ABSTRACT
It is well known that the aberrant expression of programmed death ligand 1 (PD-L1) on tumor cells impairs antitumor immunity. To date, in hepatocellular carcinoma (HCC), the relationship between PD-L1 expression and host-tumor immunity is not well defined. Here, the expression levels of PD-L1 and CD8+ T cell infiltration were analyzed by immunohistochemistry (IHC) in formalin fixed paraffin embedded (FFPE) specimens from 167 HCC patients undergoing resection. A significant positive association was found between PD-L1 expression and the presence of CD8+ T cell (p < 0.0001). Moreover, constitutive PD-L1 protein expression was not detected by western blot in HepG2, Hep3B, and 7402 HCC cancer cell lines; but co-cultured these cell lines with INFγ, a cytokine produced by activated CD8+ T cells, remarkably upregulated PD-L1 expression. In fresh frozen HCC specimens, INFγ was found to be significantly correlated with PD-L1 and CD8+ gene expression, as evaluated by quantitative reverse transcriptase polymerase chain reaction (RT-PCR). These findings indicate that increased PD-L1 level may represent an adaptive immune resistance mechanism exerted by tumor cells in response to endogenous antitumor activity. Both increased intratumoral PD-L1 and CD8+ were significantly associated with superior DFS (CD8+: p = 0.03; PD-L1: p = 0.023) and OS (CD8+: p = 0.001 and PD-L1: p = 0.059), but PD-L1 expression was not independently prognostic. In conclusions, PD-L1 upregulation is mainly induced by activated CD8+ cytotoxic T cells pre-existing in HCC milieu rather than be constitutively expressed by the tumor cells, and it is a favorable prognostic factor for HCC.
Abbreviations
| AJCC | = | American Joint Committee On Cancer |
| DFS | = | disease-free survival |
| DMEM | = | Dulbecco's Modified Eagle Medium |
| FFPE | = | formalin fixed paraffin embedded |
| HCC | = | hepatocellular carcinoma |
| IHC | = | immunohistochemistry |
| INFγ | = | interferon-γ |
| MDSC | = | myeloid-derived suppressor cell |
| OS | = | overall survival |
| PD-L1 | = | programmed death ligand 1 |
| qPCR | = | quantitative polymerase chain reaction |
| TAM | = | tumor-associated macrophages |
| TGF-β | = | transforming growth factor-betal |
| TIL | = | tumor-infiltrating lymphocyte |
| Treg | = | regulatory T cells |
| UICC | = | Union for International Cancer Control. |
Introduction
The development of hepatocellular carcinoma (HCC) is a process with accumulation of a large number of genomic alterations. These changes not only contribute to the malignant transformation but also create neoantigens, which could elicit defensive immune responses, and thereby exert some control on tumor growth.Citation1 Indeed, the presence of antigen specific CD8+ T cells in HCC was demonstrated to be associated with better prognosis after curative resection.Citation2 Numerous attempts to exploit adoptive immunotherapy in HCC (e.g., vaccines, cytokines, adoptive cell transfer) have been made in the past, but the success obtained is limited.Citation3 It is increasingly recognized that cancer immunoresistance and immune escape may play important roles in tumor progression and pose obstacles for immunotherapy. In tumors, some immune cells, such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs); a spectrum of inhibitory cytokines (for example, IL-10 and TGF-β); and immune inhibitory receptors and ligands, collectively constitute a suppressive milieu, which contributes to immune evasion.Citation4
The PD-1/PD-L1 immune checkpoint pathway has been identified as a key immune escape mechanism utilized by tumors. The binding of PD-L1 to its receptors can induce T-cell anergy or exhaustion and mediate the inhibition of local immune responses, thus shielding the tumor from T-cell-mediated killing.Citation5,6 In support of this notion, PD-1/PD-L1 blockades have shown remarkable clinical efficacy in several cancers, such as melanoma, non-small cell lung carcinoma (NSCLC), renal cell carcinoma, ovarian cancer, and bladder cancer.Citation7-9 The impressive clinical results achieved by blocking this inhibitory pathway, are revolutionizing cancer immunotherapy.
To predict the response to and optimize PD-1/PD-L1 blockade therapy, it is essential to understand the mechanisms controlling PD-L1 expression. Two general mechanisms have been purposed for the regulation of PD-L1 by tumor cells.Citation10 The first is innate immune resistance, which implies that constitutive expression of PD-L1 can be driven by some intrinsic cellular changes associated with carcinogenesis, leading to reduction of immune cell infiltration and represents a statue of total immune evasion.Citation11,12 The second mechanism is adaptive immune resistance, which means that tumors upregulate PD-L1 as an adaptive response to endogenous antitumor immunity. It may reflect the presence of immunosurveillance existing in cancers but not total immune evasion. More and more studies have revealed that PD-L1 is characteristically associated with intratumoral immune infiltrates; Inflammatory cytokines, especially INFγ secreted by tissue-recruiting immune cells, can induce PD-L1 upregulation in various cell types, including tumor cells.Citation13-17 Both mechanisms may co-exist to regulate PD-L1 expression with distinctly relative contributions in consideration of intratumoral genetic heterogeneity; PD-L1 upregulation can reflect either lack or presence of antitumor immunity in specific tumor microenvironment. Therefore, theoretically, higher PD-L1 expression can be correlated with either worse or improved prognosis across different tumor types.Citation13,18,19
Currently, in HCC, there are very limited data reporting the prevalence of PD-L1 expression. Based on previous two studies, the elevated PD-L1 expression has long been widely considered as a poor prognostic factor for HCC.Citation3,20-24 It seems that innate immune resistance mechanism plays a more important role in HCC immune escape mediated by PD-L1. However, there was no direct evidence demonstrating the association between PD-L1 and impaired local antitumor immunity; unvalidated antibodies were used to assess PD-L1 expression.Citation25,26 Thus, their results may not completely reflect the actual expression of PD-L1.
The precise mechanism controlling PD-L1 expression is crucial for the development of effective approaches to PD-1 blockade therapy for patients with HCC. The aim of this study was to definitively characterize PD-L1 expression in HCC and uncover the relationship between PD-L1 expression and host-tumor immunity.
Results
Clinical profiles of the patients
Patient and tumor characteristics are described in . 176 patients had tumor samples and adequate clinical data for evaluation for PD-L1 expression and CD8+ TIL in tumor cells (). All the patients undergone intended of radical excision and only 90 cases received completely curative resection based on intraoperative found and postoperative pathology; Post-recurrent treatments including reoperation (n = 11), chemoembolization (n = 56), and regional therapy (n = 23), were administered as appropriate. At last follow-up, 64/90 patients had relapsed after curative surgery, including 44 intrahepatic recurrences, 8 extrahepatic metastases, and 12 cases with both events.
Table 1. Patients Clinicopathological features.
PD-L1 expression pattern in HCC tissue samples
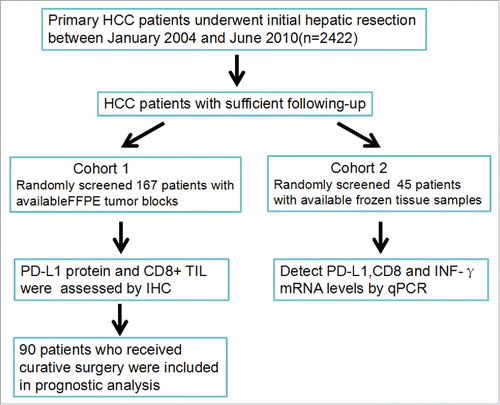
Among all HCC specimens, staining for PD-L1 was membranous and present in most cases within the tumor compartment based on morphology. As shown in , three major expression patterns were observed by IHC: absence of PD-L1 (), focal expression of PD-L1 on cancer cells (), and diffuse expression (). PD-L1 expression on tumor cells was negative (defined as <5% PD-L1+ cells) for 143 patients (86%) and positive for 24 patients (14%). For most HCC cases, PD-L1-positive cells were evenly scattered throughout the specimens, similar to their expression in melanoma and Merkel cell carcinoma.Citation13,15
Figure 2. PD-L1 expression pattern in FFPE samples stained with anti-PD-L1 antibody. (A) Placenta was used as a positive control for PD-L1 expression. (B) Isotype control. (C) Samples displaying pattern 1 exhibited negative expression in HCC cells. Original magnification, ×200. (D) Samples exhibiting pattern 2 displayed focal staining, original magnification, ×200. (E) Original magnification of the boxed area shown in (D), ×400 (F) Samples displaying pattern 3 exhibited diffuse PD-L1 expression (> 40% tumor PD-L1). Original magnification, ×200.
Correlations between PD-L1 and clinicopathological features
To gain further insights into the association of PD-L1 with tumor biology, clinicopathological features were compared with PD-L1 expression. As shown in , no significant association was identified between PD-L1 and various clinicopathological features, including age, gender, clinicopathological TNM stage, differentiated degree, and HBV status.
Table 2. Association of PD-L1 expression and clinicopathological features of HCC patients.
Correlation between PD-L1 expression and tumor-infiltrating CD8+ T lymphocytes
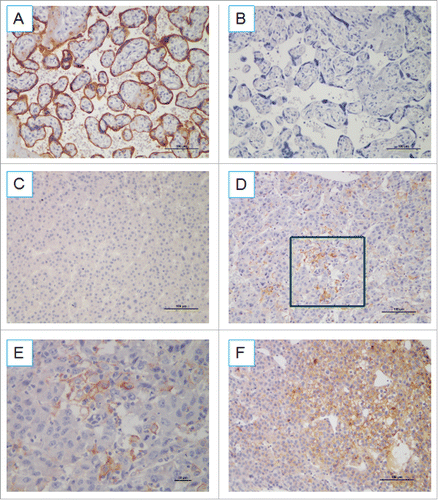
The expression of PD-L1 on tumor cells suppresses the proliferation and effector function of CD8+ T cells and leads to immune evasion in HCC. To determine whether PD-L1 expression indeed reflects host-tumor immunity, we evaluated CD8+ TILs. Interestingly, PD-L1 expression was found to be proportional to the CD8+ infiltrate () and that the number of CD8+ T lymphocytes was significantly higher in samples with positive rather than negative PD-L1 expression (p < 0.0001, ). Of note, in the 24 tumor PD-L1 positive specimens, four cases showed PD-L1 staining not proportional to the CD8+ infiltrate (), and one case exhibited diffuse PD-L1 expression with little CD8+ infiltration (Fig. S1).
Figure 3. Immunohistochemical staining of human HCC tissues using anti-PD-L1 and CD8+ Abs. (A) FFPE tissue sections were analyzed by IHC for PD-L1 expression on tumor cells and CD8+ cell infiltration. PD-L1 positivity was defined as ≥ 5%, and the number of CD8+ cells was assessed in five distinct microscopic field (×200). (B) Tumors were classified as PD-L1+ and PD-L1− and analyzed for the amount of CD8+.
INFγ and PD-L1 expression in HCC
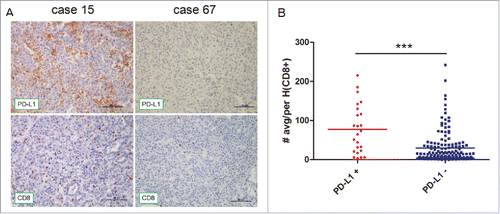
To elucidate the underling mechanism behind the association between elevated expression of PD-L1 and abundant CD8+ infiltration in HCC, the expression of PD-L1, INFγ (a primary inducer driving PD-L1 expression), and CD8 genes was quantitatively evaluated in surgically excised tumor specimen. The quantitative reverse transcription–polymerase chain reaction (qRT-PCR) revealed a significant positive correlation among the expression of CD8, PD-L1, and INFγ (). This is also supported by the result as regards to CD8+, INFγ, and PD-L1 expression in para-carcinoma compared their tumor counterparts in 17 HCC patients (Fig. S2). However, the other subsets of TILs including CD4+, NK (CD56) as well as macrophage (CD68) were not correlated with INFγ or PD-L1 (Table S1). In addition, constitutive PD-L1 protein expression was not detectable by Western blot in HCC cancer cell lines, including HepG2, Hep3B, and 7402. Stimulation of the various cancer cell lines with 20 ng/mL INFγ for 24 h resulted in the induction of high levels of PD-L1 protein expression ().
Figure 4. Correlation among the relative expression of PD-L1, CD8+, and INFγ, in tumorous tissue as determined by quantitative polymerase chain reaction and induction of PD-L1 by INFγ stimulation in vitro. Correlation studies were performed for (A) PD-L1 and CD8+ (B) PD-L1 and INFγ (C) INFγ and CD8+ in 45 HCC patients' tumor tissues, GAPDH was used as an internal control. r : Spearman's correlation coefficient. (D) Western blot detection of PD-L1 expression in HCC cell lines in the presence or absence of 20 ng/mL INFγ for 24 h. Total protein (50 µg) was loaded in each lane.
Association of PD-L1 expression and CD8+ TIL with prognosis in HCC patients
PD-L1 expression in the tumor microenvironment appears to reflect antitumor immunity and adaptive immune resistance by the tumor. We next examined whether tumor growth might be partially restrained in PD-L1+ HCC. Considering partial patients only undergone palliative surgery, we exclude this population in the subsequent prognostic analysis to accurately evaluate the role of immune system to prevent tumor progression after surgery. In total, 90 patients received curative resection, including 15 cases with PD-L1 positive. The expression level of PD-L1 protein was strongly associated with superior clinical outcome of the patients. Favorable DFS intervals were highlighted by Kaplan–Meier curves (p = 0.023; ) for the patients with positive PD-L1 expression compared to those with negative expression. Moreover, Kaplan–Meier analysis revealed the trend of the patients expressing PD-L1 for superior OS outcome (p = 0.059; ). The Kaplan–Meier curve and log-rank test also highlighted that the level of CD8+ T lymphocyte infiltration had significantly better DFS (p = 0.03; ) and OS (p = 0.001; ) compared to those with low CD8+ T lymphocyte infiltration, which is consistent with previous study by others, confirming the role of the cytotoxic T cell in the eradication of HCC.Citation1,27 Multivariate analysis including gender, age, TNM stage, tumor grade, vascular invasion, tumor size, tumor number, and HBV status, were performed for the assessment of the independent prognostic value of CD8+ and PD-L1 (). CD8+ T lymphocyte infiltration in primary tumor was an independent predictor with improved DFS(HR, 0.559; CI, 0.321–0.971; p = 0.039) and OS(HR, 0.329; CI, 0.148–0.731; p = 0.006), but not PD-L1 expression (HR, 0.655; CI, 0.299–1.436; p = 0.291; for DFS; HR, 0.527; CI, 0.153–1.818; p = 0.311; for OS).
Figure 5. PD-L1 and CD8+ density are correlated with superior disease-free survival (DFS) and overall survival (OS) of HCC patients. (A, B) Kaplan–Meier curves for the analysis of HCC patients. (A) DFS and (B) OS according to PD-L1 protein levels. p values were calculated by log-rank test. (C, D) Kaplan–Meier curves for the analysis of (C) DFS and (D) OS in HCC patients according to CD8+ density. p values were calculated by log-rank test.
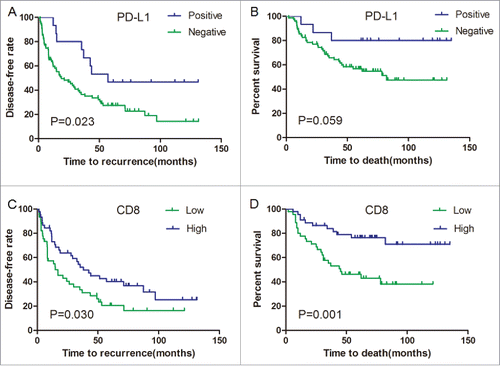
Table 3. Univariate and multivariate analyses for DFS.
Table 4. Univariate and multivariate analyses for OS.
Discussion
Many solid tumors were found to express the B7-protein PD-L1, which interacts with the PD-1 receptor and causes T-cell anergy.Citation10 Conventional view deems PD-L1 represent an immune-inhibitory ligand driven up by constitutive oncogene, and PD-L1 overexpression is believed to reflect an impaired local immune.Citation11 In HCC, two reports have been published to assess the clinical significance of PD-L1 expression, consistently indicating that high PD-L1 expression is a poor prognostic factor.Citation20,21 However, neither study provided the direct evidence supporting the association of high PD-L1 expression with impaired antitumor immunity. Interestingly, our study showed that tumors expressing high level of PD-L1 were more likely to be infiltrated with abundant CD8+ T-cells; cox regression model showed a positive association between PD-L1 expression and favorable prognosis in HCC. This seemingly paradoxical observation, in which expression of an immunosuppressive molecule correlates with enhanced antitumor immunity, support the new view that PD-L1 expression is an adaptive mechanism to escape endogenous antitumor immunity and reflect the presence of immune surveillance.Citation13,14,28
The probable contributing factors to the inconformity between our data and previous studies in HCC may involve in the antibody and assay variability as well as interpretative subjectivity. First, evaluating the expression of PD-L1 in the previous study on tissue microarray might underestimate or overestimate the PD-L1 expression levels due to intratumoral heterogeneity. In our study, the whole-tissue sections were used to assess PD-L1 staining. Second, PD-L1 is a type I transmembrane molecule and inhibits lymphocyte function when it is engaged by its receptors. It is believed that cell surface expression of PD-L1 is the most immediately biologically relevant; however, the previous two studies did not distinguish cytoplasmic and membranous patterns of PD-L1 staining.Citation29 More importantly, a newly developed anti-PD-L1 antibody (clone E1L3N, Cell Signaling Technology) validated by a recent ASCO poster (http://meetinglibrary.asco.org/content/132010-144), was used in this study, instead of previous ones.Citation25,26 The specificity and reproducibility of this newly developed anti-PD-L1 antibodies were also confirmed in current and other studies.Citation30-32 Our results are in agreement with those reports in non-small cell carcinomas, Merkel cell carcinoma and metastatic melanoma using the widely acknowledged monoclonal antibody clone 5H1.Citation13,15,26 Recently, Schalper et al. quantifying in situ PD-L1 mRNA by means of a novel antibody-independent and tissue compartment-specific assay, also demonstrate that higher expression of PD-L1 was significantly associated with increased inflammatory response and better outcome in breast cancer.Citation17
INFγ, typically produced by activated T cells, is believed to be a primary cytokine driving PD-L1 expression. A previous study in a murine melanoma model has demonstrated that the induction of immunosuppressive elements such as PD-L1, IDO1, and Tregs in the tumor microenvironment depends on the presence of CD8+ T cells and INFγ.Citation14 To elucidate the possible mechanism that underlies the association between PD-L1 expression and CD8+, the expression of INFγ, CD8+, and PD-L1 genes was quantitatively analyzed in human HCC specimens; INFγ is significantly correlated with PD-L1 and CD8+ gene expression. Moreover, experiments performed ex vivo failed to detect PD-L1 protein in HCC cell lines, whereas co-culture of tumor cells with recombinant INFγ led to remarkable expression of PD-L1. These findings collectively indicated that cytotoxic lymphocyte cells recruited into the tumor microenvironment can release factors driving PD-L1 expression as a negative feedback mechanism, leading to a form of adaptive immune resistance exerted by HCC. Given the role of memory and effector CD8+ cells in preventing tumor invasion and metastasis, the survival advantage of HCC patients with PD-L1 positive should be related to the concomitant increase in CD8+ T cell infiltration.Citation1 Likely due to the association between PD-L1 expression and CD8+ T cell content, PD-L1 was not completely independently correlated with DFS and OS in multivariate analysis.
In virus-associated malignancy, PD-L1 expression likely reflects both a host reaction to the tumor and the chronic inflammatory environment triggered by the presence of virus. It has been demonstrated that PD-L1 was found to be upregulated in EBV+ nasopharyngeal carcinoma, MCPyV virus+ Merkel cell carcinoma and HPV+ head and neck squamous cell carcinoma.Citation15,33,34 However, the PD-L1 expression did not differ between HBV+ and HBV− HCC from our results. We postulate that the balance between immune activation and tolerance may also depend on organs, cancer or virus types. Anyway, blockade of the PD-1/PD-L1 pathway has been shown to restore virus-specific T-cell function and facilitate virus clearance. It is a promising therapeutic regimen not only for virus-associated malignancies, but also for clearing the underlying infection.
Notably, in the 24 tumor PD-L1 positive specimens, four cases showed PD-L1 staining not proportional to infiltrating immune cells, which indicated that intrinsic cellular changes triggered by carcinogenesis might also be involved in PD-L1 expression. Some studies have reported that PD-L1 can be expressed constitutively on cancer cells through poorly characterized oncogenic signaling pathways such as PTEN silencing and Akt activation.Citation11,12 In chronically inflamed livers, genetic and epigenetic changes underlie oncogenic transformation are common, some intrinsic cancerous changes may drive PD-L1 constitutively expression. Meanwhile, genomic alterations can also generate neoantigens capable of eliciting defensive immune responses. Thus, both intrinsic and extrinsic mechanisms may operate independently or concordantly to jointly determine the PD-L1 level in the tumor microenvironment.
These findings help us to explain why immunogenic HCC cells fail to be controlled by the immune system, even when boosted by antigen-based cancer vaccines. Cancer cells can take advantage of the upregulation of PD-L1, which is intrinsic to the immune system, to down-modulate T-cell activation and ultimately shut down immune responses. PD-1/PD-L1 inhibitor therapy should be interpreted as protection or potentiation of ongoing immunity rather than the generation of de novo immunity against tumor cell. PD-1 inhibitor nivolumab has been showed promising outcomes for the treatment of advanced HCC.Citation35 However, only a proportion of patients respond to the treatment, we speculated that clinical response to PD-1/PD-L1 blockade may end up being restricted to cases in which PD-L1 expression is associated with abundant CD8+ T cell infiltrate in HCC.
There are some limitations in this work. One major weakness is that inevitable selection bias may exist in this retrospective analysis. Another is that our method is unable to precisely distinguish PD-L1 expression on macrophages and monocytes from PD-L1 cancer cell expression within the tumor compartment. Theoretically, PD-L1 expression by any cell type could exert local immunosuppressive effects, and therapeutic targeting of PD-L1+ macrophages can tip the balance in favor of antitumor immunity.Citation36-39 Methods such as flow cytometry and newer in situ multiplex methods currently under development might facilitate the determination of PD-L1 expression in different cell types in the tumor microenvironment.
In conclusion, the presented data support the view that PD-L1 expression is mainly stimulated by activated CD8+ cytotoxic T cells pre-existing in the HCC milieu rather than be constitutively expressed by the tumor cells, and PD-L1 is a favorable prognostic factor for HCC following resection.
Materials and methods
Cell lines
Three HCC cell lines, HepG2, 7402, and Hep3B (American Type Culture Collection), were cultured at 37°C, 5% CO2 in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin in the presence or absence of INFγ (20 ng/mL).
Patients and specimens
The study protocol was approved by the Ethics Committee of Sun Yat-sen University Cancer Center, and written informed consent was obtained from all patients. Between 2004 and 2010, a total of 167 patients who underwent initial resection of HCC were randomly selected and analyzed. The surgically resected specimens were fixed in formalin and embedded in paraffin for routine histological diagnosis. Patients did not receive any preoperative treatment. Clinical and follow-up details were analyzed for all patients. Clinical stage was evaluated according to the 7th edition AJCC/UICC tumor-node-metastasis (TNM) classification system.Citation40 Tumor differentiation was classified by the Edmondson grading system. Subsequent prognostic analysis was performed for those patients receiving curative hepatic resection (complete removal of all the tumors). Disease-free survival (DFS) and overall survival (OS) were calculated from the date of surgery to event (either recurrence or death) or until the last follow-up (censored).
Immunohistochemistry (IHC)
FFPE specimens were cut into 5-μm sections and mounted on glass slides. For each specimen, CD8+ (1:100, ZSGB-BIO, Beijing, China) and PD-L1 (1:100, #13684, Cell Signaling Technology) staining were performed according to standard protocols. Briefly, after the specimens were dewaxed, hydrated, and washed, endogenous peroxidase was blocked (0.3% H2O2 for 10 min), and microwave antigen retrieval was performed in Tris-EDTA (pH 9.0). The slides were then incubated with blocking serum at room temperature for 30 min and with the primary antibody at 4°C overnight. The sections were serially rinsed, incubated with secondary antibodies, and visualized with DAB following counterstaining with hematoxylin.
Evaluation of immunostaining parameters
Two independent pathologists blinded to the clinical data examined immunohistochemical slides. The proportion of PD-L1-positive cells was evaluated as the percentage of total tumor cells, and PD-L1 tumor positivity was defined by membrane staining of ≥ 5% of tumor cells, in accordance with previous studies.Citation13,15 CD8+ T lymphocytes were counted in a microscopic field at 200×; the five most representative areas were selected, and the average count was calculated. The median values were used as the cut-off in subsequent analyses.
Reverse transcription-polymerase chain reaction
Tumor samples were harvested and immediately snap-frozen in liquid nitrogen. Total RNA was extracted using TRI Reagent (Sigma-Aldrich), and complementary DNA (cDNA) was synthesized using 2 µg of total RNA and the ImProm-II Reverse Transcription System (Promega) according to the manufacturer's directions. Real-time PCR analysis was conducted with diluted cDNA and Fast SYBR Green Master Mix (Promega). The sequences of the primers were as follows:
CD8+
Forward: ATGGCCTTACCAGTGACCG
Reverse: AGGTTCCAGGTCCGATCCAG
INFγ
Forward: TCGGTAACTGACTTGAATGTCCA
Reverse: TCGCTTCCCTGTTTTAGCTGC
PD-L1
Forward: TGGCATTTGCTGAACGCATTT
Reverse: TGCAGCCAGGTCTAATTGTTTT
GAPDH
Forward: CTCCTCCTGTTCGACAGTCAGC
Reverse: CCCAATACGACCAAATCCGTT
Expression relative to the reference gene GAPDH was calculated as 2-ΔCt, where ΔCt is Ctgene-Ctctrl. If undetectable, Ct was given a value of 40.
Western blot analysis
Whole cells protein lysates were quantified using the BioRad protein assay kit (BioRad). Protein extracts were fractionated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 5% milk in TBST for 1 h at room temperature and probed with antibodies. Rabbit monoclonal anti-PD-L1 (1:1000, #13684, Cell Signaling Technology) was used as the primary antibody, HRP-conjugated goat anti-rabbit IgG (1:2500, sc-2004, Santa Cruz Biotechnology) was used as the secondary antibody and rabbit monoclonal anti-GAPDH (1:2500, #2118, Cell Signaling Technology) was used as an internal control.
Statistical analysis
All statistical analyses were performed using SPSS 15.0 statistical software, and a p value (two-sided) <0.05 was considered statistically significant. The chi-square test and Fisher's exact test were used to analyze the association between immune parameters and clinicopathological features. For the gene expression analysis, the correlations between the mRNA expression of PD-L1 and CD8+ and INFγ were analyzed using Spearman's rank correlation. Comparisons of tumor-infiltrating CD8+ T lymphocyte counts were conducted using Student's t test and ANOVA. The prognostic significance of PD-L1 expression and CD8+ T cell infiltration was evaluated using the Kaplan–Meier method and compared by the log-rank test. The Cox-regression model was used to perform univariate analyses, and multivariate analysis was performed on all factors with p less than 0.10.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1181252_supplementary_materials.zip
Download Zip (7 MB)Acknowledgments
We thank all patients who participated in this study. We thank Aihua Lin for helpful advice and expertise in data analysis.
Funding
This work was primarily supported by a grant from Guangdong province natural science foundation of China (Nos.2014A030313191).
References
- Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 2014; 59:1415-26; PMID:24002931; http://dx.doi.org/10.1002/hep.26731
- Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y, Kagaya T, Yamashita T, Honda M, Kaneko S. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology 2011; 53:1206-16; PMID:21480325; http://dx.doi.org/10.1002/hep.24149
- Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2015; 12:681-700; PMID:26484443; http://dx.doi.org/10.1038/nrgastro.2015.173
- Butt AQ, Mills KH. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene 2014; 33:4623-31; PMID:24141774; http://dx.doi.org/10.1038/onc.2013.432
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26:677-704; PMID:18173375; http://dx.doi.org/10.1146/annurev.immunol.26.021607.090331
- Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother 2007; 56:739-45; PMID:17195077; http://dx.doi.org/10.1007/s00262-006-0272-1
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455-65; PMID:22658128; http://dx.doi.org/10.1056/NEJMoa1200694
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200690
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515:563-7; PMID:25428504; http://dx.doi.org/10.1038/nature14011
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:22437870; http://dx.doi.org/10.1038/nrc3239
- Atefi M, Avramis E, Lassen A, Wong DJ, Robert L, Foulad D, Cerniglia M, Titz B, Chodon T, Graeber TG et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res 2014; 20:3446-57; PMID:24812408; http://dx.doi.org/10.1158/1078-0432.CCR-13-2797
- Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 2007; 13:84-8; PMID:17159987; http://dx.doi.org/10.1038/nm1517
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37; PMID:22461641; http://dx.doi.org/10.1126/scitranslmed.3003689
- Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013; 5:200ra116; PMID:23986400; http://dx.doi.org/10.1126/scitranslmed.3006504
- Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, Xu H, Nayar SK, Wang TS, Sidransky D et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res 2013; 1:54-63; PMID:24416729; http://dx.doi.org/10.1158/2326-6066.CIR-13-0034
- Vassilakopoulou M, Avgeris M, Velcheti V, Kotoula V, Rampias T, Chatzopoulos K, Perisanidis C, Kontos CK, Giotakis AI, Scorilas A et al. Evaluation of PD-L1 Expression and Associated Tumor-Infiltrating Lymphocytes in Laryngeal Squamous Cell Carcinoma. Clin Cancer Res 2015; PMID:26408403; http://dx.doi.org/10.1158/1078-0432.CCR-15-1543
- Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res 2014; 20:2773-82; PMID:24647569; http://dx.doi.org/10.1158/1078-0432.CCR-13-2702
- Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005; 11:2947-53; PMID:15837746; http://dx.doi.org/10.1158/1078-0432.CCR-04-1469
- Massi D, Brusa D, Merelli B, Ciano M, Audrito V, Serra S, Buonincontri R, Baroni G, Nassini R, Minocci D et al. PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann Oncol 2014; 25:2433-42; PMID:25223485; http://dx.doi.org/10.1093/annonc/mdu452
- Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009; 15:971-9; PMID:19188168; http://dx.doi.org/10.1158/1078-0432.CCR-08-1608
- Umemoto Y, Okano S, Matsumoto Y, Nakagawara H, Matono R, Yoshiya S, Yamashita Y, Yoshizumi T, Ikegami T, Soejima Y et al. Prognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I-positive hepatocellular carcinoma after curative hepatectomy. J Gastroenterol 2015; 50:65-75; PMID:24509608; http://dx.doi.org/10.1007/s00535-014-0933-3
- Hato T, Goyal L, Greten TF, Duda DG, Zhu AX. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology 2014; 60:1776-82; PMID:24912948; http://dx.doi.org/10.1002/hep.27246
- Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF. The yin and yang of evasion and immune activation in HCC. J Hepatol 2015; 62:1420-9; PMID:25733155; http://dx.doi.org/10.1016/j.jhep.2015.02.038
- Greten TF, Wang XW, Korangy F. Current concepts of immune based treatments for patients with HCC: from basic science to novel treatment approaches. Gut 2015; 64:842-8; PMID:25666193; http://dx.doi.org/10.1136/gutjnl-2014-307990
- Rimm D, Schalper K, Pusztai L. Unvalidated antibodies and misleading results. Breast Cancer Res Treat 2014; 147:457-8; PMID:25086631; http://dx.doi.org/10.1007/s10549-014-3061-0
- Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L, Rimm DL. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014; 94:107-16; PMID:24217091; http://dx.doi.org/10.1038/labinvest.2013.130
- Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, Weber A, Slankamenac K, Poon RT, Yang H et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 2012; 61:427-38; PMID:21930732; http://dx.doi.org/10.1136/gutjnl-2011-300509
- Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, Xu H, Meeker AK, Fan J, Cheadle C et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin Cancer Res 2015; 21:3969-76; PMID:25944800; http://dx.doi.org/10.1158/1078-0432.CCR-15-0244
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999; 5:1365-9; PMID:10581077; http://dx.doi.org/10.1038/70932
- Twyman-Saint VC, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015; 520:373-7; PMID:25754329; http://dx.doi.org/10.1038/nature14292
- Schultheis AM, Scheel AH, Ozretic L, George J, Thomas RK, Hagemann T, Zander T, Wolf J, Buettner R. PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer 2015; 51:421-6; PMID:25582496; http://dx.doi.org/10.1016/j.ejca.2014.12.006
- Katsuya Y, Fujita Y, Horinouchi H, Ohe Y, Watanabe S, Tsuta K. Immunohistochemical status of PD-L1 in thymoma and thymic carcinoma. Lung Cancer 2015; 88:154-9; PMID:25799277; http://dx.doi.org/10.1016/j.lungcan.2015.03.003
- Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 2013; 19:3462-73; PMID:23674495; http://dx.doi.org/10.1158/1078-0432.CCR-13-0855
- Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 2013; 73:1733-41; PMID:23288508; http://dx.doi.org/10.1158/0008-5472.CAN-12-2384
- El-Khoueiry AB, Melero I, Crocenzi TS, Welling TH, Yau TC, Yeo W, Chopra A, Grosso JF, Lang L, Anderson J, et al. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma(HCC):CA209-040 [abstract]. J Clin Oncol, 2015 ASCO Annual Meeting Proceedings 2015, 33 (suppl).
- Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003; 9:562-7; PMID:12704383; http://dx.doi.org/10.1038/nm863
- Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 2009; 206:1327-37; PMID:19451266; http://dx.doi.org/10.1084/jem.20082173
- Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res 2009; 69:8067-75; PMID:19826049; http://dx.doi.org/10.1158/0008-5472.CAN-09-0901
- Heeren AM, Kenter GG, Jordanova ES, de Gruijl TD. CD14(+) macrophage-like cells as the linchpin of cervical cancer perpetrated immune suppression and early metastatic spread: A new therapeutic lead. Oncoimmunology 2015; 4:e1009296; PMID:26155430; http://dx.doi.org/10.1080/2162402X.2015.1009296
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17:1471-4; PMID:20180029; http://dx.doi.org/10.1245/s10434-010-0985-4