ABSTRACT
In human breast cancer cells, the presence of cytoplasmic dots positive for microtubule-associated proteins 1A/1B light chain 3B (LC3B) indicates enhanced autophagic flux and favorable prognosis. LC3B+ puncta within malignant cells positively correlate with the intratumoral abundance of CD8+ cytotoxic T lymphocytes, yet negatively correlate with the frequency of local FOXP3+ regulatory T cells and CD68+ tumor-associated macrophages, resulting in an improvement of CD8+/FOXP+ or CD8+/CD68+ ratios.
We recently characterized the impact of autophagy on patient survival in breast cancer. Autophagic flux was measured indirectly, by counting the frequency of malignant cells containing cytoplasmic dots (puncta) that stained positively for the autophagosomes/autophagolysome marker microtubule-associated proteins 1A/1B light chain 3B (LC3B). The frequency of cells with LC3B+ puncta negatively correlated with the staining intensity of the autophagic substrate Sequestosome 1 (SQSTM1, p62), suggesting that LC3B+ puncta indeed reflect autophagic flux.Citation1 In two independent cohorts of breast cancer patients comprising a total of 1,700 individuals, the absence of autophagic flux in malignant cells correlated with poor survival after adjuvant chemotherapy.Citation1
Autophagy is considered a predominantly oncosuppressive mechanism, since this natural cellular process of cytoplasmic self-renewal preserves normal cellular metabolism and homeostatic functions with positive effects on genomic stability and barriers against oncogenesis (such as senescence).Citation2 Beyond these cell-autonomous effects, autophagy in malignant cells plays a major role in assuring anticancer immunosurveillance.Citation3 Thus, suppression of autophagy by knockout of the essential autophagy-related gene Atg5 can accelerate KRAS-induced lung cancer oncogenesis through local inhibition of the immune response.Citation4 Moreover, knockdown of Atg5 and Atg7 in tumor cells abolished their capacity to induce anticancer immune responses when they were killed in vitro by anthracyclines and oxaliplatin and then injected into mice.Citation3 Similarly, tumors depleted of Atg5 or Atg7 (by transfection with specific shRNAs) or BRAF-induced melanomas rendered deficient for Atg7 (by conditional knockout) became resistant against chemotherapy due to their incapacity to stimulate a tumor growth-inhibitory anticancer immune response.Citation3,5
Why do autophagy-resistant tumors escape from tumor immunosurveillance ? One of the most important chemotactic factors that is released from cancers upon chemotherapy-induced stress in vivo is adenosine triphosphate (ATP). Extracellular ATP acts on purinergic receptors, in particular P2Y2 receptors, to attract myeloid cells into the tumor bed.Citation6,7 Autophagy-deficient cells exhibit a reduced ATP release, presumably due to a defect in lysosomal exocytosis.Citation8 In addition, autophagy-deficient cancer cells may activate a transcriptional program that includes the overexpression of CD39, an ectoenzyme that catalyzes the first step of the degradation of immunostimulatory ATP into immunosuppressive adenosine.Citation4 Adenosine acts on adenosinergic receptors to favor an increase in the frequency of immunosuppressive regulatory T cells (Tregs) with a CD25+ FOXP3+ phenotype. In the model of ATG5-deficient KRAS-induced lung carcinogenesis, inhibition of CD39, inhibition of adenosinergic receptors or depletion of Tregs restores local immunosurveillance and avoids accelerated oncogenesis.Citation4 These findings provide mechanistic insights on the link between deficient autophagy and reduced anticancer immunosurveillance.
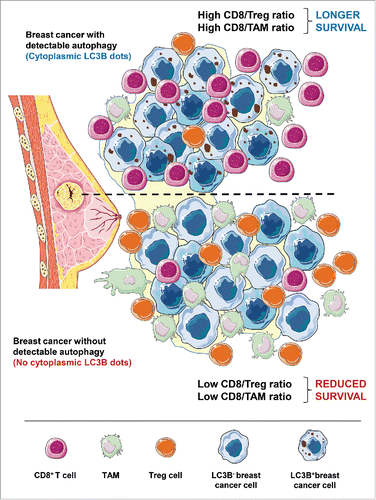
Breast cancer is under strong immunosurveillance, as indicated by the fact that the density, composition and function of tumor-infiltrating immune cells (which altogether determines the so-called immune contexture) dictate the prognosis of patients, be they treated by adjuvant or neo-adjuvant chemotherapy.Citation9 Driven by the aforementioned results obtained in mouse models, we decided to evaluate the possible impact of autophagy in breast cancer cells on the tumor microenvironment. For this, we quantified the intratumoral and peritumoral density of CD8+ cytotoxic T lymphocytes (CTL), FOXP3+ Tregs and yet another immunosuppressive cell type, namely CD68+ tumor-associated macrophages (TAMs). As expected,Citation9 we found that the ratio of CTL over Tregs (the CD8+/FOXP3+ ratio) or that of CTL over TAMs (the CD8+/CD68+ ratio) had a major impact on progression-free and overall patient survival, meaning that a favorable ratio predicted a positive outcome. In the next step, these parameters were correlated with the frequency of malignant cells with clearly discernible LC3B+ puncta. Indeed, we observed a strongly positive correlation between LC3B+ puncta and the density of the intratumoral CTL infiltrate, contrasting with a significant negative correlation between LC3B+ puncta and both immunosuppressive leukocytes subpopulations (Tregs and TAMs). Logically, this corresponds to a marked positive correlation between LCD3B+ puncta and the CD8+/FOXP+ and CD8+/CD68+ ratios ().Citation10
Altogether, these results support that the aforementioned results obtained in mouse models can be extrapolated to human malignancies (or at least to human breast cancer). It should be noted that high expression of CD39 and that of another ectoenzyme, CD73, which operates downstream of CD39 to generate adenosine, also constitutes a negative prognostic marker in breast cancer,Citation9 suggesting that the entire molecular cascade (deficient autophagy reduced ATP release and increase conversion of ATP into adenosine adenosinergic receptor-dependent recruitment of Tregs failing immunosurveillance) delineated in mice might apply to mammary carcinoma as well.
It will be interesting to see whether the artificial stimulation of autophagy, the inhibition of CD39 of CD73, the blockade of adenosinergic receptors or the depletion of Tregs will constitute novel avenues for resuscitating failing immunosurveillance in cancer patients. One possibility that should deserve further scrutiny consists in administering pharmacological agents aiming at the local induction of such effects in the tumor, thereby avoiding systemic toxicity. Such a strategy would profit from the mobility of many immune cell types, meaning that the local induction of an antineoplastic response would probably be sufficient to induce distant (abscopal) effects against distant metastases.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Ladoire S, Penault-Llorca F, Senovilla L, Dalban C, Enot D, Locher C, Prada N, Poirier-Colame V, Chaba K, Arnould L et al. Combined evaluation of LC3B puncta and HMGB1 expression predicts residual risk of relapse after adjuvant chemotherapy in breast cancer. Autophagy 2015; 11:1878-90; PMID:26506894; http://dx.doi.org/10.1080/15548627.2015.1082022
- Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J, Gewirtz DA, Karantza V et al. Autophagy in malignant transformation and cancer progression. EMBO J 2015; 34:856-80; PMID:25712477; http://dx.doi.org/10.15252/embj.201490784
- Ma Y, Galluzzi L, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity 2013; 39:211-27; PMID:23973220; http://dx.doi.org/10.1016/j.immuni.2013.07.017
- Rao S, Tortola L, Perlot T, Wirnsberger G, Novatchkova M, Nitsch R, Sykacek P, Frank L, Schramek D, Komnenovic V et al. A dual role for autophagy in a murine model of lung cancer. Nat Commun 2014; 5:3056; PMID:24445999; http://dx.doi.org/10.1038/ncomms4056
- Michaud M, Xie X, Bravo-San Pedro JM, Zitvogel L, White E, Kroemer G. An autophagy-dependent anticancer immune response determines the efficacy of melanoma chemotherapy. Oncoimmunology 2014; 3:e944047; PMID:25610726; http://dx.doi.org/10.4161/21624011.2014.944047
- Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 2013; 38:729-41; PMID:23562161; http://dx.doi.org/10.1016/j.immuni.2013.03.003
- Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013; 39:74-88; PMID:23890065; http://dx.doi.org/10.1016/j.immuni.2013.06.014
- Martins I, Wang Y, Michaud M, Ma Y, Sukkurwala AQ, Shen S, Kepp O, Metivier D, Galluzzi L, Perfettini JL, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ 2014; 21:79-91; PMID:23852373; http://dx.doi.org/10.1038/cdd.2013.75
- Kroemer G, Senovilla L, Galluzzi L, Andre F, Zitvogel L. Natural and therapy-induced immunosurveillance in breast cancer. Nat Med 2015; 21:1128-38; PMID:26444637; http://dx.doi.org/10.1038/nm.3944
- Ladoire S, Enot D, Senovilla L, Ghiringhelli F, Poirier-Colame V, Chaba K, Semeraro M, Chaix M, Penault-Llorca F, Arnould L et al. The presence of LC3B puncta and HMGB1 expression in malignant cells correlate with the immune infiltrate in breast cancer. Autophagy 2016; 12(5):864-75; PMID:26979828; http://dx.doi.org/10.1080/15548627.2016.1154244