ABSTRACT
The immune system plays critical roles in tumor prevention, but also in its initiation and progression. Tumors are subjected to immunosurveillance, but cancer cells generate an immunosuppressive microenvironment that favors their escape from immune-mediated elimination. During chronic inflammation, immune cells can contribute to the formation and progression of tumors by producing mitogenic, prosurvival, proangiogenic and lymphangiogenic factors. Thyroid cancer is the most frequent type of endocrine neoplasia and is the most rapidly increasing cancer in the US. In this review, we discuss recent findings on how different immune cells and mediators can contribute to thyroid cancer development and progression.
Abbreviations
| Ang | = | angiopoietin |
| ATC | = | anaplastic thyroid carcinoma |
| CTL | = | cytotoxic T lymphocytes |
| DCs | = | dendritic cells |
| DTC | = | differentiated thyroid carcinoma |
| EMT | = | epithelial-to-mesenchymal transition |
| γδ | = | gamma delta |
| GM-CSF | = | granulocyte-macrophage colony-stimulating factor |
| IDO | = | Indoleamine 2,3-dioxygenase |
| IFN | = | interferon |
| IL | = | interleukin |
| ILC | = | innate lymphoid cell |
| iNOS | = | inducible nitric oxide synthase |
| MC | = | mast cell |
| MDSC | = | myeloid-derived suppressor cell |
| MMP | = | metalloproteinase |
| NK | = | natural killer |
| NLR | = | neutrophil-to-lymphocyte ratio |
| PD-L1 | = | Programmed Death-Ligand 1 |
| PDTC | = | poorly differentiated thyroid cancer |
| PTC | = | papillary thyroid cancer |
| ROS | = | reactive oxygen species |
| SCF | = | stem cell factor |
| TAM | = | tumor-associated macrophage |
| TAMC | = | tumor-associated mast cell |
| TAN | = | tumor-associated neutrophil |
| TC | = | thyroid cancer |
| TCR | = | T cell receptor |
| TEM | = | Tie2-expressing monocyte |
| Th | = | T helper cell |
| TNF | = | tumor necrosis factor |
| Treg | = | regulatory T cell |
| VEGF | = | vascular endothelial growth factor. |
Introduction
In 1863, Rudolf Virchow first observed leukocyte infiltration in neoplastic tissues and suggested a link between inflammation and cancer.Citation1 Epidemiological studies and animal models have demonstrated that cancer and inflammation are closely related. Several human cancers arise from inflammatory responses due to infectious agents. However, also tumors without a microbial background present signs of “smoldering inflammation”.Citation1 In addition, according to the cancer immunosurveillance theory, immune cells can recognize and eliminate transformed cells.Citation2 In fact, mice lacking recombination activating gene-1 (RAG-1) or RAG-2, devoid of B, T and NK lymphocytes, more efficiently developed spontaneous and carcinogen-induced tumors.Citation3
Thyroid cancer (TC) is the most rapidly increasing cancer in the US. The association between chronic inflammation and TC has long been recognized. Indeed, a mixture of immune cells and mediators has been described in TC, associated with tumor progression and patient clinical outcome.Citation4 The purpose of this review is to discuss recent findings investigating how the immune system can affect TC development and progression and its influence on patient clinical outcome.
Thyroid cancer (TC)
Epidemiology and risk factors
TC is the most frequent type of cancer of the endocrine system, accounting for approximately 90% of the endocrine malignancies and for 70% of deaths due to endocrine cancers. During the past 5 y TC incidence increased. Risk factors for TC include being female, having a history of goiter or thyroid nodule, a family history of TC, low-iodine diet, radiation exposure and obesity.Citation5
Molecular pathogenesis
Thyroid follicular cells can give rise to well-differentiated papillary or follicular (DTC), poorly differentiated (PDTC) or anaplastic thyroid carcinomas (ATC). Papillary thyroid carcinoma (PTC) accounts for 80–85% of TCs, tends to invade lymphatic vessels and displays a high incidence of regional lymph node metastasis. Follicular thyroid carcinoma (FTC) comprises 5–10% of TCs. DTCs show favorable outcome with therapies. However, PDTC displays an intermediate differentiation and a more aggressive phenotype. ATC accounts for 2–5% of TCs and is one of the most aggressive and lethal human malignancies.Citation5
PTC is associated with mutations in genes that encode for effectors involved in the mitogen-activated protein kinase (MAPK) and the PI3K-AKT pathway, including RET (encoding proto-oncogene tyrosine-protein kinase receptor ret), RAS genes (which encode small GTPases) and BRAF (encoding serine/threonine kinase B-raf).Citation6 A comprehensive multiplatform analysis of 496 PTCs found that the most common genetic alterations were found in BRAF and RAS, suggesting their pivotal role in driving the PTC initiation.Citation7 This study identified two major PTC subsets: BRAF-like PTCs are less differentiated, highly heterogeneous and display a high expression of genes downstream MAPK activation; RAS-like tumors are better differentiated, with a common follicular histology and more favorable outcome. FTC often displays the PAX8/PPARγ rearrangement, a fusion between paired domain transcription factor 8 and the peroxisome proliferator-activated receptor γ genes. Somatic mutations of PTEN and of PIK3CA have also been reported in FTC, and germline mutations of PTEN can result in FTC arising in the context of Cowden syndrome. PDTC and ATC carry activating mutations in PIK3CA, TP53 and CTNNB1 (β-catenin). AKT1 mutations have been reported in metastatic TC. In addition to these mutations, novel genetic alterations have been recently identified in TC, including ETV6/NTRK3, STRN/ALK and TERT.Citation6
Soluble mediators in TC
Soluble mediators in cancer-related immune network include cytokines, chemokines, angiogenic and lymphangiogenic factors that influence several aspects of cancer growth and progression.
Cytokines
Cytokines are mainly produced by tumor-infiltrating immune cells, but are also secreted by thyroid follicular cells. Cytokines play a role in the pathogenesis of autoimmune thyroid disease and contribute to several aspects of TC initiation and growth.Citation4
IL-1 promotes tumor growth through the induction of prometastatic genes (e.g., matrix metalloproteinases), angiogenic molecules (VEGF, CXCL8/IL-8, etc.) and cytokines (IL-6, TNF-α, and TGF-β).Citation8 IL-1 stimulated thyroid cell proliferation and the in vitro production of IL-8.Citation9 In a study performed on 115 patients affected by a variety of thyroid conditions and 30 controls, IL-1β serum concentrations allowed discrimination between individuals with atrophic thyroiditis and those with PTC, which displayed the highest and the lowest values, respectively.Citation10
IL-4 and IL-13 are closely related cytokines that play overlapping and distinct roles in type 2 immunity. Primary sources of IL-4 include human Th2 cells, basophils and T follicular helper cells (Tfh).Citation11,12 Exposure to IL-4 or IL-13 induces alternative (M2) activation of macrophages and tumor-associated macrophages (TAMs) in TC displayed an M2-like phenotype.Citation13,14 Recently, a link between ionizing radiation (IR), IL-13 and oxydative stress-induced DNA damage in thyreocytes has been observed. Exposure of thyreocytes to IR caused IL-13 production, which stimulated ROS increase through the activation of p38 MAPK. This mechanism might be responsible for genetic instability and emergence of neoplastic clones in TC.Citation15
IL-10 is a pleiotropic cytokine, which can favor a shift toward Th2 response in the tumor microenvironment.Citation16 TAMs release IL-10,Citation13 and tumor cells themselves also produce IL-10.Citation17 In a study performed on peripheral blood from 30 patients with PTC and multinodular goiter (MNG) as well as 30 patients with MNG alone, IL-10 circulating levels were higher in PTC associated with goiter compared to MNG alone.Citation18 Malignant epithelial cells purified from papillary, follicular and anaplastic human thyroid carcinomas produced in vitro IL-4 and IL-10, which increased the expression of the anti-apoptotic proteins Bcl-2, Bcl-xL, cFLIP and PED/PEA-15 in TC cells.Citation19,20
IL-24 belongs to the IL-10 gene family and is a potent inhibitor of cancer cell proliferation.Citation21 IL-24 was induced by RET/PTC3 expression in thyreocytes of RET/PTC3 transgenic mice and it was involved in autocrine growth/survival of RET/PTC3-expressing thyroid cells supporting its role in early cellular transformation. However, its expression decreased in poorly differentiated mouse tumors, paralleling the loss of RET/PTC3 expression along with tumor progression, thus supporting a role for IL-24 as tumor suppressor factor.Citation22
IL-6 is a cytokine involved in the regulation of cell proliferation, survival and metabolism.Citation23 In an in vitro model of human TC and mast cell (MC) lines, TC-derived conditioned media (CM) induced upregulation of IL-6 in human MCs.Citation24 Moreover, IL-6 contributed to epithelial-to-mesenchymal transition (EMT) and stemness features of TC.Citation25
TGF-β is overexpressed in aggressive cancers.Citation26 Tumors induce dendritic cells (DCs) to secrete TGF-β, which promotes regulatory T cell (Treg) expansion. TGF-β-mediated suppression of antitumor T cells is due to Foxp1 overexpression in CD8+ T cells.Citation26 In a murine model of transgenic BRAF mouse, thyreocytes and TAMs produced TGF-β, which was responsible for the acquisition of EMT features and invasiveness of TC cells.Citation27 In addition, TGF-β overexpression in sections of human PTCs correlated with tumor invasiveness, regardless of BRAF mutations.Citation28
IL-17A is produced mainly by CD4+ Th17 cells but IL-17-producing CD8+ T cells (Tc17 cells) have also been identified in humans.Citation21 Th17 and Tc17 cells have been found in many human cancers and IL-17 was associated with both protumor or antitumor responses.Citation21,29 The protumorigenic effects of IL-17 include the promotion of angiogenesis, survival and the recruitment of Myeloid-derived suppressor cells (MDSCs). TC patients display higher Th17 and lower Tc17 cell proportion in peripheral blood compared to controls. The serum concentration of IL-17 was increased in TC patients and correlated with the percent of circulating Th17 cells.Citation29 In addition, IL17RA polymorphisms negatively correlated with the development and the bilaterality of PTC in a study performed on 94 PTC patients and 260 controls in Korean population.Citation30
IL-21 is highly expressed, but not exclusively, by Tfh cells and Th9 cells.Citation31 Th9 cells can directly induce tumor cell death or limit tumor growth by production of IL-9 and IL-21.Citation32 In a case-control study performed on 615 TC patients and 600 controls in Chinese population, IL-21 promoter polymorphism was associated with increased risk of TC development.Citation33
All three classes of IFNs, type I (IFN-α/β), type II (IFNγ), and type three (IFN-λ/s), can induce apoptosis of tumor cells and control the circuits underlying cancer immunosurveillance.Citation34 IFNγ promotes Th1 cell differentiation and classical macrophage M1 polarization.Citation13 Type I IFNs induce DC maturation, enhance cytotoxicity of CD8+ T and NK cells and decrease Treg immunosuppression.Citation35 In TC, type I and type II IFNs in vitro induced the expression of MHC-I molecules on human TC cell lines, thus impeding TC immunoevasion and potentiating TC susceptibility to immune destruction.Citation36
Chemokines
Chemokines are a family of more than 50 functionally related small molecules with chemoattractant and cytokine-like functions. In addition to inducing chemotaxis, chemokines can modulate angiogenesis and lymphangiogenesis.Citation37
Chemokines can be produced by TC cells, following the activation of the MAPK pathways by the RET/PTC, RAS and BRAF oncogenic drivers.Citation38,39 Thyroid cells produce several CXC chemokines, including CXCL1, CXCL8/IL-8, CXCL9, CXCL10 and CXCL11, in basal conditions and/or under the influence of specific stimuli.Citation40, Citation41
A wide variety of thyroid tumor cell lines derived from PTC and ATC release CXCL8/IL-8, in basal conditions as well as under inflammatory stimuli, such as IL-1 and TNF-α.Citation9,42 Exogenous induction of RET/PTC1 oncogene in primary normal human thyreocytes induced the expression of CCL2, CCL20 and CXCL8/IL-8 genes. Moreover, CCL20 and CXCL8/IL-8 were upregulated in clinical samples of PTC.Citation39 Muzza and co-workers compared CCL2 and CXCL8/IL-8 gene expression in tissues from PTCs, thyroiditis and normal thyroid. Interestingly, PTCs displayed the highest expression of CCL20 and CXCL8/IL-8 compared to normal tissues and thyroiditis, regardless the genetic lesion beard by the tumor or the presence/absence of thyroiditis. Moreover, CXCL8/IL-8 expression in thyroiditis was similar to normal tissues, suggesting a prominent role in cancer-related inflammation and a minor role in thyroiditis. However, no association was found between CXCL8/IL-8 expression and patient outcome.Citation43 In a model of orthotopic TC xenograft in nude mice, CXCL8/IL-8 was involved in tumor growth and progression, at least in part due to the activation of the NF-κB pathway.Citation44
In addition to TC themselves, also the tumor-infiltrating immune cells can be a source of chemokines. Indeed, MC-derived CXCL8/IL-8 in vitro induced EMT features and stemness in human TC cells.Citation25 Moreover, TAMs purified from PTC patients released CXCL8/IL-8, which was responsible for PTC cell invasion and metastasis in vivo.Citation45
PTC and ATC cell lines autocrinously produce CXCL1/GRO-α and CXCL10/IP10, in basal conditions and under inflammatory stimuli, thus self-sustaining proliferation and invasiveness.Citation38,41, 46 In addition, MC-derived CXCL1/GRO-α and CXCL10/IP10 further increased TC cell proliferation through the engagement of CXCR2 and CXCR3 on TC cells.Citation24,38
CXCL12/SDF-1 and its novel receptor CXCR7 have been found in PTC patients.Citation47 Interestingly, CXCL12 expression was exclusively found in PTCs compared to other thyroid lesions and displayed high sensitivity, specificity, positive and negative predictive values. For these reasons CXCL12 has been proposed as a novel diagnostic marker for PTC.Citation48 From a functional point of view, the CXCL12/SDF-1-CXCR7 axis promoted in vitro TC cell line proliferation and invasion.Citation49 Also CCL20 was found to promote TC cell invasion and migration in vitro, presumably due to the activation of NF-κB-dependent upregulation of MMP-3.Citation50
We have recently found that the oncolytic adenovirus dl922-947 reduced CXCL8/IL-8 and CCL2 production by ATC cell lines.Citation51 Interestingly, dl922-947 treatment impaired ATC-induced angiogenesis and favored the switch of tumor macrophages toward an M1 phenotype. These results indicate that dl922-947 treatment, along with its role in inducing TC cell death, impacts on ATC immune microenvironment.
Angiogenic factors
The formation of blood and lymphatic vessels is a complex process, requiring a finely tuned balance between stimulatory and inhibitory signals such as VEGFs, Angiopoietins (Angs), chemokines, oxygen sensors and many others.Citation52
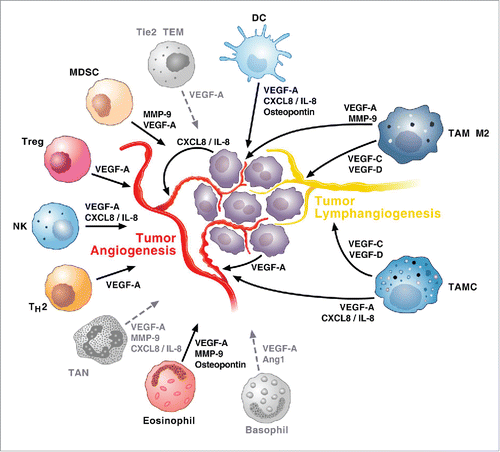
Several TC-infiltrating immune cells can impact tumor angiogenesis and lymphangiogenesis (). For instance, MCs are a major source of proangiogenic (VEGF-A and -B) and lymphangiogenic (VEGF-C and -D) factors.Citation53 In addition, thyroid MCs modulated angiogenesis through the release of CXCL8/IL-8.Citation25 Human macrophages are also a major source of both angiogenic and lymphangiogenic factors Citation54 as well as of several proangiogenic enzymes such as MMP-9, Cox-2 and iNOS.Citation13 Tumor-associated NK cells produce VEGF-A and CXCL8/IL-8.Citation55 Tumor-associated DCs release VEGF-A, CXCL8/IL-8 and osteopontin.Citation56 Human eosinophils promote angiogenesis Citation57 and eosinophilia can be observed in ATC patients.Citation58 Tregs play a role in maintaining tolerance in TC and drive tumor angiogenesis through the release of VEGF-A.Citation59 MDSCs promote tumor angiogenesis through the release of VEGF-A and MMP-9.Citation60 Tie2 TEM,Citation61 human basophils Citation62 and human neutrophils are a source of proangiogenic factors, but their role in TC is unknown.
Figure 1. Hypothetical diagram depicting immune orchestration of tumor angiogenesis and lymphangiogenesis in TC. Immune cells infiltrating TCs can play two complementary roles at the same time. On one hand, several immune cells can modulate angiogenesis thus favoring tumor growth. On the other hand, TAMs and TAMCs, can play a dual role modulating not only angiogenesis but also lymphangiogenesis thus contributing to the formation of metastasis. The role of Tie2+ TEM, TAN and basophils (gray) in angiogenesis/lymphangiogenesis has been demonstrated in several human cancers and in chronic inflammatory disorders, but not yet in human TCs.
Immune cells in TC microenvironment
The tumor microenvironment, which consists of immune cells, fibroblasts, blood and lymphatic vessels, endothelial cell progenitors and extracellular matrix components (ECM), plays a critical role in tumor initiation/progression. Normal tissue microenvironment can suppress malignancy, while certain pathogenetic tissue features can induce tumor progression.Citation63,64
TAMs: Tumor-associated macrophages
Macrophages are the most represented leukocytes in solid tumors and are highly plastic cells.Citation65 Under the influence of IFNγ, macrophages undergo classical M1 polarization, characterized by an immunostimulatory phenotype. In contrast, IL-4 or IL-13 induces alternative M2 phenotype, which promotes tumor angiogenesis and suppresses immune responses.Citation13
The majority of studies investigating the role of TAM in TC correlate TAM infiltration with clinico-pathological features. Indeed, TAMs are increased in TC and positively correlate with de-differentiation.Citation66 In PTCs, TAMs correlated with lymph-node metastasis,Citation45 larger tumor size Citation67 and reduced survival.Citation68 In PDTC, TAM density correlates with capsular invasion and extrathyroid extension.Citation66 TAMs represent more than 50% of immune cells in ATCs forming a “microglia-like” in close contact with cancer cells.Citation69 In diffuse sclerosing variant of PTC, M2-like macrophages can be found in lymphatic emboli and correlated with tumor cell lymphatic invasion.Citation70
In a murine model of transgenic BRAF(V600E)-induced thyroid carcinogenesis, tumors displayed a high TAM infiltration due to the increased expression of CSF-1 and CCL2 by tumor cells. In this model, TAMs displayed an M2-like phenotype, characterized by high production of Arginase-1, CCL22 and IL-10 and low levels of IL-12. TAM depletion reduced tumor growth in established tumors. In addition, Csf–/– BRAF transgenic mice displayed a reduction in tumor growth.Citation14 Accordingly, TAMs purified from human PTC displayed a higher expression of IL-10 and CD206 compared to peripheral blood monocytes Citation71 and promoted invasiveness of TC cell lines in vitro through to the production of CXCL8/IL-8.Citation45
Dendritic cells (DCs)
Tumor-infiltrating DCs generally display an immature phenotype, with impaired antigen presentation ability.Citation72 S100+ (mature and immature), CD1a+ (immature), and CD83+ (mature) DCs are increased in human PTC compared to normal thyroid tissue.Citation73 CM obtained from primary cultures of PTC induced chemotaxis of peripheral blood monocyte-derived DCs. Hepatocyte Growth Factor (HGF) enhanced this chemotactic activity through the engagement of the receptor Met on TC cells.Citation74 PTCs expressed the DC chemotactic molecule MIP-1α and DCs expressed CCR6, thus suggesting a role for TC cells in recruiting DCs.Citation75 PTC displayed a higher CD1a+ DC infiltration compared to FTC and adenomas.Citation75 In contrast, a reduced or absent DC infiltration in PDTC and ATC was described in comparison to DTC.Citation76
TAMCs: Tumor-associated mast cells
Beyond their pivotal roles in allergy, defense against parasites and immune regulation,Citation77 MCs are involved in tumor biology.Citation78 In a cohort of 96 PTC patients, tumor-associated MC density was higher than in healthy thyroids and correlated with tumor extracapsular extension.Citation24 A higher MC density was also found in FTC compared with adenomas and correlated with extracapsular extension.Citation79 TC cell line CM induced in vitro MC chemotaxis through the release of VEGF-A,Citation24 which activated the VEGFRs on human MCs.Citation53,80 In addition, TC cells activated MCs to release cytokines (IL-6, TNF-α, GM-CSF) and chemokines (CXCL10/IP10 and CXCL1/Gro-α). In turn, MCs promoted TC cell proliferation through CXCL1/GROα and CXCL10/IP10. In an in vivo model of TC xenograft, MCs were recruited at the tumor site and accelerated tumor growth, enhancing tumor vascularization and cell proliferation. These effects were inhibited by cromolyn, a MC inhibitor.Citation24 MCs also induced TC cell EMT mainly through the release of CXCL8/IL-8, thus sustaining TC invasiveness and stem cell features.Citation25
TANs: Tumor associated neutrophils
Neutrophils participate to the early phases of inflammation and resistance against extracellular pathogens.Citation81 However, a role for neutrophils in cancer has been envisaged. Indeed, a new paradigm has been proposed, by which mouse neutrophils can be polarized toward an antitumor N1 or a protumor N2 phenotype in response to signals derived from the microenvironment.Citation82 In humans, the ratio between peripheral blood neutrophil and lymphocyte count (Neutrophil-to-Lymphocyte Ratio—NLR) has been proposed as an index of systemic inflammation and has been found to be associated with tumor development.Citation83 A higher NLR was associated with a larger tumor size and higher risk of recurrence in TC patients, but failed to distinguish patients with benign or malignant nodules.Citation84 In contrast, an increased NLR has been found in TC compared with benign lesions and healthy controls.Citation85 No correlation was found with patient disease-free survival or risk of occult metastasis.Citation86 In a recent study, NLR failed to discriminate benign and malignant lesions.Citation87 Thus, the diagnostic and prognostic significance of NRL in TCs remains uncertain. No studies are so far available investigating the occurrence, roles and prognostic significance of tumor-associated neutrophils in TC.
MDSCs: Myeloid-derived suppressor cells
Cancer development is accompanied by the expansion of a heterogeneous population of myeloid cells, namely MDSCs, with immunosuppressive and cancer-promoting properties.Citation88 MDSCs are classically distinguished between Monocytic (Mo-MDSCs) and Granulocytic (G-MDSCs) subsets based on morphological and phenotypical aspects.Citation88 Peripheral blood MDSC levels were higher in patients with ATC compared to healthy controls and correlated with the serum level of IL-10, thus suggesting a correlation between MDSCs and systemic immunosuppression.Citation89 The only study investigating the significance of TC-infiltrating MDSCs failed to find an association between MDSC density and patient survival.Citation90 Indeed, in a wide cohort of histological samples from 398 TC patients (253 PTCs and 13 FTCs) MDSCs were identified as CD11b+ and CD33+ cells. Intratumoral MDSC count did not correlate with patient clinic-pathological features.Citation90 Unfortunately, the identification of human MDSCs is complicated by the lack of specific marker. Moreover, it has been suggested that several human MDSC phenotypes can be endowed with suppressive activity.Citation91 Thus, further studies are required to better define the roles and functions of different MDSC phenotypes in TC.
NK: Natural killer cells
Natural killer (NK) cells are a family of innate immune cells, which play a central role in antiviral immunity and tumor immunosurveillance. Subtypes of human NK cells have been described on the basis of relative surface expression of CD16 and CD56. CD56dim CD16+ NK cells display a higher cytotoxic activity, whereas CD56bright CD16−/low NK cells are more efficient in cytokine production.Citation92 Flow cytometry analysis on tissue samples and peripheral blood from PTC and MNG patients showed that tumor-infiltrating NK cells were increased in PTCs compared to goiters and healthy thyroids, whereas no differences were found in peripheral blood NK cells. In PTC patients, NK cell infiltration negatively correlated with disease stage.Citation93 A flow cytometry analysis performed on blood and tissue samples from patients with PTC, MNG and healthy controls displayed an increased infiltration of the immunoregulatory subset of NK cells CD56bright in PTC samples compared to MNG. CD56bright immunoregulatory NK cells inversely correlated with the disease stage, whereas cytotoxic NK cells positively correlated with the disease stage in PTC patients. These findings suggest that a modulation of cell phenotype can occur in the TC microenvironment.Citation94
From the functional point of view, ATC cell lines were sensitive to lysis by PBMC-derived NK cells.Citation95 NK cell-mediated lysis of ATC was dependent on the expression of the activating receptor NKG2D on NK cells and its ligands ULBP2/5/6 on ATC cells. Indeed, ATC cell lines and TC derived from human fine-needle aspiration (FNA) samples from ATC patients expressed ULB2/5/6, whereas non-malignant thyroid tissue did not. Interestingly, ATC cell lines induced NK cell migration through the activation of the CXCL10-CXCR3 axis. Flow cytometry analysis of NK cells from FNA and peripheral blood of ATC patients displayed a suppressed phenotype, characterized by a lower percentage of CD56dim cells and CXCR3+ cells and a reduced NKG2D expression, compared to peripheral blood NK cells. PGE2 was identified as the main candidate responsible for ATC-mediated NK cell suppression.Citation95 This study indicates that ATC could be a target for NK cell-based adoptive cell therapy, paving the way to novel immune-mediated therapeutic approach to ATC.
A recent experimental study addressed the role of IL-12 immunotherapy in PTC. In a knock-in mouse model Braf oncogene was expressed under the control of the thyroid peroxidase promoter (LSL-BrafV600E /TPO Cre mice). These mice spontaneously developed thyroid tumors at 5 weeks of age. IL-12 gene therapy and recombinant IL-12 reduced tumor growth, restored the follicular architecture of the gland and improved the survival of tumor-bearing mice. Interestingly, IL-12 improved the cytotoxic activity of CD8+ T cells and NK cells, and increased the infiltration of M1 macrophages compared to alternative M2 macrophages within the tumors.Citation96
Natural killer T cells (NKT), γδ T cells and innate lymphoid cells (ILCs)
NKT cells are a heterogeneous lymphoid population, which recognizes lipid antigens presented by CD1d. NKT are important for tumor immunosurveillance. Two NKT subsets have been identified: type I induce lysis of tumor cells directly via a perforin/granzyme B-mediated mechanism or indirectly via the activation of NK cells and DCs; type II show immunosuppressive activity by IL-13 secretion.Citation97 NKT subsets have not been characterized in TC.
γδ T lymphocytes, expressing a γδ TCR, are not MHC-restricted and do not recognize peptide antigens. The contribution of γδ T cells in tumor immunosurveillance is controversial. Zitvogel's group demonstrated that IL-17-producing γδ T cells play a prominent role in chemotherapy-induced anticancer immune responses.Citation98 The role of tumor-infiltrating γδ cells in TC is still unknown.
Innate Lymphoid Cells (ILCs) lack TCR but functionally resemble effector T cells. ILCs include three subsets of cytokine-producing helper cells: group 1 ILCs produce IFNγ and include conventional NK cells; group 2 produces Th2-type cytokines (e.g. IL-4, IL-5 and IL-13); group 3 ILCs comprises several distinct cell subsets.Citation99 In a mouse model of melanoma, ILCs promote tumor rejection.Citation100 Studies are required to understand the role of ILC subsets in TCs.
CD8+ cytotoxic T cells
CD8+ cytotoxic T lymphocytes (CTLs) recognize and attack tumor cells expressing tumor antigens.Citation101 In a wide immunohistochemical characterization of the immune cell infiltrate in patients with chronic lymphocytic thyroiditis concurrent with DTC, a high CD8+ T lymphocyte infiltration was associated with improved disease free survival.Citation90 By contrast, in a recent study conducted in a wide cohort of DTC patients, including papillary and follicular subtypes, immunohistochemical analysis of tumor samples revealed that combined enrichment of CD8+ cells and Cox-2 overexpression correlated with the highest risk of disease relapse. In the majority of tumor samples analyzed (68%), CD8+ cells were granzyme B negative, reflecting a state of anergy.Citation102
A low intratumoral CD8+/Foxp3+ ratio was found in human BrafV600E PTC, associated with increased expression of the immunosuppressive molecules Arginase-1, Indoleamine 2,3-dioxygenase (IDO) and PD-L1. The latter findings suggest a Braf-driven tumor-promoting microenvironment.Citation103
CD4+ cells
CD4+ T helper (Th) lymphocytes are a heterogeneous population. Th1-mediated immunity is generally considered as antitumoral, while polarized Th2 and/or Treg activity is believed protumorigenic.Citation104 This simplistic view is complicated by the plasticity of Th differentiation, which can be modulated by the microenvironment.
Increasing evidence highlights the antitumor potential of CD4+ Th1 cells in murine models and humans.Citation105 However, in TC, the extent of tumor-infiltrating CD4+ cells does not appear to predict patient outcome.Citation90,102 No differences were found between PTC and MNG patients, with respect to tissue or peripheral blood CD4+ cell frequencies.Citation18 Interestingly, a double negative CD4− CD8− lymphocyte population has been recently identified in TC. This double negative lymphocyte population was identified as the dominant cell type in PTC and was more abundant in PTC than in thyroid autoimmunity.Citation106 These cells ex vivo released IFNγ and IL-17, thus resembling effector cells.
Tregs shut down antitumor immune response via the production of IL-10 or by expressing immunosuppressive molecules, including CTLA4, GITR, PD-1 and stimulate angiogenesis through the production of VEGF-A.Citation107 Moreover, increased PD-1+ T cells in metastatic lymph nodes correlated with a more aggressive TC.Citation108 In a small PTC patient dataset, Foxp3+ Tregs and VEGF, evaluated by immunohistochemistry, were found in PTC samples and Treg infiltration correlated with disease stage and lymph node metastasis.Citation109 A higher Treg density was also observed in PTC samples compared with nodular goiter, and positively correlated to the stage of the disease.Citation93 Accordingly, in a study performed on 124 tissue samples from PTC microcarcinomas, high infiltration of Foxp3+ Treg cells was associated with aggressive features of PTC microcarcinoma.Citation110 Moreover, increased Foxp3+ and reduced CD3+ tumor-infiltrating lymphocytes correlated with IDO expression. Similarly to PD-L1, IDO is overexpressed in different tumors and is associated with activation of Foxp3+ Tregs and downregulation of cytotoxic cellular immunity in the tumor microenvironment.Citation110
A higher percentage of Foxp3+ T cells and ICOS+ Treg cells were found in tissues, but not in peripheral blood, of PTC patients with MNG compared to MNG alone.Citation18 In PTC plus MNG, tissue ICOS+ Foxp3+ T cells were increased in advanced stages and metastatic tumors. Tissue ICOS+ Foxp3+ T cell numbers correlated with tissue plasmocytoid DCs, which favor an immunosuppressive microenvironment.Citation18
CD4+ IL17+ T cells (Th17) cells can exert protumor or antitumor functions depending on tissue microenvironment.Citation21 Only one paper examined the prevalence and distribution of Th17 cells in TC samples. In peripheral blood and tissue samples of PTC patients, increased Th17 levels were found compared to healthy controls, whereas the percentage of CD8+ IL-17+ T cells (Tc17) in the peripheral blood was reduced. The frequency of peripheral blood Th17 cells was positively correlated with the IL-17 serum level, while no correlation between serum level of IL-17 and Tc17 cells was found. Peripheral blood Th17 cells inversely correlated with tumor size.Citation29
Tfh cells are characterized by the expression of CXCR5 and Bcl6. Tfh cells promote immunoglobulin production by B lymphocytes through the release of IL-21.Citation31 Tfh cells can also be involved in the immune response to human cancer.Citation111 Studies investigating the presence and functions of Tfh subsets in TC are still missing.
IL-9-producing CD4+ helper T cells (Th9 cells) are a subset of CD4+ helper T cells with proinflammatory functions and anticancer properties in vivo.Citation112 The release of IL-9 has been proposed to account for the anticancer efficacy of these cells. Moreover, Th9 cells release IL-21, which promotes the production of IFNγ and tumor elimination by CD8+ T cells and NK cells.Citation113 The relevance of Th9 cells in thyroid oncogenesis remains to be elucidated.
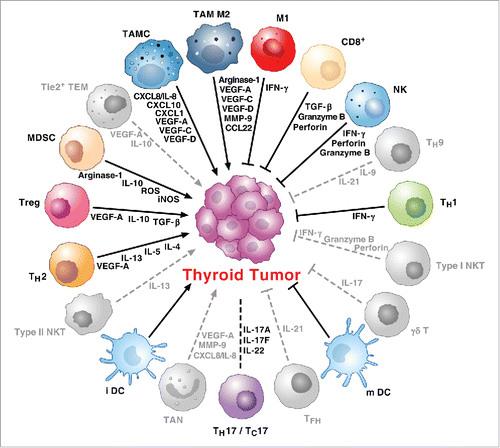
schematically illustrates a hypothetical immune network in TC.
Figure 2. Hypothetical scheme of immune network in TC. The immune network in thyroid cancer is a complex and dynamic system characterized by multiple interactions between tumor cells and a wide spectrum of immune cells. Tumor-infiltrating immune cells interact each other and with tumor cells in a complex synergistic and opposite manner still largely unknown. Several cytokines, chemokines, angiogenic and lymphangiogenic factors, derived from both immune cells and tumor cells, enrich the complexity of the inflammatory tumor microenvironment. The roles of Tie2+ TEM, NKT cells, TAN, Tfh cells, γδ T cells and Th9 cells (gray) have been demonstrated in several other human cancers or are under investigation in human TCs. Protumor or antitumor activities of Th17 and Tc17 cells are context dependent (dashed line).
Conclusions and perspectives
During recent years the incidence of TC has increased and it now represents approximately 90% of all endocrine malignancies. While the prognosis of DTC is favorable, PDTC and ATC are among the most lethal human malignancies. The tumor microenvironment plays a critical role in tumor initiation and progression. A normal tissue microenvironment can suppress malignancy, while certain pathogenetic tissue features are critical for tumor development.Citation63,54 A myriad of immune cells are present in the TC microenvironment and in some cases are associated with patient outcome.Citation66,68 However, only studies assessing the functional role of TAMs and TAMCs in the TC microenvironment have provided evidence for their protumorigenic role.Citation14,24 The majority of these studies were merely qualitative and quantitative analyses of immune cells. Moreover, the presence and functional role(s) of subsets of immune cells (e.g., neutrophils, NKT and γδ T cells, ILC, Tfh, Th9), known to be relevant for tumor initiation/growth, have not yet been investigated in the microenvironment of different types of TC.
Genetic alterations in the RET/PTC-RAS-BRAF-MAPK or in the PI3K-AKT signaling pathways, which represent “driver” mutations (i.e., required for cancer cell growth/survival) have been identified in TC.Citation6 Driver mutations represent prognostic biomarkers and promising new targets for the treatment of TC. Kinase inhibitors targeting RET or BRAF can induce either stable disease or partial responses in PTC and FTC metastatic patients, but are ineffective in ATC.Citation114 Thus, novel therapies for these TC histotypes are needed. A possible therapeutic approach is represented by drugs targeting tumor stromal cells including immune cells and/or the interactions between immune cells and cancer cells.
Angiogenesis and lymphangiogenesis play an essential role in tumor growth and in the formation of metastasis. TC cells, TAMs, TAMCs and other immune cells are a major source of proangiogenic and lymphangiogenic molecules.Citation53,54 Interestingly, immune cells and tumors can produce pro- and anti-angiogenic isoforms of VEGFs.Citation62 A deeper knowledge of the balance between these isoforms could lead to the discovery of novel targets that can be manipulated to block TC growth.
Most of the in vivo experimental studies of TC have been performed with athymic nude mice models. Functional studies on MCs conducted with these models have demonstrated a protumorigenic role of MCs in human TC.Citation24,25 These results suggest the possibility that targeting these cells and/or their mediators could be useful to interfere with TC proliferation, angiogenesis, invasive ability and stemness.Citation24,25 Genetically modified mouse models that recapitulate multistep TC tumorigenesis should be employed to better characterize the role of different immune cell subsets in TC. For instance, the protumorigenic role of macrophages in TC has been established by using thyroid-targeted BRAF(V600E) mice in which macrophage deletion has been obtained by either pharmacological or genetic tools.Citation14 Targeting macrophages has been shown effective for blocking tumor growth.Citation115 This experimental approach could be also useful in TC.
TC cells and immune cells are a major source of several protumorigenic and proangiogenic cytokines/chemokines.Citation53 Targeting these mediators could be exploited to block TC growth, since this strategy has been already developed for other tumors.Citation116 Moreover, boosting anticancer immune responses by blocking immunosuppressive molecules (TGF-β, IL-10, CTLA-4, PD-1 and PD-L1) expressed either by cancer cells or by tumor-infiltrating immune cells Citation117 and cytolytic viruses approaches Citation51 appear promising therapeutic strategies in different tumors, including TC.
In conclusion, insights into the molecular mechanisms regulating immune networks as well as angiogenesis/lymphangiogenesis in different types of TC could result in the discovery of diagnostic and prognostic markers and novel targets in this common endocrine malignancy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors apologize to the many authors who have contributed importantly to this field and whose work has not been cited due to space and citation restrictions. We thank Fabrizio Fiorbianco for the figures.
Funding
Supported in part by grants from Regione Campania CISI-Lab Project, CRèME Project, and TIMING Project.
References
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436-44; PMID:18650914; http://dx.doi.org/10.1038/nature07205
- Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest 2008; 118:1991-2001; PMID:18523649; http://dx.doi.org/10.1172/JCI35180
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001; 410:1107-11; PMID:11323675; http://dx.doi.org/10.1038/35074122
- Cunha LL, Marcello MA, Ward LS. The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocrine-related cancer 2014; 21:R85-R103; PMID:24302667; http://dx.doi.org/10.1530/ERC-13-0431
- Carling T, Udelsman R. Thyroid cancer. Ann Rev Med 2014; 65:125-37; PMID:24274180; http://dx.doi.org/10.1146/annurev-med-061512-105739
- Hsiao SJ, Nikiforov YE. Molecular approaches to thyroid cancer diagnosis. Endocrine-related cancer 2014; 21:T301-13; PMID:24829266; http://dx.doi.org/10.1530/ERC-13-0165
- Cancer Genome Atlas Research N. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014; 159:676-90; PMID:25417114; http://dx.doi.org/10.1016/j.cell.2014.09.050
- Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity 2013; 39:1003-18; PMID:24332029; http://dx.doi.org/10.1016/j.immuni.2013.11.010
- Weetman AP, Bennett GL, Wong WL. Thyroid follicular cells produce interleukin-8. J Clin Endocrinol Metab 1992; 75:328-30; PMID:1619027; http://dx.doi.org/10.1210/jcem.75.1.1619027\
- Kammoun-Krichen M, Bougacha-Elleuch N, Mnif M, Bougacha F, Charffedine I, Rebuffat S, Rebai A, Glasson E, Abid M, Ayadi F et al. IL-1beta a potential factor for discriminating between thyroid carcinoma and atrophic thyroiditis. Eur Cytokine Netw 2012; 23:101-6; PMID:22995155; http://dx.doi.org/10.1684/ecn.2012.0312
- Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Ann Rev Immunol 2013; 31:317-43; PMID:23298208; http://dx.doi.org/10.1146/annurev-immunol-032712-095906
- Genovese A, Borgia G, Bjorck L, Petraroli A, de Paulis A, Piazza M, Marone G. Immunoglobulin superantigen protein L induces IL-4 and IL-13 secretion from human Fc epsilon RI+ cells through interaction with the kappa light chains of IgE. J Immunol 2003; 170:1854-61; PMID:12574351; http://dx.doi.org/10.4049/jimmunol.170.4.1854
- Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013; 229:176-85; PMID:23096265; http://dx.doi.org/10.1002/path.4133
- Ryder M, Gild M, Hohl TM, Pamer E, Knauf J, Ghossein R, Joyce JA, Fagin JA. Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid cancer progression. PloS one 2013; 8:e54302; PMID:23372702; http://dx.doi.org/10.1371/journal.pone.0054302
- Ameziane-El-Hassani R, Talbot M, de Souza Dos Santos MC, Al Ghuzlan A, Hartl D, Bidart JM, De Deken X, Miot F, Diallo I, de Vathaire F et al. NADPH oxidase DUOX1 promotes long-term persistence of oxidative stress after an exposure to irradiation. Proc Natl Acad Sci U S A 2015; 112:5051-6; PMID:25848056; http://dx.doi.org/10.1073/pnas.1420707112
- Mannino MH, Zhu Z, Xiao H, Bai Q, Wakefield MR, Fang Y. The paradoxical role of IL-10 in immunity and cancer. Cancer letters 2015; 367:103-7; PMID:26188281; http://dx.doi.org/10.1016/j.canlet.2015.07.009
- Li C, Li H, Jiang K, Li J, Gai X. TLR4 signaling pathway in mouse Lewis lung cancer cells promotes the expression of TGF-beta1 and IL-10 and tumor cells migration. Bio-medical materials and engineering 2014; 24:869-75; PMID:24211974; http://dx.doi.org/10.3233/BME-130879
- Yu H, Huang X, Liu X, Jin H, Zhang G, Zhang Q, Yu J. Regulatory T cells and plasmacytoid dendritic cells contribute to the immune escape of papillary thyroid cancer coexisting with multinodular non-toxic goiter. Endocrine 2013; 44:172-81; PMID:23264145; http://dx.doi.org/10.1007/s12020-012-9853-2
- Todaro M, Zerilli M, Ricci-Vitiani L, Bini M, Perez Alea M, Maria Florena A, Miceli L, Condorelli G, Bonventre S, Di Gesu G et al. Autocrine production of interleukin-4 and interleukin-10 is required for survival and growth of thyroid cancer cells. Cancer Res 2006; 66:1491-9; PMID:16452205; http://dx.doi.org/10.1158/0008-5472.CAN-05-2514
- Stassi G, Todaro M, Zerilli M, Ricci-Vitiani L, Di Liberto D, Patti M, Florena A, Di Gaudio F, Di Gesu G, De Maria R. Thyroid cancer resistance to chemotherapeutic drugs via autocrine production of interleukin-4 and interleukin-10. Cancer Res 2003; 63:6784-90; PMID:14583474
- Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O'Mahony L, Palomares O et al. Interleukins, from 1 to 37, and interferon-gamma: receptors, functions, and roles in diseases. J Allergy Clin Immunol 2011; 127:701-21 e1-70; PMID:21377040; http://dx.doi.org/10.1016/j.jaci.2010.11.050
- Shinohara S, Rothstein JL. Interleukin 24 is induced by the RET/PTC3 oncoprotein and is an autocrine growth factor for epithelial cells. Oncogene 2004; 23:7571-9; PMID:15326486; http://dx.doi.org/10.1038/sj.onc.1207964
- Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015; 16:448-57; PMID:25898198; http://dx.doi.org/10.1038/ni.3153
- Melillo RM, Guarino V, Avilla E, Galdiero MR, Liotti F, Prevete N, Rossi FW, Basolo F, Ugolini C, de Paulis A et al. Mast cells have a protumorigenic role in human thyroid cancer. Oncogene 2010; 29:6203-15; PMID:20729915; http://dx.doi.org/10.1038/onc.2010.348
- Visciano C, Liotti F, Prevete N, Cali G, Franco R, Collina F, de Paulis A, Marone G, Santoro M, Melillo RM. Mast cells induce epithelial-to-mesenchymal transition and stem cell features in human thyroid cancer cells through an IL-8-Akt-Slug pathway. Oncogene 2015; 34:5175-86; PMID:25619830; http://dx.doi.org/10.1038/onc.2014.441
- Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol 2010; 10:554-67; PMID:20616810; http://dx.doi.org/10.1038/nri2808
- Knauf JA, Sartor MA, Medvedovic M, Lundsmith E, Ryder M, Salzano M, Nikiforov YE, Giordano TJ, Ghossein RA, Fagin JA. Progression of BRAF-induced thyroid cancer is associated with epithelial-mesenchymal transition requiring concomitant MAP kinase and TGFbeta signaling. Oncogene 2011; 30:3153-62; PMID:21383698; http://dx.doi.org/10.1038/onc.2011.44
- Eloy C, Santos J, Cameselle-Teijeiro J, Soares P, Sobrinho-Simoes M. TGF-beta/Smad pathway and BRAF mutation play different roles in circumscribed and infiltrative papillary thyroid carcinoma. Virchows Arch 2012; 460:587-600; PMID:22527019; http://dx.doi.org/10.1007/s00428-012-1234-y
- Jiang G, Ma S, Wei Y, Wu Y, Yu X, Liu H. The prevalence and distribution of Th17 and Tc17 cells in patients with thyroid tumor. Immunology letters 2014; 162:68-73; PMID:25068436; http://dx.doi.org/10.1016/j.imlet.2014.07.005
- Lee YC, Chung JH, Kim SK, Rhee SY, Chon S, Oh SJ, Hong IK, Eun YG. Association between interleukin 17/interleukin 17 receptor gene polymorphisms and papillary thyroid cancer in Korean population. Cytokine 2015; 71:283-8; PMID:25484349; http://dx.doi.org/10.1016/j.cyto.2014.11.011
- Crotty S. A brief history of T cell help to B cells. Nat Rev Immunol 2015; 15:185-9; PMID:25677493; http://dx.doi.org/10.1038/nri3803
- Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo VK et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med 2012; 18:1248-53; PMID:22772464; http://dx.doi.org/10.1038/nm.2856
- Xiao M, Hu S, Tang J, Zhang L, Jiang H. Interleukin (IL)-21 promoter polymorphism increases the risk of thyroid cancer in Chinese population. Gene 2014; 537:15-9; PMID:24389496; http://dx.doi.org/10.1016/j.gene.2013.12.050
- Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol 2015; 15:405-14; PMID:26027717; http://dx.doi.org/10.1038/nri3845
- Schiavoni G, Mattei F, Gabriele L. Type I Interferons as Stimulators of DC-Mediated Cross-Priming: Impact on Anti-Tumor Response. Front Immunol 2013; 4:483; PMID:24400008; http://dx.doi.org/10.3389/fimmu.2013.00483
- Angell TE, Lechner MG, Jang JK, LoPresti JS, Epstein AL. MHC class I loss is a frequent mechanism of immune escape in papillary thyroid cancer that is reversed by interferon and selumetinib treatment in vitro. Clin Cancer Res 2014; 20:6034-44; PMID:25294906; http://dx.doi.org/10.1158/1078-0432.CCR-14-0879
- Bosisio D, Salvi V, Gagliostro V, Sozzani S. Angiogenic and antiangiogenic chemokines. Chem Immunol Allergy 2014; 99:89-104; PMID:24217604; http://dx.doi.org/10.1159/000353317
- Melillo RM, Castellone MD, Guarino V, De Falco V, Cirafici AM, Salvatore G, Caiazzo F, Basolo F, Giannini R, Kruhoffer M et al. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest 2005; 115:1068-81; PMID:15761501; http://dx.doi.org/10.1172/JCI200522758
- Borrello MG, Alberti L, Fischer A, Degl'innocenti D, Ferrario C, Gariboldi M, Marchesi F, Allavena P, Greco A, Collini P et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci U S A 2005; 102:14825-30; PMID:16203990; http://dx.doi.org/10.1073/pnas.0503039102
- Rotondi M, Coperchini F, Chiovato L. CXCL8 in thyroid disease: from basic notions to potential applications in clinical practice. Cytokine & growth factor reviews 2013; 24:539-46; PMID:24011840; http://dx.doi.org/10.1016/j.cytogfr.2013.08.001
- Antonelli A, Ferrari SM, Fallahi P, Piaggi S, Di Domenicantonio A, Galleri D, Santarpia L, Basolo F, Ferrannini E, Miccoli P. Variable modulation by cytokines and thiazolidinediones of the prototype Th1 chemokine CXCL10 in anaplastic thyroid cancer. Cytokine 2012; 59:218-22; PMID:22633083; http://dx.doi.org/10.1016/j.cyto.2012.04.042
- Rotondi M, Coperchini F, Pignatti P, Magri F, Chiovato L. Metformin reverts the secretion of CXCL8 induced by TNF-alpha in primary cultures of human thyroid cells: an additional indirect anti-tumor effect of the drug. J Clin Endocrinol Metab 2015; 100:E427-32; PMID:25590211; http://dx.doi.org/10.1210/jc.2014-3045
- Muzza M, Degl'Innocenti D, Colombo C, Perrino M, Ravasi E, Rossi S, Cirello V, Beck-Peccoz P, Borrello MG, Fugazzola L. The tight relationship between papillary thyroid cancer, autoimmunity and inflammation: clinical and molecular studies. Clin Endocrinol 2010; 72:702-8; PMID:20447069; http://dx.doi.org/10.1111/j.1365-2265.2009.03699.x
- Bauerle KT, Schweppe RE, Lund G, Kotnis G, Deep G, Agarwal R, Pozdeyev N, Wood WM, Haugen BR. Nuclear factor kappaB-dependent regulation of angiogenesis, and metastasis in an in vivo model of thyroid cancer is associated with secreted interleukin-8. J Clin Endocrinol Metab 2014; 99:E1436-44; PMID:24758177; http://dx.doi.org/10.1210/jc.2013-3636
- Fang W, Ye L, Shen L, Cai J, Huang F, Wei Q, Fei X, Chen X, Guan H, Wang W et al. Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis 2014; 35:1780-7; PMID:24608042; http://dx.doi.org/10.1093/carcin/bgu060
- Antonelli A, Ferrari SM, Fallahi P, Frascerra S, Piaggi S, Gelmini S, Lupi C, Minuto M, Berti P, Benvenga S et al. Dysregulation of secretion of CXC alpha-chemokine CXCL10 in papillary thyroid cancer: modulation by peroxisome proliferator-activated receptor-gamma agonists. Endocrine-related cancer 2009; 16:1299-311; PMID:19755523; http://dx.doi.org/10.1677/ERC-08-0337
- Liu Z, Sun DX, Teng XY, Xu WX, Meng XP, Wang BS. Expression of stromal cell-derived factor 1 and CXCR7 in papillary thyroid carcinoma. Endocrine Pathol 2012; 23:247-53; PMID:23070788; http://dx.doi.org/10.1007/s12022-012-9223-x
- Chung SY, Park ES, Park SY, Song JY, Ryu HS. CXC motif ligand 12 as a novel diagnostic marker for papillary thyroid carcinoma. Head & neck 2014; 36:1005-12; PMID:23784811; http://dx.doi.org/10.1002/hed.23404
- Liu Z, Yang L, Teng X, Zhang H, Guan H. The involvement of CXCR7 in modulating the progression of papillary thyroid carcinoma. J Surg Res 2014; 191:379-88; PMID:24814201; http://dx.doi.org/10.1016/j.jss.2014.04.016
- Zeng W, Chang H, Ma M, Li Y. CCL20/CCR6 promotes the invasion and migration of thyroid cancer cells via NF-kappa B signaling-induced MMP-3 production. Exp Mol Pathol 2014; 97:184-90; PMID:24984269; http://dx.doi.org/10.1016/j.yexmp.2014.06.012
- Passaro C, Borriello F, Vastolo V, Somma SD, Scamardella E, Gigantino V, Franco R, Marone G, Portella G. The oncolytic virus dl922-947 reduces IL-8/CXCL8 and MCP-1/CCL2 expression and impairs angiogenesis and macrophage infiltration in anaplastic thyroid carcinoma. Oncotarget 2015; 7(2):1500-15; PMID:26625205; http://dx.doi.org/10.18632/oncotarget.6430
- Loffredo S, Staiano RI, Granata F, Genovese A, Marone G. Immune cells as a source and target of angiogenic and lymphangiogenic factors. In: Marone G, Granata F, eds. Angiogenesis, Lymphangiogenesis and Clinical Implications Chem Immunol Allergy. Basel: Karger 2014; 99:15-36; PMID:24217601; http://dx.doi.org/10.1159/000353316
- Detoraki A, Staiano RI, Granata F, Giannattasio G, Prevete N, de Paulis A, Ribatti D, Genovese A, Triggiani M, Marone G. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J Allergy Clin Immunol 2009; 123:1142-9, 9 e1-5; PMID:19275959; http://dx.doi.org/10.1016/j.jaci.2009.01.044
- Granata F, Frattini A, Loffredo S, Staiano RI, Petraroli A, Ribatti D, Oslund R, Gelb MH, Lambeau G, Marone G et al. Production of vascular endothelial growth factors from human lung macrophages induced by group IIA and group X secreted phospholipases A2. J Immunol 2010; 184:5232-41; PMID:20357262; http://dx.doi.org/10.4049/jimmunol.0902501
- Bruno A, Focaccetti C, Pagani A, Imperatori AS, Spagnoletti M, Rotolo N, Cantelmo AR, Franzi F, Capella C, Ferlazzo G et al. The proangiogenic phenotype of natural killer cells in patients with non-small cell lung cancer. Neoplasia 2013; 15:133-42; PMID:23441128; http://dx.doi.org/10.1593/neo.121758
- Curiel TJ, Cheng P, Mottram P, Alvarez X, Moons L, Evdemon-Hogan M, Wei S, Zou L, Kryczek I, Hoyle G et al. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res 2004; 64:5535-8; PMID:15313886; http://dx.doi.org/10.1158/0008-5472.CAN-04-1272
- Nissim Ben Efraim AH, Levi-Schaffer F. Roles of Eosinophils in the Modulation of Angiogenesis. In: Marone G, Granata F, eds. Angiogenesis, Lymphangiogenesis and Clinical Implications. Chem Immunol Allergy. Basel: Karger, 2014:138-54.
- Shiraishi J, Koyama H, Seki M, Hatayama M, Naka M, Kurajoh M, Okazaki H, Shoji T, Moriwaki Y, Yamamoto T et al. Anaplastic thyroid carcinoma accompanied by uncontrollable eosinophilia. Internal medicine 2015; 54:611-6; PMID:25786451; http://dx.doi.org/10.2169/internalmedicine.54.3446
- Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 2011; 475:226-30; PMID:21753853; http://dx.doi.org/10.1038/nature10169
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 2008; 8:618-31; PMID:18633355; http://dx.doi.org/10.1038/nrc2444
- De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer cell 2005; 8:211-26; PMID:16169466; http://dx.doi.org/10.1016/j.ccr.2005.08.002
- de Paulis A, Prevete N, Fiorentino I, Rossi FW, Staibano S, Montuori N, Ragno P, Longobardi A, Liccardo B, Genovese A et al. Expression and functions of the vascular endothelial growth factors and their receptors in human basophils. J Immunol 2006; 177:7322-31; PMID:17082651; http://dx.doi.org/10.4049/jimmunol.177.10.7322
- Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 2011; 17:320-9; PMID:21383745; http://dx.doi.org/10.1038/nm.2328
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science (New York, NY 2013; 339:286-91; PMID:23329041; http://dx.doi.org/10.1126/science.1232227
- Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol 2013; 228:1404-12; PMID:23065796; http://dx.doi.org/10.1002/jcp.24260
- Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocrine-related cancer 2008; 15:1069-74; PMID:18719091; http://dx.doi.org/10.1677/ERC-08-0036
- Kim S, Cho SW, Min HS, Kim KM, Yeom GJ, Kim EY, Lee KE, Yun YG, Park do J, Park YJ. The expression of tumor-associated macrophages in papillary thyroid carcinoma. Endocrinol Metab 2013; 28:192-8; PMID:24396678; http://dx.doi.org/10.3803/EnM.2013.28.3.192
- Jung KY, Cho SW, Kim YA, Kim D, Oh BC, Park do J, Park YJ. Cancers with Higher Density of Tumor-Associated Macrophages Were Associated with Poor Survival Rates. J Pathol Transl Med 2015; 49:318-24; PMID:26081823; http://dx.doi.org/10.4132/jptm.2015.06.01
- Caillou B, Talbot M, Weyemi U, Pioche-Durieu C, Al Ghuzlan A, Bidart JM, Chouaib S, Schlumberger M, Dupuy C. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PLoS One 2011; 6:e22567; PMID:21811634; http://dx.doi.org/10.1371/journal.pone.0022567
- Chang WC, Chen JY, Lee CH, Yang AH. Expression of decoy receptor 3 in diffuse sclerosing variant of papillary thyroid carcinoma: correlation with M2 macrophage differentiation and lymphatic invasion. Thyroid 2013; 23:720-6; PMID:23186064; http://dx.doi.org/10.1089/thy.2012.0261
- Qing W, Fang WY, Ye L, Shen LY, Zhang XF, Fei XC, Chen X, Wang WQ, Li XY, Xiao JC et al. Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid 2012; 22:905-10; PMID:22870901; http://dx.doi.org/10.1089/thy.2011.0452
- Dudek AM, Martin S, Garg AD, Agostinis P. Immature, Semi-Mature, and Fully Mature Dendritic Cells: Toward a DC-Cancer Cells Interface That Augments Anticancer Immunity. Front Immunol 2013; 4:438; PMID:24376443; http://dx.doi.org/10.3389/fimmu.2013.00438
- Hilly O, Koren R, Raz R, Rath-Wolfson L, Mizrachi A, Hamzany Y, Bachar G, Shpitzer T. The role of s100-positive dendritic cells in the prognosis of papillary thyroid carcinoma. Am J Clin Pathol 2013; 139:87-92; PMID:23270903; http://dx.doi.org/10.1309/AJCPAKYDO56NKMYZ
- Scarpino S, Stoppacciaro A, Ballerini F, Marchesi M, Prat M, Stella MC, Sozzani S, Allavena P, Mantovani A, Ruco LP. Papillary carcinoma of the thyroid: hepatocyte growth factor (HGF) stimulates tumor cells to release chemokines active in recruiting dendritic cells. Am J Pathol 2000; 156:831-7; PMID:10702399; http://dx.doi.org/10.1016/S0002-9440(10)64951-6
- Tsuge K, Takeda H, Kawada S, Maeda K, Yamakawa M. Characterization of dendritic cells in differentiated thyroid cancer. J Pathol 2005; 205:565-76; PMID:15714595; http://dx.doi.org/10.1002/path.1731
- Ugolini C, Basolo F, Proietti A, Vitti P, Elisei R, Miccoli P, Toniolo A. Lymphocyte and immature dendritic cell infiltrates in differentiated, poorly differentiated, and undifferentiated thyroid carcinoma. Thyroid 2007; 17:389-93; PMID:17542668; http://dx.doi.org/10.1089/thy.2006.0306
- Marone G, Galli SJ, Kitamura Y. Probing the roles of mast cells and basophils in natural and acquired immunity, physiology and disease. Trends Immunol 2002; 23:425-7; PMID:12200056; http://dx.doi.org/10.1016/S1471-4906(02)02274-3
- Marone G, Varricchi G, Loffredo S, Granata F. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur J Pharmacol 2015; 778:146-51; PMID:25941082; http://dx.doi.org/10.1016/j.ejphar.2015.03.088.
- Proietti A, Ugolini C, Melillo RM, Crisman G, Elisei R, Santoro M, Minuto M, Vitti P, Miccoli P, Basolo F. Higher intratumoral expression of CD1a, tryptase, and CD68 in a follicular variant of papillary thyroid carcinoma compared to adenomas: correlation with clinical and pathological parameters. Thyroid 2011; 21:1209-15; PMID:22007938; http://dx.doi.org/10.1089/thy.2011.0059
- Detoraki A, Granata F, Staibano S, Rossi FW, Marone G, Genovese A. Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy 2010; 65:946-58; PMID:20415716; http://dx.doi.org/10.1111/j.1398-9995.2010.02372.x
- Jaillon S, Galdiero MR, Del Prete D, Cassatella MA, Garlanda C, Mantovani A. Neutrophils in innate and adaptive immunity. Semin Immunopathol 2013; 35:377-94; PMID:23553214; http://dx.doi.org/10.1007/s00281-013-0374-8
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009; 16:183-94; PMID:19732719; http://dx.doi.org/10.1016/j.ccr.2009.06.017
- Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg 2010; 200:197-203; PMID:20122680; http://dx.doi.org/10.1016/j.amjsurg.2009.08.041
- Liu CL, Lee JJ, Liu TP, Chang YC, Hsu YC, Cheng SP. Blood neutrophil-to-lymphocyte ratio correlates with tumor size in patients with differentiated thyroid cancer. J Surg Oncol 2013; 107:493-7; PMID:22996403; http://dx.doi.org/10.1002/jso.23270
- Seretis C, Gourgiotis S, Gemenetzis G, Seretis F, Lagoudianakis E, Dimitrakopoulos G. The significance of neutrophil/lymphocyte ratio as a possible marker of underlying papillary microcarcinomas in thyroidal goiters: a pilot study. Am J Surg 2013; 205:691-6; PMID:23388425; http://dx.doi.org/10.1016/j.amjsurg.2012.08.006
- Lang BH, Ng CP, Au KB, Wong KP, Wong KK, Wan KY. Does preoperative neutrophil lymphocyte ratio predict risk of recurrence and occult central nodal metastasis in papillary thyroid carcinoma? World journal of surgery 2014; 38:2605-12; PMID:24809487; http://dx.doi.org/10.1007/s00268-014-2630-z
- Cho JS, Park MH, Ryu YJ, Yoon JH. The neutrophil to lymphocyte ratio can discriminate anaplastic thyroid cancer against poorly or well differentiated cancer. Annals of surgical treatment and research 2015; 88:187-92; PMID:25844352; http://dx.doi.org/10.4174/astr.2015.88.4.187
- Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 2010; 40:2969-75; PMID:21061430; http://dx.doi.org/10.1002/eji.201040895
- Suzuki S, Shibata M, Gonda K, Kanke Y, Ashizawa M, Ujiie D, Suzushino S, Nakano K, Fukushima T, Sakurai K et al. Immunosuppression involving increased myeloid-derived suppressor cell levels, systemic inflammation and hypoalbuminemia are present in patients with anaplastic thyroid cancer. Mol Clin Oncol 2013; 1:959-64; PMID:24649277; http://dx.doi.org/10.3892/mco.2013.170
- Cunha LL, Morari EC, Guihen AC, Razolli D, Gerhard R, Nonogaki S, Soares FA, Vassallo J, Ward LS. Infiltration of a mixture of immune cells may be related to good prognosis in patients with differentiated thyroid carcinoma. Clin Endocrinol 2012; 77:918-25; PMID:22738343; http://dx.doi.org/10.1111/j.1365-2265.2012.04482.x
- Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 2014; 1319:47-65; PMID:24965257; http://dx.doi.org/10.1111/nyas.12469
- Wang F, Tian Z, Wei H. Genomic expression profiling of NK cells in health and disease. Eur J Immunol 2015; 45:661-78; PMID:25476835; http://dx.doi.org/10.1002/eji.201444998
- Gogali F, Paterakis G, Rassidakis GZ, Kaltsas G, Liakou CI, Gousis P, Neonakis E, Manoussakis MN, Liapi C. Phenotypical analysis of lymphocytes with suppressive and regulatory properties (Tregs) and NK cells in the papillary carcinoma of thyroid. J Clin Endocrinol Metab 2012; 97:1474-82; PMID:22399513; http://dx.doi.org/10.1210/jc.2011-1838
- Gogali F, Paterakis G, Rassidakis GZ, Liakou CI, Liapi C. CD3(−)CD16(−)CD56(bright) immunoregulatory NK cells are increased in the tumor microenvironment and inversely correlate with advanced stages in patients with papillary thyroid cancer. Thyroid 2013; 23:1561-8; PMID:23721357; http://dx.doi.org/10.1089/thy.2012.0560
- Wennerberg E, Pfefferle A, Ekblad L, Yoshimoto Y, Kremer V, Kaminskyy VO, Juhlin CC, Hoog A, Bodin I, Svjatoha V et al. Human anaplastic thyroid carcinoma cells are sensitive to NK cell-mediated lysis via ULBP2/5/6 and chemoattract NK cells. Clin Cancer Res 2014; 20:5733-44; PMID:25212604; http://dx.doi.org/10.1158/1078-0432.CCR-14-0291
- Parhar RS, Zou M, Al-Mohanna FA, Baitei EY, Assiri AM, Meyer BF, Shi Y. IL-12 immunotherapy of Braf(V600E)-induced papillary thyroid cancer in a mouse model. Lab Invest 2016; 96:89-97; PMID:26501867; http://dx.doi.org/10.1038/labinvest.2015.126
- Robertson FC, Berzofsky JA, Terabe M. NKT cell networks in the regulation of tumor immunity. Front Immunol 2014; 5:543; PMID:25389427; http://dx.doi.org/10.3389/fimmu.2014.00543
- Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med 2011; 208:491-503; PMID:21383056; http://dx.doi.org/10.1084/jem.20100269
- Artis D, Spits H. The biology of innate lymphoid cells. Nature 2015; 517:293-301; PMID:25592534; http://dx.doi.org/10.1038/nature14189
- Eisenring M, vom Berg J, Kristiansen G, Saller E, Becher B. IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46. Nat Immunol 2010; 11:1030-8; PMID:20935648; http://dx.doi.org/10.1038/ni.1947
- Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol 2002; 20:323-70; PMID:11861606; http://dx.doi.org/10.1146/annurev.immunol.20.100201.131730
- Cunha LL, Marcello MA, Nonogaki S, Morari EC, Soares FA, Vassallo J, Ward LS. CD8+ tumour-infiltrating lymphocytes and COX2 expression may predict relapse in differentiated thyroid cancer. Clin Endocrinol 2015; 83:246-53; PMID:25130519; http://dx.doi.org/10.1111/cen.12586
- Angell TE, Lechner MG, Jang JK, Correa AJ, LoPresti JS, Epstein AL. BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid 2014; 24:1385-93; PMID:24955518; http://dx.doi.org/10.1089/thy.2014.0134
- Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev 2010; 21:3-10; PMID:20005150; http://dx.doi.org/10.1016/j.cytogfr.2009.11.002
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010; 207:637-50; PMID:20156971; http://dx.doi.org/10.1084/jem.20091918
- Imam S, Paparodis R, Sharma D, Jaume JC. Lymphocytic profiling in thyroid cancer provides clues for failure of tumor immunity. Endocrine-related cancer 2014; 21:505-16; PMID:24623740; http://dx.doi.org/10.1530/ERC-13-0436
- Wolf D, Sopper S, Pircher A, Gastl G, Wolf AM. Treg(s) in Cancer: Friends or Foe? J Cell Physiol 2015; 230:2598-605; PMID:25913194; http://dx.doi.org/10.1002/jcp.25016
- French JD, Kotnis GR, Said S, Raeburn CD, McIntyre RC, Jr, Klopper JP, Haugen BR. Programmed death-1+ T cells and regulatory T cells are enriched in tumor-involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J Clin Endocrinol Metab 2012; 97:E934-43; PMID:22466343; http://dx.doi.org/10.1210/jc.2011-3428
- French JD, Weber ZJ, Fretwell DL, Said S, Klopper JP, Haugen BR. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J Clin Endocrinol Metab 2010; 95:2325-33; PMID:20207826; http://dx.doi.org/10.1210/jc.2009-2564
- Ryu HS, Park YS, Park HJ, Chung YR, Yom CK, Ahn SH, Park YJ, Park SH, Park SY. Expression of indoleamine 2,3-dioxygenase and infiltration of FOXP3+ regulatory T cells are associated with aggressive features of papillary thyroid microcarcinoma. Thyroid 2014; 24:1232-40; PMID:24742251; http://dx.doi.org/10.1089/thy.2013.0423
- Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013; 123:2873-92; PMID:23778140; http://dx.doi.org/10.1172/JCI67428
- Kaplan MH, Hufford MM, Olson MR. The development and in vivo function of T helper 9 cells. Nat Rev Immunol 2015; 15:295-307; PMID:25848755; http://dx.doi.org/10.1038/nri3824
- Vegran F, Berger H, Boidot R, Mignot G, Bruchard M, Dosset M, Chalmin F, Rebe C, Derangere V, Ryffel B et al. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat Immunol 2014; 15:758-66; PMID:24973819; http://dx.doi.org/10.1038/ni.2925
- Ferrari SM, Fallahi P, Politti U, Materazzi G, Baldini E, Ulisse S, Miccoli P, Antonelli A. Molecular Targeted Therapies of Aggressive Thyroid Cancer. Front Endocrinol 2015; 6:176; PMID:26635725; http://dx.doi.org/10.3389/fendo.2015.00176
- Germano G, Frapolli R, Simone M, Tavecchio M, Erba E, Pesce S, Pasqualini F, Grosso F, Sanfilippo R, Casali PG et al. Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res 2010; 70:2235-44; PMID:20215499; http://dx.doi.org/10.1158/0008-5472.CAN-09-2335
- Wright KT, Giardina C, Vella AT. Therapeutic targeting of the inflammome. Biochem Pharmacol 2014; 92:184-91; PMID:25204592; http://dx.doi.org/10.1016/j.bcp.2014.08.027
- Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3–potential mechanisms of action. Nat Rev Immunol 2015; 15:45-56; PMID:25534622; http://dx.doi.org/10.1038/nri3790
