ABSTRACT
Purpose: CD169 was first identified on macrophages (Mφ) and linked to antigen presentation. Here, we showed CD169 expression on some CD8+ T lymphocytes in regional lymph nodes (LNs) and investigated the function and clinical relevance of CD169+CD8+ T cells in tumor-draining LNs of colorectal cancer (CRC) patients. Experimental design: Fresh tumor-draining LN tissues from 39 randomly enrolled patients were assessed by flow cytometry for activation and differentiation of CD169+CD8+ T cells and T cell-mediated killing of tumor cells. In total, 114 tumor-draining LN paraffin sections from CRC patients were analyzed by multiple-color immunofluorescence for CD169+CD8+ T cell distribution and clinical values. The prognostic significance of CD169+CD8+ T cells was evaluated by Kaplan–Meier analysis. Results: A fraction of CD8+ T cells in regional LNs, but not peripheral blood, tonsils, or tumors, expressed surface CD169. In situ detection of draining LNs revealed preferential localization of CD169+CD8+ T cells to subcapsular sinus and interfollicular regions, closely associated with CD169+ Mφ. CD169+CD8+ T cell ratios were significantly lower in peri-tumor LNs than distant-tumor LNs. CD169+CD8+ T cells predominantly expressed activation markers (CD69, HLA-DR, PD-1) with slightly lower CD45RA and CD62L levels. They produced high granzyme B, perforin, TNF-α, and IFNγ levels, and promoted tumor-killing efficiency ex vitro. Moreover, CD169+CD8+ T cells infiltrating tumor-draining LNs decreased with disease progression and were strongly associated with CRC patient survival. Conclusions: We identified novel activated/cytolytic CD169+CD8+ T cells selectively present in regional LNs, potentially serving as a powerful prognostic factor and indicator for selecting patients for immunotherapy.
Abbreviations
| APCs | = | antigen-presenting cells |
| CRC | = | colorectal cancer |
| DAPI | = | 40-6-diamidino-2-phenylin-dole |
| DFS | = | disease-free survival |
| distant-LN | = | distant-tumor LN |
| FBS | = | fetal bovine serum |
| HLA-DR | = | human leukocyte antigen DR |
| IFNγ | = | interferon γ |
| LNs | = | lymph nodes |
| Mφ | = | macrophages |
| OS | = | overall survival |
| PBMCs | = | peripheral blood mononuclear cells |
| PBS | = | phosphate-buffered saline |
| PD-1 | = | programmed cell death protein 1 |
| peri-LN | = | peri-tumor LN |
| PI | = | propidium iodide |
| TNF-α | = | tumor necrosis factor α |
Introduction
Colorectal cancer (CRC) is one of the most common malignancies with a high incidence of death for both men and women.Citation1 Lymph node (LN) metastasis is a key step in disease progression and serves as an important parameter in CRC staging.Citation2-6 Adjuvant chemotherapy is recommended for patients with LN metastases in order to prevent tumor recurrence. Thus, adequate LN evaluation is critical for prognosis and treatment of patients with CRC.Citation2,3,Citation6-9
Regional LNs preferentially accommodate naive lymphocytes and allow these cells to efficiently encounter antigen-presenting cells (APCs) that bring in antigens from surrounding tissues.Citation10,11 Efficient tumor priming in the draining LN can produce antitumor immunity; however, a modulated LN microenvironment can also provide hospitable soil for the seeding and proliferation of tumor cells that lead to metastases.Citation12,13 CD8+ T cells play an important effector role in orchestrating host antitumor immunity through their capacity to recognize tumor antigens and confer superior immunological protection against tumors.Citation14,15 Accordingly, a favorable prognostic role of tumor-infiltrating CD8+ T cells has been well established in CRC patients.Citation16,17 Despite the presence of CD8+ T cells in the primary tumor, a lower percentage of effector CD8+ T cells has also been found in the metastatic or pre-metastatic LN of CRC patients, suggesting that cell-mediated immunity also depends on the localization and functional role of CD8+ T cells in the tumor-draining LN.Citation18,19 Thus, defining the potential CD8+ T cell subsets in LNs is essential to understanding their roles and mechanisms in tumor immunopathogenesis.
CD169, also known as sialoadhesin or sialic acid binding immunoglobulin-like lectin, is the founding member of the Siglec superfamily. It was first identified as a sheep erythrocyte binding receptor in bone marrow macrophages and found to be involved in cell–cell adhesion.Citation20 In mouse LNs, anti-CD169 antibodies label subcapsular sinus and medullary macrophages (Mφ), and these CD169+ Mφ are poised to rapidly encounter pathogens, antigens, and exosomes that reach the LN.Citation21-25 A recent study demonstrated that CD169+ Mφ could effectively generate CTL responses by cross-priming CD8+ T cells, which in turn, conferred immunity against re-exposure to the malignant cells.Citation26 The expression of CD169 in tumor-draining LN and its association with survival in CRC patients has been recently reportedCitation27; however, the specific composition and function of CD169+ cells in tumor-draining LNs of cancer patients remain largely unknown. In the current study, we addressed this issue in CRC and identified a novel T cell subset, CD169+CD8+ T cells. We further investigated their function and clinical relevance and demonstrated that these cells have a highly activated and cytolytic phenotype and are positively associated with patient survival.
Results
CD169+CD8+ T cells accumulate in regional LN and decrease with disease progression in CRC patients
To evaluate the potential role of CD169 in tumor immunopathology, we initially applied immunofluorescence staining to examine the in situ expression of this molecule in the tumor-draining LN from CRC patients. CD169+ cells were enriched in the LN sinus, which is the hotspot for CD68+ Mφ ( and S1). Consistent with previous reports, CD169 protein was mainly expressed on CD68+ Mφ in the LN sinus.Citation27,35 Interestingly, multi-staining confocal microscopy analysis revealed a certain proportion of CD169+ cells were CD3+CD8+ T cells ( and S2). Notably, these CD169+CD8+ T cells usually were in close contact with CD169+CD68+ Mφ ().
Figure 1. CD169+CD8+ T cells are selectively present in the regional LNs and decreased with progressive stages in CRC patients. (A) Immunofluorescence microscopy of tumor-draining LN sections from CRC patients stained with anti-CD169, anti-CD8+ and anti-CD68 monoclonal antibodies. Enlargements show examples of CD169+CD8+ cells (arrowhead) closely associated with CD169+CD68+ cells. (B–E) FACS analysis of CD169+CD8+ T cells in fresh lymphocytes isolated from control donors with hemangioma (n = 2) or congenital megacolon (n = 2) and CRC patients (n = 25). Lymphocytes were gated as CD45+CD3+CD14− cells. Representative dot plots of at least three individuals from more than three independent experiments (B), and the statistical analysis (C–E) of these samples are shown. The continuous and dashed horizontal bars in C and D represent median values. Results are expressed as means ± SEM.
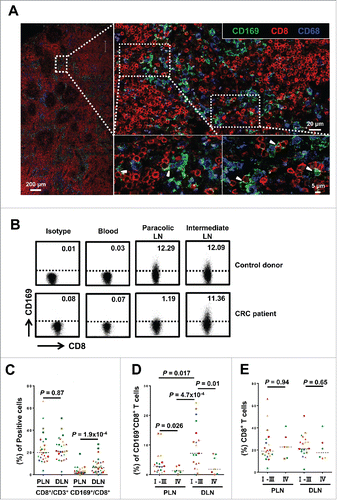
To confirm the existence of CD169+CD8+ T cells, we used flow cytometry to examine these cells on circulating leukocytes and tissue-infiltrating leukocytes isolated from LN tissues of CRC patients and control donors. A noteworthy population of CD45+CD14−CD3+ CD8+CD169+ T cells was detected in the LN tissues, but not in the peripheral blood or tonsils ( and Fig. S3A). In addition, the CD169+CD8+ T cells were also present in LN suspensions from patients with other types of diseases (Fig. S3B). These data suggested that the CD169+CD8+ T cells were selectively present in the regional LN tissue.
The regional LN can be divided into different groups according to their location and distance from the primary lesions.Citation36 In CRC, the number and distribution of LN metastases can significantly impact the survival of patients.Citation2,37 Therefore, we further examined paired paracolic and intermediate LN tissues from patients with various stages of CRC. Compared with intermediate LN, the proportion of CD169+CD8+ T cell in the paracolic LN was significantly decreased in CRC patients but not in control donors (n = 25, p < 0.0001; ). Moreover, we found that ratios of CD169+CD8+ T cells in both peri-LN and distant-LN were lower in advanced stage CRC patients (stages IV; n = 7) than those in early stages (stages I, II, and III; n = 18; p = 0.026 for peri-LN and p = 0.01 for distant-LN; ). In addition, no difference was observed in the proportion of CD8+ T cells between peri-LN and distant-LN (p = 0.87; ) or between patients in advanced stage and those in early stages (p = 0.94 for peri-LN and p = 0.65 for distant-LN; ). Collectively, the results indicated that we have identified the CD169+CD8+ T cell subset that was selectively present in the regional LNs and decreased with progressive stages in CRC patients.
Phenotypic characteristics of CD169+ CD8+ T cells
To determine the phenotypic and activation status of CD169+CD8+ T cells, we first analyzed the expression profiles of CD45RA and CD62L in LN tissues from 25 CRC patient via multi-parameter flow cytometry ( and S4). Most CD169+CD8+ T cells expressed low levels of CD45RA and the LN-homing receptor CD62L as compared with CD169−CD8+ T cells, indicating that they were mostly effector memory cells (CD45RA−CD62L−). Moreover, when gated on the memory CD8+ T cells (CD45RA−), we found that memory CD169+CD8+ T cells showed higher expression of CD127 (interleukin-7 receptor-α, which is associated with long-term survival in T lymphocytes) than CD169− counterparts (Fig. S6).
Figure 2. Phenotypic features of CD169+CD8+ T cells in the tumor-draining LN from CRC patients. The phenotypic characteristics of CD169+CD8+ T cells from fresh CRC tissues of peri-LN (PLN, A) or of distant-LN (DLN, B) were determined by flow cytometry. Data for perforin, granzyme B, TNF-α, and IFNγ were acquired in cells stimulated with Leukocyte Activation Cocktail (BD PharMingen, San Diego, CA) at 37°C for 5 h. Results are presented as means ± SEM of three separate experiments. Significant differences are indicated (*p < 0.05; **p < 0.01; ***p < 0.001).
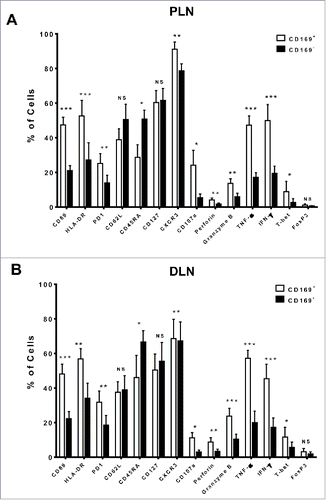
We also found that the CD169+CD8+ T cells had higher expression of the activation markers CD69, PD-1, and HLA-DR, and the expression pattern of CD169 was positively correlated with the activation markers CD69 and HLA-DR (Fig. S5). In addition, CD169+CD8+ T cells secreted significantly high levels of cytotoxic effector molecules (granzyme B, perforin) and had strong abilities to produce the cytokines TNF-α and IFNγ. Similar phenotypic features were also found in CD169+CD8+ T cells isolated from tumor-draining LNs of gastric carcinoma and cervical carcinoma patients (Figs. S6 and S7). Taken together, the results showed that CD169+CD8+ T cells exhibited a highly activated phenotype with an increased cytotoxic potential phenotype in the tumor-draining LNs of cancer patients.
Quantification of T cell mediated-tumor cell killing
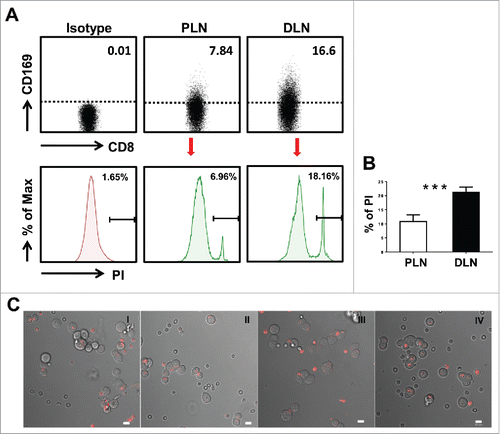
To verify the cytotoxic function of CD169+CD8+ T cells, we next measured the tumor cell killing efficiency using T cells isolated from tumor-draining LNs of CRC patients. The effector T cells and target tumor cells were incubated and harvested for analysis after 5 h. The relative percentage of PI-positive cells in CFSE labeled-target HCT116 tumor cells was used to compare the killing efficiency. As expected, T cells isolated from the distant-LN group with a higher proportion of CD169+CD8+ T cells showed a higher killing rate of tumor cells compared with the peri-LN group, which contained a lower proportion of CD169+CD8+ T cells (). To further visualize T-tumor cell conjugate formation, T cells from the distant-LN were labeled with CellTracker™ before being mixed with an equal proportion of T cells isolated from the peri-LN and then co-cultured with HCT116 tumor cells. The T cells confronted with target tumor cells, which could represent an increase in T-target cell conjugates, were mostly from the distant-LN tissues which contained a high frequency of CD169+CD8+ T cells (). These results indicated that the presence of CD169+CD8+ T cells in the tumor-draining LN could increase the T cell mediated-tumor killing efficacy in CRC patients.
Figure 3. Visualization of target cell killing by co-culture of T cells from tumor-draining LN with tumor cells. (A) FACS analysis of CD169+CD8+ T cells on fresh lymphocytes isolated from peri-LN or distant-LN tissues of CRC patients (upper). Lymphocytes were gated as CD45+CD3+CD14− cells. T cells (effector cell, E) from tumor-draining LN were assessed for non-specific cytotoxicity against the HCT116 colorectal cancer cell line (target cells, T) in vitro by a CFSE-based assay (E:T = 5:1). PI staining was used to discriminate viable and intact cells from dead subpopulations. Cells were co-cultured and harvested for analysis after 5 h of incubation. (B) The histograms depict the viable PI positive target dead cell subpopulations. Results are representative of at least three different CRC patients. Paired t-test (*p < 0.05; **p < 0.01; ***p < 0.001). (C) T cells isolated from distant-LN stained with Cell Tracker™ Red were mixed with T cells from peri-LN and then co-cultured with target cells (HCT116) in vitro. T cell-mediated killing of target cells was monitored by time-lapse epifluorescence and bright field microscopy. The combination of bright field and fluorescence microscopy allowed the detection of interactions between T cells and target cells.
Prognostic significance of CD169+CD8+ T cells in CRC patients
To address whether the CD169+CD8+ T cells present in the tumor-draining LN are associated with CRC progression, we analyzed the relevant clinical information and correlated the data with the location and density of these cells in the LN tissues of 114 patients. CD8+ and CD169 labeled by double-color immunofluorescence staining were scanned and quantified with an image analysis workstation (). Using the median value, densities of CD169+ cells, CD8+ T cells, and CD169+CD8+ T cells in the subcapsular sinus or interfollicular region allowed the stratification of patients into groups. We first determined if any significant associations existed between clinical characteristics and CD169+CD8+ T cell density. The results showed that both in the peri-LN and distant-LN regions, these cells positively correlated with tumor stage, nodal and distant metastases, and Dukes' stage (p < 0.0001; ).
Figure 4. Prognostic significance of CD169+CD8+ T cells in CRC patients. (A) (Left) A representative example of CD169 and CD8+ immunofluorescence staining of a tumor-draining LN section from CRC patients. CD8+ T cells (red), CD169 cells (green), and nuclei (blue) are shown. (Right) Digital image analyzed with the Nuance image software. (B–D) Cumulative OS and DFS times were calculated by the Kaplan–Meier method and analyzed by the log-rank test. The patients were divided into two groups according to the median value of CD169+CD8+ T cells in peri-LN (B), distant-LN (C), and combined regions (D).
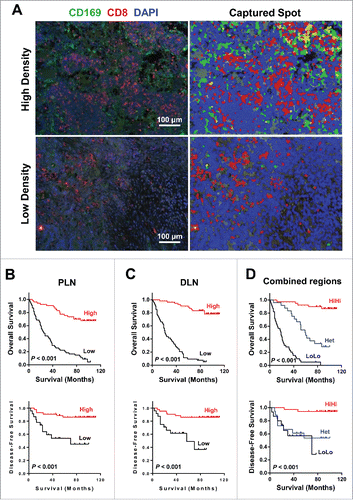
Table 1. Characteristics of patients.
Table 2. Association of CD169+CD8+ T cells density with clinicopathologic characteristics.
Kaplan–Meier survival curves were then plotted to investigate the association with survival. Positive correlations were detected between CD169+CD8+ T cell density and OS (p < 0.001) and DFS (p < 0.001) in both the peri-LN and distant-LN regions (). We further investigated whether the combined analysis of LN regions could improve the prediction of patient survival. For each cell type (CD169+CD8+, CD169+, and CD8+), the combined analysis of peri-LN plus distant-LN regions (high density in both regions (HiHi) versus low density in both regions (LoLo)) increased the accuracy of prediction of DFS and OS time ( and S8). Patients with a lower density of CD169+CD8+ cell in both the peri-LN and distant-LN had significantly shorter OS and DFS than patients with a higher density (median OS, 19 versus 53 mo; median DFS, 6 versus 23 mo; ).
The multivariate Cox proportional hazards analysis was performed, and variables that were associated with survival by univariate analysis were adopted as covariates. In multivariate analysis, the CD169+CD8+PLN/CD169+CD8+DLN patterns emerged as an independent prognostic factor of both OS (HR, 0.262; 95% CI, 0.162–0.424; p < 0.0001) and DFS (HR, 0.443; 95% CI, 0.265–0.742; p = 0.002; ). These results suggested that the location and density of CD169+CD8+ T cells was significantly associated with CRC progression and could serve as a powerful predictor of patient survival.
Table 3. Univariate and multivariate analyses of factors associated with survival and recurrence.
Discussion
LNs are secondary lymphoid organs that are important for the initiation of adaptive immune responses, including the induction of antitumor immunity.Citation7,38,Citation39 In the present study, we identified the novel CD169+CD8+ T cells, which were selectively concentrated in the LN tissues from both cancer patients and control donors, but rarely found in the primary tumors, peripheral blood, or control tonsils. CD169+CD8+ T cells are effector memory cells that have a highly activated and cytolytic phenotype, and their levels were decreased in the paracolic LN close to the primary tumor. Moreover, high levels of CD169+CD8+ T cells in LNs were significantly associated with good prognosis in CRC patients.
Adaptive immune responses against infection, grafted organs, or tumors are usually generated in the draining LN. CD8+ T cells are important effector T lymphocytes for antitumor immunity by exerting direct and indirect cytotoxicity against tumor cells.Citation40-43 Our results demonstrated an unrecognized subpopulation of CD8+ T cells in the regional LNs. These CD169+CD8+ T cells were found to have a highly activated phenotype which expressed higher levels of CD69, PD-1, and HLA-DR. Most CD169+CD8+ T cells are effector memory cells (CD45RA−CD62L−) with a strong ability to secrete cytotoxic effector cytokines (granzyme B, perforin) and immune regulators (TNF-α, IFNγ). We also found that T cells from tumor-draining LNs of CRC patients containing a greater proportion of CD169+CD8+ T cells demonstrated strong killing rates of tumor cells in the co-culture system. Moreover, the density of CD169+CD8+ T cells in the draining LN predicted better survival of CRC patients. Taken together, the results suggest that the presence of CD169+CD8+ T cells in the tumor-draining LN represents an activated antitumor response in the primary tumor site that can suppress tumor progression.
Tumor invasion into the regional LN is a key step in disease progression.Citation2,37,Citation44 However, tumors may manipulate the LN microenvironment to provide hospitable soil for the seeding and proliferation of tumor cells.Citation12 In the current study, we observed that levels of CD169+CD8+ T cells were significantly lower in peri-LN than distant-LN, and the ratios of CD169+CD8+ T cells in both peri-LN and distant-LN were higher in early stage CRC patients than those in advanced stages. Considering that the peri-LN is anatomically the nearest group to the tumor nest, the downregulation of CD169+CD8+ T cell ratios suggests the necessity for the tumor environment to impair the antitumor functions. Moreover, we found that the decreased density of CD169+CD8+ T cell was associated with LN and distant metastasis, and reduced survival of CRC patients. These data suggest that CD169+CD8+ T cells in the regional LN may provide an immune defense against tumor invasion.
CD169 is the founding member of the Siglec superfamily of proteins that are often involved in cell–cell adhesion. Studies in murine systems have shown that CD169+ Mφ contribute to antigen retention and cross-presentation of antigens to CD8+ T cells in the LN, thereby functioning as powerful APCs that significantly contribute to CTL responses.Citation21,23,Citation26 The mechanism of generating CD169+CD8+ T cells is presently unknown. T lymphocytes located adjacent to CD169+ Mφ have been shown to be stained for CD169 due to blebs from CD169+ Mφ undergoing fragmentation.Citation45 We found that CD169+CD8+ T cells in the tumor-draining LN were activated with the ability to secrete cytotoxic effector cytokines, suggesting that these cell are involved in antitumor responses. Therefore, we speculate that CD8+ T cells may have acquired CD169+ Mφ-derived membrane blebs during antigen cross-priming. Additionally, tumors have been suggested to significantly modulate the microenvironment of LNs.Citation37,38,Citation40 Thus, a better understanding of the mechanism of tumor-modulating CD169+CD8+ T cells may be used to develop new cancer immunotherapies.
Current clinical practice focuses on histological examination of LNs for the presence of a tumor, ignoring their immune microenvironments.Citation46,47 Our results emphasized that the immune profile changes in tumor-draining LNs may represent a sensitive indicator of tumor progression. The decrease of CD169+CD8+ T cells in tumor-draining LN indicates that the tumor may selectively modulate the context of the antitumor response to invasion and metastasis. Thus, the presence of CD169+CD8+ T cells in the tumor-draining LN can be a potential predictive factor to facilitate selection of patients who may benefit from immune therapies when the clinical response is critically reliant on the antitumor immunity of T cells.
Materials and methods
Patients and specimens
Tumor-draining LNs or peripheral blood samples were obtained from 139 patients with pathologically confirmed CRC at the Cancer Center of Sun Yat-Sen University. We grouped epicolic/epirectal and paracolic/pararectal LNs as peri-tumor LN (peri-LN), and intermediate and principal/central LNs as distant-tumor LN (distant-LN), for CRC patients according to their localization.Citation28,29 Paired peri-LN and distant-LN tissues, as well as blood samples from 25 patients (Group 1) who underwent surgical resection between 2012 and 2014 were used for isolation of peripheral blood mononuclear cells (PBMCs) and leukocytes. Fresh regional LN tissues were obtained from patients with hepatocellular carcinoma (n = 2), bile duct carcinoma (n = 2), gastric cancer (n = 3), cervical cancer (n = 3), hemangioma (n = 2), and congenital megacolon (n = 2). None of the patients received anticancer therapy before sampling.
Archived, formalin-fixed, paraffin-embedded tissues obtained from 114 patients (Group 2) who underwent curative resection between November 2003 and December 2006 with complete follow-up data were included in the survival analysis. These LN samples from CRC patients were cut into 5-µm sections and processed for immunofluorescence staining as described previously.Citation30 Clinical stages were classified according to the International Union Against Cancer (UICC) TNM classification (7th Edition, 2010). None of the patients had neoadjuvant therapies before surgery. Individuals with concurrence of autoimmune disease, HIV, and syphilis were excluded. Overall survival (OS) was defined as the interval between the dates of surgery and death or the last follow-up. Disease-free survival (DFS) was defined as the interval between the dates of surgery and recurrence or the last follow-up. The clinical characteristics of the patients are summarized in .
All samples were anonymously coded in accordance with local ethical guidelines (as stipulated by the Declaration of Helsinki), and written informed consent was obtained. The protocol was approved by the Review Board of Sun Yat-sen University.
Isolation of leukocytes
Peripheral leukocytes were isolated by Ficoll density gradient centrifugation.Citation31 Fresh infiltrating leukocytes were obtained as described elsewhere.Citation32 Fresh tissue-infiltrating leukocytes from LNs were obtained based on a modification of a previously described procedure.Citation33 In short, LN tissues were chopped into small pieces and digested in RPMI 1640 supplemented with 0.05% collagenase IV (Sigma-Aldrich), 0.002% DNase I (Roche), and 10% fetal bovine serum (FBS) (HyClone Laboratories) at 37°C for 20 min. Dissociated cells were filtered through a 400-µm mesh and separated by Ficoll centrifugation. The leukocytes were washed and resuspended in phosphate-buffered saline (PBS) supplemented with 2 mM EDTA and 1% FBS for cell sorting or FACS analysis.
Flow cytometry
Leukocytes were stained with fluorochrome-conjugated monoclonal antibodies for CD45RA, CD62L, CD14, CD3, CD8+, CD69, CD107a, programmed cell death protein 1 (PD-1), human leukocyte antigen DR (HLA-DR), CD43, CCR6, CXCR3, CD169 (BD or eBioscience), or control antibody. For intracellular cytokine staining, T cells were stimulated for 4 h with Leukocyte Activation Cocktail (BD Biosciences, San Diego, CA), followed by staining with CD3, CD4+, and CD8+, fixation, permeabilization with IntraPre Reagent (Beckman Coulter) and finally stained with antibodies to perforin, granzymes B, IL-10, IL-17, TNF-a, and IFNγ. Data were acquired on a Gallios flow cytometer (Beckman Coulter) and analyzed with Kaluza software.
Tumor cell lines
A human colorectal carcinoma cell line (HCT116) was obtained from American Type Culture Collection (Manassas, VA) and tested for mycoplasma contamination using a single-step PCR method. Cells were maintained in DMEM supplemented with 10% FBS and incubated at 37°C with 5% CO2.
CFSE-based cytotoxicity assay
After washing with PBS, the target cell (HCT116) suspensions were resuspended at 2 × 107 cells/mL and labeled with 5 µM CFSE for 15 min at 37°C. The reaction was stopped by the addition of an equal volume of FBS, followed by a 2-min incubation at room temperature. After washing, the CFSE-labeled target cells were resuspended in RPMI 1640 supplemented with 10% FBS and directly used for co-culture in the presence or absence of T lymphocytes. The cell concentration was adjusted to 1 × 106 cells/mL for plating in 24-well plates. T lymphocytes were obtained from peri-LN and distant-LN of CRC patients with or without staining with CellTracker™ Red CMTPX (Molecular Probes) added at different effector-to-target ratios and further co-cultured at 37°C for 5 h. The cells were harvested for quantitative analysis of the subpopulations by flow cytometry. To stain for dead cells, propidium iodide (PI, 1 µg/mL) was added, and samples were mixed properly and directly analyzed. Images were captured by using a scanning confocal microscope (LSM780 META, ZEISS, Jena, Germany).
Confocal microscopic analysis
Frozen LN tissue sections were stained with sheep anti-human CD169 (R&D Systems, Minneapolis, MN, USA), mouse anti-human CD3 or mouse anti-human CD68 (Thermo Fisher Scientific, Waltham, MA, USA) and rabbit anti-human CD8+ (Thermo Fisher Scientific), followed by specimen-paired immunofluorescence secondary antibodies (Life Technologies, Carlsbad, CA, USA). Nuclei were stained with 40-6-diamidino-2-phenylin-dole (DAPI) (Vector Laboratories). Images were viewed and assessed using a scanning confocal microscope (LSM780 META, ZEISS) and analyzed with LSM780 META software.
Immunofluorescence staining
For multiple-color immunofluorescence staining, formalin-fixed, paraffin-embedded sections were deparaffinized and rehydrated. Antigen retrieval was performed by microwave treatment in EDTA buffer pH 9.5, and blockage of non-specific antibody binding was carried out with 5% BSA. Sections were then incubated with anti-human CD169 and CD8+ overnight at 4°C, followed by specimen-paired immunofluorescence secondary antibodies. Negative controls were generated by replacing primary antibodies with isotype-matched antibodies. Slides were mounted with VECTASHIELD mounting media containing DAPI (Vector Laboratories) and analyzed on a fluorescent imaging microscope.
Automated image acquisition and quantification
To quantify the multiple-color immunostaining, the Vectra-Inform image analysis (Perkin-Elmer Applied Biosystems, Foster City, CA) was performed as described in previous studies.Citation34 Briefly, the slides were loaded onto the Vectra slide scanner following the protocol. Representative areas for each LN were collected on 2–5 fields from each slide at a constant magnification of 200×. The stained slides were captured with the Nuance VIS-FL Multispectral Imaging System (Perkin-Elmer Applied Biosystems). The spectrum for each chromogen was determined on single-stained control slides. A spectral unmixing algorithm separated the grayscale images representing each spectral component quantitatively. The images were analyzed with a standard fluorescence-field scanning protocol by InForm software. The proportions of cells in different populations were calculated (e.g., the proportion of CD169+ cells which were CD8+ lymphocytes was calculated as: (number of CD169+CD8+ cells)/(number of CD169+CD8+ cells + CD169−CD8+ cells).
Statistical analysis
All data were summarized as means ± SEM and analyzed with SPSS software (IBM). Comparisons between groups were performed by Student's t-test. Correlations between variables were determined by linear regression analysis. Survival was estimated by the Kaplan–Meier method and compared by the log-rank test. Multivariate analysis of prognostic factors for DFS and OS was performed using the Cox proportional hazards model. The χ2 test was used to test for relationships between categorical variables. Values of p < 0.05 (two-tailed) were considered significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1177690_s02.pptx
Download MS Power Point (10.9 MB)Funding
This work was supported by a grant from National Natural Science Foundation of China (No. 81101861; 81502459), National High Technology Research and Development Program of China (863 Program, No.2012AA02A506), Health Medical Collaborative Innovation Program of Guangzhou (No. 201400000001-3 and 201400000001-4), Guangdong Science and Technology Plan Projects (No. 2013B021800146) and Specialized Research Fund for the Doctoral Program of Higher Education (No. 20110171120100).
Reference
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87-108; PMID:25651787; http://dx.doi.org/10.3322/caac.21262
- Ceelen W, Van Nieuwenhove Y, Pattyn P. Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol 2010; 17:2847-55; PMID:20559741; http://dx.doi.org/10.1245/s10434-010-1158-1
- Chou JF, Row D, Gonen M, Liu YH, Schrag D, Weiser MR. Clinical and pathologic factors that predict lymph node yield from surgical specimens in colorectal cancer: a population-based study. Cancer 2010; 116:2560-70; PMID:20499400; http://dx.doi.org/10.1002/cncr.25032
- Lee KY. Lymph node ratio for nodal staging in colorectal cancer - a promising, but premature tool. J Korean Soc Coloproctol 2011; 27:224-5; PMID:22102970; http://dx.doi.org/10.3393/jksc.2011.27.5.224
- Qiu HB, Chen G, Keshari RP, Luo HY, Fang W, Qiu MZ, Zhou ZW, Xu RH. The extramural metastasis might be categorized in lymph node staging for colorectal cancer. BMC Cancer 2011; 11:414; PMID:21943144; http://dx.doi.org/10.1186/1471-2407-11-414
- Resch A, Langner C. Lymph node staging in colorectal cancer: old controversies and recent advances. World J Gastroenterol 2013; 19:8515-26; PMID:24379568; http://dx.doi.org/10.3748/wjg.v19.i46.8515
- Buettner M, Bode U. Lymph node dissection–understanding the immunological function of lymph nodes. Clin Exp Immunol 2012; 169:205-12; PMID:22861359; http://dx.doi.org/10.1111/j.1365-2249.2012.04602.x
- Contassot E, Preynat-Seauve O, French L, Huard B. Lymph node tumor metastases: more susceptible than primary tumors to CD8(+) T-cell immune destruction. Trends Immunol 2009; 30:569-73; PMID:19837632; http://dx.doi.org/10.1016/j.it.2009.08.001
- Nagtegaal ID, Quirke P, Schmoll HJ. Has the new TNM classification for colorectal cancer improved care? Nat Rev Clin Oncol 2012; 9:119-23; PMID:22009076; http://dx.doi.org/10.1038/nrclinonc.2011.157
- van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol 2010;10:664-74; PMID:20706277; http://dx.doi.org/10.1038/nri2832
- Houston SA, Cerovic V, Thomson C, Brewer J, Mowat AM, Milling S. The lymph nodes draining the small intestine and colon are anatomically separate and immunologically distinct. Mucosal Immunol 2015; 9:468-78; PMID:26329428; http:/dx.doi.org/10.1038/mi.2015.77
- Qian CN, Berghuis B, Tsarfaty G, Bruch M, Kort EJ, Ditlev J, Tsarfaty I, Hudson E, Jackson DG, Petillo D et al. The. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res 2006; 66:10365-76; PMID:17062557; http://dx.doi.org/10.1158/0008-5472.CAN-06-2977
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565-70; PMID:21436444; http://dx.doi.org/10.1126/science.1203486
- Zhang W, Zhang C, Li W, Deng J, Herrmann A, Priceman SJ, Liang W, Shen S, Pal SK, Hoon DS et al. CD8+ T-cell immunosurveillance constrains lymphoid premetastatic myeloid cell accumulation. Eur J Immunol 2015; 45:71-81; PMID:25310972; http://dx.doi.org/10.1002/eji.201444467
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353:2654-66; PMID:16371631; http://dx.doi.org/10.1056/NEJMoa051424
- Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 2013; 39:11-26; PMID:23890060; http://dx.doi.org/10.1016/j.immuni.2013.07.008
- Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 2009; 27:5944-51; PMID:19858404; http://dx.doi.org/10.1200/JCO.2008.19.6147
- Nakamura K, Ninomiya I, Oyama K, Inokuchi M, Kinami S, Fushida S, Fujimura T, Kayahara M, Ohta T. Evaluation of immune response according to the metastatic status in the regional lymph nodes in patients with gastric carcinoma. Oncol Rep 2010; 24:1433-41; PMID:21042737; http://dx.doi.org/10.3892/or_00001003
- Wu X, Zhang H, Xing Q, Cui J, Li J, Li Y, Tan Y, Wang S. PD-1(+) CD8(+) T cells are exhausted in tumours and functional in draining lymph nodes of colorectal cancer patients. Br J Cancer 2014; 111:1391-9; PMID:25093496; http://dx.doi.org/10.1038/bjc.2014.416
- Klaas M, Crocker PR. Sialoadhesin in recognition of self and non-self. Semin Immunopathol 2012; 34:353-64; PMID:22450957; http://dx.doi.org/10.1007/s00281-012-0310-3
- Martinez-Pomares L, Gordon S. CD169+ macrophages at the crossroads of antigen presentation. Trends Immunol 2012; 33:66-70; PMID:22192781; http://dx.doi.org/10.1016/j.it.2011.11.001
- Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood 2014; 123:208-16; PMID:24255917; http://dx.doi.org/10.1182/blood-2013-03-489732
- Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, Kanagawa O, Fujii S, Tanaka M. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity 2011; 34:85-95; PMID:21194983; http://dx.doi.org/10.1016/j.immuni.2010.12.011
- Barral P, Polzella P, Bruckbauer A, van Rooijen N, Besra GS, Cerundolo V, Batista FD. CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nat Immunol 2010; 11:303-12; PMID:20228797; http://dx.doi.org/10.1038/ni.1853
- Kastenmuller W, Torabi-Parizi P, Subramanian N, Lammermann T, Germain RN. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell 2012; 150:1235-48; PMID:22980983; http://dx.doi.org/10.1016/j.cell.2012.07.021
- Bernhard CA, Ried C, Kochanek S, Brocker T. CD169+ macrophages are sufficient for priming of CTLs with specificities left out by cross-priming dendritic cells. Proc Natl Acad Sci USA 2015; 112:5461-6; PMID:25922518; http://dx.doi.org/10.1073/pnas.1423356112
- Ohnishi K, Komohara Y, Saito Y, Miyamoto Y, Watanabe M, Baba H, Takeya M. CD169-positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer Sci 2013; 104:1237-44; PMID:23734742; http://dx.doi.org/10.1111/cas.12212
- General rules for clinical and pathological studies on cancer of the colon, rectum and anus. Part I. Clinical classification. Japanese research society for cancer of the colon and rectum. Jpn J Surg 1983; 13:557-73; PMID:6672390; http://dx.doi.org/10.1007/BF02469505
- Grinnell RS. Lymphatic metastases of carcinoma of the colon and rectum. Ann Surg 1950; 131:494-506; PMID:17859481; http://dx.doi.org/10.1097/00000658-195004000-00004
- Kuang DM, Zhao Q, Xu J, Yun JP, Wu C, Zheng L. Tumor-educated tolerogenic dendritic cells induce CD3epsilon down-regulation and apoptosis of T cells through oxygen-dependent pathways. J Immunol 2008; 181:3089-98; PMID:18713979; http://dx.doi.org/10.4049/jimmunol.181.5.3089
- Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 2009; 206:1327-37; PMID:19451266; http://dx.doi.org/10.1084/jem.20082173
- Zhao Q, Kuang DM, Wu Y, Xiao X, Li XF, Li TJ, Zheng L. Activated CD69+ T cells foster immune privilege by regulating IDO expression in tumor-associated macrophages. J Immunol 2012; 188:1117-24; PMID:22184722; http://dx.doi.org/10.4049/jimmunol.1100164
- Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 2013; 38:187-97; PMID:23260195; http://dx.doi.org/10.1016/j.immuni.2012.09.020
- Li L, Yan J, Xu J, Liu CQ, Zhen ZJ, Chen HW, Ji Y, Wu ZP, Hu JY, Zheng L et al. CXCL17 expression predicts poor prognosis and correlates with adverse immune infiltration in hepatocellular carcinoma. PLoS One 2014; 9: e110064; PMID:25303284; http://dx.doi.org/10.1371/journal.pone.0110064
- Saito Y, Ohnishi K, Miyashita A, Nakahara S, Fujiwara Y, Horlad H, Motoshima T, Fukushima S, Jinnin M, Ihn H et al. Prognostic significance of CD169+ lymph node sinus macrophages in patients with malignant melanoma. Cancer Immunol Res 2015; 3:1356-63; PMID:26297710; http://dx.doi.org/10.1158/2326-6066.CIR-14-0180
- Fujii TY, Tabe R, Yajima S, Yamaguchi S, Tsutsumi T, Asao & H. Kuwano. Process of distant lymph node metastasis in colorectal carcinoma: implication of extracapsular invasion of lymph node metastasis. BMC Cancer 2011; 11: 216; PMID:21635742; http://dx.doi.org/10.1186/1471-2407-11-216
- Kawada K, Taketo MM. Significance and mechanism of lymph node metastasis in cancer progression. Cancer Res 2011; 71:1214-8; PMID:21212413; http://dx.doi.org/10.1158/0008-5472.CAN-10-3277
- Pereira ER, Jones D, Jung K, Padera TP. The lymph node microenvironment and its role in the progression of metastatic cancer. Semin Cell Dev Biol 2015; 38:98-105; PMID:25620792; http://dx.doi.org/10.1016/j.semcdb.2015.01.008
- Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer 2012; 12:210-9; PMID:22362216; http://dx.doi.org/10.1038/nrc3186
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14:1014-22; PMID:24048123; http://dx.doi.org/10.1038/ni.2703
- Chandrasekaran S, King MR. Microenvironment of tumor-draining lymph nodes: opportunities for liposome-based targeted therapy. Int J Mol Sci 2014; 15:20209-39; PMID:25380524; http://dx.doi.org/10.3390/ijms151120209
- Fransen MF, Arens R, Melief CJ. Local targets for immune therapy to cancer: tumor draining lymph nodes and tumor microenvironment. Int J Cancer 2013; 132:1971-6; PMID:22858832; http://dx.doi.org/10.1002/ijc.27755
- Sewald X, Ladinsky MS, Uchil PD, Beloor J, Pi R, Herrmann C, Motamedi N, Murooka TT, Brehm MA, Greiner DL et al. Retroviruses use CD169-mediated trans-infection of permissive lymphocytes to establish infection. Science 2015; 350:563-7; PMID:26429886; http://dx.doi.org/10.1126/science.aab2749
- Wong KP, Poon JT, Fan JK, Law WL. Prognostic value of lymph node ratio in stage III colorectal cancer. Colorectal Dis 2011; 13:1116-22; PMID:20874800; http://dx.doi.org/10.1111/j.1463-1318.2010.02435.x
- Gray EE, Friend S, Suzuki K, Phan TG, Cyster JG. Subcapsular sinus macrophage fragmentation and CD169+ bleb acquisition by closely associated IL-17-committed innate-like lymphocytes. PLoS One 2012; 7: e38258; PMID:22675532; http://dx.doi.org/10.1371/journal.pone.0038258
- Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol 2010; 28:264-71; PMID:19949014; http://dx.doi.org/10.1200/JCO.2009.24.0952
- Compton C. C. Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol 2003; 16:376-88; PMID:12692203; http://dx.doi.org/10.1097/01.MP.0000062859.46942.93
