety, pharmacokinetics, and biological pharmacodynamics of the immunocytokine EMD 273066 (huKS-IL2): results of a phase I trial in patients with prostate cancer. J Immunother 2004; 27:232-9; PMID:15076141; http://dx.doi.org/10.1097/00002371-200405000-00008
- Puskas J, Skrombolas D, Sedlacek A, Lord E, Sullivan M, Frelinger J. Development of an attenuated interleukin-2 fusion protein that can be activated by tumour-expressed proteases. Immunology 2011; 133:206-20; PMID:21426339; http://dx.doi.org/10.1111/j.1365-2567.2011.03428.x
- Deknuydt F, Bioley G, Valmori D, Ayyoub M. IL-1beta and IL-2 convert human Treg into T(H)17 cells. Clin Immunol 2009; 131:298-307; PMID:19211307; http://dx.doi.org/10.1016/j.clim.2008.12.008