ABSTRACT
The first-line standard of care for patients with metastatic colorectal cancer (mCRC) is FOLFIRI (irinotecan, levo-leucovorin, 5-fluorouracil (5-FU)) plus bevacizumab. With the renewed interest in cancer immunotherapy with agents such as vaccines, checkpoint inhibitors and immune modulators, the possibility exists for the use of one or more of these immunotherapeutics in the first-line setting and thus in combination with the FOLFIRI and bevacizumab regimen. Studies were undertaken to study the effects of FOLFIRI and bevacizumab therapy on peripheral T-cell subsets, and to determine if there are any associations between these subsets and response to therapy. Peripheral blood mononuclear cell subsets of patients with mCRC (n = 23) were analyzed prior to and during therapy. While there were differences among patients, the majority of patients showed either a minimal change or an increase in CD4+ T cell to regulatory T cell (Treg) ratios during therapy, as well as either minimal change or a decrease in Treg suppressive activity during therapy. There was also an association (p = 0.036) between a decrease in Treg frequency during FOLFIRI therapy and overall survival, and an association (p = 0.037) between the frequency of Tregs prior to therapy and progression-free survival. Responders to the chemotherapy by RECIST criteria also had a greater decrease in Tregs during therapy vs. pre-therapy (p = 0.0064) as compared to non-responders. While the number of mCRC patients undergoing chemotherapy in this study is relatively small, it provides the rationale for the use of immunotherapeutics in this first-line metastatic setting.
Abbreviations
| ALP | = | alkaline phosphatase |
| CEA | = | carcinoembryonic antigen |
| CI | = | confidence interval |
| CRC | = | colorectal cancer |
| FDA | = | Food and Drug Administration |
| 5-FU | = | 5-fluorouracil |
| FOLFIRI | = | irinotecan, levo-leucovorin, 5-fluorouracil |
| LDH | = | lactate dehydrogenase |
| MAb | = | monoclonal antibody |
| mCRC | = | metastatic colorectal cancer |
| MMR, mismatch repair; MSI | = | microsatellite instability |
| MVA | = | Modified Vaccinia Ankara strain |
| OS | = | overall survival |
| PANVAC | = | rV-CEA-MUC1-TRICOM (B7.1, ICAM-1, LFA-3) |
| PBMC | = | peripheral blood mononuclear cell |
| PD-1 | = | programmed cell death protein-1 |
| PD-L1 | = | programmed cell death protein-1 ligand |
| PFS | = | progression-free survival |
| PR | = | partial response |
| RECIST | = | Response Evaluation Criteria in Solid Tumors |
| SD | = | stable disease |
| Tregs | = | regulatory T cells |
Introduction
Several immunotherapeutic agents have recently been approved by the Food and Drug Administration (FDA) for a range of human cancers, including melanoma, non-small cell and squamous cell lung cancer, and renal cancer. While colorectal cancer (CRC) remains a leading cause of cancer death, to date no immunotherapeutics have been approved for CRC therapy. This is paradoxical in a way, since the elegant studies of Galon and FridmanCitation1-4 and othersCitation5-7 have shown that the presence of an immune infiltrate in primary CRC tumors is an excellent positive prognostic indicator.
A minority (15%) of sporadic CRC tumors possess microsatellite instability (MSI), whereas almost all cases of hereditary non-polyposis CRC do.Citation8 The combination of MSI positivity and a lymphocytic infiltrate has been shown to be a favorable prognostic indicator.Citation9 These MSI positive tumors have been shown to respond clinically to monoclonal antibodies (MAbs) directed against the programmed cell death protein-1 (PD-1)/programmed cell death protein-1 ligand (PD-L1) axis. The vast majority of metastatic CRC (mCRC) tumors, however, have shown only limited responses to these checkpoint inhibitors. One contributing factor could be that metastatic lesions, which have escaped first-, second- or third-line chemotherapy are at this point “non-inflamed,” i.e., devoid or having minimal immune infiltrate.
The standard of care for first-line therapy in patients with mCRC is the regimen consisting of FOLFIRI (irinotecan, levo-leucovorin, 5-fluorouracil (5-FU)) and bevacizumab. One potential therapeutic approach would be to employ immunotherapeutics such as checkpoint inhibitors or vaccines early in the metastatic process, i.e., in the first-line metastatic setting in combination with FOLFIRI. In this case, it would be necessary to interrogate whether the FOLFIRI regimen with bevacizumab would be deleterious to the CRC patients' immune system, be immune inert or enhance certain immune components.
In the studies reported here, we have analyzed various immune subsets in the peripheral blood mononuclear cells (PBMCs) of 23 mCRC patients both prior to and during first-line FOLFIRI therapy. While there were differences among patients, the majority of patients showed either a minimal change or an increase in CD4+ T cell to regulatory T-cell (Treg) ratios during therapy, as well as either minimal change or a decrease in Treg suppressive activity during therapy. There was also an association (p = 0.036) between the decrease in Treg frequency during FOLFIRI therapy and overall survival (OS), and an association (p = 0.037) between the frequency of Tregs prior to therapy and progression-free survival (PFS). While the number of patients (n = 23) is relatively small, these studies provide the rationale for the combined use of immunotherapeutic agents such as vaccines, immune modulators such as immunocytokines, and/or checkpoint inhibitor MAbs with FOLFIRI plus bevacizumab in first-line therapy in patients with mCRC.
Results
Clinical outcome
Twenty-three patients with first-line metastatic colon or rectal cancer () were enrolled in the study. All underwent FOLFIRI therapy, consisting of irinotecan (180 mg/m2 day 1), levo-leucovorin (200 mg/m2 day 1), 5-FU (400 mg/m2 bolus day 1 and 2400 mg/m2 continuous infusion over 48 h), and bevacizumab (5 mg/kg day 1). This treatment schedule was repeated every 2 weeks. Peripheral blood samples were collected from each patient prior to the start of cycle I, and after 30 d, i.e. prior to initiation of the third cycle. As shown in , cytofluorometric analysis of PBMCs revealed no statistical differences between the PBMCs collected at baseline and post 30 d of therapy in terms of PBMC amount, percent of CD4+ cells relative to total PBMCs, total number of CD4+ T cells, percent of CD8+ cells relative to total PBMCs, total number of CD8+ T cells, percent of Tregs relative to total PBMCs, total number of Tregs, or the ratios between CD4+ effectors and Tregs, CD8+ effectors and Tregs, or CD4+ and CD8+ effectors. A waterfall plot of the change in the ratio between CD4+ effector T cells and regulatory T cells (CD4+:Treg ratio) in the course of FOLFIRI therapy is shown in . Increases in the CD4+:Treg ratio >25% were seen in 10/23 (43%) patients, while decreases in this ratio of >25% were seen in only 3/23 (13%) patients. Ten of 23 patients had minimal changes (an increase or decrease <25%) in the CD4+:Treg ratio. To assess any factors that could contribute to the variability between patients, the change in CD4+:Treg ratio was divided at the median, and the two cohorts were compared (Table S1). There were significant differences regarding age (p = 0.0353, patients who had a greater than the median change in the CD4+:Treg ratio were older, median age 74 y vs. median age 65 y, respectively); moreover, there were also significant changes in the number of liver metastases (p = 0.0475, patients who had a greater increase in the CD4+:Treg ratio had fewer metastases, 1.6 vs. 2.3, respectively).
Figure 1. Therapy-induced changes in the ratio of CD4+ effectors to regulatory T cells (Tregs), and in the immunosuppressive activity of Tregs in patients with colorectal cancer. (A) Waterfall plot of the change in the ratio between CD4+ effector T cells and regulatory T cells (CD4+:Treg ratio) in the course of FOLFIRI therapy in patients (n = 23) with first-line metastatic colorectal cancer. (B) Enough peripheral blood mononuclear cells (PBMCs) from 13 patients were available to measure suppressive activity of Tregs. Waterfall plot of the change in suppressive activity of Tregs after FOLFIRI therapy, compared to baseline, in patients with first-line metastatic colorectal cancer. Peripheral blood samples were collected at baseline and after 30 d of therapy.
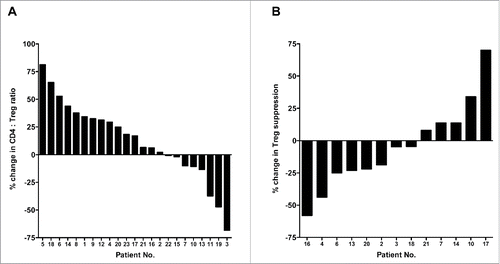
Table 1. Patient demographics and clinical characteristics.
Table 2. Evaluation of immune cell subsets in colorectal cancer patients prior to and after 30 d of standard-of-care anticancer therapy.
Sufficient PBMCs from 13/23 patients were available to conduct a functional study of the suppressive activity of Tregs at baseline and after 30 d of therapy. A waterfall plot of the changes in suppressive activity is shown in . Six of 13 (46%) patients showed more than a 10% decrease in suppressive activity, while 4/13 (31%) showed a ≥10% increase.
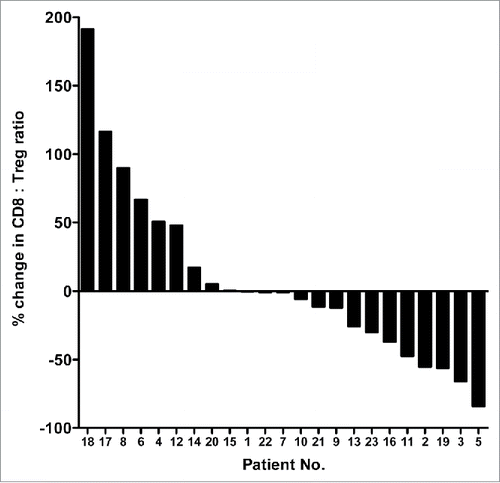
We also evaluated the change in the ratio between CD8+ effector T cells and regulatory T cells (CD8+:Treg ratio). As seen in , there were similar numbers of patients showing an increase or decrease in the CD8+:Treg ratio. To assess any factors that could contribute to the variability between patients, the change in CD8+:Treg ratio was divided at the median, and the two cohorts were compared (Table S1). There was a significant difference regarding the time from progressive disease to death (p = 0.0312, patients who had a greater than the median change in the CD8+:Treg ratio had a longer time from progressive disease to death, 15.6 vs. 6.8 mo, respectively).
Figure 2. Therapy-induced changes in the ratios of CD8+ effectors to regulatory T cells in patients with colorectal cancer. Waterfall plot of the change in the ratio between CD8+ effector T cells and regulatory T cells (CD8+:Treg ratio) in the course of FOLFIRI therapy in patients with first-line metastatic colorectal cancer (n = 23). Peripheral blood samples were collected at baseline and after 30 d of therapy.
The clinical characteristics and outcome of the individual patients enrolled on the study are shown in . Primary tumor biopsy samples were available from 15 patients. Analysis of the levels of CD3+, CD8+ and CD4+ are shown in . Only two of these patients were MSI positive. We evaluated whether there was any association between the frequency of Tregs prior to therapy and progression-free survival (PFS) as seen in . The median PFS for patients with <2.5% Tregs of PBMCs at baseline was 23.3 months, and for patients with >2.5% Tregs of PBMCs at baseline, PFS was 10.7 months (p = 0.037, n = 23, 3 patients were censored) (). We also evaluated if there was any clinical benefit associated with the change in Treg frequency during FOLFIRI therapy. As seen in , the median OS for patients with any decrease in Tregs after 30 d treatment was 43.3 mo, and for patients with any increase in Tregs after treatment 23.3 mo (p = 0.036, n = 23). It should be noted that the median decrease in Treg frequency was −26% (), and the median increase was +23% ().
Figure 3. Association between the frequency of regulatory T cells (Tregs) in peripheral blood mononuclear cells (PBMCs) at baseline and progression-free survival (PFS) after FOLFIRI therapy in patients with colorectal cancer. Patients were categorized based on their frequency of Tregs (< or > 25% of PBMCs) before therapy. A Kaplan–Meier curve is shown for these two groups and the association with PFS. The median PFS for patients with <2.5% Tregs pre-treatment was 23.3 months, and for patients with >2.5% Tregs pre-treatment 10.7 months (p = 0.037, n = 23, 3 patients were censored).
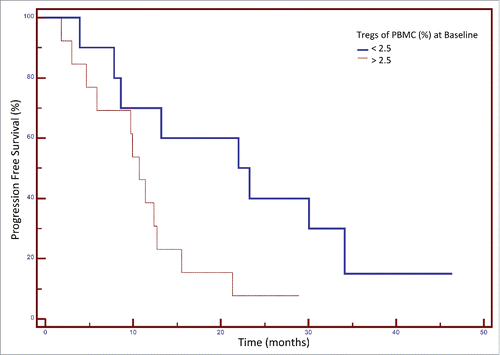
Figure 4. Association between the change in regulatory T cell (Treg) frequency in peripheral blood mononuclear cells (PBMCs) after FOLFIRI therapy and overall survival (OS) in patients with colorectal cancer. Patients were categorized based on any decrease or increase in Treg frequency after 30 d of FOLFIRI therapy. (A) A Kaplan–Meier curve is shown for these two groups and the association with OS. The median OS for patients with decreased Tregs after 30 d treatment was 43.3 mo, and for patients with increased Tregs after treatment, OS was 23.3 mo (p = 0.036, n = 23). The change in Treg frequency was found to be an independent predictive variable for OS by stepwise multiple regression analysis. (B) The median decrease in Treg frequency was −26%. (C) The median increase in Treg frequency was +23%.
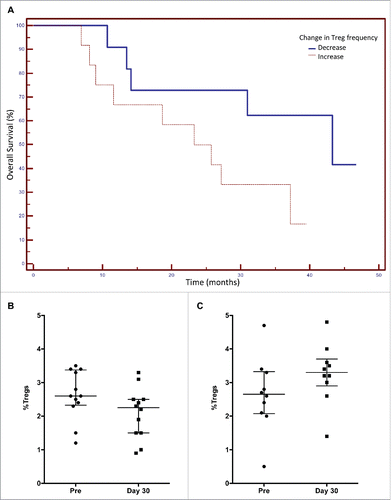
Table 3. Clinical characteristics of patients enrolled on the study
We also correlated the levels of Tregs with antitumor response to therapy by RECIST (Response Evaluation Criteria in Solid Tumors) criteria. We found that 12/23 patients displayed a partial response (PR) by RECIST, 7/23 patients had stable disease (SD), and 4/23 had progressive disease (). It is interesting to note that there was a trend in that of the 12 responders by RECIST criteria on the study, 67% (8/12) displayed Treg levels <2.5% at baseline, whereas only 18% (2/11) of the non-responders by RECIST criteria (including those patients with stable disease) had equivalent low levels of Tregs (p = 0.019). In addition, 75% of the responders displayed a decreased frequency of Tregs in peripheral blood after 30 d of therapy, whereas only 18% of the 11 non-responders by RECIST criteria on the trial had a decrease in Tregs (p = 0.0064 with Pearson test, χ2 = 7.4). There were no other significant differences between responders and non-responders regarding age, gender, CEA, CA19.9, alkaline phosphatase (ALP), lactate dehydrogenase (LDH), one vs. > 1 metastases, mucinous or non-mucinous tumor, or grade at diagnosis.
We employed multiple regression analysis to define which variables were associated with OS. As seen in , only Tregs at baseline and LDH at baseline were statistically significant, with a trend for the change in Tregs at 30 d. Forward stepwise multiple regression analysis showed that Tregs at baseline, baseline LDH, and the change in Tregs after 30 d were independent predictive factors for OS ().
Table 4. Multiple regression analysis of variables associated with overall survival.
Discussion
While no immune therapy has been approved by the FDA for patients with CRC, there has been some evidence of immune-mediated interventions in Phase II clinical studies. For example, the PANVAC vaccine, consisting of rV-CEA-MUC1-TRICOM (B7.1, ICAM-1, LFA-3) as a primary vaccination and multiple boosts with rF-CEA-MUC1-TRICOM, has been administered to patients with CRC metastatic to the liver or lung post-metastasectomy. Survival was approximately 95% at 2 y and approximately 90% at 4 y. A contemporary control group displayed identical time to progression as the vaccinated group, but OS was approximately 50% at 4 y, which is comparable to other reported studies in this population.Citation10,11 An adenovirus-CEA vaccineCitation12 and an MVA-5T4 (TroVax) vaccineCitation13 also reported some evidence of clinical benefit in patients with advanced mCRC. While very few RECIST responses have been observed in non-MSI mCRC patients treated with MAbs directed against the checkpoint inhibitors anti-PD1/PDL1, several patients have shown long-term stable disease post-therapy. New immunotherapy approaches for metastatic CRC are clearly warranted. One approach would be to inflame the tumor microenvironment with a vaccine directed against CRC antigens or neo-epitopes followed by the use of checkpoint inhibitor antibodies. Other approaches to inflame the tumor microenvironment or decrease suppressive cell subsets include the use of radiation, chemotherapy, immunocytokines or an IDO (indoleamine-2,3-dioxygenase) inhibitor. Reduction of immune inhibitory factors such as IL8 and TGF-β could also be employed in combination therapies.
The majority of immunotherapy trials in CRC have been carried out in patients with advanced disease who have already received multiple regimens of chemotherapy. Previous studies have revealedCitation14 that there is an inverse correlation between the number of prior chemotherapy regimens and the ability of mCRC patients to generate immune responses to vaccine. Prior clinical studies have shown that administration of docetaxel to patients with metastatic prostate cancer, as well as that of cisplatin plus vinorelbine to non-small cell lung cancer patients, showed a significant increase in the ratio between effector T cells and Tregs and a reduction in the immunosuppressive activity of Tregs. Treatment of breast cancer patients with tamoxifen had minimal effect on Tregs, while sunitinib exerted differential effects on Tregs among patients with metastatic renal cancer.Citation15-19 Preclinical studies have shown that Tregs are more susceptible to certain chemotherapy regimens compared to CD4+ effector cells.Citation20,21
One obvious strategy would be to deliver an immunotherapeutic early in the metastatic process. This would require administering the immunotherapeutic in combination with the first-line therapy, i.e., FOLFIRI plus bevacizumab. The study reported here was undertaken to determine if this first-line chemotherapy regimen would have an adverse, neutral or enhancing effect on the patients' (n = 23) immune system. We show here for the first time that for the majority of patients, the FOLFIRI regimen either had minimal effect or a positive effect on the effector CD4+:Treg ratios and a reduction in Treg suppressive function. Responders to the chemotherapy by RECIST criteria also had a greater decrease in Tregs during therapy vs. pre-therapy (p = 0.0064) as compared to non-responders. There was also an association (p = 0.037) between the frequency of Tregs prior to therapy and PFS, and an association (p = 0.036) with the decrease in Treg frequency as a consequence of FOLFIRI therapy and OS. It is unknown at this time why there was a lack of association between clinical outcome and CD8+ T cells. Further studies using activation markers for both CD4+ and CD8+ cells are planned.
It has also been reported that certain chemotherapeutic agents can induce immunogenic cell death leading to a more “inflamed” tumor microenvironment and T-cell infiltrate. Moreover, several chemotherapeutic agents (e.g., 5-FU) have been shown to induce immunogenic modulation, where the phenotype of the tumor cell is modified to make it more susceptible to immune-mediated attack. It would also be of value to further study in preclinical models the mechanisms involved in the use of FOLFOX or FOLFIRI ± bevacizumab in combination with various immunotherapy regimens.
While the number (n = 23) of mCRC patients undergoing first-line chemotherapy in this study is relatively small, it provides the rationale for the use of immunotherapeutics in this setting.
Materials and methods
Patients and anticancer treatments
Twenty-three patients with first-line metastatic colon or rectal cancer were enrolled in the study at the University of Rome Tor Vergata, Rome, Italy. All underwent FOLFIRI therapy, consisting of irinotecan (180 mg/m2 day 1), levo-leucovorin (200 mg/m2 day 1), 5-FU (400 mg/m2 bolus day 1 and 2400 mg/m2 continuous infusion over 48 h) and bevacizumab (5 mg/kg day 1). This treatment schedule was repeated every 2 weeks. Peripheral blood samples were collected prior to the start of cycle I, and after 30 d. All patients signed an informed consent form. The procedures were conducted in accordance with the Helsinki Declaration of 1975 in the study at Tor Vergata University Clinical Center, Rome, Italy.
Assessment of response
Radiologic assessment was performed with a contrast enhanced thorax-abdomen pelvis CT scan or MRI at baseline, within 30 d before chemotherapy began, and every 3 mo thereafter, during treatment. Radiologic response and disease progression were defined according to RECIST 1.0 criteria. Disease response was defined as a decrease of at least 30% in the sum of the longest diameter of target lesions, as compared to the baseline. Early changes in immune cells, after 1 mo of therapy, were correlated with the best radiologic response in accordance with RECIST criteria.
Collection of PBMC
PBMCs were isolated by Ficoll (MP Biomedicals, Santa Ana, CA) density gradient separation, washed twice and cryopreserved in 90% heat-inactivated human AB serum and 10% DMSO in liquid nitrogen at a concentration of 1 × 107 cells/mL until assayed.
Flow cytometry analysis
Cryopreserved PBMCs were analyzed by four-color flow cytometry for phenotypic characterization of Tregs as described by Vergati et al.Citation22 Cells were resuspended in staining buffer (PBS containing 3% fetal bovine serum) and stained for 30 min at 4°C with FITC-conjugated anti-CD4+ (BD PharMingen, San Jose, CA), phycoerythrin-conjugated anti-CD25 (BD) and PerCP Cy5.5-conjugated anti-CD127 (eBioscience, San Diego, CA). FoxP3 intracellular staining was done on the cells stained with anti-CD4+, anti-CD25 and anti-CD127. Cells were fixed and permeabilized using a fix/perm buffer (eBioscience) according to the manufacturer's instructions, then labeled with Allophycocyanin-conjugated anti-FoxP3 (eBioscience) or its isotype control as a negative control. Flow cytometry was performed on a FACSCalibur (BD Biosciences): 5 × 104 events were acquired and data were analyzed using CellQuest software (BD Biosciences). To determine the percentage of Tregs, lymphocytes were gated by plotting forward vs. side scatter. The CD4+ population was gated first, followed by the CD25+CD127neg population and finally the FoxP3+ population was gated into the CD4+/CD25+/CD127neg population. Tregs are thus defined as the CD4+/CD25+/CD127neg/ FoxP3+ population.
CD4+CD25high T-cell enrichment
CD4+CD25high T cells were enriched using a CD4+CD25+ Treg isolation kit (Miltenyi Biotec, San Diego, CA), with modifications to the manufacturer's instructions. After CD4+ T cells were negatively enriched, positive selection for CD25high T cells was done on the negatively selected CD4+ T cells. In order to achieve a consistently high CD25high purity rate, the amount of CD25 antibody microbeads was decreased by 70% and the incubation time was decreased by 15%. The CD25high fraction was collected by eluting twice the cells through a magnetic separation (LS) column to further enrich for CD4+CD25high T cells.
Treg suppression assay
RPMI 1640 medium supplemented with 10% AB serum, 100 units/mL of penicillin, 100 μg/mL of streptomycin (Mediatech, Manassas, VA), and 2 mmol/L of L-glutamine (Mediatech) was used for T-cell culture. Responder CD4+CD25neg T cells were labeled with 2 μM CFSE (Sigma, St. Louis, MO). In suppression assays, to assess CD4+CD25high T cells' suppressive capacity, 1 × 104 CFSE-labeled responder CD4+CD25− T cells were cultured alone or cocultured with 1 × 104 CD4+CD25high T cells in the presence of Mitomycin-treated T-cell-depleted PBMC as antigen-presenting cell, and were stimulated with 0.5 μg/mL plate-bound anti-CD3+ antibody (OKT3; eBioscience) in 96-well round-bottom plates.Citation21 Proliferation of CFSE-labeled cells was assessed by flow cytometry after 4 d of culture. The percent suppression was calculated using the following formula: [(percent of proliferating effector cells (CD4+CD25neg) when cultured alone minus the percent of proliferating CFSE-diluting effector cells in the presence of suppressor cells (CD4+CD25hi) at a 1:1 ratio)/percent of proliferating responder cells when cultured alone] × 100.
Immunohistochemistry
Tissue sections of formalin-fixed, paraffin-embedded, colon carcinomas were tested for CD3+, CD4+, CD8+, MLHl and MSH6 expression using the Ventana BenchMark XT automated staining platform with the ultraView Universal DAB Detection Kit (Roche Diagnostics GmbH, Mannheim, Germany).Citation23 For each slide, three to five random fields were evaluated by two pathologists in an independent blinded manner, with an interobserver variability <5%. Tumoral lymphocyte infiltration was characterized by evaluation of CD3+, CD4+, CD8+ immunostainings performed in a traditional microscope-based manner at 40x magnification. For each slide, three to five random fields were evaluated and graded arbitrarily, considering density of each T-cell subset (cells/mm2) according to the observed mean values as follows: CD3+ (slight: <67 cells/mm2, moderate between 67 and 670 cells/mm2, and severe >670 cells/mm2), CD4+ (slight: <30 cells/mm2, moderate between 30 and 300 cells/mm2, and severe >300 cells/mm2) and CD8+ (slight: <35 cells/mm2, moderate between 35 and 350 cells/mm2, and severe >350 cells/mm2). DNA mismatch repair (MMR) deficiency leading to MSICitation24 was analyzed by immunohistochemistry,Citation25 and tumors with MSI-high were defined as those demonstrating an MMR protein expression less than 10%.Citation24 Staining of nuclei of adjacent normal crypts, stromal cells and lymphocytes was used as internal positive controls.
Statistical analysis
Statistical analysis was performed using the non-parametric Wilcoxon test or Friedman test with Dunn's multiple comparison for paired samples (GraphPad Software, La Jolla, CA). The probabilities of survival and progression-free survival as a function of time were established with the Kaplan–Meier method, with 95% confidence intervals (CIs) around the median established with a reflected CI approach. Multiple regression analysis was performed in a forward stepwise fashion. A p value <0.01 was considered statistically significant, and a p value <0.05 was considered a trend.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1188243_s02.docx
Download MS Word (16.3 KB)Acknowledgments
The authors thank Debra Weingarten for her editorial assistance in the preparation of the manuscript.
Funding
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. This work has been partially carried out within the Ph.D. program in “Medicina Sperimentale e dei Sistemi” XXXI Ciclo, Tor Vergata University, Rome, Italy.
References
- Camus M, Tosolini M, Mlecnik B, Pages F, Kirilovsky A, Berger A, Costes A, Bindea G, Charoentong P, Bruneval P et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res 2009; 69:2685-93; PMID:19258510; http://dx.doi.org/10.1158/0008-5472.CAN-08-2654
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/10.1126/science.1129139
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353:2654-66; PMID:16371631; http://dx.doi.org/10.1056/NEJMoa051424
- Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 2009; 27:5944-51; PMID:19858404; http://dx.doi.org/10.1200/JCO.2008.19.6147
- Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. Tumour inflammatory infiltrate predicts survival following curative resection for node-negative colorectal cancer. Eur J Cancer 2009; 45:2138-45; PMID:19409772; http://dx.doi.org/10.1016/j.ejca.2009.04.011
- Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. Comparison of the prognostic value of inflammation-based pathologic and biochemical criteria in patients undergoing potentially curative resection for colorectal cancer. Ann Surg 2009; 249:788-93; PMID:19387324; http://dx.doi.org/10.1097/SLA.0b013e3181a3e738
- Turner N, Wong HL, Templeton A, Tripathy S, Whiti Rogers T, Croxford M, Jones I, Sinnathamby M, Desai J, Tie J et al. Analysis of local chronic inflammatory cell infiltrate combined with systemic inflammation improves prognostication in stage II colon cancer independent of standard clinicopathologic criteria. Int J Cancer 2016; 138:671-8; PMID:26270488; http://dx.doi.org/10.1002/ijc.29805
- Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998; 58:5248-57; PMID:9823339
- Chang EY, Dorsey PB, Frankhouse J, Lee RG, Walts D, Johnson W, Anadiotis G, Johnson N. Combination of microsatellite instability and lymphocytic infiltrate as a prognostic indicator in colon cancer. Arch Surg 2009; 144:511-5; PMID:19528382; http://dx.doi.org/10.1001/archsurg.2009.40
- Lyerly HK, Hobeika A, Niedzwiecki D, Osada T, Marshall J, Garrett CR, Chang DZ, Aklilu M, Crocenzi TS, Cole DJ et al. A dendritic cell-based vaccine effects on T-cell responses compared with a viral vector vaccine when administered to patients following resection of colorectal metastases in a randomized phase II study. 2011 ASCO Annual Meeting. J Clin Oncol 2011; 29(suppl): abstr 2533
- Morse M, Niedzwiecki D, Marshall J, Garrett CR, Chang DZ, Aklilu M, Crocenzi TS, Cole DJ, Dessureault S, Hobeika A et al. Survival rates among patients vaccinated following resection of colorectal cancer metastases in a phase II randomized study compared with contemporary controls. 2011 ASCO Annual Meeting. J Clin Oncol 2011; 29(suppl): abstr 3557
- Balint JP, Gabitzsch ES, Rice A, Latchman Y, Xu Y, Messerschmidt GL, Chaudhry A, Morse MA, Jones FR. Extended evaluation of a phase 1/2 trial on dosing, safety, immunogenicity, and overall survival after immunizations with an advanced-generation Ad5 [E1-, E2b-]-CEA(6D) vaccine in late-stage colorectal cancer. Cancer Immunol Immunother 2015; 64:977-87; PMID:25956394; http://dx.doi.org/10.1007/s00262-015-1706-4
- Harrop R, Connolly N, Redchenko I, Valle J, Saunders M, Ryan MG, Myers KA, Drury N, Kingsman SM, Hawkins RE et al. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin Cancer Res 2006; 12:3416-24; PMID:16740766; http://dx.doi.org/10.1158/1078-0432.CCR-05-2732
- von Mehren M, Arlen P, Gulley J, Rogatko A, Cooper HS, Meropol NJ, Alpaugh RK, Davey M, McLaughlin S, Beard MT et al. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res 2001; 7:1181-91; PMID:11350882
- Adotevi O, Pere H, Ravel P, Haicheur N, Badoual C, Merillon N, Medioni J, Peyrard S, Roncelin S, Verkarre V et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother 2010; 33:991-8; PMID:20948437; http://dx.doi.org/10.1097/CJI.0b013e3181f4c208
- Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, Wood L, Elson P, Garcia J, Dreicer R et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res 2008; 14:6674-82; PMID:18927310; http://dx.doi.org/10.1158/1078-0432.CCR-07-5212
- Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, Rini B, Finke JH, Cohen PA. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res 2010; 70:3526-36; PMID:20406969; http://dx.doi.org/10.1158/0008-5472.CAN-09-3278
- Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 2009; 15:2148-57; PMID:19276286; http://dx.doi.org/10.1158/1078-0432.CCR-08-1332
- Roselli M, Cereda V, di Bari MG, Formica V, Spila A, Jochems C, Farsaci B, Donahue R, Gulley JL, Schlom J et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology 2013; 2:e27025; PMID:24353914; http://dx.doi.org/10.4161/onci.27025
- Farsaci B, Higgins JP, Hodge JW. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy. Int J Cancer 2012; 130:1948-59; PMID:21633954; http://dx.doi.org/10.1002/ijc.26219
- Gameiro SR, Caballero JA, Higgins JP, Apelian D, Hodge JW. Exploitation of differential homeostatic proliferation of T-cell subsets following chemotherapy to enhance the efficacy of vaccine-mediated antitumor responses. Cancer Immunol Immunother 2011; 60:1227-42; PMID:21544650; http://dx.doi.org/10.1007/s00262-011-1020-8
- Vergati M, Cereda V, Madan RA, Gulley JL, Huen NY, Rogers CJ, Hance KW, Arlen PM, Schlom J, Tsang KY. Analysis of circulating regulatory T cells in patients with metastatic prostate cancer pre- vs. post-vaccination. Cancer Immunol Immunother 2011; 60:197-206; PMID:20976449; http://dx.doi.org/10.1007/s00262-010-0927-9
- Ferlosio A, Arcuri G, Doldo E, Scioli MG, De Falco S, Spagnoli LG, Orlandi A. Age-related increase of stem marker expression influences vascular smooth muscle cell properties. Atherosclerosis 2012; 224:51-7; PMID:22857896; http://dx.doi.org/10.1016/j.atherosclerosis.2012.07.016
- Bertagnolli MM, Niedzwiecki D, Compton CC, Hahn HP, Hall M, Damas B, Jewell SD, Mayer RJ, Goldberg RM, Saltz LB et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803. J Clin Oncol 2009; 27:1814-21; PMID:19273709; http://dx.doi.org/10.1200/JCO.2008.18.2071
- Garcia-Solano J, Conesa-Zamora P, Carbonell P, Trujillo-Santos J, Torres-Moreno DD, Pagan-Gomez I, Rodriguez-Braun E, Perez-Guillermo M. Colorectal serrated adenocarcinoma shows a different profile of oncogene mutations, MSI status and DNA repair protein expression compared to conventional and sporadic MSI-H carcinomas. Int J Cancer 2012; 131:1790-9; PMID:22287190; http://dx.doi.org/10.1002/ijc.27454
