ABSTRACT
Studies of sequential anti-CD137/anti-CD20 therapy have previously shown that the efficacy of anti-CD20 was heavily reliant upon anti-CD137; however, the exact mechanism of the anti-B-cell lymphoma efficacy, and whether this correlates with enhanced adverse effects or toxicity, had not been elucidated. Here, we observed that sequential anti-CD137 administration with anti-CD20 resulted in a synergistic therapy, largely dependent upon Fc receptors (FcR), to prolong survival in an experimental B-cell lymphoma therapy model. Tumor suppression was accompanied by B cell depletion, which was not dependent on one activating FcR. Surprisingly, the B-cell activating factor (BAFF) was elevated in the plasma of mice receiving anti-CD137 alone or in combination with anti-CD20, while a selective increase in some plasma cytokines was also noted and triggered by anti-CD137. These effects were independent of activating FcR. Sustained treatment of advanced lymphoma revealed increased lymphocyte infiltrates into the liver and a significant decrease in the metabolic capability of the liver in mice receiving anti-CD137. Importantly, these effects were not exacerbated in mice receiving the anti-CD20/CD137 combination, and elevations in classical liver damage markers such as alanine aminotransferase (ALT) were less than that caused by the lymphoma itself. Thus, combined anti-CD20/anti-CD137 treatment increases the therapeutic index of anti-CD20 or anti-CD137 alone. These mouse data were corroborated by ongoing clinical development studies to assess safety, tolerability and pharmacodynamic activity of human patients treated by this approach. Together, these data support the use of this sequential antibody therapeutic strategy to improve the efficacy of rituximab in B-cell lymphoma patients.
Introduction
B-cell lymphoma is the most common type of Non-Hodgkin's lymphoma (NHL) in Europe and North America, where it accounts for 3% of all cancer-related deaths.Citation1 During lymphoid differentiation, B-lymphocyte antigen CD20 expression progressively increases with B-cell maturation. CD20 is not expressed by the early B-cell progenitors or plasma cells, but is expressed by most B-cell lymphomas.Citation2 Rituximab, a chimeric monoclonal antibody (mAb) against CD20 antigen, has shown great potential for B-lymphoma depletion in pre-clinical models, and is now frequently used for the treatment of patients with relapsed or refractory B-cell lymphoma. However, to achieve the optimal anticancer effect, rituximab is routinely used in combination with classical chemotherapy agents (e.g. cyclophosphamide, doxorubicin, vincristine, and prednisone).Citation1 An important consideration regarding conventional chemotherapy is its non-selective mechanism of action and resultant high incidence of systemic side effects (e.g., blood disorders, constipation, fatigue, pain, gastrointestinal disorders, infertility, nausea, liver damage, etc.) that directly impact on the quality of life of patients undergoing this treatment.Citation3,4 Thus, there is impetus to consider combinations of anti-CD20 mAbs with immunotherapy, provided these are safe. Further, the mechanism of action of rituximab is mediated via antibody-dependent cell-mediated cytotoxicity (ADCC), which triggers host anticancer responses by natural killer (NK) cells and macrophages through Fc receptors (FcR).Citation5,6
Recently, we have shown that inhibition of the killer immunoglobulin-like receptor (KIR) by anti-KIR receptor mAbs induced an enhanced anti-lymphoma response by NK cells when combined with anti-CD20 mAb.Citation7 In this light, agonists of co-stimulation (directed against CD27, CD28, CD137, ICOS, OX40) have also been extensively studied for optimization of immune cell antitumor functions.Citation8 Ligation of these receptors with specific mAbs results in an enhanced antitumor immune response involving different and complex mechanisms of action. For instance, ligation of the co-receptor CD137 (or 4-1BB), a cell surface glycoprotein member of the tumor necrosis factor (TNF) receptor superfamily that regulates multiple immune cell subsets including dendritic cells, NK cells, CD4+, CD8+ and regulatory T cells, promotes enhancement of immune cell activation (for a review, see Citation9). Our previous studies have also shown that anti-CD137 mAb augments the efficacy of several tumor-targeting mAbs, including anti-CD20 treatment in CD20+ B-cell lymphomas, anti-EGFR (Cetuximab) in colorectal cancer, and anti-HER2 (Trastuzumab) in HER2-expressing breast cancers.Citation10-12 Despite these positive pre-clinical data, the side effects of anti-CD137 alone and in combination with anti-CD20 have been poorly characterized, and concerns have remained about confidently delivering this combination to human B-cell lymphoma patients. Anti-CD137 treatment (weekly injections of 200 μg of anti-CD137, or isotype control, for three weeks) of BALB/c or C57BL/6 strains of mice was shown to induce anemia, blood lymphopenia, lymphadenopathy, hepatitis/hepatomegaly, increased infiltration of lymphocytes into the liver (e.g. NK cells, CD8 T cells, and B cells), splenomegaly, and thrombocytopenia.Citation13 These symptoms were mediated via TNF-α, type I interferons (IFNs), and IFNγ. However, the same study also suggested that anti-CD137 treatment is unlikely to induce an autoimmune response, since the symptoms in mice resolved following cessation of treatment.Citation13 A fully human IgG4 anti-CD137 antibody is under development with signs of clinical activity and cases of liver toxicity that seem to be on target and dose dependent.Citation14,15
Although antitumor benefit of combinatorial therapy with anti-CD20 plus anti-CD137 in mice was previously demonstrated using an in vivo subcutaneous B-cell lymphoma transplant system,Citation10 here we showed in a systemic model of B-cell lymphoma using anti-CD20 (clone 5D2, mouse IgG2a) Citation16 and anti-CD137 (clone 3H3, rat IgG2a) Citation17 that the combination could synergistically enhance survival. FcRs can specifically bind to the Fc portion of immunoglobulins, and activate cellular signal transduction through a γ chain subunit. In mice, four different FcRs have been described: FcγRI (activating), FcγRIIb (inhibitory), FcγRIII (activating), FcγRIV (activating).Citation18 Considering that the optimized anticancer effects of anti-CD137 and anti-CD20 therapies are mainly triggered by NK cells and macrophages,Citation5,10 we used FcR-deficient mice to assess the effects of conventionally expressed activating FcRs (FcγRIII and FcγRIV) that specifically bind to murine or rat IgG2a class of antibodies,Citation19,20 and using FcRγ chain-deficient mice which lack signaling in all FcRs as a positive control. Combination treatment with anti-CD20 and anti-CD137 suppressed experimental systemic B cell-lymphoma in an FcRIV-dependent manner; however, accompanying B-cell depletion was not dependent on any one activating FcR. Sustained treatment of advanced lymphoma revealed increased lymphocyte infiltrates into the liver and a significant decrease in the metabolic capability of the liver in mice receiving anti-CD137. Importantly, these effects were not exacerbated in mice receiving the anti-CD20/CD137 combination, and elevations in classical liver damage markers were less than that caused by the lymphoma itself. Thus, combined anti-CD20/anti-CD137 treatment increases the therapeutic index of anti-CD20 or anti-CD137 alone with negligible toxicity.
Results
Combination of anti-CD20/anti-CD137 treatment suppresses B-cell lymphoma via an FcRIV-dependent mechanism
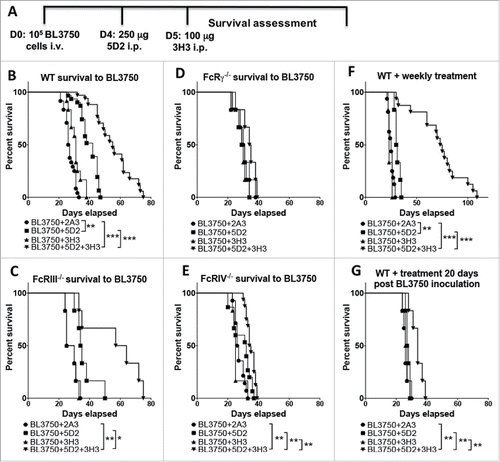
We examined the combination of anti-CD20 (clone 5D2) and anti-CD137 (clone 3H3) in mice injected intravenously with 105 BL3750 cells (). The combination of anti-CD20 (Day 4 post tumor inoculation) and anti-CD137 (Day 5) in the systemic BL3750 tumor inoculation model led to a significant survival benefit that was superior to anti-CD20 or anti-CD137 monotherapies (). Anti-CD20 alone significantly enhanced survival as compared to isotype control IgG (cIg), but anti-CD137 did not (). To examine which specific FcR was contributing to anti-CD20/CD137 and anti-CD20 survival benefit, we explored the treatment outcomes in FcRIII−/−, FcRIV−/−, and FcγR−/− mice. While the absence of FcRIII did not affect the anti-CD20/CD137 combination or anti-CD20 (), the survival benefit of this combination or anti-CD20 alone was completely abolished in mice lacking FcRIV () or FcγR (). We next assessed whether repetitive weekly treatment following the day 4/5 treatment with anti-CD20/CD137 combination therapy, or either of the respective monotherapies, further enhanced the survival benefit. However, weekly treatments did not appear to greatly improve the survival outcome and no mice survived combination treatment (). This data indicates the relative intransigence of systemic disease as opposed to subcutaneous disease where cures can be obtained.Citation10 When the commencement of combination therapy was delayed until day 20 (after tumor inoculation), most of its benefit was lost ().
Figure 1. Treatment with αCD20 and αCD137 enhances survival against B lymphoma. (A) Tumor injection and treatment design. 1 × 105 BL3750 cells were injected intravenously at day (D) 0. Control Ig (2A3, 250 μg i.p.) or anti-CD20 (clone 5D2, 250 μg i.p.) was administered on day 4, and anti-CD137 (clone 3H3, 100 μg i.p.) either alone or in combination on day 5 in C57BL6 wild-type (WT) and various gene-targeted mice (FcRIII−/−, FcRIV−/−, and FcγR−/−). (B) Results are pooled from three independent experiments (n (2A3) = 24; n (5D2) = 31; n (3H3) = 12; n (5D2+3H3) = 34). Statistical analysis was calculated using Mantel–Cox test. **p < 0.01 and ***p < 0.001 as indicated. (C) Results representative from one experiment, n = 6 each group. *p < 0.05 and **p < 0.01 as indicated. (D) Results representative from one experiment, n = 6 each group. (E) Results are pooled from two independent experiments (n (2A3) = 14; n (5D2) = 15; n (3H3) = 6; n (5D2+3H3) = 16). Statistical analysis was calculated using Mantel–Cox test. **p < 0.01 as indicated. (F) Tumor inoculation (1 × 105 BL3750 cells, i.v.) on D0 was followed by administration of anti-CD20 (clone 5D2, 250 μg i.p.) on day 4, and anti-CD137 (clone 3H3, 100 μg i.p.) at day 5, either alone or in combination in C57BL6 WT mice. Antibody treatment was then applied weekly until sacrifice. Data shown is pooled from two independent experiments (n (2A3) = 16, n (5D2) = 6, n (3H3) = 6, n (5D2+3H3) = 16). **p < 0.001, and ***p < 0.001 as indicated. (G) WT mice survival after single treatment with anti-CD20 (clone 5D2, 250 μg i.p. 20 d after BL3750 inoculation) and anti-CD137 (clone 3H3, 100 μg i.p. 21 d after BL3750 inoculation). Results are representative of one experiment, n = 6, each group. **p < 0.01 as indicated.
Combination of anti-CD20/anti-CD137 therapy reduces lymphocyte/B cell numbers
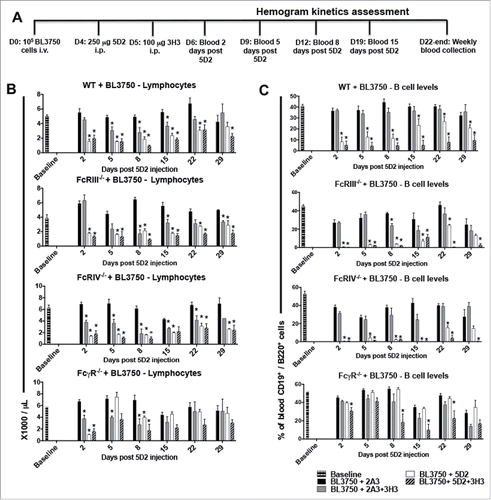
To examine B-cell depletion, we measured peripheral blood lymphocyte and B-cell numbers in BL3750-bearing mice following combination anti-CD20/CD137 therapy compared to anti-CD20 or anti-CD137 monotherapies using the schedule indicated (). Lymphocyte counts () and B-cell numbers () were significantly lower in BL3750-bearing WT mice treated with anti-CD20 or combined anti-CD20/CD137 therapies within several days (by day 2 after anti-CD20 injection), in contrast to treatment with the isotype control. Lymphocyte counts and B-cell numbers remained low in these treated groups for 8–20 d and were starting to return to normal levels in WT mice at the experimental endpoint (29 d post anti-CD20 inoculation). BL3750-bearing FcR-deficient mice were also used to assess whether lymphocyte and B-cell reductions were associated with a specific FcR pathway. FcRIII−/− and FcRIV−/− mice receiving anti-CD20/CD137 combination or anti-CD20 alone both demonstrated a significant reduction in lymphocyte and B-cell numbers (). It was only in FcγR−/− mice, lacking all activating FcR, that B cell depletion was compromised. This redundancy between FcγRIII and FcγRIV for normal B-cell depletion by anti-CD20/CD137 or anti-CD20 was interesting considering that B-cell lymphoma clearance was only FcγRIV dependent. Anti-CD137 was capable of transiently reducing peripheral blood lymphocyte numbers (not B cells) from at least day 5 post anti-CD20 inoculation (anti-CD137 one day later) until day 15 in WT mice (). None of the FcRs appeared important in this depletion by anti-CD137 mAb. Using the same schedule of treatment (Fig. S1A), lymphocyte depletion was also observed in mice not inoculated prior with BL3750 lymphoma cells (Fig S1B–C). Comparatively, minor neutropenia and monocyte level changes were detected in these same mice (Fig. S1D–E), and no depletion was detected for basophil, eosinophil, platelet and red blood cell (RBC) levels (Fig. S2). In addition, we failed to observe any change in basophil, eosinophil, monocyte, neutrophil, platelet and RBC levels in BL3750-bearing WT or FcR-deficient mice (Fig. S3–6).
Figure 2. Treatment with αCD20 and αCD137 reduces blood B-cell numbers in mice bearing B-cell lymphoma. Blood samples were collected for hemogram assessment according to the experimental design shown in (A). 1 × 105 BL3750 cells were injected i.v. at D0, and followed by treatment with control Ig (2A3, 250 μg i.p.) or anti-CD20 (clone 5D2, 250 μg i.p.) on day 4, and anti-CD137 (clone 3H3, 100 μg i.p.) either alone or in combination on day 5 in C57BL6 WT or various FcR-deficient mice, as indicated. Peripheral blood lymphocyte (B), and B cell numbers (C). Results are representative of one experiment n = 6 per group. Statistical analysis was calculated using Multiple T tests using the Holm–Sidak Method for multiple comparisons, where *p < 0.05 was considered statistically significant between respective anti-CD137 (clone 3H3), anti-CD20 (clone 5D2), 5D2 + 3H3 groups versus (2A3) isotype control-treated mice.
Combination of anti-CD20 and anti-CD137 therapy increases systemic cytokine levels
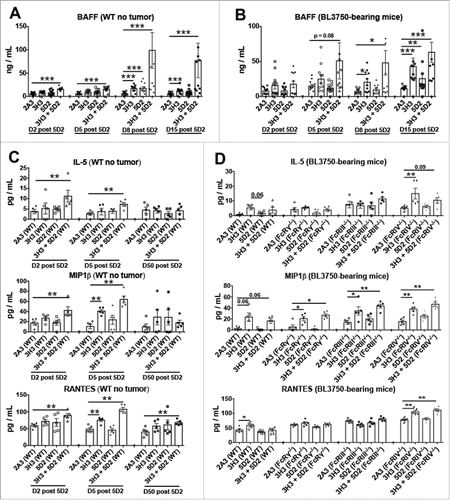
Terrier and colleagues Citation21 and Pranzatelli and colleagues Citation22 previously reported an increase in B-cell activating factor (BAFF) levels in lymphoma patients as the result of B-cell loss induced by rituximab treatment. To investigate whether BAFF was induced by the anti-CD20/CD137 treatment in mice with or without BL3750 tumors, we screened plasma samples. BAFF levels were increased by both anti-CD20 and anti-CD137 monotherapies, but more dramatically and earlier by the anti-CD20/CD137 combination therapy in both BL3750-injected and naive WT mice (). This likely reflects the level of normal B cell and B-cell lymphoma elimination liberating surface BAFF into the serum. It is also well known that chemokines are able to attract cells into specific compartments, and that pro-inflammatory cytokines may trigger tissue damage. Analysis of serum in the same mice revealed that Interleukin (IL)-5, Macrophage Inflammatory Protein (MIP)1β, and RANTES were primarily increased in tumor-free WT mice following combination anti-CD20/CD137 treatment (). However, in BL3750 lymphoma-bearing mice, only MIP1β was reproducibly increased in all groups of mice treated with anti-CD137 alone or anti-CD20/CD137 combination. Similar results were observed in various FcR-deficient mice, further suggesting that the increase in serum MIP1β was due to anti-CD137 (). We failed to detect any changes in plasma G-CSF, GM-CSF, KC, IL-2, IL-6, IL-17A, IP-10, MCP-1, MIG, MIP-1α, and TNFα levels.
Figure 3. Treatment with αCD20 and αCD137 induces BAFF and cytokines in plasma with or without B lymphoma inoculation. Control Ig (2A3, 250 μg i.p.) or anti-CD20 (clone 5D2, 250 μg i.p.) was administered on day (D) 0, and anti-CD137 (clone 3H3, 100 μg i.p.) either alone or in combination on D1 in C57BL6 wild-type (WT) or gene-targeted mice. In some experiments (B, D), 1 × 105 BL3750 cells were injected intravenously on day (D) 0, and control Ig (2A3, 250 μg i.p.) or anti-CD20 (clone 5D2, 250 μg i.p.) was administered on day (D) 4, and anti-CD137 (clone 3H3, 100 μg i.p.) either alone or in combination on D5 in WT or gene-targeted mice. (A) and (B) BAFF assessment from plasma of mice at D2, D5, D8 and D15 post anti-CD20 treatment. Data is representative of two experiment, n = 5 each group (n = 10 total).
Leukocyte infiltration into organs post anti-CD20 and anti-CD137
Considering that MIP1β and RANTES, both ligands for the CCR5 receptor, are chemokines that can attract several different immune cell subsets,Citation23 and based on the observation by Dubrot and colleagues of T cell recruitment to the liver of mice treated with anti-CD137,Citation24 we next assessed the levels of specific immune cells in different compartments during mono-/combination therapy using flow cytometry. B cells, monocytes/macrophages, neutrophils, NK cells, T CD4+ and T CD8+ cells were gated as exemplified in Fig. S7, from peripheral blood, bone marrow (BM), liver and spleen compartments of mice post combination or monotherapy treatments with anti-CD20 and anti-CD137. As expected, 5 d post anti-CD20 treatment, both anti-CD20 monotherapy and combination anti-CD20/CD137 therapy induced B-cell depletion, which was recovered 50 d post treatment in the BM and spleen (Fig. S8). In these same compartments, no difference was observed in monocyte/macrophages, NK cell, or T CD4+ and CD8+ cells. However, neutrophil counts were elevated in the spleen after anti-CD137 treatment, both with monotherapy (anti-CD137) and combination therapy (anti-CD20/CD137) (Fig. S8). Similar to the BM and spleen compartments, peripheral blood and liver B-cell counts were also decreased 5 d post treatment, and were recovered 50 d post combination (anti-CD20/CD137) or monotherapy (anti-CD20) treatments (Fig. S9). In peripheral blood, anti-CD137 treatment induced a decrease in the numbers of B cells and T CD4+ and T CD8+ cells. By contrast, a significant increase in the levels of monocytes, neutrophils, NK cells, T CD4+, and T CD8+ cells in liver was observed 5 d post treatment. Cell numbers largely returned to normal 50 d post combination (anti-CD20/CD137) or monotherapy (anti-CD137) treatments (Fig. S9).
Sustained combination of anti-CD20 and anti-CD137 therapy attenuates liver damage compared with anti-CD137 monotherapy
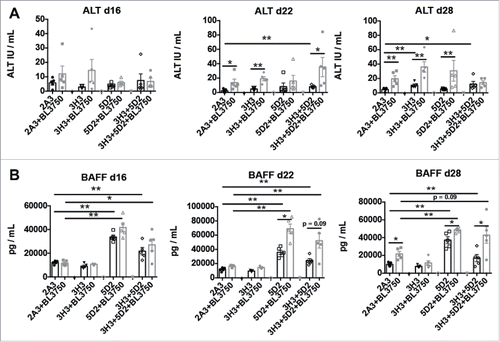
We next devised a more clinically relevant model consistent with advanced disease and late sustained treatment by monotherapy (anti-CD20 and anti-CD137) or combination therapy (administered three times—14, 18, and 24 d post BL3750 cell inoculation) (). Blood samples were acquired 16, 22, and 28 d post tumor inoculation for serum analysis, and livers were acquired at the end point (day 28) for assessment of liver damage. At the end point, using histology (H&E stain) we observed that livers with or without BL3750 tumors displayed visibly increased lymphocyte infiltration post anti-CD137 or anti-CD20/CD137 treatment (). We next performed a lactic dehydrogenase (LDH) reaction on the frozen sections from the endpoint liver samples. The LDH stain reveals the metabolic activity of a tested tissue, which loses LDH activity when inflamed and/or damaged Citation25,26 (). We observed that LDH quantification revealed a significant decrease in metabolic capability of liver samples after anti-CD137 monotherapy, compared with the controls. Surprisingly, livers from the combination therapy group (anti-CD20/CD137) or anti-CD20 monotherapy group did not display a similar reduction in metabolic capability. The presence or absence of tumor did not affect the liver damage outcome in this model (). Furthermore, Dubrot and colleagues previously reported that anti-CD137 treatment in naive mice triggered Caspase 3+ cells in liver tissue sections, indicative of apoptotic cells as a consequence of liver damage.Citation24 Here, we also detected more Caspase 3+ cells in the livers of mice treated with anti-CD137, while the presence of the BL3750 tumor itself also enhanced the Caspase 3 positivity in isotype control-treated mice (Fig. S10). Alanine aminotransferase (ALT) is a circulating biomarker that frequently correlates with liver damage, and its measurement in blood samples from patients is routinely used to assess the function of this organ in various disease states.Citation27 Dubrot and colleagues observed that anti-CD137 treatment in mice increased ALT levels, correlating with liver damage in their model.Citation24 We also assessed the plasma samples from the same mice depicted in . Here, we observed that plasma ALT levels increased slightly on days 22 and 28 post BL3750 lymphoma inoculation, but this increase was due to lymphoma and was independent of the treatment administered (). Plasma sampling also revealed that BAFF levels were elevated when anti-CD20 treatment was applied. Curiously, BAFF elevation was higher in the anti-CD20 monotherapy group than in the combination treatment group, particularly in the absence of BL3750 lymphoma cells ().
Figure 4. Sustained combination treatment of advanced tumor burden. (A) Experimental schedule of sustained 2A3, 3H3, 5D2, or 5D2 plus 3H3 treatment against advanced BL3750 lymphoma burden (treatment starting 14 d post BL3750 cells i.v. inoculation) as indicated. (B) H&E staining of livers 28 d after BL3750 inoculation reveals increased lymphocyte infiltration in 3H3 and 3H3 plus 5D2 treatment groups. Representative images from liver are shown. Scale bar = 1000 μm. Lymphocyte infiltrates (Ly) were observed in some treatments. (C) Lactic dehydrogenase reaction with eosin as background stain was performed in liver frozen sections (unfixed samples). Slides were scanned using 40x scan magnification. The Aperio Algorithm: Positive Pixel Count V9 was used to quantify positive pixels. A Hue Value = 0.65 was set up to exclude the background stain (eosin) from analysis and reveal the LDH stain intensity by pixels quantification (where yellow selected pixels = weak positive pixels; orange = positive pixels; red = strong positive pixels). (D) Representative LDH images from liver are shown in the first line according the treatment group, pixel selection by image analysis is represented in the line below. Scale bar = 1000 μm. (E): Weak, positive and strong positive pixel quantification from one field/liver/group are represented. Statistical analysis was performed using a Mann–Whitney test, **p < 0.01; n = 5.
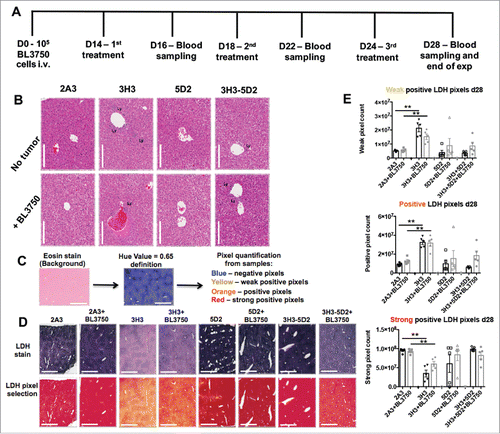
Figure 5. Circulating BAFF and ALT cell levels in mice after sustained combination therapy treatment of advanced lymphoma. Blood samples were collected for plasma assessment according to the experimental design shown in (A) BAFF was measured in plasma samples collected at day 16, day 22 and day 28 after tumor injection. Statistical analysis was performed using Mann–Whitney test, *p < 0.05 and **p < 0.01; n = 5 per group. (B) ALT was measured in plasma samples collected at the same time points after tumor injection. Statistical analysis was performed using Mann–Whitney test, *p < 0.05 and **p < 0.01; n = 5 per group.
Anti-CD137 at low doses or in combination with rituximab has demonstrated safety in clinical trials
To corroborate our preclinical findings with clinical trial observations, we compiled safety data for two clinically available anti-CD137 mAbs (urelumab from Bristol Myers Squibb and PF-05082566 from Pfizer) from past and ongoing clinical trials as monotherapy or in combination with rituximab (). High doses of anti-CD137 mAb monotherapy (≥1 mg/kg) were associated with a high incidence of moderate-to-severe hepatic toxicity, as measured by increases in serum ALT and aspartate aminotransferase (AST) levels. The most frequent treatment-related adverse events (AEs) were increased ALT and AST (27% each with urelumab). Additionally, high doses were associated with more frequent serious AEs (SAEs) (the most common being increased ALT and AST), discontinuation of therapy (16% with urelumab), and two treatment-related deaths due to hepatic toxicity. On the other hand, low doses of anti-CD137 mAb monotherapy (0.1 and 0.3 mg/kg) were associated with less frequent increases in liver transaminases (8–14% with urelumab), lower rates of therapy discontinuation (9–11% with urelumab), and no treatment-related deaths. Preliminary data from an ongoing trial of combination therapy of anti-CD137 mAb and rituximab in patients with NHL show a favorable safety profile, with no liver toxicity in any of the 38 patients enrolled and no AE beyond Grade 3. Collectively, these results suggest that anti-CD137 mAb at low doses or in combination with rituximab is associated with the lowest probability of liver toxicity and a safer clinical profile.
Table 1. Clinical trials of anti-CD137 mAb as monotherapy or in combination with rituximab.
Discussion
The immune system plays a key role in protecting against tumor initiation and cancer progression, and also contributes to immunopathology.Citation28 Although benefits of boosting the immune response against cancer are well recognized, several combinatorial therapy approaches are currently studied to test whether they are able to trigger a synergistic anticancer response by targeting cancer cells directly and boosting the immune system's antitumor response.Citation29-31 Considering the significant benefits of synergistic antitumor response against experimental B-cell lymphoma after combining a conventional B-cell lymphoma antibody (anti-CD20) with an enhancer of immune response (anti-CD137),Citation10 a new clinical trial is currently recruiting patients to assess the clinical benefit of combinatorial treatment with rituximab (anti-CD20) and urelumab (anti-CD137) for relapsed, refractory, or high-risk untreated chronic lymphocytic leukemia (CLL) patients (ClinicalTrials.gov identifier: NCT02420938).
Our results herein suggest that the combinatorial therapy benefit is dependent on FcγRIV specifically. Previous studies using a subcutaneous lymphoma have indicated that the anti-CD20 and anti-CD137 combination was critically dependent upon NK cells and clodronate-sensitive myeloid cells.Citation10 FcγRIV expression is restricted to myeloid cells and neutrophils,Citation20 so collectively the data might suggest that myeloid/granulocytes, rather than NK cells, are the major effectors in our model. However, the effector may vary according to the tumor site and microenvironment. Importantly, any associated effects of anti-CD137 monotherapy by itself, such as changes in lymphocyte levels or elevation of plasma IL-5, MIP1β or RANTES, were not associated with any FcRs. These results suggest that anti-CD137 directly regulates immune response via CD137 ligation, in an activating FcR-independent mechanism. Additionally, anti-CD137 treatment only displayed a beneficial antitumor effect when applied in combination with anti-CD20 therapy in our B-cell lymphoma model.
Regardless of antitumor benefit (anti-CD20 monotherapy), antitumor enhanced benefit (anti-CD20 and anti-CD137 combination therapy), or absence of modulation of antitumor response (anti-CD137), we observed systemic effects on circulating leukocytes and related cytokines in all the treatment conditions. Both increased BAFF and MIP1β levels associated with changes in different circulating leukocyte levels were already observed as a consequence of rituximab treatment in human B-cell lymphoma patients.Citation21,22,32 Considering that B cells are the most populous immune cells in the circulation, and that their depletion is followed by a late-onset recovery post treatment, Terrier and colleagues showed that patients treated with rituximab displayed neutropenia as a consequence of hematopoietic lineage competition due to high circulating BAFF levels inducing B-cell recovery.Citation21 In concert, Pranzatelli and colleagues also corroborated that patients treated with rituximab had increased levels of BAFF, in parallel to other cytokines and chemokines.Citation22
CD137 stimulation has already been shown to be associated with increased expression in levels of IL-5 Citation33 and RANTES.Citation34 Both MIP1-β and RANTES are chemokines that activate CCR5 and modulate the migratory activity of several lymphocytes,Citation23 while IL-5 can modulate the migratory activity of granulocytes such as eosinophils.Citation33 A previous study also showed a direct increase in liver lymphocyte infiltration following anti-CD137 (both blocking and non-blocking antibody clones) treatment, which also correlates with liver damage, demonstrated by indicators such as elevated serum transaminases and apoptotic cell events in liver sections.Citation24 In addition, the liver has already been described as the B cell and B-cell lymphoma depletion site targeted by anti-CD20 therapies via a liver macrophage phagocytosis-dependent mechanism.Citation35 In concert with these findings, we have observed a decrease in circulating lymphocyte levels, an increase in circulating cytokines and chemokines, and an increase in liver lymphocyte infiltration in our model. It has already been reported that human myeloid cells also express CD137, and its ligation can trigger an enhanced monocyte-derived cytokine secretion and cellular function.Citation36 One possible mechanism of the systemic consequences observed during monotherapies or combinatorial therapies, including increased circulating cytokines/chemokines and altered circulating and liver leukocyte populations, may be due to enhanced B cell and B-cell lymphoma phagocytosis mediated by a FcγRIV+ phagocytic cell population of resident macrophages in the liver, as well as enhanced cytokine/chemokine secretion from this compartment, directly affecting the circulatory leukocytes. For example, CCR5 ligands (RANTES and MIP1β) have already been associated with liver inflammation progression from steatosis to cirrhosis in non-alcoholic fatty liver disease. In the same study, authors demonstrated that experimental treatment with Maraviroc, a low toxicity antagonist of CCR5, could prevent liver inflammation and leukocyte chemotaxis to this compartment during liver disease.Citation37
However, CD137 ligation was shown to not only activate myeloid, T and NK cells, but also CD137+ endothelial cells, increasing their blood vessel permeability and allowing activated lymphocytes to migrate to the inflammatory tissues.Citation38 Another possible mechanism of the systemic consequences observed in this study may be due to activation of peripheral leukocyte subsets as a consequence of anti-CD137 treatment, as well as permeabilization of blood vessels, which aids these cells to migrate to the B cell/B-cell lymphoma depletion site. In agreement with Dubrot and colleagues,Citation24 we also observed that lymphocyte migration to the liver following anti-CD137 treatment may enhance liver toxicity, and does not contribute to therapeutic efficacy against tumor cells. Importantly, the combinatorial anti-CD137 plus anti-CD20 therapy did not enhance liver toxicity in our model. These results are in agreement with clinical findings reported in and highlight the potential of combination therapy to not only improve response rates but to also alleviate toxicity.
In summary, combination treatment with anti-CD20 and anti-CD137 suppressed experimental systemic B-cell lymphoma far more effectively than either monotherapy and in an FcRIV-dependent manner. Tumor suppression was accompanied by B-cell depletion, which was not dependent on one activating FcR. BAFF was elevated in the plasma of mice receiving anti-CD137 alone or in combination with anti-CD20, while a selective increase in some plasma cytokines was also noted and triggered by anti-CD137. These effects were independent of activating FcR. Sustained treatment of advanced lymphoma revealed increased lymphocyte infiltrates into the liver and a significant decrease in the metabolic capability of the liver in mice receiving anti-CD137. Importantly, these effects were not exacerbated in mice receiving the anti-CD20/CD137 combination. Our study results suggest that anti-CD137 treatment may trigger liver toxicity, which may be modulated by using an optimal dosage of treatment, independent of B cell/B-cell lymphoma depletion due to anti-CD20 treatment. Deciphering which of the treatment consequences are contributing to toxicity, and are not essential for therapeutic success, may suggest complementary therapeutic strategies to prevent undesirable side effects associated with the therapeutic benefit of combinatorial treatment. Evaluation of anti-CD137 safety data from clinical trials showed that significant liver toxicity was strongly associated with monotherapy at doses ≥1 mg/kg, while lower doses were demonstrated to be safe and efficacious. Additional studies are necessary to find out which specific lymphocyte subset is responsible for causing anti-CD137-associated liver toxicity and to investigate escalation of dose-effect for an optimal antibody concentration treatment with minimal liver side effects. These studies further support the continued clinical evaluation of anti-CD137 as monotherapy and in combination with other immunotherapies such as rituximab.
Materials and methods
Mice
C57BL/6J wild-type (WT) mice were purchased from the Walter and Eliza Hall Institute of Medical Research and housed at the QIMR Berghofer Medical Research Institute. FcRIII−/−, FcRIV−/−, and FcRγ−/−mice have been previously described,Citation19,20,39 and were bred and maintained on a C57BL/6J background at the QIMR Berghofer Medical Research Institute. All mice used were between the ages of 6 and 14 weeks, and all experiments were approved by QIMR Berghofer Medical Research Institute animal ethics committee.
Cell culture
The mouse CD20+ B-cell line BL3750, a C57BL/6J B-cell lymphoma, was developed in a cMyc transgenic mouse,Citation5 and obtained by Professor Thomas Tedder (Duke University, North Carolina, USA). BL3750 was cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μM 2-mercaptoethanol (Sigma-Aldrich). Cells were grown in suspension culture at 37°C in 5% CO2.
In vivo tumor transplantation and mAb therapy
As previously described,Citation5 1 × 105 BL3750 cells were injected i.v. into the tail vein of mice. In vivo mAb treatment was performed as previously described.Citation10 For most experiments, on day 4 post BL3750 lymphoma inoculation, anti-CD20 mAb (clone 5D2, Genentech) Citation16 or isotype control (2A3, Bio X Cell) was administered (250 μg i.p. per treatment). On day 5 post BL3750 lymphoma inoculation, anti-CD137 mAb (3H3, Bio X Cell) was administered (100 μg i.p. per injection). A minimum of five mice per group was assessed for survival benefit in each experiment. For sustained treatment using a high tumor burden setting, 1 × 105 BL3750 cells were injected i.v. into the tail vein of mice. As indicated, isotype control (2A3) and anti-CD20 mAb (5D2) was given on days 14, 18, and 24 post BL3750 inoculation (250 μg i.p. per treatment), while anti-CD137 mAb (3H3) was given administered on days 15, 19, and 25 post BL3750 inoculation (100 μg i.p. per injection).
Hemogram analysis
Blood samples from mice, with or without tumor, were taken at several time points (Day 0 (baseline), and 2, 5, 8, 15, 22, 29, 36, 42, and 50 d post 5D2 antibody treatment) for hemogram or cytokine analysis by retro-orbital bleeding into 0.5 mL K3EDTA MiniCollect Tubes (Greiner Bio-One). Samples were then subsequently analyzed with a Hemavet Counter (Drew Scientific) as previously described,Citation40 to enumerate the following cell types: basophils, eosinophils, lymphocytes, monocytes, neutrophils, platelets, and RBCs. For the sustained treatment of advanced BL3750 lymphoma burden, blood was sampled on days 16, 22, and 28 post tumor injection.
Flow cytometry analysis
Single cell suspensions from various organs were prepared as previously described,Citation41 and incubated for at least 15 min in Fc blocking buffer (2.4G2 antibody). Cells were then stained with the following antibodies: anti-mouse- B220 (RA3-6B2), -CD4 (GK1.5), -CD8 (SK1), -CD19 (6D5), -Ly6C (HK1.4), -Ly6G (1A8), -NKp46 (29A1.4), -NK1.1 (PK136), -TCRβ (H57-597) purchased from either eBiosciences or Biolegend. Zombie Yellow, or Zombie UV, and Fixable Viability Kit (Biolegend) were used to assess viability. Leukocyte quantification was assessed by addition of Liquid Counting Beads (BD Biosciences) prior to flow cytometric acquisition using LSR II Fortessa Flow Cytometer (BD Biosciences). Analysis was performed using FlowJo (Treestar) software.
Cytokine and ALT detection
Cytokines from plasma isolated throughout the duration of in vivo assays were detected using Cytometric Bead Array (CBA) technology (BD Biosciences) according to the manufacturer's instructions: G-CSF, GM-CSF, KC, IL-2, IL-5, IL-6, IL-17A, MCP-1, MIG, MIP-1α, MIP-1β, RANTES, and TNFα. Similarly, plasma cytokines were detected using Duoset ELISA kits following manufacturer's instructions (R&D Systems): BAFF and IP-10. Liquid ALT (SGPT) Reagent Set from Pointe Scientific was used according to manufacturer's instructions to read ALT absorbance per sample.
Liver histopathology
Mouse livers from sustained treatment of advanced BL3750 lymphoma burden were sampled 28 d post tumor injection. For Caspase-3 immunohistochemistry (IHC) and hematoxin-eosin (HE) stains, one fresh liver lobe was fixed in formalin 10%-buffered saline for 48 h and paraffin-processed. For LDH histochemistry, one fresh liver lobe was included in OCT media and frozen for cryo-sections. H&E stain was performed by a program set up in a Leica XL Stainer (Leica), as routinely processed by the QIMR Berghofer's Histology Facility.
Caspase-3 IHC: Endogenous peroxidase activity was blocked by incubating the sections in 1.0% H2O2 in PBS for 5 min. Background sniper (Biocare Medical) + 2% BSA were applied for 20 min. Excess normal sniper was decanted from the sections, and the anti-Caspase 3 antibody (Clone ASP175, Cell Signaling Technology) diluted 1:80 in Sniper + 2% BSA applied for 60 min at room temperature. Sections were washed in three changes of PBS for 1 min each. MACH2 anti-rabbit HRP secondary (Biocare Medical) was applied for 30 min at room temperature. Sections were washed in three changes of PBS for 1 min each. Color was developed in Vector Dab Substrate Kit (Vector labs) for 5 to 10 min. Sections were washed in gently running tap water for 5–10 min to remove excess chromogen. Sections were lightly counterstained in Mayers' haematoxylin, then dehydrated through ascending graded alcohols, cleared in xylene, and mounted using DePeX mounting media (Sigma Aldrich). Slides were scanned using an Aperio XT slide scanner using 40x scan magnification. For the image analysis and staining quantification, the Aperio Algorithm: Positive Pixel Count V9 was used to quantify positive pixels from one section per liver sample. A Hue Value = 0.1 was set up to select DAB (Caspase 3) staining and quantify the respective pixels (selected as yellow by the image analysis) from different groups.
LDH histochemistry: This procedure is a modified staining procedure for lactate dehydrogenase described by Balough et al.Citation25,26 7 μm frozen sections were dried overnight. Sections were washed in distilled water to remove OCT. Sections were placed in LDH incubation medium (5 mL Veronal Buffer pH 7.4, 2.5 mg Nitro Blue Tetrazolium Chloride (NBTC), 1.5 mg Beta-Nicotinamided Adenine Dinucleotide (NADH), 15.0 mg Lactic Acid-Sodium Salt) 3 to 12 h at 4oC in the dark, and then allowed to come to room temperature and rinsed in Veronal Acetate Buffer for 15 min. Slides were then washed in running water for 5 min and washed in 90% ethanol 1 min. Slides were counterstained in 0.3% alcoholic Eosin for 2–5 s (1 or 2 dips), dehydrated through 100% ethanol, and then dried. Cover slips with Biocare Medical Ecomount mountant (Biocare Medical) were added to mount slides. Slides were scanned using an Aperio XT slide scanner using 40x scan magnification. For image analysis, the Aperio Algorithm: Positive Pixel Count V9 was used to quantify positive pixels per liver section/sample. A Hue Value = 0.65 was set up to exclude the background stain (Eosin) from analysis and reveal the LDH stain intensity by pixels quantification (where yellow selected pixels = weak positive pixels; orange = positive pixels; red = strong positive pixels).
Patients, study design and assessment
Please refer to anti-CD137 monotherapy clinical trials (NCT00309023, NCT00612664, NCT01471210, and NCT01307267) and anti-CD137/Rituximab combination trials (NCT01775631 and NCT01307267) on ClinicalTrials.gov for details on study design, patient eligibility and assessment studies.
Statistical analysis
Statistical analysis was achieved using GraphPad Prism or SPSS software. Data was considered to be statistically significant where the p value was less than 0.05. For mouse models, statistical tests used were the Multiple T tests using Holm–Sidak Method for multiple comparisons, the Mann–Whitney test, and the Mantel–Cox Log Rank test for survival.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
F.S.F.G., H.E.K., and M.J.S. designed research, supervised work, and wrote the paper. F.S.F.G., S.B., A.M., C.C., and M.J.S. performed the research. F.S.F.G., H.E.K., and M.J.S. analyzed the data.
KONI_A_1192740_s02.pdf
Download PDF (1.8 MB)Acknowledgments
We thank Clay Winterford, Deborah Knight, Leesa Wockner, Liam Town, Nigel Waterhouse, Steven Lane, Tam Nguyen for providing technical support, reagents, discussion, comments, and advice in this project. The authors acknowledge Genentech for providing 5D2 mAb and Tom Tedder for the BL3750 lymphoma cells. This manuscript is dedicated to the memory of our colleague and friend, Dr. Holbrook Edwin Kohrt, who was a distinguished clinician scientist in the field of cancer immunotherapy.
Funding
M.J.S was supported by a National Health and Medical Research Council (NH&MRC) Senior Research Fellowship (1078671). F.S.F.G. was supported by a NH&MRC Early Career Fellowship (1088703), a National Breast Cancer Foundation (NBCF) Fellowship (PF-15-008), and Cancer Cure Australia Priority-Driven Young Investigator Project Grant (1082709).
References
- Maloney DG. Anti-CD20 antibody therapy for B-cell lymphomas. N Eng J Med 2012; 366:2008-16; PMID:22621628; http://dx.doi.org/10.1056/NEJMct1114348
- Blombery PA, Wall M, Seymour JF. The molecular pathogenesis of B-cell non-Hodgkin lymphoma. Eur J Haematol 2015; 95:280-93; PMID:25996166; http://dx.doi.org/10.1111/ejh.12589
- Azim HA, Jr, de Azambuja E, Colozza M, Bines J, Piccart MJ. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Annals Oncol 2011; 22:1939-47; PMID:21289366; http://dx.doi.org/10.1093/annonc/mdq683
- Stone JB, DeAngelis LM. Cancer-treatment-induced neurotoxicity-focus on newer treatments. Nat Rev Clin Oncol. 2016 Feb; 13(2):92–105; PMID:26391778; http://dx.doi.org/10.1038/nrclinonc.2015.152
- Minard-Colin V, Xiu Y, Poe JC, Horikawa M, Magro CM, Hamaguchi Y, Haas KM, Tedder TF. Lymphoma depletion during CD20 immunotherapy in mice is mediated by macrophage FcgammaRI, FcgammaRIII, and FcgammaRIV. Blood 2008; 112:1205-13; PMID:18495955; http://dx.doi.org/10.1182/blood-2008-01-135160
- Lim SH, Levy R. Translational medicine in action: anti-CD20 therapy in lymphoma. J Immunol 2014; 193:1519-24; PMID:25086174; http://dx.doi.org/10.4049/jimmunol.1490027
- Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, Fuseri N, Bonnafous C, Czerwinski D, Rajapaksa A et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood 2014; 123:678-86; PMID:24326534; http://dx.doi.org/10.1182/blood-2013-08-519199
- Sanmamed MF, Pastor F, Rodriguez A, Perez-Gracia JL, Rodriguez-Ruiz ME, Jure-Kunkel M, Melero I. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Seminars Oncol 2015; 42:640-55; PMID:26320067; http://dx.doi.org/10.1053/j.seminoncol.2015.05.014
- Yonezawa A, Dutt S, Chester C, Kim J, Kohrt HE. Boosting cancer immunotherapy with anti-CD137 antibody therapy. Clin Cancer Res 2015; 21:3113-20; PMID:25908780; http://dx.doi.org/10.1158/1078-0432.CCR-15-0263
- Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, Müller A, Pachynski R, Czerwinski D, Coutre S et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood 2011; 117:2423-32; PMID:21193697; http://dx.doi.org/10.1182/blood-2010-08-301945
- Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, Colevas AD, Weng WK, Clarke MF, Carlson RW et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest 2012; 122:1066-75; PMID:22326955; http://dx.doi.org/10.1172/JCI61226
- Kohrt HE, Colevas AD, Houot R, Weiskopf K, Goldstein MJ, Lund P, Mueller A, Sagiv-Barfi I, Marabelle A, Lira R et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest 2014; 124:2668-82; PMID:24837434; http://dx.doi.org/10.1172/JCI73014
- Niu L, Strahotin S, Hewes B, Zhang B, Zhang Y, Archer D, Spencer T, Dillehay D, Kwon B, Chen L et al. Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice. J Immunol 2007; 178:4194-213; PMID:17371976; http://dx.doi.org/10.4049/jimmunol.178.7.4194
- Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Seminars Oncol 2010; 37:508-16; PMID:21074066; http://dx.doi.org/10.1053/j.seminoncol.2010.09.008
- Makkouk A, Chester C, Kohrt HE. Rationale for anti-CD137 cancer immunotherapy. Eur J Cancer 2016; 54:112-9; PMID:26751393; http://dx.doi.org/10.1016/j.ejca.2015.09.026
- Sarikonda G, Sachithanantham S, Manenkova Y, Kupfer T, Posgai A, Wasserfall C, Bernstein P, Straub L, Pagni PP, Schneider D et al. Transient B-cell depletion with anti-CD20 in combination with proinsulin DNA vaccine or oral insulin: immunologic effects and efficacy in NOD mice. PloS One 2013; 8:e54712; PMID:23405091; http://dx.doi.org/10.1371/journal.pone.0054712
- Wu ZQ, Khan AQ, Shen Y, Wolcott KM, Dawicki W, Watts TH, Mittler RS, Snapper CM. Four-1BB (CD137) differentially regulates murine in vivo protein- and polysaccharide-specific immunoglobulin isotype responses to Streptococcus pneumoniae. Infect Immun 2003; 71:196-204; PMID:12496166; http://dx.doi.org/10.1128/IAI.71.1.196-204.2003
- Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012; 119:5640-9; PMID:22535666; http://dx.doi.org/10.1182/blood-2012-01-380121
- Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, Schmidt RE, Sandor M, Capel PJ, Daëron M et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity 1996; 5:181-8; PMID:8769481; http://dx.doi.org/10.1016/S1074-7613(00)80494-X
- Nimmerjahn F, Lux A, Albert H, Woigk M, Lehmann C, Dudziak D, Smith P, Ravetch JV. FcgammaRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc Natl Acad Sci U S A 2010; 107:19396-401; PMID:20974962; http://dx.doi.org/10.1073/pnas.1014515107
- Terrier B, Ittah M, Tourneur L, Louache F, Soumelis V, Lavie F, Casadevall N, Candon S, Hummel A, Mariette X et al. Late-onset neutropenia following rituximab results from a hematopoietic lineage competition due to an excessive BAFF-induced B-cell recovery. Haematologica 2007; 92:e20-3; PMID:17405749; http://dx.doi.org/10.3324/haematol.11031
- Pranzatelli MR, Tate ED, Travelstead AL, Verhulst SJ. Chemokine/cytokine profiling after rituximab: reciprocal expression of BCA-1/CXCL13 and BAFF in childhood OMS. Cytokine 2011; 53:384-9; PMID:21211990; http://dx.doi.org/10.1016/j.cyto.2010.12.004
- Mueller A, Strange PG. The chemokine receptor, CCR5. Int J Biochem Cell Biol 2004; 36:35-8; PMID:14592532; http://dx.doi.org/10.1016/S1357-2725(03)00172-9
- Dubrot J, Milheiro F, Alfaro C, Palazon A, Martinez-Forero I, Perez-Gracia JL, Morales-Kastresana A, Romero-Trevejo JL, Ochoa MC, Hervás-Stubbs S et al. Treatment with anti-CD137 mAbs causes intense accumulations of liver T cells without selective antitumor immunotherapeutic effects in this organ. Cancer Immunol Immunotherapy 2010; 59:1223-33; PMID:20336294; http://dx.doi.org/10.1007/s00262-010-0846-9
- Balogh K, Dudley RH, Cohen RB. Oxidative enzyme activity in skeletal cartilage and bone. Lab Invest 1961; 10:839-45
- Sherwood ME, Flotte TJ. Improved staining method for determining the extent of thermal damage to cells. Lasers Surg Med 2007; 39:128-31; PMID:17163480; http://dx.doi.org/10.1002/lsm.20450
- Sookoian S, Pirola CJ. Alanine and aspartate aminotransferase and glutamine-cycling pathway: their roles in pathogenesis of metabolic syndrome. World J Gastroenterol 2012; 18:3775-81; PMID:22876026; http://dx.doi.org/10.3748/wjg.v18.i29.3775
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565-70; PMID:21436444; http://dx.doi.org/10.1126/science.1203486
- Krasnova Y, Putz EM, Smyth MJ, Souza-Fonseca-Guimaraes F. Bench to bedside: NK cells and control of metastasis. Clin Immunol 2015; PMID:26476139; pii: S1521-6616(15)30050-4; http://dx.doi.org/10.1016/j.clim.2015.10.001
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015; 14:561-84; PMID:26228759; http://dx.doi.org/10.1038/nrd4591
- Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol 2016; 13:143-58; PMID:26598942; http://dx.doi.org/10.1038/nrclinonc.2015.209
- Wasmuth JC, Fischer HP, Sauerbruch T, Dumoulin FL. Fatal acute liver failure due to reactivation of hepatitis B following treatment with fludarabine/cyclophosphamide/rituximab for low grade non-Hodgkin's lymphoma. Eur J Med Res 2008; 13:483-6; PMID:19008178
- Cho YS, Kwon B, Lee TH, Kim TB, Moon KA, La S, Lee J, Lee SD, Oh YM, Moon HB. 4-1 BB stimulation inhibits allergen-specific immunoglobulin E production and airway hyper-reactivity but partially suppresses bronchial eosinophilic inflammation in a mouse asthma model. Clin Exp Allergy 2006; 36:377-85; PMID:16499650; http://dx.doi.org/10.1111/j.1365-2222.2006.02445.x
- Vinay DS, Lee SJ, Kim CH, Oh HS, Kwon BS. Exposure of a distinct PDCA-1+ (CD317) B cell population to agonistic anti-4-1BB (CD137) inhibits T and B cell responses both in vitro and in vivo. PloS One 2012; 7:e50272; PMID:23185591; http://dx.doi.org/10.1371/journal.pone.0050272
- Montalvao F, Garcia Z, Celli S, Breart B, Deguine J, Van Rooijen N, Bousso P. The mechanism of anti-CD20-mediated B cell depletion revealed by intravital imaging. J Clin Invest 2013; 123:5098-103; PMID:24177426; http://dx.doi.org/10.1172/JCI70972
- Kienzle G, von Kempis J. CD137 (ILA/4-1BB), expressed by primary human monocytes, induces monocyte activation and apoptosis of B lymphocytes. Int Immunol 2000; 12:73-82; PMID:10607752; http://dx.doi.org/10.1093/intimm/12.1.73
- Perez-Martinez L, Perez-Matute P, Aguilera-Lizarraga J, Rubio-Mediavilla S, Narro J, Recio E, Ochoa-Callejero L, Oteo JA, Blanco JR. Maraviroc, a CCR5 antagonist, ameliorates the development of hepatic steatosis in a mouse model of non-alcoholic fatty liver disease (NAFLD). J Antimicrobial Chemotherapy 2014; 69:1903-10; PMID:24651825; http://dx.doi.org/10.1093/jac/dku071
- Palazon A, Teijeira A, Martinez-Forero I, Hervas-Stubbs S, Roncal C, Penuelas I, Dubrot J, Morales-Kastresana A, Pérez-Gracia JL, Ochoa MC et al. Agonist anti-CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes. Cancer Res 2011; 71:801-11; PMID:21266358; http://dx.doi.org/10.1158/0008-5472.CAN-10-1733
- Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell 1994; 76:519-29; PMID:8313472; http://dx.doi.org/10.1016/0092-8674(94)90115-5
- Bruedigam C, Bagger FO, Heidel FH, Paine Kuhn C, Guignes S, Song A, Austin R, Vu T, Lee E, Riyat S et al. Telomerase inhibition effectively targets mouse and human AML stem cells and delays relapse following chemotherapy. Cell Stem Cell 2014; 15:775-90; PMID:25479751; http://dx.doi.org/10.1016/j.stem.2014.11.010
- Souza-Fonseca-Guimaraes F, Young A, Mittal D, Martinet L, Bruedigam C, Takeda K, Andoniou CE, Degli-Esposti MA, Hill GR, Smyth MJ. NK cells require IL-28R for optimal in vivo activity. Proc Natl Acad Sci U S A 2015; 112:E2376-84; PMID:25901316; http://dx.doi.org/10.1073/pnas.1424241112
- Sznol M, Hodi FS, Margolin K, McDermott DF, Ernstoff MS, Kirkwood JM, Wojtaszek C, Feltquate D, Logan T. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA). J Clin Oncol 2008; 26:3007; Abstract number:TPS3107
- Melero I, Gangadhar TC, Kohrt HE, Segal NH, Logan T, Urba WJ, Hodi FS, Ott PA, Perez-Garcia JL, Wolchok JD et al. A phase I study of the safety, tolerability, pharmacokinetics, and immunoregulatory activity of urelumab (BMS-663513) in subjects with advanced and/or metastatic solid tumors and relapsed/refractory B-cell non-Hodgkin's lymphoma (B-NHL). J Clin Oncol 2013; 32
- Segal NH, L T, Hodi FS, McDermott D, Melero I, Hamid O, Schmidt H, Robert C, Chiarion-Sileni V, Ascierto PA et al. Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Submitted 2016
- Kohrt HE, Godwin JE, Lossos IS, Williams ME, Timmerman J, Link BK, Goldberg SM, McGirr A, Kurland JF, Wigginton JM et al. A phase Ib, open-label, multicenter study of urelumab (BMS-663513) in combination with rituximab in subjects with relapsed/refractory B-cell malignancies. J Clin Oncol 2013; 31; Abstract number:TPS3108
- Segal NH, Gopal AK, Bhatia S, Kohrt HE, Levy R, Pishvaian MJ, Houot R, Bartlett N, Nghiem P, Kronenberg SA. A phase 1 study of PF-05082566 (anti-4-1BB) in patients with advanced cancer. J Clin Oncol 2014; Suppl; abstr 3007; PMID:24934783
- Gopal AK, Bartlett NL, Levy R, Houot R, Smith SD, Segal NH, Thall AD, Mugundu G, Huang B, Davis C et al. A phase I study of PF-05082566 (anti-4-1BB) + rituximab in patients with CD20+ NHL. J Clin Oncol 2015; Suppl; abstr 3004
