ABSTRACT
Checkpoint blockade has demonstrated promising antitumor responses in approximately 10–40% of patients. However, the majority of patients do not make a productive immune response to their tumors and do not respond to checkpoint blockade. These patients may benefit from an effective vaccine that stimulates high-avidity T cell responses in combination with checkpoint blockade. We have previously shown that incorporating TRP-2 and gp100 epitopes into the CDR regions of a human IgG1 DNA (ImmunoBody®: IB) results in significant tumor regression both in animal models and patients. This vaccination strategy is superior to others as it targets antigen to antigen-presenting cells and stimulates high-avidity T cell responses. To broaden the application of this vaccination strategy, 16 NY-ESO-1 epitopes, covering over 80% of HLA phenotypes, were incorporated into the IB (SCIB2). They produced higher frequency and avidity T cell responses than peptide vaccination. These T cells were of sufficient avidity to kill NY-ESO-1-expressing tumor cells, and in vivo controlled the growth of established B16-NY-ESO-1 tumors, resulting in long-term survival (35%). When SCIB2 was given in combination with Treg depletion, CTLA-4 blockade or PD-1 blockade, long-term survival from established tumors was significantly enhanced to 56, 67 and 100%, respectively. Translating these responses into the clinic by using a combination of SCIB2 vaccination and checkpoint blockade can only further improve clinical responses.
Abbreviations
| Ab | = | antibody |
| APC | = | antigen-presenting cells |
| CDR | = | complementary determining region |
| CTLA-4 | = | cytotoxic T lymphocyte associated protein-4 |
| ELISA | = | enzyme-linked immunosorbent assay |
| Elispot | = | enzyme-linked immunospot |
| EP | = | electroporation |
| FBS | = | fetal bovine serum |
| FcR | = | Fc receptor |
| IFA/CFA | = | Incomplete/complete Freund's adjuvant |
| PBMCs | = | peripheral blood mononuclear cells |
| PD-1 | = | programmed cell death protein 1 |
| PD-L1 | = | programmed death ligand 1 |
| PI | = | proliferation index |
| Treg | = | regulatory T cells |
Introduction
Checkpoint inhibitors have reformed cancer treatments of melanoma, with 20–30% of patients responding to ipilimumab (CTLA-4 blockade) and to PD1/PD-L1 blockade.Citation1,2 More recently, among previously untreated patients with metastatic melanoma, anti-PD-1 alone or combined with anti-CTLA-4 resulted in significantly longer progression-free survival than anti-CTLA-4 alone.Citation3 Although the responses are promising, not all melanoma patients respond and the responses in other cancers have not been as high. Recent studies show that the response to checkpoint blockade may be related to the frequency of neo-epitopes that stimulate new T cell responses that have not been tolerized.Citation4,5 However, the majority of patients are non-responders and do not have the appropriate mutations, so they fail to stimulate a productive immune response. These patients may benefit from an effective vaccine that stimulates high-avidity CD4+ and CD8+ responses in combination with checkpoint blockade. This is not a new concept as the initial trials with ipilimumab used gp100 peptide vaccination. However, the problem with peptide vaccinations is that they do not generate T cells with sufficient avidity to clear the tumor.Citation6
Due to its restricted normal expression and widespread tumor expression (including esophageal, lung, liver, ovarian, melanoma, bladder, prostate and breast cancer),Citation7-9 NY-ESO-1 is an ideal target for a tumor therapy. Indeed, T cell responses restricted through multiple HLA alleles have been reported,Citation10-12 allowing a cancer vaccine that targets this antigen to be applicable to both a broad range of patients with different HLA types and also to a wide range of tumors. A variety of vaccination approaches have been tried with NY-ESO-1, including administration of synthetic peptide, recombinant protein and DNA encoding full-length NY-ESO-1Citation13-15 but they have failed to control tumor growth.Citation13,16,17 This may be related to induction of low-avidity T cell responses with restricted ability to recognize tumor cells and the profoundly immunosuppressive tumor environment which further restricts T cell activity.
We have previously shown that a DNA plasmid encoding T cell epitopes within the complementary determining regions (CDR)Citation18 of a human IgG1 antibody (ImmunoBody®) stimulates high-avidity T cell responses.Citation19 Electroporation (EP) increases DNA uptake over 1,000-fold and has an adjuvant effect resulting from local tissue damage and the subsequent expression of pro-inflammatory cytokines.Citation20,21 The ImmunoBody® acts by direct presentation of the DNA within antigen-presenting cells and cross-presentation of secreted protein via the high-affinity FcγR1 receptor (CD64). When comparing DNA and protein immunization of ImmunoBody®, the DNA gave higher frequency and avidity responses suggesting direct presentation of the DNA within antigen-presenting cells. However, experiments in CD64-knockout mice but not CD32-knockout mice induced lower frequency and avidity T responses in wild-type mice suggesting that cross-presentation of secreted protein via the high-affinity FcγR1 receptor (CD64) was important. Although either presentation induces T cell responses, it is only the combination that induces T cells with sufficiently high avidity to kill tumor cells.Citation18,19 This was further validated by comparison of the same ImmunoBody® DNA expressing Fab or whole antibody molecules, which showed much weaker responses in the absence of Fc. We have also replaced human IgG1 from the same DNA backbone vector with moIgG2a; both huIgG1 and moIgG2a can stimulate immune responses in mice.Citation17 SCIB1 is an ImmunoBody® encoding a human IgG1 antibody, with three epitopes from gp100 and one from TRP-2 engineered into its CDR regions, which has entered clinical trials. A clinical study in stage III/IV melanoma patients with tumor present at study entry showed that SCIB1 could induce T cell responses in 10/11 patients with no associated toxicity. Overall survival was 19 mo with patients showing clinical responses including two partial responses and stable disease. Results were even more dramatic in patients with fully resected disease as they all showed a T cell response and are still alive with a current median observation time of 3 y. Two- and three-year recurrence-free survival for stage III resected patients was 89 and 67%, respectively, and for stage IV resected patients, it was 71% at both time points.Citation22
In this study, we report the validation of SCIB2 which encodes NY-ESO-1 T cell epitopes in the CDR regions of the human IgG1 vector. This approach stimulates higher avidity T cell responses than peptide and demonstrates the advantage of combining both high-avidity T cell responses with checkpoint blockade. The combination of SCIB2 with PD1 blockade led to complete tumor regression.
Results
Melanoma patients demonstrate NY-ESO-1-specific T cell responses
Previous studies suggest NY-ESO-1-specific T cell responses could be detected in peripheral blood mononuclear cells (PBMCs) from cancer patients.Citation23 We selected 16 epitopes from NY-ESO-1 covering the most common class I and class II haplotypes and confirmed that melanoma patients show T cell responses to these peptides. 10 of 11 (90%) patients responded to one or more of the CD8+ epitopes () and 9 of 11 (82%) to one or more of the CD4+ epitopes (). The clinicopathology and HLA types are shown in Table S1. This data suggested that inclusion of these epitopes within SCIB2 would provide a vaccine that could target the majority of HLA types. summarizes the HLA-restricted NY-ESO-1 epitopes that have been incorporated into SCIB2.
Figure 1. Peripheral blood lymphocytes (PBMC) from melanoma patients show spontaneous response to selected NY-ESO-1 peptides. The in vitro proliferation assay was performed on PBMC from melanoma patients following 10 d incubation with NY-ESO-1 (A) CD8+ peptides (NY-ESO-1 83–91, 88–96, 157–165, 158–166) and (B) CD4+ peptides (NY-ESO-1 87–111 and 119–143).
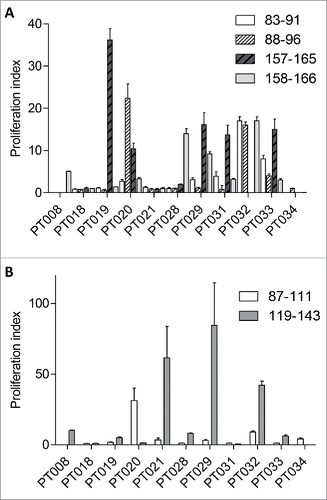
Table 1. NY-ESO-1 incorporated epitopes.
Immunization with SCIB2 generates strong NY-ESO-1-specific CD8+ and CD4+ responses in HLA transgenic mice
HHDII (HLA-A*0201) transgenic mice immunized with SCIB2 showed high-frequency responses to the CD8+ T cell epitope NY-ESO-1 157–165, and the CD4+ T cell epitopes 87–111 and 119–143 over background control () confirmed by in vitro CD8+ and CD4+ depletion, respectively (Figs. S1A and B). In this instance, the CD4+-mediated responses were I-Ab restricted (Fig. S1B).
Figure 2. Epitope-specific responses generated in HHDII and HHDII/DR1 mice immunized with SCIB2. Splenocytes from SCIB2-immunized HHDII mice (A) and HHDII/DR1 mice (B) were analyzed by IFNγ Elispot to show the frequency of responses to NY-ESO-1 157–165, 87–111 and 119–143. Graph shows pooled data from >3 experiments in which n = 3. (C) Splenocytes from immunized HHDII and HHDII/DR1 mice were assayed for avidity to NY-ESO-1 157–165 peptide by measuring responses to increasing concentration of peptides in IFNγ Elispot assay. (D) After 6 d in vitro stimulation, cytotoxicity of NY-ESO-1 157–165-specific T cells on tumor lines were assessed by 51Cr-release assay at a 50:1 effector: target ratio. (E) Granzyme B release from splenocytes of HHDII mice immunized with SCIB2. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05. Data show mean and SD and are representative of at least two experiments where n ≥ 3.
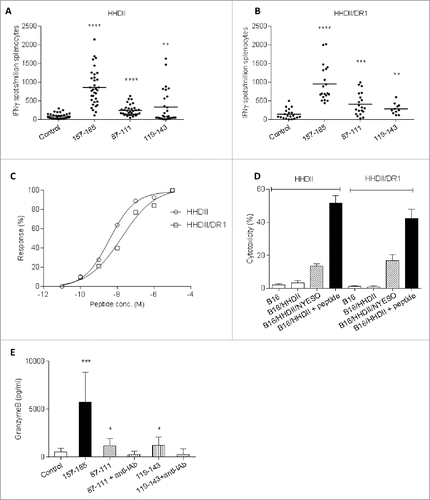
To assess the immune response in a mouse with only human MHC, we immunized HHDII/DR1 mice that have human class I HLA*0201 and human class II (HLA-DR1) and no mouse MHC. As illustrated in , T cells from immunized HHDII/DR1 mice show significantly higher epitope-specific responses to NY-ESO-1 157–165 over background control. This is consistent with Fig. S1C showing paired response between background control and NY-ESO-1 157–165. SCIB2-immunized mice also showed significantly higher antigen-specific responses to 87–111 and 119–143, indicating that the 87–111 and 119–143 sequences also induce responses restricted through HLA-DR1 (). Addition of HLA-DR-blocking Ab into the assay confirmed that these responses were HLA-DR-restricted CD4+ responses (Fig. S1D). To assess if the DNA vector alone could act as an adjuvant and generate NY-ESO-1-specific immune responses, mice were immunized with vector expressing the human IgG1 antibody with no NY-ESO-1 epitopes inserted. The empty antibody vector did not generate any NY-ESO-1-specific IFNγ responses (Fig. S1E). In addition, no responses to irrelevant peptides were observed in SCIB2-immunized mice (Fig. S1F).
High-avidity T cell (3.8 × 10−9, 1.7 × 10−8) responses were demonstrated by titration of the NY-ESO-1 157–165 peptide in both HHDII and HHDII/DR1 mice (). These high-avidity T cell responses result in killing of NY-ESO-1-positive tumor cells (B16/HHDII/NY-ESO-1 cells) but not of HLA-mismatched or antigen-negative control cells (). This data demonstrated that SCIB2 can be used in vivo to stimulate strong CD8+ T cell responses that are capable of tumor cell lysis in vitro as well as induction of CD4+ T cell responses.
To further assess the cytotoxic potential of the encoded epitopes, splenocytes from mice immunized with SCIB2 were incubated with NY-ESO-1 peptides in vitro for 40 h and the supernatants were analyzed by granzyme B ELISA. All three epitopes stimulated significant amounts of granzyme B including the CD4+ epitopes NY-ESO-1 87–111 and 119–143 epitopes, these responses could be completely blocked by mouse MHC class II blocking Ab ().
SCIB2 induces higher avidity CD8+ responses than peptide vaccination
Many clinical trials using NY-ESO-1 vaccines have failed to show clinical benefits in patients. To determine whether SCIB2 was likely to be more potent, the frequency and avidity of T cell responses generated from vaccination with SCIB2 and conventional peptide immunization were compared. SCIB2 immunization stimulated higher frequency T cell responses specific for the NY-ESO-1 157–165 epitope than peptide immunization (SCIB2 vs. peptide p = 0.0004) (). The functional avidity, as measured by peptide titration, showed that SCIB2 (9 × 10−9 M) generated a 100-fold higher avidity than peptide (10−6 M) immunized mice ().
Figure 3. Immune responses and cytotoxicity induced in HHDII mice immunized with SCIB2 or NY-ESO-1 peptides. Comparison of (A) frequency and (B) normalized avidity responses to NY-ESO-1 157–165 peptide measured in IFNγ Elispot assay from mice immunized with either SCIB2 or peptide. (C) Cytotoxicity of splenocytes assessed by 51Cr-release assay. Data are shown at effector to target ratio of 50:1. **p < 0.01. Data are presented as mean and SD and are representative of at least two experiments in which n ≥ 3.
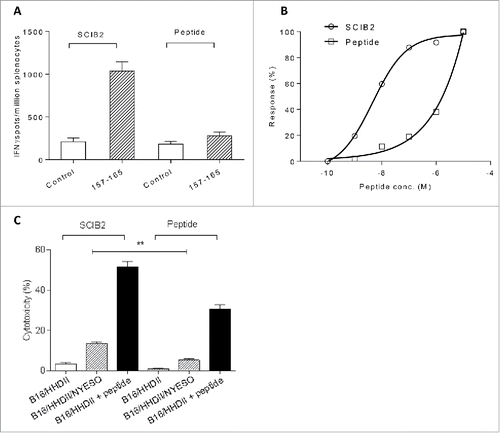
Differences in functional avidity have been directly correlated with antitumor activity. NY-ESO-1 157–165-specific T cells induced by immunization with either SCIB2 or with peptide were able to specifically recognize peptide pulsed tumor targets; however, SCIB2 immunization was the only approach which showed recognition and lysis of naturally processed and presented NY-ESO-1 antigen on tumor cells (). Overall, this demonstrated that NY-ESO-1 157–165-specific T cells generated from mice immunized with SCIB2 were more efficient at targeting tumor cells in vitro.
Immunization with SCIB2 generates strong antitumor immunity
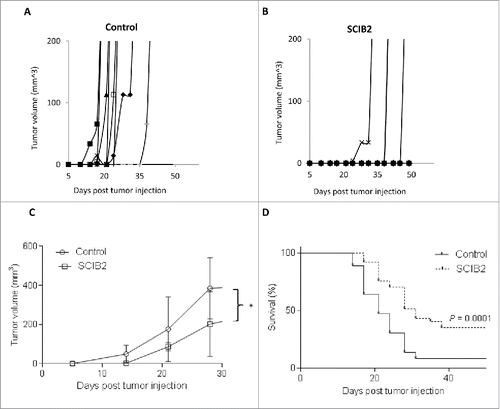
To see if the enhanced in vitro recognition of tumor targets was also reflected in vivo, mice with established B16/HHDII/NY-ESO-1 tumors were immunized with SCIB2. Mice immunized with SCIB2 demonstrate a significant delay in tumor growth (p < 0.05) () and 35% mice remain tumor-free with long-term survival (p = 0.0001) ().
Figure 4. SCIB2 stimulates strong antitumor immunity. Individual (A and B) and average (C) tumor growth curves and percentage survival (D) of HHD II mice challenged with 2.5 × 104 B16/HHDII/NY-ESO-1 tumor cells at day 0 and immunized with SCIB2 at days 4, 11 and 18. *p < 0.05. Lack of survival was defined as tumor size > 528 mm3. (A) and (B) are representative of data from one study in which n = 10. (C) and (D) are representative of data from at least two independent studies in which n = 10 each study.
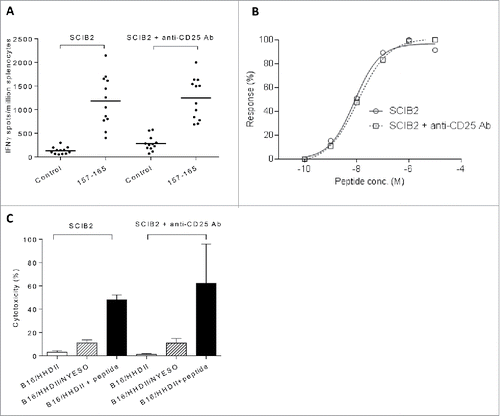
T cell responses induced by combining SCIB2 with anti-CD25 Ab or checkpoint blockade
Results from clinical studies have suggested that Treg depletion or checkpoint blockade can enhance endogenous T cell responses and/or recover immune responses within the tumor environment. Initially, the effect of Treg depletion or checkpoint blockade on SCIB2 immune responses was assessed. We have shown that anti-CD25 Ab could effectively eliminate Tregs in vivo. The percentage of CD4+CD25+ FOXP3+ cells was reduced from 0.53% of CD4+ cells to 0.00% after administration of anti-CD25 Ab. However, there was also a depletion of CD4+CD25+ cells from 1.78 to 0.13% suggesting some activated CD4+ cells may also have been depleted. Therefore, prior to the antitumor studies, it was important to assess the effect of anti-CD25 Ab on SCIB2-induced T cell responses. Similar frequencies and avidities of CD8+ epitope-specific responses were observed with SCIB2 in the presence or absence of anti-CD25 Ab (). The cytotoxicity and IFNγ release ( and Fig. S2) by SCIB2-induced T cells was also similar in the presence or absence of anti-CD25 Ab. This data implies that anti-CD25 Ab had little effect upon the induction of NY-ESO-1-specific CD8+ responses.
Figure 5. T cell responses induced by combining SCIB2 with Treg depletion. (A–C) Mice were immunized with SCIB2 in combination with anti-CD25 Ab. Splenocytes from immunized mice were assayed at day 20 for (A) frequency of immune responses against 10 µg/mL of NY-ESO-1 157–165 peptide. Graph shows pooled data from >3 experiments in which n = 3. (B) Normalized avidity by titration of NY-ESO-1 157–165 peptide by IFNγ Elispot assay. (C) After 6 d in vitro stimulation with NY-ESO-1 157–165 peptide, CD8+ T cells were assessed by 51Cr-release cytotoxicity assay. Data is shown at an effector to target ratio of 50:1. Data show mean and SD and are representative of at least two experiments, where n ≥ 3.
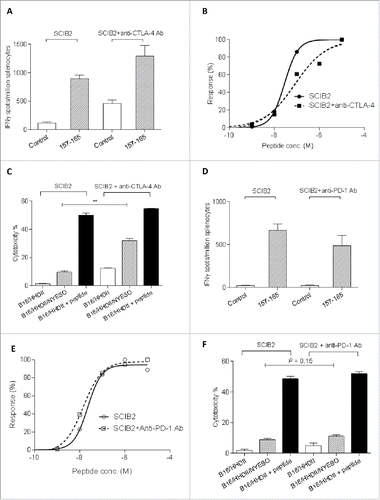
To determine if CTLA-4 or PD-1 blockade could enhance SCIB2-induced T cell responses, mice were immunized with SCIB2 in the presence or absence of anti-CTLA-4 or anti-PD-1 Ab. Anti-CTLA-4 and anti-PD-1 Ab did not significantly enhance the frequency () or avidity () of the CD8+ T cell responses. However, when splenocytes from SCIB2/anti-CTLA-4-immunized mice were incubated in vitro with NY-ESO-1 157 peptide, they showed significantly enhanced tumor killing of targets expressing NY-ESO-1 when compared to SCIB2 alone immunized mice (). This suggests that anti-CTLA-4 Ab could enhance CTL cytotoxicity. In contrast, anti-PD-1 Ab did not enhance the CTL cytotoxicity killing over tumor targets ().
Figure 6. T cell responses induced by combining SCIB2 with checkpoint inhibitors. Mice were immunized with SCIB2 in combination with the anti-CTLA-4 Ab (A–C) or anti-PD-1 Ab (D–F). (A, D) Frequency of Immune responses against 10 µg/ mL NY-ESO-1 157–165 peptide and (B, E) avidity by titration of NY-ESO-1 157–165 peptide were measured by IFNγ Elispot assay. (C, F) After 6 d in vitro stimulation with NY-ESO-1 157–165 peptide, CD8+ T cells were assessed for the ability to kill B16/HHDII/NY-ESO-I target cells by 51Cr-release cytotoxicity assay. Data are shown at an effector to target ratio of 50:1. **p < 0.01. Data are presented as mean and SD. Data are representative of at least two experiments in which n ≥ 3.
Anti-CD25 Ab and checkpoint blockade enhance the antitumor effect of SCIB2
As addition of anti-CD25 Ab or checkpoint blockade did not enhance the SCIB2 vaccine-induced T cell responses, we assessed if these treatments could enhance tumor rejection. In order to ensure that the vaccine-induced T cells entering the tumor were protected from Treg depletion/blocking or checkpoint inhibitors, administration of anti-CD25 Ab was initiated at the same time as tumor challenge and the checkpoint blockade antibodies were given at the same time as vaccination.
The combination of anti-CD25 Ab and vaccination with SCIB2 was assessed in a therapeutic antitumor model (HHDII mice challenged with 2.5 × 104 B16/HHDII/NY-ESO-1 tumor cells). Administration of anti-CD25 Ab at the same time as tumor challenge was not sufficient for tumor rejection (p > 0.05) (). However, mice immunized with SCIB2 demonstrate enhanced survival compared to control (p = 0.001) (). While the combination of anti-CD25 Ab and SCIB2 vaccination resulted in further inhibition of tumor growth compared to vaccination with SCIB2 (p = 0.0484) with 56% of mice showing long-term tumor-free survival ().
Figure 7. Antitumor effect of combination of SCIB2 and Treg depletion and SCIB2 with CTLA-4 or PD-1 blockade. HHDII mice were implanted with B16/HHDII/NY-ESO-1 cells and treated with anti-CD25 Ab, SCIB2 or both. (A) Percentage survival of mice challenged with low-dose (2.5 × 104) tumor and immunized with SCIB2 and anti-CD25 Ab alone or in combination. Con vs. SCIB2 (**p = 0.001); SCIB2 vs. SCIB2+anti-CD25Ab depletion (*p = 0.0484); Con vs. SCIB2+anti-CD25 Ab (***p = 0.0006). (B) Percentage survival rate of mice challenged with high-dose (1.5 × 105) tumor. Con vs. SCIB2 (p > 0.05); Con vs. anti-CD25 Ab (p > 0.05); SCIB2 vs. SCIB2+anti-CD25 Ab depletion (*p = 0.03); anti-CD25 Ab vs SCIB2+anti-CD25Ab (*p = 0.04); (C) Survival of mice challenged with 2.5 × 104 tumor cells and immunized with SCIB2 and anti-CTLA-4 Ab alone or in combination. Con vs. anti-CTLA-4 Ab (***p = 0.0003); Con vs. SCIB2 (****p < 0.0001); anti CTLA-4 Ab vs. anti-CTLA-4 Ab plus SCIB2 (p > 0.05); SCIB2 vs. SCIB2 plus anti-CTLA-4 Ab (p > 0.05); (D) shows survival of mice challenged with 1 × 105 tumor cells and immunized with SCIB2 and anti-CTLA-4 Ab alone or in combination. Con vs. SCIB2 (*p = 0.021); SCIB2 vs. SCIB2+anti-CTLA-4 Ab (*p = 0.02); anti-CTLA-4 Ab vs. SCIB2 + anti-CTLA-4 Ab (*p = 0.05); Con vs. SCIB2+anti-CTLA-4 Ab (****p < 0.0001); (E) Survival of mice challenged with 5 × 104 tumor cells and immunized with SCIB2 and anti-PD-1 Ab alone or in combination. Con vs. SCIB2 (*p = 0.037); Con vs. anti-PD-1 Ab ( p = 0.111); Con vs. SCIB2+anti-PD-1 Ab (***p = 0.0003); SCIB2 vs. SCIB2+anti-PD-1 Ab (*p = 0.0177); anti-PD-1 Ab vs. SCIB2+anti-PD-1 Ab (*p = 0.0177). Lack of survival was defined as tumor size > 528 mm3. Each curve represents at least 10 mice per group.
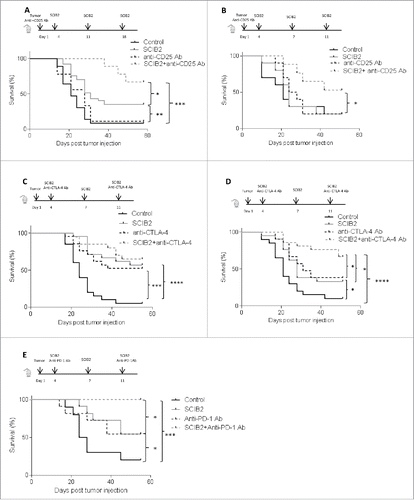
To further assess the efficacy of combined vaccines, HHDII mice were given a higher tumor load of B16/HHDII/NY-ESO-1 tumor cells (1.5 × 105) than in the previous experiment. SCIB2 alone and anti-CD25 Ab alone failed to show any therapeutic advantage compared to control () against these high tumor load. However, SCIB2 showed synergy with anti-CD25 Ab with 50% long-term survival (). The combination group showed a significantly higher survival rate than the single therapies (SCIB2 vs. SCIB2 plus anti-CD25 Ab, p = 0.03; and anti-CD25 vs. SCIB2 plus anti-CD25, p = 0.04).
The combination of anti-CTLA-4 Ab and vaccination with SCIB2 was assessed in the therapeutic antitumor model. In the lower dose model (2.5 × 104 cells), anti-CTLA-4 Ab and SCIB2 alone significantly delayed tumor growth with long-term survival of more than 50% (). This was further enhanced to 65% improvement of survival when these modalities were combined (). Using a higher tumor cell dose (1 × 105 cells), both single therapy groups still showed a significant survival advantage over control (). However, the combination therapy induced antitumor responses in 67% mice which is significantly enhanced when compared to single therapies (control vs. SCIB2, p = 0.021; SCIB2 vs. SCIB2+anti-CTLA-4 Ab, p = 0.02; anti-CTLA-4 Ab vs. SCIB2 + anti-CTLA-4 Ab, p = 0.05; control vs. SCIB2+anti-CTLA-4 Ab, p < 0.0001) ().
The antitumor effect of combination of PD-1-blocking antibody and vaccination with SCIB2 was then assessed. Mice were challenged with (5 × 104) B16/HHDII/NY-ESO-1 tumor cells; anti-PD-1 Ab and SCIB2 alone delayed tumor growth with long-term survival of 50% (). This was further enhanced to 100% improvement of survival when these modalities were combined (control vs. SCIB2, p = 0.037; control vs. anti-PD-1 Ab, p = 0.11; SCIB2 vs. SCIB2+anti-PD-1 Ab, p = 0.0177; anti-PD-1 Ab vs. SCIB2 + anti- PD-1 Ab, p = 0.0177; control vs. SCIB2+anti- PD-1 Ab, p = 0.0003).
Discussion
NY-ESO-1 is a cancer testis antigen that has a restricted normal expression but is expressed by a wide range of tumors.Citation7-9 It is very immunogenic and many patients have endogenous responses to this antigenCitation11,12,24,52 as we confirmed in our in vitro studies from melanoma patients. We showed T cell response to one or more of 16 T cell epitopes in 82–90% of patients. Despite the presence of spontaneous T cell responses, the majority of patients still die of their disease. One of the key factors may be failure to generate high-avidity T cells that can lyse tumor cells. We have previously demonstrated the superiority of the vaccine approach of encoding antigenic epitopes within a human antibody IgG1 framework for the induction of high-avidity CD8+ and CD4+ responses.Citation18,19 SCIB2 was therefore designed to encode these 16 NY-ESO-1 epitopes, which are nested within four regions of NY-ESO-1, in the CDR regions of the human IgG1 Ab ImmunoBody® vector.
SCIB2 was tested in HHDII (HLA-A*0201) transgenic mice models. It not only elicited a higher frequency and avidity response to NY-ESO-1 157 than peptide vaccination but also generated CD8+ responses with the ability to recognize naturally processed and presented NY-ESO-1 antigen on the surface of tumor cells. This is consistent with our previous findings that the frequency and avidity of responses from SCIB1 was significantly higher than that from peptide immunization.Citation19 In addition, SCIB2 induced high-frequency CD4+ responses in HHDII and HHDII/DR1 transgenic mice confirming I-Ab and demonstrating HLA-DR1 restriction.Citation10,12,25-27 Furthermore, the induced CD4+ cells released granzyme B that could be completely blocked by MHC class II Ab suggesting that they were cytotoxic CD4+ responses. Recent studies demonstrate CD4+ T cells can possess cytotoxicity abilities and are able to lyse virus-infected and tumor cells.Citation28,29 NY-ESO-1 87–111-specific CD4+ CTL lines from cancer patients have also been shown to recognize autologous APC loaded with protein or transfected with NY-ESO-1 cDNA.Citation12 MHC II expression can be induced in majority of cells, including melanoma by IFNγ; it can also be constitutively expressed in some melanomas.Citation30-32 The effect of both CD8+ and CD4+ responses on tumor therapy was measured using the HHDII transgenic mouse model. As NY-ESO-1 does not have a murine homolog, the human NY-ESO-1 was cloned into the B16/HHDII cell line. As a foreign antigen, it was anticipated that it may induce an antitumor response in the absence of vaccination. However, NY-ESO-1-expressing tumors still grew rapidly in the majority of mice. In contrast, the strong cytotoxic high-avidity NY-ESO-1-specific CD8+ and CD4+ T cell responses induced by SCIB2 led to long-term survival in 35% of mice. In this study, we did not investigate if the antitumor effect induced by SCIB2 was CD4+ and/or CD8+ T cells mediated, this will be examined in a future study. We have shown both CD8+ and CD4+ responses to NY-ESO-1 epitopes encoded within SCIB2 DNA constructs could be generated in patients which gives a good indication that SCIB2-stimulated responses in humans may involve both CD8+ and CD4+ T cells.
Tumors are well known to promote an anti-inflammatory suppressive environment and recent data has demonstrated that high-avidity CD8+ T cells are tolerized within the tumor environment rendering them non-functional.Citation33 We report that SCIB2-mediated antitumor responses were further enhanced in combination with Treg depletion/blocking. Anti-CD25 Ab has been used in many studies to deplete/block Tregs in murine models and reports suggest an antitumor role as a monotherapy or combined with vaccines.Citation34,35 However, its antitumor efficacy as a monotherapy was limited in aggressive tumor models, including B16.Citation64 Daclizumab, a human anti-CD25 Ab has been shown to reduce the presence of Tregs in peripheral blood; however, its antitumor therapeutic effect has yet to be determined as it can also eliminate activated T cells.Citation36 A recent clinical trial demonstrates the synergistic effect of cyclophosphamide with multiple peptide vaccines in renal cancer patients with prolonged survival.Citation37 This effect was not observed in the absence of immune responses which suggest the importance of both immune response and immunomodulatory mechanisms.
A vaccination that generates high-avidity T cells could be used in cancer treatments along with checkpoint inhibitors to remove the brakes and unleash the full potential of the T cell response. Indeed, we have shown here that in a preclinical model, the combination of CTLA-4 with SCIB2 resulted in an enhanced antitumor response. This is in contrast to other studies showing that when it is given as monotherapy, anti-CTLA-4 Ab failed to reject tumor in several models (B16, SM1 mammary carcinoma, EL4 lymphoma, M109 lung cancer).Citation38 CTLA-4 binds to CD80/86 with higher affinity than CD28 and inhibits the activating signals. Using our immunization regime of co-vaccinating with the initial and third dose of vaccine, we showed no increase in the frequency or the avidity of the SCIB2-induced immune response but did see good additive antitumor responses. This is in line with other studies showing that T cell responses can be suppressed within the tumor environment and anti-CTLA-4 Ab can reactivate T cells at the tumor site.Citation39
In our preclinical model, the combination of anti-PD-1 with SCIB2 resulted in an enhanced antitumor response. Studies in the B16 tumor model in C57Bl/6 mice have failed to show an effect with PD-1 blockade alone.Citation40 This may be a reflection of its low immunogenicity and/or the fact it does not normally express PD-L1 in vivo. Previous studies indicate that the PD-L1 level within the tumor environment can be elevated by infiltrating activated T cells secreting IFNγ.Citation41,42 PD-L1 expression within tumor environment can be related to poor prognosis in various cancers.Citation43-45 High levels of PD-L1 expression in the tumor microenvironment have also been correlated with a better response rate to PD-1/PD-L1 blockade in cancer patients.Citation46 However, some patients whose tumors do not express PD-L1 also respond to PD-1/PD-L1 blockade. PD-L1 can also be expressed on infiltrating monocytes and endothelial cells and the role these cells play in checkpoint inhibition remains unclear. It is suggested that induction of PD-L1 in the melanoma tumor microenvironment may suppress T cells mediated antitumor immune responses by engaging PD-1 on the tumor-infiltrating T cellsCitation41 and may also promote iTreg induction, proliferation and immune suppressive function.Citation48 Recent studies showed, in addition to blocking negative pathway in immune cells, the checkpoint inhibitors (anti CTLA-4 and anti PD-1 Abs) also eliminates Treg.Citation40,49,50 Within the tumor environment, depletion of Tregs by anti-CTLA-4 is dependent on the presence of Fcγ receptor-expressing receptor macrophage.Citation50 This is consistent with our results in which anti-CTLA-4 and anti-PD-1 Abs failed to increase the immune response to SCIB2 but did enhance its antitumor response with 100% of mice showing long-term survival with the SCIB2/anti-PD-1 combination.
In conclusion, this study shows that targeting NY-ESO-1 epitopes to antigen-presenting cells results in high-avidity and high-frequency T cell immune responses, with potent antitumor responses, which were further enhanced by checkpoint blockade. The 100% tumor survival with the combination of SCIB2 and PD-1 blockade is especially promising as PD-1 blockade is known to have lower associated toxicity when compared to CTLA-4 blockade. SCIB2 will now be rapidly translated into the clinic where it is anticipated that patients with low tumor burden may benefit from SCIB2 alone but patients with more bulky disease may benefit from combinations of SCIB2 with checkpoint blockade.
Materials and methods
Vaccine and expression plasmids
In brief, to generate SCIB2 oligonucleotides encoding the HLA-A24 epitope 158–166 (LLMWITQCF) and HLA-A2-restricted epitope 157–165 (SLLMWITQC) were incorporated into the CDRH1 and CDRH2 of the vector pDCOrig, respectively, as previously described.Citation18 The NY-ESO-1 amino acid sequence 83–111 (PESRLLEFYLAMPFATPMEAELARRSLAQ) was cloned into the CDRH3 site. NY-ESO-1 amino acid sequence 119–143 (PGVLLKEFTVSGNILTIRLTAADHR) was inserted into the CDRHL1 site within the light chain.
To construct the mammalian double expression plasmid that encodes murine TAP2 and NY-ESO-1, NY-ESO-1 was amplified from the IMAGE clones 40146393 obtained from Genservice with forward and reverse primers that incorporated a BamH1/XhoI site, respectively. On sequence confirmation, full-length NY-ESO-1 was ligated into the BamHI/Xho1 multiple cloning site of the double expression vector in replacement of the light chain. Murine TAP2 (after site-directed mutagenesis of the image clone 6530488 to remove a HindIII site from encoding sequence and insertion before the start codon) was cloned into the expression vector pOrigHIB using HindIII/EcoRV, then transferred in replacement of the heavy chain using HindIII/AvrII into the double expression vector alongside full length NY-ESO-1.
To generate the HHD plasmid, cDNA was synthesized from total RNA isolated from EL4-HHD cells. This was used as a template to amplify HHD using the forward and reverse primers and subcloned into pCR2.1. The HHD chain, comprising of a human HLA-A2 leader sequence, the human β2-microglobulin (β2M) molecule covalently linked via a glycine serine linker to the α 1 and 2 domains of human HLA-0201 MHC class 1 molecule and the α3, transmembrane and cytoplasmic domains of the murine H-2Db class 1 molecule, was then inserted into the EcoRV/HindIII sites of the mammalian expression vector pCDNA3.1 obtained from Invitrogen.
In order to knockdown expression of murine β2M and murine MHC class II in the cell line B16F10, RNA interference was utilized. Complimentary oligonucleotides that target sequence 266 of murine β2M and 159 of murine MHC class II were annealed and inserted separately into pCDNA6.2 GW miR (Invitrogen). The pre-miRNA expression cassette containing miRNA 266 was excised using BamHI/XhoI and ligated into the XhoI/BglII site of pCDNA6.2 GW miR 159 in order to chain the two miRNAs and express them in one primary transcript within the same vector.
Cell lines
B16F10 cell line (ATCC) was maintained in RPMI (Cambrex) with 10% FBS (Sigma). B16F10 cells were transfected successively using Lipofectamine transfection reagent (Invitrogen) with expression vectors encoding full-length NY-ESO-1 and Murine TAP2, HHDII and a siRNA to knockdown expression of murine MHC class II and murine β2M. Transfected cells were selected by growth in the presence of Zeocin (300 µg/ mL), G418 (500 µg/ mL) and Blasticidin (4 µg/ mL), respectively. Lines were cloned by limiting dilution and expression was confirmed by flow cytometry.
Mice and immunizations
Animal work was carried out under a Home Office approved project license at Nottingham Trent University. HLA-A2/DR1 (HHDII/DR1) or HLA-A2 (HHDII) transgenic mice on a C57Bl/6 background (Pasteur Institute) were used between 6 and 16 weeks of age. HHDII mice are murine class I deficient and express the human HLA-A2 allele. HHDII/DR1 mice are deficient in both murine MHC class I and II and replaced with the human HLA-A2 and DR1 alleles. Synthetic peptide SLLMWITQC (NY-ESO-1 157–165) was emulsified with IFA/CFA combination (Invivogen). Peptide (25 μg/immunization) was injected via the subcutaneous route at the base of the tail. DNA was coated onto 1.0 μm gold particles (Bio-Rad) using the manufacturer's instructions and administered intradermally by the Helios Gene Gun (Bio-Rad). Each mouse received 1 μg DNA/immunization into the shaved abdomen. Depletion of Tregs was performed by intraperitoneal injection of 400 μg anti-CD25 Ab (PC61) (BioXcell) 4 d prior to the first immunization. Mice were immunized at day 1, 8 and 15 and spleens removed at day 20 for analysis, unless otherwise stated. The half-life of the hamster anti-CTLA-4 (9H10) Ab in vivo has been shown to be 3–4 d.Citation51 The rat anti PD-1 (RPM1-14) Ab has a slightly longer half-life and is usually administered every 4–7 d. For consistency, we gave both checkpoint blockade antibodies weekly with the first and last SCIB2 administration. Anti-CTLA-4 Ab (9H10) or anti-PD-1 Ab (RMP1-14) (BioXcell) was injected at 200 or 250 μg intraperitoneally at day 1 and 15.
Proliferation assay
PBMC from melanoma patients were isolated by Ficol-Hypaque (Sigma) gradient centrifugation. In brief, PBMC (1.5 × 106cell/well) were stimulated with peptide (10 μg/ mL) for 10 d at 37°C. The cultured PBMC were then incubated with 3H-thymidine (0.0185 MBq/well) for 8 h at 37°C. The cultures were harvested onto unifilter plates and incorporation of 3H-thymidine was determined by β-scintillation counting. The results were assessed by calculating the proliferation index (PI) as the ratio of the mean of counts per minutes (cpm) of epitope-stimulated to the mean of unstimulated cultures. The proliferative assay was considered positive when PI > 2.5.
Ex vivo Elispot assay
Elispot assays were performed using murine IFNγ capture and detection reagents according to the manufacturer's instructions (Mabtech AB). Detailed method was described previously.Citation19 Synthetic peptides SLLMWITQC (NY-ESO-1 157–165), LLEFYLAMPFATPMEAELARRSLAQ (NY-ESO-1 87–111) and PGVLLKEFTVSGNILTIRLTAADHR (NY-ESO-1 119–143) (at a variety of concentrations) were used in these assays. Anti-mouse MHC-II IAb blocking Ab (M5/114.15.2) was purchased from ebioscience. Anti HLA-DR blocking Ab (L243) was obtained from Biolegend.
Re-stimulation in vitro
Splenocytes (5 × 106/ mL) were co-cultured at 37°C with irradiated, peptide-pulsed LPS blast. LPS blasts were obtained by activating splenocytes (1.5 × 106/ mL) with 25 μg/ mL LPS (Sigma) and 7 μg/ mL dextran sulfate (Pharmacia) for 48 h at 37°C. Before use, 2 × 107 LPS blasts were labeled with 10 μg/ mL synthetic peptide for 1 h. Anti CTLA-4 Ab and PD-1 Ab were used at 40 μg/mL. After six days, cultures were assays for cytotoxic activity in a 51Cr-release assay.
CTL assay
Target cells were labeled for 90 min with 1.85 MBq sodium (51Cr) chromate (Amersham) in the presence or absence of 1 μg/mL (NY-ESO-1 157) peptides. Targets cells (5 × 103/well) were then incubated with different numbers of effector cells in a final volume of 200 μL. After 4 h incubation at 37°C, 50 μL of supernatants were transferred to a Lumaplate (Perkin Elmer). Plates were then read on a Topcount Microplate Scintillation Counter (Packard). Percentage-specific lysis was calculated using the following formula: specific lysis = 100 × [(experimental release minus spontaneous release)/(maximum release minus spontaneous release)].
Granzyme B ELISA
Supernatant from ex vivo IFNγ Elispot assays was analyzed using a granzyme B Elisa kit according to manufacturer's instruction (R&D system).
Tumor studies
In the therapeutic model, HLA-A2 transgenic (HHDII) mice were challenged by subcutaneous injection with 2.5 × 104 B16/HHDII/NY-ESO-1 cells at day 1. Anti-CD25 Ab (400 µg/mouse) was injected intraperitoneally concurrent with tumor cell implant on day 1. The vaccination groups were immunized with DNA bullets intradermally using the Helios Gene Gun for three consecutive weeks at day 4, 11 and 18. For a more aggressive tumor model, 1.5 × 105 B16/HHDII/NY-ESO-1 cells was injected on day 1. For checkpoint blockade tumor model, the vaccine groups were immunized with DNA bullets at day 4, 7 and 11. Anti-CTLA-4 Ab (200 µg/mouse; BioXcell) or anti-PD-1 (250 µg/mouse, BioXcell) was injected intraperitoneally on day 4 and 11. The end point for tumor therapy studies was when tumor exceeded 528 mm3.
Statistical analysis
Comparative analysis of the Elispot, Elisa, CTL killing assay and tumor size results was performed by applying the student's t test with values of p calculated accordingly. Paired Wilcoxon ranking test was used to compare peptide with media Elispot responses from the same mice. Comparison of avidity curves and tumor growth was performed by applying the two-way ANOVA and survival studies were assessed by Log Rank test using the Graphpad Prism 5.0 software (GraphPad Software, Inc.). p < 0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
The authors wish to disclose that Lindy G. Durrant is a director of Scancell Ltd and W. X., R. L. M., V. A. B., P. S., and B. G. are employees of Scancell Ltd.
KONI_A_1169353_s02.zip
Download Zip (242.6 KB)Acknowledgments
The authors would like to thank Dr Ian Daniels and Dr Katherine Cook for their help in proofreading the manuscript. The authors wish to thank H. Yagita for permission to use anti-PD-1 Ab.
Funding
This work was funded by Scancell Ltd.
References
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200690
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373:23-34; PMID:26027431; http://dx.doi.org/10.1056/NEJMoa1504030
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014; 371:2189-99; PMID:25409260; http://dx.doi.org/10.1056/NEJMoa1406498
- Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 2014; 515:577-81; PMID:25428507; http://dx.doi.org/10.1038/nature13988
- Brentville VA, Metheringham RL, Gunn B, Durrant LG. High avidity cytotoxic T lymphocytes can be selected into the memory pool but they are exquisitely sensitive to functional impairment. PloS One 2012; 7:e41112; PMID:22829916; http://dx.doi.org/10.1371/journal.pone.0041112
- Lee L, Wang RF, Wang X, Mixon A, Johnson BE, Rosenberg SA, Schrump DS. NY-ESO-1 may be a potential target for lung cancer immunotherapy. Cancer J Sci Am 1999; 5:20-5; PMID:10188057
- Akcakanat A, Kanda T, Tanabe T, Komukai S, Yajima K, Nakagawa S, Ohashi M, Hatakeyama K. Heterogeneous expression of GAGE, NY-ESO-1, MAGE-A and SSX proteins in esophageal cancer: Implications for immunotherapy. Int J Cancer 2006; 118:123-8; PMID:16003736; http://dx.doi.org/10.1002/ijc.21219
- Nicholaou T, Ebert L, Davis ID, Robson N, Klein O, Maraskovsky E, Chen W, Cebon J. Directions in the immune targeting of cancer: lessons learned from the cancer-testis Ag NY-ESO-1. Immunol Cell Biol 2006; 84:303-17; PMID:16681828; http://dx.doi.org/10.1111/j.1440-1711.2006.01446.x
- Zarour HM, Maillere B, Brusic V, Coval K, Williams E, Pouvelle-Moratille S, Castelli F, Land S, Bennouna J, Logan T et al. NY-ESO-1 119-143 is a promiscuous major histocompatibility complex class II T-helper epitope recognized by Th1- and Th2-type tumor-reactive CD4+ T cells. Cancer Res 2002; 62:213-8; PMID:11782380
- Yamaguchi H, Tanaka F, Ohta M, Inoue H, Mori M. Identification of HLA-A24-restricted CTL epitope from cancer-testis antigen, NY-ESO-1, and induction of a specific antitumor immune response. Clin Cancer Res 2004; 10:890-6; PMID:14871964; http://dx.doi.org/10.1158/1078-0432.CCR-1086-3
- Mandic M, Castelli F, Janjic B, Almunia C, Andrade P, Gillet D, Brusic V, Kirkwood JM, Maillere B, Zarour HM. One NY-ESO-1-derived epitope that promiscuously binds to multiple HLA-DR and HLA-DP4 molecules and stimulates autologous CD4+ T cells from patients with NY-ESO-1-expressing melanoma. J Immunol 2005; 174:1751-9; PMID:15661941; http://dx.doi.org/10.4049/jimmunol.174.3.1751
- Jager E, Gnjatic S, Nagata Y, Stockert E, Jager D, Karbach J, Neumann A, Rieckenberg J, Chen YT, Ritter G et al. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc Natl Acad Sci USA 2000; 97:12198-203; PMID:11027314; http://dx.doi.org/10.1073/pnas.220413497
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011; 29:917-24; PMID:21282551; http://dx.doi.org/10.1200/JCO.2010.32.2537
- Jager E, Karbach J, Gnjatic S, Neumann A, Bender A, Valmori D, Ayyoub M, Ritter E, Ritter G, Jager D et al. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci USA 2006; 103:14453-8; PMID:16984998; http://dx.doi.org/10.1073/pnas.0606512103
- Bender A, Karbach J, Neumann A, Jager D, Al-Batran SE, Atmaca A, Weidmann E, Biskamp M, Gnjatic S, Pan L et al. LUD 00-009: phase 1 study of intensive course immunization with NY-ESO-1 peptides in HLA-A2 positive patients with NY-ESO-1-expressing cancer. Cancer Immun 2007; 7:16; PMID:17944437
- Chen Q, Jackson H, Shackleton M, Parente P, Hopkins W, Sturrock S, MacGregor D, Maraskovsky E, Tai TY, Dimopoulos N et al. Characterization of antigen-specific CD8+ T lymphocyte responses in skin and peripheral blood following intradermal peptide vaccination. Cancer Immun 2005; 5:5; PMID:15755075
- Metheringham RL, Pudney VA, Gunn B, Towey M, Spendlove I, Durrant LG. Antibodies designed as effective cancer vaccines. mAbs 2009; 1:71-85; PMID:20046577; http://dx.doi.org/10.4161/mabs.1.1.7492
- Pudney VA, Metheringham RL, Gunn B, Spendlove I, Ramage JM, Durrant LG. DNA vaccination with T-cell epitopes encoded within Ab molecules induces high-avidity anti-tumor CD8+ T cells. Eur J Immunol 2010; 40:899-910; PMID:20039301; http://dx.doi.org/10.1002/eji.200939857
- Chiarella P, Massi E, De Robertis M, Sibilio A, Parrella P, Fazio VM, Signori E. Electroporation of skeletal muscle induces danger signal release and antigen-presenting cell recruitment independently of DNA vaccine administration. Expert Opin Biol Ther 2008; 8:1645-57; PMID:18847301; http://dx.doi.org/10.1517/14712598.8.11.1645
- van Drunen Littel-van den Hurk S, Hannaman D. Electroporation for DNA immunization: clinical application. Expert Rev Vaccines 2010; 9:503-17; PMID:20450325; http://dx.doi.org/10.1586/erv.10.42
- Poulam M, Patel CO CM, Lorigan P, Plummer R, Hannaman D, Cunnell M, Metheringham R, Brentville V, Daniel I, Machado L et al. An adjuvant clinical trial of SCIB1, a DNA vaccine that targets dendritic cells in vivo, in fully resected melanoma patients. ASCO Annual Meet 2015
- Jager E, Nagata Y, Gnjatic S, Wada H, Stockert E, Karbach J, Dunbar PR, Lee SY, Jungbluth A, Jager D et al. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc Natl Acad Sci USA 2000; 97:4760-5; PMID:10781081; http://dx.doi.org/10.1073/pnas.97.9.4760
- Bioley G, Dousset C, Yeh A, Dupont B, Bhardwaj N, Mears G, Old LJ, Ayyoub M, Valmori D. Vaccination with recombinant NY-ESO-1 protein elicits immunodominant HLA-DR52b-restricted CD4+ T cell responses with a conserved T cell receptor repertoire. Clin Cancer Res 2009; 15:4467-74; PMID:19531622; http://dx.doi.org/10.1158/1078-0432.CCR-09-0582
- Zarour HM, Storkus WJ, Brusic V, Williams E, Kirkwood JM. NY-ESO-1 encodes DRB1*0401-restricted epitopes recognized by melanoma-reactive CD4+ T cells. Cancer Res 2000; 60:4946-52; PMID:10987311
- Ayyoub M, Pignon P, Dojcinovic D, Raimbaud I, Old LJ, Luescher I, Valmori D. Assessment of vaccine-induced CD4 T cell responses to the 119-143 immunodominant region of the tumor-specific antigen NY-ESO-1 using DRB1*0101 tetramers. Clin Cancer Res 2010; 16:4607-15; PMID:20670945; http://dx.doi.org/10.1158/1078-0432.CCR-10-1485
- Lopes L, Dewannieux M, Gileadi U, Bailey R, Ikeda Y, Whittaker C, Collin MP, Cerundolo V, Tomihari M, Ariizumi K et al. Immunization with a lentivector that targets tumor antigen expression to dendritic cells induces potent CD8+ and CD4+ T-cell responses. J Virol 2008; 82:86-95; PMID:17959670; http://dx.doi.org/10.1128/JVI.01289-07
- MacArthur GJ, Wilson AD, Birchall MA, Morgan AJ. Primary CD4+ T-cell responses provide both helper and cytotoxic functions during Epstein-Barr virus infection and transformation of fetal cord blood B cells. J Virol 2007; 81:4766-75; PMID:17314172; http://dx.doi.org/10.1128/JVI.02608-06
- Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 2007; 109:5346-54; PMID:17327412; http://dx.doi.org/10.1182/blood-2006-10-051318
- Deffrennes V, Vedrenne J, Stolzenberg MC, Piskurich J, Barbieri G, Ting JP, Charron D, Alcaide-Loridan C. Constitutive expression of MHC class II genes in melanoma cell lines results from the transcription of class II transactivator abnormally initiated from its B cell-specific promoter. J Immunol 2001; 167:98-106; PMID:11418637; http://dx.doi.org/10.4049/jimmunol.167.1.98
- Mach B, Steimle V, Martinez-Soria E, Reith W. Regulation of MHC class II genes: lessons from a disease. Ann Rev Immunol 1996; 14:301-31; PMID:8717517; http://dx.doi.org/10.1146/annurev.immunol.14.1.301
- Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science 1994; 265:106-9; PMID:8016643; http://dx.doi.org/10.1126/science.8016643
- Zhu Z, Singh V, Watkins SK, Bronte V, Shoe JL, Feigenbaum L, Hurwitz AA. High-avidity T cells are preferentially tolerized in the tumor microenvironment. Cancer Res 2013; 73:595-604; PMID:23204239; http://dx.doi.org/10.1158/0008-5472.CAN-12-1123
- Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 1999; 59:3128-33; PMID:10397255
- Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med 2001; 194:823-32; PMID:11560997; http://dx.doi.org/10.1084/jem.194.6.823
- Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci 2009; 1174:99-106; PMID:19769742; http://dx.doi.org/10.1111/j.1749-6632.2009.04939.x
- Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 2012; 18:1254-61; PMID:22842478; http://dx.doi.org/10.1038/nm.2883
- Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun 2013; 13:5; PMID:23390376
- Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity 1999; 11:483-93; PMID:10549630; http://dx.doi.org/10.1016/S1074-7613(00)80123-5
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA 2010; 107:4275-80; PMID:20160101; http://dx.doi.org/10.1073/pnas.0915174107
- Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013; 5:200ra116; PMID:23986400; http://dx.doi.org/10.1126/scitranslmed.3006504
- Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer 2015; 112:1501-9; PMID:25867264; http://dx.doi.org/10.1038/bjc.2015.101
- Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006; 66:3381-5; PMID:16585157; http://dx.doi.org/10.1158/0008-5472.CAN-05-4303
- Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010; 116:1757-66; PMID:20143437; http://dx.doi.org/10.1002/cncr.24899
- Dolan DE, Gupta S. PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control 2014; 21:231-7; PMID:24955707
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515:563-7; PMID:25428504; http://dx.doi.org/10.1038/nature14011
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320-30; PMID:25399552; http://dx.doi.org/10.1056/NEJMoa1412082
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009; 206:3015-29; PMID:20008522; http://dx.doi.org/10.1084/jem.20090847
- Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol 2009; 21:1065-77; PMID:19651643; http://dx.doi.org/10.1093/intimm/dxp072
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013; 210:1695-710; PMID:23897981; http://dx.doi.org/10.1084/jem.20130579
- Rohlman D, Punj S, Pennington J, Bradford S, Kerkvliet NI. Suppression of acute graft-versus-host response by TCDD is independent of the CTLA-4-IFN-gamma-IDO pathway. Toxicol Sci 2013; 135:81-90; PMID:23798565; http://dx.doi.org/10.1093/toxsci/kft140
- Jager E, Chen YT, Drijfhout JW, Karbach J, Ringhoffer M, Jager D, Arand M, Wada H, Noguchi Y, Stockert E et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med 1998; 187:265-70; PMID:9432985; http://dx.doi.org/10.1084/jem.187.2.265
- Chen JL, Dunbar PR, Gileadi U, Jager E, Gnjatic S, Nagata Y, Stockert E, Panicali DL, Chen YT, Knuth A et al. Identification of NY-ESO-1 peptide analogues capable of improved stimulation of tumor-reactive CTL. J Immunol 2000; 165:948-55; PMID:10878370; http://dx.doi.org/10.4049/jimmunol.165.2.948
- Valmori D, Dutoit V, Lienard D, Rimoldi D, Pittet MJ, Champagne P, Ellefsen K, Sahin U, Speiser D, Lejeune F et al. Naturally occurring human lymphocyte antigen-A2 restricted CD8+ T-cell response to the cancer testis antigen NY-ESO-1 in melanoma patients. Cancer research 2000; 60:4499-506; PMID:10969798
- Chen Q, Jackson H, Parente P, Luke T, Rizkalla M, Tai TY, Zhu HC, Mifsud NA, Dimopoulos N, Masterman KA et al. Immunodominant CD4+ responses identified in a patient vaccinated with full-length NY-ESO-1 formulated with ISCOMATRIX adjuvant. Proc Natl Acad Sci USA 2004; 101:9363-8; PMID:15197261; http://dx.doi.org/10.1073/pnas.0403271101
- Zhao RY, Mifsud NA, Xiao K, Chan KF, Oveissi S, Jackson HM, Dimopoulos N, Guillaume P, Knights AJ, Lowen T et al. A novel HLA-B18 restricted CD8+ T cell epitope is efficiently cross-presented by dendritic cells from soluble tumor antigen. PloS One 2012; 7:e44707; PMID:22970293; http://dx.doi.org/10.1371/journal.pone.0044707
- Benlalam H, Linard B, Guilloux Y, Moreau-Aubry A, Derre L, Diez E, Dreno B, Jotereau F, Labarriere N. Identification of five new HLA-B*3501-restricted epitopes derived from common melanoma-associated antigens, spontaneously recognized by tumor-infiltrating lymphocytes. J Immunol 2003; 171:6283-9; PMID:14634146; http://dx.doi.org/10.4049/jimmunol.171.11.6283
- Jager E, Karbach J, Gnjatic S, Jager D, Maeurer M, Atmaca A, Arand M, Skipper J, Stockert E, Chen YT et al. Identification of a naturally processed NY-ESO-1 peptide recognized by CD8+ T cells in the context of HLA-B51. Cancer Immun 2002; 2:12; PMID:12747757
- Gnjatic S, Nagata Y, Jager E, Stockert E, Shankara S, Roberts BL, Mazzara GP, Lee SY, Dunbar PR, Dupont B et al. Strategy for monitoring T cell responses to NY-ESO-1 in patients with any HLA class I allele. Proc Natl Acad Sci USA 2000; 97:10917-22; PMID:11005863; http://dx.doi.org/10.1073/pnas.97.20.10917
- Mizote Y, Taniguchi T, Tanaka K, Isobe M, Wada H, Saika T, Kita S, Koide Y, Uenaka A, Nakayama E. Three novel NY-ESO-1 epitopes bound to DRB1*0803, DQB1*0401 and DRB1*0901 recognized by CD4 T cells from CHP-NY-ESO-1-vaccinated patients. Vaccine 2010; 28(32):5338-5346; PMID:20665979; http://dx.doi.org/10.1016/j.vaccine.2010.05.044
- Jager E, Jager D, Karbach J, Chen YT, Ritter G, Nagata Y, Gnjatic S, Stockert E, Arand M, Old LJ et al. Identification of NY-ESO-1 epitopes presented by human histocompatibility antigen (HLA)-DRB4*0101-0103 and recognized by CD4(+) T lymphocytes of patients with NY-ESO-1-expressing melanoma. J Exp Med 2000; 191:625-30; PMID:10684854; http://dx.doi.org/10.1084/jem.191.4.625
- Knights AJ, Nuber N, Thomson CW, de la Rosa O, Jager E, Tiercy JM, van den Broek M, Pascolo S, Knuth A, Zippelius A. Modified tumour antigen-encoding mRNA facilitates the analysis of naturally occurring and vaccine-induced CD4 and CD8 T cells in cancer patients. Cancer Immunol Immunother 2009; 58:325-38; PMID:18663444; http://dx.doi.org/10.1007/s00262-008-0556-8
- Matsuzaki J, Qian F, Luescher I, Lele S, Ritter G, Shrikant PA, Gnjatic S, Old LJ, Odunsi K. Recognition of naturally processed and ovarian cancer reactive CD8+ T cell epitopes within a promiscuous HLA class II T-helper region of NY-ESO-1. Cancer Immunol Immunother 2008; 57:1185-95; PMID:18253733; http://dx.doi.org/10.1007/s00262-008-0450-4
- Nagai H, Horikawa T, Hara I, Fukunaga A, Oniki S, Oka M, Nishigori C and Ichihashi M. In vivo elimination of CD25+ regulatory T cells leads to tumor rejection of B16F10 melanoma, when combined with interleukin-12 gene transfer. Experimental dermatology. 2004; 13(10):613-620; PMID:15447721
