ABSTRACT
We have previously reported that tumor antigen-specific DNA vaccination in mice led to an increase in IFNγ-secreting T cells and an increase in tumor expression of PD-L1. Further, we demonstrated that increasing the encoded antigen's MHC-binding affinity led to increased PD-1 expression on antigen-specific CD8+ T cells. Together these phenomena provided resistance to antitumor immunization that was abrogated with PD-1/PD-L1 blockade. We consequently sought to determine whether similar regulation occurred in human patients following antitumor immunization. Using clinical samples from prostate cancer patients who were previously immunized with a DNA vaccine, we analyzed changes in checkpoint receptor expression on antigen-specific CD8+ T cells, the effect of PD-1 blockade on elicited immune responses, and for changes in checkpoint ligand expression on patients' circulating tumor cells (CTCs). We observed no significant changes in T-cell expression of PD-1 or other checkpoint receptors, but antigen-specific immune responses were detected and/or augmented with PD-1 blockade as detected by IFNγ and granzyme B secretion or trans vivo DTH testing. Moreover, PD-L1 expression was increased on CTCs following vaccination, and this PD-L1 upregulation was associated with the development of sustained T-cell immunity and longer progression-free survival. Finally, similar results were observed with patients treated with sipuleucel-T, another vaccine targeting the same prostate antigen. These findings provide in-human rationale for combining anticancer vaccines with PD-1 blocking antibodies, particularly for the treatment of prostate cancer, a disease for which vaccines have demonstrated benefit and yet PD-1 inhibitors have shown little clinical benefit to date as monotherapies.
Introduction
The field of cancer immunotherapy has seen remarkable growth and renewed momentum in the past few years due to many major successes, most notably the development and clinical success of T-cell checkpoint inhibitors.Citation1 Because tumor cells are derived from normal host cells, they can maintain or hijack autoimmunity defense mechanisms to repress the function of T cells that might otherwise attempt to eliminate malignant cells, creating a major barrier to the development of productive antitumor immune responses by immunotherapy.Citation2,3 Blockade of some of these regulatory molecules (notably PD-1 and CTLA-4), alone or in combination, has been shown to be remarkably effective at treating multiple cancer types.Citation4-8 At the time of this writing, the US FDA has currently approved three different checkpoint inhibitors (ipilimumab, nivolumab, and pembrolizumab) for treating melanoma, renal cell cancer, and non-small cell lung cancer, and approvals for several other malignancies are anticipated.Citation9-14
Because these specific agents act on T cells (instead of on tumor cells directly), these agents have activity to treat a wide variety of malignancies that use checkpoint ligand expression to avoid immune detection. However, there are also some diseases in which these have shown little or no efficacy as monotherapies. Prostate cancer is one disease for which there has been little evidence of benefit following treatment with PD-1 blockade alone.Citation5,15 These observations suggest that there might be differences in the pre-existing T-cell populations among patients with different types of cancer. Indeed, groups have observed that the malignancies most responsive to PD-1 pathway blockade tend to have high DNA mutation rates, potentially causing these patients to have a higher frequency of pre-existing T cells specific for mutant tumor epitopes that might be preferentially PD-1-regulated.Citation16-18 Some groups have also shown an association between higher levels of PD-L1 expression in the tumor microenvironment and a response to PD-1 blockade.Citation5,19,20 Because PD-L1 is an IFNγ responsive gene, this suggests that patients with high PD-L1 (and therefore likely to respond to PD-1 blockade) are those patients with high levels of tumor-antigen specific IFNγ-secreting T cells.Citation21 These data suggest the necessity of pre-existing T cells specific for one or more tumor epitopes in the determination of clinical response to PD-1 blockade. This further suggests that checkpoint blockade might be most effective when combined with a method to increase the frequency of these tumor antigen-specific T cells.
While many such methods have been employed in a non-antigen-specific fashion (e.g. by the use of chemotherapy, radiation therapy, or hormonal therapy Citation22-25), tumor antigen-specific vaccination or adoptive transfer of antigen-specific CD8+ T cells may provide the most direct means of eliciting or supplying tumor-specific T cells.Citation26,27 In fact, several different groups have recently demonstrated in animal models that combining antitumor vaccines with checkpoint blockade could increase the antitumor efficacy of these vaccines. Our group recently demonstrated that a DNA vaccine encoding high-affinity epitopes elicited CD8+ T cells with high PD-1 expression and an inferior antitumor response unless vaccination was combined with PD-1 blockade, or by targeting tumors incapable of expressing PD-L1.Citation28 Fu and colleagues demonstrated that an IFNγ-inducing recombinant GM-CSF vaccine (TEGVAX) plus anti-PD-1 elicited a stronger antitumor response than either one alone.Citation29 In both studies, it was found that PD-L1 expression on tumors was increased following vaccination due to an increase in tumor antigen-specific IFNγ-secreting T cells.
Since we have previously observed that patients with prostate cancer developed persistent IFNγ-secreting T-cell immune responses following vaccination, and yet tumors continued to progress (albeit at a potentially slower rate), we questioned whether a similar mechanism of immune regulation might have occurred in these patients; namely, that the IFNγ-secreting T cells elicited via vaccination induced elevated levels of PD-L1 expression on tumors, leading to a possible means of tumor immune escape. Using samples previously collected from patients with advanced prostate cancer treated with a DNA vaccine encoding prostatic acid phosphatase (PAP), we observed no changes in T-cell checkpoint receptors on antigen-specific CD8+ T cells following vaccination. However, using in vitro and trans vivo methods, we found that immune responses to PAP were detected and/or augmented when combined with PD-1 blockade. Moreover, we detected increased expression of PD-L1 on CTCs following vaccination, and we found that higher expression correlated with the development of antigen-specific IFNγ-secreting immune responses. Similar findings were also observed in patients treated with sipuleucel-T, an FDA-approved immunotherapy for prostate cancer which targets the same PAP antigen. Together, these data provide substantial evidence to support combining antitumor vaccines with a PD-1 pathway inhibitor in clinical trials, an approach we are currently pursuing using this DNA vaccine (NCT02499835). In addition, our findings suggest that dynamic monitoring of PD-L1 expression on CTCs could be a simple means to assess antitumor immunity induced by different therapies.
Results
PD-1-regulated PAP-specific T cells are elicited in patients following DNA vaccination
We have previously conducted a Phase I clinical trial in which patients with castrate-resistant, non-metastatic prostate cancer were treated at least 6 times biweekly with a DNA vaccine encoding PAP.Citation30 Cryopreserved peripheral blood mononuclear cell (PBMC) samples from these patients were used to assess for changes in T-cell checkpoints and ligands associated with the development of antigen-specific immunity. We first analyzed the expression of various immune checkpoint receptors on antigen-specific T cells elicited via vaccination. PBMC from 6 HLA-A2+ patients were stained with tetramers specific for two HLA-A2-restricted PAP epitopes, p112-120 and p299-307,Citation34 and expression of PD-1, BTLA, TIM-3, LAG-3, and CD160 was then assessed on these PAP-specific CD8+ T cells via flow cytometry. As shown in , we observed no significant changes in the expression of any of these checkpoint receptors over the course of vaccination. However, given that this approach was limited to HLA-A2 restricted PAP-specific T cells in a small number of patients, we next utilized functional assays to analyze the effect of checkpoint regulation on antigen-specific immune responses following vaccination in all patients. PBMC collected 1 y post-treatment were cultured in vitro with recombinant PAP protein (or ovalbumin as a negative control, Fig. S1) in combination with antibodies blocking PD-1 or TIM-3; ELISA was used to quantify cytokines secreted. demonstrates that antigen-specific secretion of both IFNγ and granzyme B was increased when PD-1 was blocked. This was determined to be due to CD8+ T cells, as IFNγ and granzyme B secretion increased following PD-1 blockade using isolated CD8+ T cells with purified autologous dendritic cells (Fig. S2). Interestingly, however, we saw a slight decrease in PAP-specific TNFα secretion when combined with PD-1 blockade. We also observed that TIM-3 blockade, although having no effect on antigen-specific Th1 cytokine production, significantly reduced the PAP-specific secretion of IL-10, a classically-inhibitory Th2 cytokine. No changes in other cytokine levels (IL-2, IL-6, MCP-1, GRO, or soluble Fas) were observed after culture in the presence of PD-1 or TIM-3 blockade (data not shown).
Figure 1. PAP-specific immune responses elicited following DNA vaccination are regulated by PD-1. (A) PBMC from HLA-A2+ patients (n = 6) were stained with tetramers for both p112-120 and p299-307, two HLA-A2-restricted PAP epitopes, and analyzed for their expression of various checkpoint molecules. Graphs show the frequency of PAP-specific CD8+ T cells (% of total CD8+) or the mean fluorescence intensity (MFI) of PD-1, TIM-3, LAG-3, BTLA, or CD160 on the surface of antigen-specific CD8+ T cells from pre-treatment, and 3 mo and 1 y post-treatment, samples. (B) 1 y post-treatment PBMC were stimulated with recombinant PAP protein in the presence of PD-1 or TIM-3 blocking antibodies (or IgG control), and cytokine secretion after 72 h (36 h for TNFα) was assessed by ELISA. Graphs show the cytokine secretion for each individual patient. *p < 0.05 using a Wilcoxon signed-rank test. (C) Pre-treatment (open circles) or 1 y post-treatment (closed circles) PBMC were injected into the footpads of SCID mice with recombinant PAP protein (or TT/D as positive control) and the indicated antibody (or IgG control), and DTH swelling responses were measured after 24 h. Results are shown for patients who did (responders, n = 4) and did not (non-responders, n = 6) develop a persistent PAP-specific T cell immune response; *p < 0.05 using a paired t test.
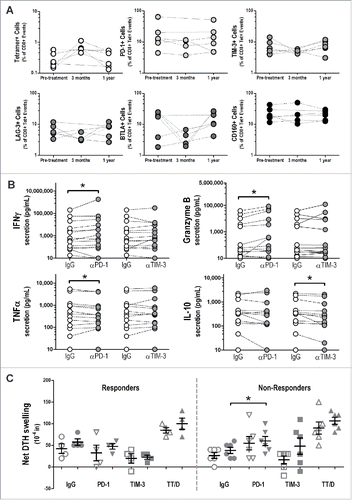
To further study the effects of checkpoint blockade on antigen-specific immunity following vaccination, we used a murine footpad trans-vivo delayed-type hypersensitivity (tvDTH) assay, as we have previously reported.Citation33 Specifically, we inoculated the footpads of SCID mice with PBMC obtained pre-treatment or after 1 y, and with recombinant PAP protein (or tetanus/diphtheria toxoid [TT/D] as a positive control) and antibodies blocking PD-1 or TIM-3 (or control IgG). Net footpad swelling was measured 24 h following inoculation as a readout of an antigen-specific inflammatory immune response elicited with or without checkpoint blockade. As shown in , while we observed a slight increase in PAP-specific net footpad swelling after immunization in patients previously determined to be long-term immune responders, no changes in PAP-specific net footpad swelling was observed in the presence of PD-1 blockade. However, in patients characterized as immune non-responders, we saw a significant increase in PAP-specific immunity when combined with PD-1 blockade.
Changes in checkpoint ligand expression on CTCs following vaccination were associated with the development of an immune response and longer progression-free survival
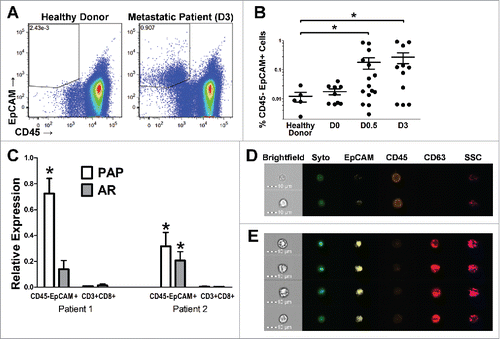
Given our findings that PD-1 blockade led to the detection or augmentation of antigen-specific T-cell function after immunization, but that there were no detectable changes in PD-1 expression on these antigen-specific T cells, we next aimed to assess the changes in expression of PD-L1 (or other known T-cell checkpoint ligands) on tumor cells following vaccination. Because patients treated on this trial had no radiographic evidence of metastases, evaluating tumor biopsy samples was not possible. Consequently, we evaluated cryopreserved PBMC samples for the presence of disseminated prostate tumor cells for which we could then evaluate the surface expression of immune regulatory markers. As shown in , we were able to detect CD45−/EpCAM+ cells in the peripheral blood of prostate cancer patients (and not in healthy donor controls), and the frequency of these events was higher in patients with castrate-resistant disease (). We further demonstrated that this population of cells contains cells of prostate origin, as they had increased expression of two prostate-specific transcripts, the androgen receptor (AR) and PAP (). Lastly, we confirmed that these events were morphologically consistent with circulating epithelial cells by using imaging cytometry, demonstrating that these cells were indeed nucleated and had membrane-localized expression of EpCAM and CD63, another marker of prostate-derived CTCs ().Citation35 These data taken together supported that this CD45−/EpCAM+ cell population, referred to as “CTC” for subsequent analysis, at least included a population of circulating prostate tumor cells.
Figure 2. Circulating tumor cells can be detected by flow cytometry in the peripheral blood of patients with advanced prostate cancer. (A–B) PBMC collected from patients with varying stages of disease (non-castrate, non-metastatic, PSA-recurrent (D0); castrate-resistant, non-metastatic, PSA-recurrent (D0.5); castrate-resistant, metastatic (D3)); or healthy donor controls were assessed for the frequency of CD45-EpCAM+ cells (CTCs) by multi-parameter flow cytometry. Shown are representative dot plots (A) or group averages (B) for the frequency of Live/CD45−/EpCAM+ cells as the percentage of total live events. (C) CTCs or CD3+/CD8+ T cells (negative control) were isolated via FACS and their expression of two prostate-specific transcripts was analyzed using quantitative PCR. Graphed is the relative mRNA expression of prostatic acid phosphatase (PAP) and the androgen receptor (AR) normalized to the housekeeping gene P0. (D, E) The morphology of the CTC population was evaluated using high-throughput single-cell fluorescence imaging. Shown are representative images of nucleated CD45−/EpCAM+/CD63+ cells (E) or CD45+ cells as control (D). All panels: *p ≤ 0.05 using a Mann–Whitney test.
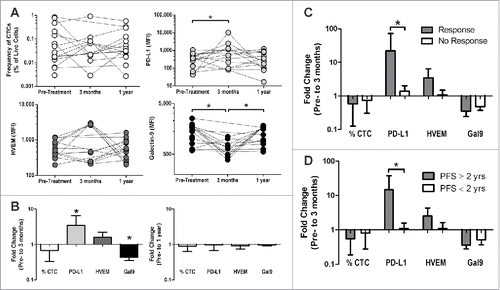
We then used flow cytometry to analyze the expression levels of PD-L1 (ligand for PD-1 and CD80), HVEM (ligand for BTLA and CD160), and Galectin-9 (a ligand for TIM3).Citation21,36-38 shows the expression levels of these three ligands on the CTCs (and the overall frequency of the CTCs) for each individual patient at baseline, and at 3 mo and 1 y post-treatment. Most notably we detected a significant increase in PD-L1 expression and significant decrease in Galectin-9 after 3 mo. However, these changes observed at 3 mo were not detectable at 1 y, suggesting that the expression of these molecules is dynamic. Panels C and D show the fold change from baseline to 3 mo for the patients previously characterized as persistent immune responders and non-responders (C) or as a function of those patients who had no radiographic progression at 2 y at the time of study conclusion (D). Similar to what we observed in preclinical studies,Citation28 we found that the patients who developed long-term PAP-specific immune responses had an upregulation of PD-L1 on their CTCs. The detection of increased PD-L1 expression was also associated with a longer time to disease progression. The changes in expression of HVEM and Galectin 9, however, were not significantly associated with either criteria.
Figure 3. Changes in checkpoint ligand expression on CTCs correlate with the development of an immune response following DNA vaccination and longer progression-free survival. CTCs from PBMC of patients treated with a DNA vaccine encoding PAP (n = 15) were assessed for the expression of various checkpoint ligands pre-treatment, during treatment (3 mo) and at 1 y post-treatment. Of note, only 9 of the 15 patients analyzed at 1 y had samples available at 3 mo available for these analyses. (A) Frequency of CTC and the mean-fluorescence intensity (MFI) of PD-L1, HVEM, and Galectin-9 on the CTC are shown for all individual patients. *p < 0.05 using a Wilcoxon signed-rank test. (B) The log-transformed fold change (post/pre) is shown for both 3 mo and 1 y post-treatment. *p < 0.05 using a one-sample Wilcoxon signed rank test against a hypothetical median of 1 (no change). (C–D) Fold change (pre-treatment to 3 mo) was assessed in patients who developed a persistent immune response or not (responder n = 3, non-responder n = 6, as defined previously, C) or in patients whose progression-free survival was ≥ 2 y (n = 4) versus < 2 y (n = 5) (D). *p < 0.05 using a Mann–Whitney test.
Similar checkpoint regulation was observed in patients treated with sipuleucel-T, an FDA approved PAP-targeting vaccine
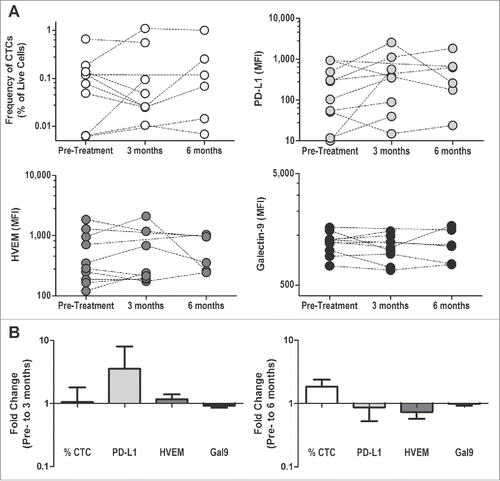
We lastly sought to identify whether these observations were exclusive to patients treated with a DNA vaccine, or if other types of antitumor vaccines elicited similar methods of regulation. For this, we analyzed PBMC samples from patients who had been treated with Provenge® (sipulecuel-T, Valeant Pharmaceuticals), a cellular immunotherapy that targets the same PAP antigen as in our studies above and has been shown to elicit PAP-specific IFNγ-secreting T cells.Citation39 As before, PBMC that had been collected from five patients 6 mo after treatment with sipuleucel-T were stimulated in vitro in the presence or absence of checkpoint receptor blockade. In these patients, contrary to what we observed in DNA-immunized patients, we observed no significant differences in the antigen-specific cytokine secretion when either PD-1 or TIM-3 was blocked (Fig. S3). However, as shown in , we observed similar (albeit not statistically significant) findings that patients treated with sipuleucel-T had a similar increase in PD-L1 expression on CTCs at 3 mo post-treatment relative to pre-treatment levels, and as before this upregulation was not detected at a later time point.
Figure 4. Treatment with sipuleucel-T leads to similar changes in checkpoint ligand expression on CTCs. CTCs from PBMC of patients treated with sipuleucel-T (n = 10) were assessed for the expression of checkpoint ligands (as above) pre-treatment, during treatment (3 mo) and at 6 mo post-treatment. Of note, samples were only available from 6 of 10 patients at 6 mo after treatment. (A) Frequency of CTC and the mean-fluorescence intensity (MFI) of PD-L1, HVEM, and Galectin-9 on the CTC are shown for all individual patients. (B) The log-transformed fold change (post/pre) is shown for both 3 mo and 6 mo post-treatment.
Discussion
Previously, we and others have shown that, in murine models, the delivery of tumor-antigen vaccines led to an upregulation of PD-L1 expression on tumors and consequently impaired antitumor immune responses, and that this regulation could be overcome by combining vaccination with antibodies blocking the PD-1/PD-L1 pathway. Therefore, in this study we sought to determine whether human cancer patients treated with antitumor vaccines developed similar responses that were regulated by this same PD-1 pathway (or by other checkpoint pathways). To that end, we analyzed samples from patients with castration-resistant non-metastatic prostate cancer, who had been previously treated in a pilot clinical trial using a DNA vaccine encoding PAP, for PD-1-regulated immune responses. We found that the PD-1 expression levels on PAP-specific T cells were not augmented following vaccination, but that both in vitro and trans vivo PAP-specific Th1 and effector-type immune responses elicited via vaccination were enhanced when combined with PD-1 antibody blockade, and that these patients had upregulated levels of PD-L1 expression on their CTCs following vaccination. We lastly observed a similar trend of PD-1-regulated immunity occurring following treatment of patients with another prostate cancer vaccine, sipuleucel-T, which similarly targets the PAP antigen.
Our findings here confirm the observations we previously reported in mice, namely that eliciting a greater frequency of tumor-antigen specific, IFNγ-secreting T cells as a result of vaccination can lead to the specific upregulation of PD-L1 on tumor cells. While certainly conceivable that other IFNγ-secreting cell populations, including NK cells, could affect PD-L1 on CTC, our observation that changes were highly associated with antigen-specific immunization and the presence of antigen-specific IFNγ-secreting cellular immune responses suggests this was mediated by T cells. That is, we demonstrated a significant correlation between increased PD-L1 expression on CTCs and the development of a long-term PAP-specific immune response, defined by the presence of persistent PAP-specific IFNγ-secreting T cells as measured by ELISPOT. This association is not necessarily surprising, given that PD-L1 expression has been linked in part to IFNγ-secreting T cells Citation40We also interestingly observed a significant correlation between increase in CTC PD-L1 expression and longer progression-free survival. This again is perhaps not surprising, given that we have previously demonstrated that the development of chronic Th1-biased antitumor immunity was associated with favorable changes in PSA kinetics, and other groups have demonstrated a more favorable prognosis for multiple tumor types if there is an infiltration of CD8+ IFNγ-secreting T cells.Citation41 Taken together, these data suggest that upregulation of PD-L1 on tumor cells can occur as a result of a productive antitumor immune response and in itself is not necessarily detrimental to an individual's disease progression. Our findings here are also consistent with what we previously reported in mice in which use of a vaccine encoding the native antigen, without changes to increase MHC class I binding affinity, did not lead to an upregulation of PD-1 on antigen-specific CD8+ T cells.
Our findings demonstrate that it is also possible to monitor the expression of PD-L1 on CTCs over the course of a therapy. To date the best characterized biomarker for a response to PD-1/L1 blockade as a monotherapy has been the expression of PD-L1 either on tumor cells or tumor-infiltrating immune cells. However, this has been limited in use due to the need for biopsy samples and the difficulty of staining formalin-fixed paraffin-embedded (FFPE) tissues for PD-L1 expression and quantifying this information. Such data may also be irrelevant for samples collected years before treatment, as is often the case for prostate cancer, if PD-L1 expression changes over time. Here, we present a much simpler, and more quantitative, approach to identify tumor-cell expression of PD-L1. Given the ease of collecting blood samples from patients this could permit tumor-cell PD-L1 expression to serve as a biomarker of response to PD-1 blockade therapy for many disease types or patients where biopsy samples are otherwise inaccessible. The dynamic detection of PD-L1 on CTC could also effectively serve as a biomarker for the presence or augmentation of tumor-specific IFNγ-secreting T cells. This could provide a simple means to assess the effects of vaccine or other therapies in generating antitumor T-cell immunity and ultimately help define personalized vaccination schedules or early biomarkers of patients likely to have clinical benefit.
We also demonstrated through both in vitro and novel trans vivo assays that PAP-specific Th1-biased immune responses were enhanced in vaccinated patients when combined with PD-1 blockade. These evaluations, conducted in the absence of tumor cells, suggest that PD-L1 on other antigen-presenting cells can affect the function of CD8+ T cells. While we did not observe an increase in PD-1 expression on PAP-specific CD8+ T cells, it is conceivable that other non-HLA-A2-restricted CD8+ T cells elicited with vaccination did have higher PD-1 expression. It is also possible that even low levels of PD-1 expression on CD8+ T cells can affect their function when engaged by its ligand, as was suggested in murine studies in which PD-1 blockade with vaccination led to greater antitumor responses even in the absence of PD-1 upregulation on vaccine-induced CD8+ T cells.Citation28 In the current study, we found that DNA-immunized patients who were previously classified as having not developed a long-term immune response were those who showed the greatest increase in immune response when combined with PD-1 blockade. This is similar to work we have previously published in which patients with earlier stage prostate cancer were shown to have pre-existing PAP-specific DTH responses that were regulated by CTLA-4.Citation33 This suggests a potential mechanism for why these patients did not develop PAP-specific immune responses, namely that some patients have pre-existing PAP-specific T cells that are already regulated by PD-1 and/or other checkpoint pathways, preventing the subsequent development of a detectable PAP-specific immune response by vaccination without using checkpoint blockade. Interestingly, we demonstrated that patients treated with sipuleucel-T did not have a similar augmentation in Th1 cytokine secretion, but that they had a similar (albeit not as robust) upregulation of PD-L1 on CTCs following immunization. This could simply be due to a low number of patient samples available for analysis. However, it also could in part be due to sipuleucel-T eliciting more of a mixed Th1 and Th2 immune response, with production of antibodies and Th2 cytokines not observed with DNA immunization.Citation42 Alternatively, the greater tumor burden in patients with more advanced prostate cancer receiving sipuleucel-T could have potentially affected the frequency of CD8+ T cells detected in the peripheral blood due to tumor trafficking.
Taken together, these finding suggest that the evaluation of CTC for PD-L1 expression may be useful for monitoring the effects of other antitumor vaccines, or other therapies that might affect tumor-associated lymphocytes, including chemotherapies, radiation therapy, or other targeted therapies. Given the remarkable success of checkpoint blockade therapy in certain disease settings, the field is rapidly moving toward combining checkpoint blockade with other proven therapies in clinical trials. These findings suggest that, by monitoring changes in checkpoint ligand and receptor expression following treatment with these various therapies, better predictions could be made regarding which specific checkpoint (if any) might be best targeted when used in combination with these other therapies. Lastly, our results suggest a clear rationale for combining this DNA vaccine with PD-1 blockade in a human clinical trial, an approach which is currently being examined in patients with castrate-resistant, metastatic prostate cancer (NCT02499835).
Materials and methods
Patient sample populations
Patient PBMC used for this study were from a previous IRB-approved clinical trial in which 17 patients with castrate-resistant, PSA-recurrent prostate cancer but with no radiographic evidence of metastases (clinical stage D0.5) were treated with a DNA vaccine encoding PAP for up to 2 y.Citation30 Samples were collected at baseline, and at 3 mo and 1 y post-treatment and cryopreserved until use. Patients were characterized as being either immune responders or non-responders based on the development of a persistent PAP-specific immune response (measured by IFNγ ELISPOT), detectable at >2 post-treatment time points, within 1 y of treatment,Citation31 and on the basis of whether or not they had evidence of radiographic progression 2 y after study initiation,Citation30 as previously reported.
PBMC from healthy male blood donors, and patients with stage D0 (PSA-recurrent but with no radiographically detectable metastases) and D3 (metastatic, castration-resistant) disease, and patients undergoing treatment with Provenge® (sipulecuel-T, Valeant Pharmaceuticals, Laval, Quebec), were collected from individuals who provided informed consent under other IRB-approved blood draw protocols.
Flow cytometry analysis
For analysis of the cell population containing CTCs, PBMC were thawed and washed 2 times in HBSS. Cells were stained with CD45-FITC (Clone HI30, BD Biosciences, San Jose, CA), EpCAM-PerCPCy5.5 (Clone EBA-1, BD Biosciences), PD-L1-PECy7 (Clone MIH1, eBioscience, San Diego, CA), HVEM-PE (Clone 122, BioLegend, San Diego, CA), and Galectin-9-APC (Clone 9M1-3, BioLegend) at a concentration of 2 tests/mL and with Ghost Dye Red-780 viability dye (Tonbo Biosciences, San Diego, CA) at a 1:1000 dilution in FACS buffer (PBS, 3% FCS, 1 mM EDTA) and analyzed on an LSR Fortessa (BD). For imaging experiments, cells were stained instead with CD45-PE-CF594, EpCAM-PE, CD63-APC (Clone MEM-259, BioLegend) and SYTO 13 nuclear dye at a 0.5 µM dilution (ThermoFisher Scientific, Waltham, MA) and were imaged using an Amnis ImageStream (EMD Millipore, Billerica, MA). CTCs were gated as Live/FSCxSSC/CD45−/EpCAM+.
For analyzing antigen-specific T cells, tetramers specific for HLA-A2 restricted epitopes p112-120 (TLMSAMTNL) and p299-307 (ALDVYNGLL) were obtained from the NIH Tetramer Core Facility (Atlanta, GA). PBMC were thawed, washed 2 times in HBSS, and then stained for 45 min at 4°C in FACS buffer containing a 1:500 dilution of tetramer. Cells were then washed and stained with CD3-BUV395 (Clone UCHT1, BD Biosciences), CD8-BV605 (Clone SK1, BD Biosciences), PD-1-PerCP-Cy5.5 (Clone EH12.2H7, BioLegend), TIM3-eFluor450 (Clone F38-2E2, eBioscience), LAG3-PE-Cy7 (Clone 3DS223H, eBioscience), BTLA-PE (Clone J168-540, BD Biosciences), CD160-AlexaFluor488 (Clone BY55, eBioscience) at a concentration of 2 tests/mL and with Ghost Dye Red-780 viability marker as before. Cells were analyzed on an LSR Fortessa and antigen-specific T cells were gated as Live/FSCxSSC/Singlet/CD3+/CD8+/Tetramer+.
Gene expression
CTCs were isolated from patient PBMC via FACS and sorted directly into cell lysis buffer. RNA was purified from these sorted CTCs (or CD3+CD8+ T cells as control) using the Dynabeads mRNA DIRECT purification kit (ThermoFisher). cDNA was synthesized using the iScript cDNA kit (BioRad, Hercules, CA) and qPCR was performed using the SsoFast EvaGreen Supermix (BioRad). Primers used were as follows: PAP (Fwd: CGGCATGGAGACCGAAGTCCC, Rev: CTGTGTGCACCGGGATGGGC), AR (Fwd: ACATCAAGGAACTCGATCGTATCATTGC, Rev: TTGGGCACTTGCACAGAGAT), and P0 (Fwd: GACAATGGCAGCATCTACAAC, Rev: GCAGACAGACACTGGCAAC). Relative expression is calculated as 2−ΔCt between the indicated transcript and the housekeeping gene P0.Citation32
In vitro stimulation and cytokine ELISAs
PBMC from patients collected 1 y post treatment were washed and resuspended at 2 × 106 cells/mL in RPMI media containing 10% human AB sera/2% Penicillin/Streptomycin/1% Sodium Pyruvate/0.1% β-mercaptoethanol. Cells were stimulated for 36–72 h with the indicated antigen (2 ug/mL recombinant human PAP, Fitzgerald Industries, Acton, MA; 2 ug/mL recombinant ovalbumin, ThermoFisher; or 5 ug/mL concanavalin A, Sigma-Aldrich, St. Louis, MO, as a positive control [data not shown]). For stimulations with purified cell populations, autologous dendritic cells were prepared by culture of adherent PBMC in X-VIVO 15 serum-free medium (Lonza, Allendale, NJ) for 6 d with 20 ng/mL GM-CSF and 10 ng/mL IL-4. CD8+ T cells were isolated using the EasySep human CD8+ T cell enrichment kit (StemCell Technologies, Vancouver, BC. Cells were then cultured at a 10:1 T-cell:DC ratio for 72 h as before. Supernatants were collected and cytokine concentrations were assessed using standard ELISA methods with the following antibody clone pairs: IFNγ (NIB42 and 4S.B3, BD), TNFα (MAb1 and MAb11, BD), Granzyme B (GB11 and GB 10, GeneTex, Irvine, CA), IL-10 (JES3-19F1 and JES3-12G8, BD).
Trans-vivo delayed-type hypersensitivity assay
Trans-vivo delayed-type hypersensitivity (tvDTH) assays were performed as previously described using PBMC from patients at baseline and 1 y post-treatment.Citation33 Briefly, 7.5 × 106 PBMC were injected into the footpad of 6–8 week old SCID mice combined with 1 µg of recombinant PAP (or tetanus/diptheria toxoid (TT/D), Sanofi Pasteur, Swiftwater, PA, as a positive control). DTH activity was measured after 24 h as the change in footpad thickness (measured in 10−4 inches) minus the swelling induced from a PBS control injection using a dial thickness gauge (Mitutoyo, Japan). One µg of blocking antibodies against PD-1 (pembrolizumab, Merck, Kenilworth, NJ), TIM-3 (F38-2E2, BioLegend), or IgG control (BioLegend) were mixed with the PBMC prior to injection where indicated.
Disclosure of potential conflicts of interest
DGM has ownership interest, receives research support, and serves as consultant to Madison Vaccines, Inc., that has licensed material described in this report. None of the other authors have relevant potential competing interests.
KONI_A_1165377_s02.docx
Download MS Word (3.9 MB)Acknowledgments
The authors thank the NIH Tetramer Facility (Atlanta, GA) for tetramer reagents, the UWCCC Flow Cytometry core facility (and NIH small instrument grants 1S10RR025483-01 and 1S100OD018202-01) for technical support, Mr. Jordan Becker for technical assistance with PBMC preparation, and Dr. Laura Johnson, Dr. Christopher Zahm, and Mr. Jordan Bloom for their helpful assistance with manuscript preparation. This work was supported by the Prostate Cancer Foundation 2014 Movember-PCF Global Treatment Sciences Challenge Award and by NIH R21-CA132267 and NRSA T32 GM07215.
References
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013; 342:1432-3; PMID:24357284; http://dx.doi.org/10.1126/science.342.6165.1432
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA 2002; 99:12293-7; PMID:12218188; http://dx.doi.org/10.1073/pnas.192461099
- Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 2005; 54:307-14; PMID:15599732; http://dx.doi.org/10.1007/s00262-004-0593-x
- Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455-65; PMID:22658128; http://dx.doi.org/10.1056/NEJMoa1200694
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200690
- Weber JS, O'Day S, Urba W, Powderly J, Nichol G, Yellin M, Snively J, Hersh E. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol 2008; 26:5950-6; PMID:19018089; http://dx.doi.org/10.1200/JCO.2008.16.1927
- Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng S et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515:558-62; PMID:25428503; http://dx.doi.org/10.1038/nature13904
- Hamid O, Robert C, Daud A, Hodi FS, Hwu W-J, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369:134-44; PMID:23724846; http://dx.doi.org/10.1056/NEJMoa1305133
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain J-F, Testori A, Grob J-J et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517-26; PMID:21639810; http://dx.doi.org/10.1056/NEJMoa1104621
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320-30; PMID:25399552; http://dx.doi.org/10.1056/NEJMoa1412082
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015; 372:2006-17; PMID:25891304; http://dx.doi.org/10.1056/NEJMoa1414428
- Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu W-J, Gangadhar TC et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014; 384:1109-17; PMID:25034862; http://dx.doi.org/10.1016/S0140-6736(14)60958-2
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015; 373:1803-13; PMID:26406148; http://dx.doi.org/10.1056/NEJMoa1510665
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28:3167-75; PMID:20516446; http://dx.doi.org/10.1200/JCO.2009.26.7609
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348:69-74; PMID:25838375; http://dx.doi.org/10.1126/science.aaa4971
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348:124-8; PMID:25765070; http://dx.doi.org/10.1126/science.aaa1348
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015; 372:2509-20; PMID:26028255; http://dx.doi.org/10.1056/NEJMoa1500596
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti-PD-1 Therapy. Clin Cancer Res 2014; 20:5064-74; PMID:24714771; http://dx.doi.org/10.1158/1078-0432.CCR-13-3271
- Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515:563-7; PMID:25428504; http://dx.doi.org/10.1038/nature14011
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26:677-704; PMID:18173375; http://dx.doi.org/10.1146/annurev.immunol.26.021607.090331
- Nadal R, Amin A, Geynisman DM, Voss MH, Weinstock M, Doyle J, Zhang Z, Viudez A, Plimack ER, McDermott DF, Motzer R, Rini B, Hammers HJ. Safety and clinical activity of vascular endothelial growth factor receptor (VEGFR)- tyrosine kinase inhibitors after programmed cell death 1 inhibitor treatment in patients with metastatic clear cell renal cell carcinoma. Ann Oncol. (in press) PMID:27059553
- Crittenden M, Kohrt H, Levy R, Jones J, Camphausen K, Dicker A, Demaria S, Formenti S. Current clinical trials testing combinations of immunotherapy and radiation. Semin Radiat Oncol 2015; 25:54-64; PMID:25481267; http://dx.doi.org/10.1016/j.semradonc.2014.07.003
- Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, Massarelli E, Hong D, Naing A, Diab A et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res 2014; 2:831-8; PMID:25187273; http://dx.doi.org/10.1158/2326-6066.CIR-14-0069
- McNeel DG, Smith HA, Eickhoff JC, Lang JM, Staab MJ, Wilding G, Liu G. Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol Immunother 2012; 61:1137-47; PMID:22210552; http://dx.doi.org/10.1007/s00262-011-1193-1
- Van den Eertwegh AJM, Versluis J, van den Berg HP, Santegoets SJAM, van Moorselaar RJA, van der Sluis TM, Gall HE, Harding TC, Jooss K, Lowy I et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 2012; 13:509-17; PMID:22326922; http://dx.doi.org/10.1016/S1470-2045(12)70007-4
- Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Donehower RC, Jaffee EM et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 2013; 36:382-9; PMID:23924790; http://dx.doi.org/10.1097/CJI.0b013e31829fb7a2
- Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG. PD-1 or PD-L1 Blockade Restores Antitumor Efficacy Following SSX2 Epitope-Modified DNA Vaccine Immunization. Cancer Immunol Res 2015; 3:946-55; PMID:26041735; http://dx.doi.org/10.1158/2326-6066.CIR-14-0206
- Fu J, Malm I-J, Kadayakkara DK, Levitsky H, Pardoll D, Kim YJ. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res 2014; 74:4042-52; PMID:24812273; http://dx.doi.org/10.1158/0008-5472.CAN-13-2685
- McNeel DG, Becker JT, Eickhoff JC, Johnson LE, Bradley ES, Pohlkamp IF, Staab MJ, Liu G, Wilding G, Olson BM. Real-Time Immune Monitoring to Guide Plasmid DNA Vaccination Schedule Targeting Prostatic Acid Phosphatase (PAP) in Patients with Castration-Resistant Prostate Cancer. Clin Cancer Res 2014; 20:3692-704; PMID:24850844; http://dx.doi.org/10.1158/1078-0432.CCR-14-0169
- Becker JT, Olson BM, Johnson LE, Davies JG, Dunphy EJ, McNeel DG. DNA vaccine encoding prostatic acid phosphatase (PAP) elicits long-term T-cell responses in patients with recurrent prostate cancer. J Immunother 2010; 33:639-47; PMID:20551832; http://dx.doi.org/10.1097/CJI.0b013e3181dda23e
- Laborda J. 36B4 cDNA used as an estradiol-independent mRNA control is the cDNA for human acidic ribosomal phosphoprotein PO. Nucleic Acids Res 1991; 19:3998; PMID:1861990; http://dx.doi.org/10.1093/nar/19.14.3998
- Olson BM, Jankowska-Gan E, Becker JT, Vignali DAA, Burlingham WJ, McNeel DG. Human prostate tumor antigen-specific CD8+ regulatory T cells are inhibited by CTLA-4 or IL-35 blockade. J Immunol 2012; 189:5590-601; PMID:23152566; http://dx.doi.org/10.4049/jimmunol.1201744
- Olson BM, Frye TP, Johnson LE, Fong L, Knutson KL, Disis ML, McNeel DG. HLA-A2-restricted T-cell epitopes specific for prostatic acid phosphatase. Cancer Immunol Immunother 2010; 59:943-53; PMID:20140431; http://dx.doi.org/10.1007/s00262-010-0820-6
- Chéry L, Lam H-M, Coleman I, Lakely B, Coleman R, Larson S, Aguirre-Ghiso JA, Xia J, Gulati R, Nelson PS et al. Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget 2014; 5:9939-51; PMID:25301725; http://dx.doi.org/10.18632/oncotarget.2480
- Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 2005; 6:1245-52; PMID:16286920; http://dx.doi.org/10.1038/ni1271
- Gonzalez LC, Loyet KM, Calemine-Fenaux J, Chauhan V, Wranik B, Ouyang W, Eaton DL. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci USA 2005; 102:1116-21; PMID:15647361; http://dx.doi.org/10.1073/pnas.0409071102
- Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol 2005; 6:90-8; PMID:15568026; http://dx.doi.org/10.1038/ni1144
- Fong L, Carroll P, Weinberg V, Chan S, Lewis J, Corman J, Amling CL, Stephenson RA, Simko J, Sheikh NA et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst 2014; 106; PMID:25255802; http://dx.doi.org/10.1093/jnci/dju268
- Eppihimer MJ, Gunn J, Freeman GJ, Greenfield EA, Chernova T, Erickson J, Leonard JP. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation 2002; 9:133-45; PMID:11932780; http://dx.doi.org/10.1080/713774061
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/10.1126/science.1129139
- McNeel DG, Gardner TA, Higano CS, Kantoff PW, Small EJ, Wener MH, Sims RB, DeVries T, Sheikh NA, Dreicer R. A transient increase in eosinophils is associated with prolonged survival in men with metastatic castration-resistant prostate cancer who receive sipuleucel-T. Cancer Immunol Res 2014; 2:988-99; PMID:25189164; http://dx.doi.org/10.1158/2326-6066.CIR-14-0073
