ABSTRACT
Cancer immunotherapy is a promising therapeutic avenue; however, in practice its efficacy is hampered by an immunosuppressive tumor microenvironment that consists of suppressive cell types like myeloid-derived suppressor cells (MDSCs). Eradication or reprogramming of MDSCs could therefore enhance clinical responses to immunotherapy. Here, we review clinically available drugs that target MDSCs, often through inhibition of STAT signaling, which is essential for MDSC accumulation and suppressive functions. Interestingly, several drugs used for non-cancerous indications and natural compounds similarly inhibit MDSCs by STAT inhibition, but have fewer side effects than anticancer drugs. Therefore, they show great potential for combination strategies with immunotherapy.
Abbreviations
| APC | = | Antigen-presenting cell |
| ATRA | = | All-trans-retinoic acid |
| DC | = | Dendritic cell |
| GM-CSF | = | Granulocyte macrophage colony-stimulating factor |
| G-MDSC | = | Granulocytic myeloid-derived suppressor cell |
| HNSCC | = | Head and neck squamous cell carcinoma |
| IFN | = | Interferon |
| iNOS | = | inducible nitric oxide synthase |
| JAK | = | Janus kinase |
| MDSC | = | Myeloid-derived suppressor cell |
| M-MDSC | = | Monocytic myeloid-derived suppressor cell |
| NOX | = | NADPH oxidase |
| PDE | = | Phosphodiesterase |
| PGE2 | = | Prostaglandin E2 |
| PPAR | = | Peroxisome proliferator-activated receptor |
| RCC | = | Renal cell carcinoma |
| ROS | = | Reactive oxygen species |
| STAT | = | Signal transducer and activator of transcription |
| TCR | = | T cell receptor |
| TGF | = | Transforming growth factor |
| TME | = | Tumor microenvironment |
| Treg | = | Regulatory T cell |
| VEGF | = | Vascular endothelial growth factor |
The immunosuppressive tumor microenvironment
In the past decade, cancer research has focused on the development of novel strategies, such as targeted therapies and immunotherapy, many of which have been approved for clinical use. These novel modalities are based on targeting specific pathways exploited by cancers using small molecule inhibitors or on empowering the immune system to eradicate cancer cells. Targeting immune checkpoints like cytotoxic T lymphocyte-associated protein 4 and programmed cell death protein 1 shows impressive results.Citation1 Other promising immunotherapies include adoptive cell transfer with tumor-infiltrating lymphocytes, vaccination with tumor-associated antigens and dendritic cell (DC)-based vaccines. Although these therapies show survival benefits and have lower incidences of lethal drug resistance than traditional chemotherapy, still not every cancer patient benefits from them.Citation2 One of the challenges that remains is generated by the tumors themselves, as they can evade immune responses by modulating the immune system in their local microenvironment.Citation3 This tumor-engineered local environment has been termed the immunosuppressive tumor microenvironment (TME), as it very effectively suppresses antitumor immune responses. Myeloid-derived suppressor cells (MDSCs) are key players in the TME and studies showing the importance of MDSCs in pathological conditions have accumulated in the past years. Many of these studies report an increased frequency of MDSCs in the blood of patients suffering from different types of cancer.Citation4,5 In addition, the presence of MDSCs in the TME is correlated with decreased efficacy of immunotherapies, including adoptive cell therapy, DC vaccination and ipilimumab treatment,Citation6-8 making MDSCs an important target for enhancing the efficacy of these therapies. This is substantiated by experiments in mice where eradication of MDSCs increased the efficacy of anticancer vaccines, adoptive cell therapy and anti-vascular endothelial growth factor (VEGF) antibody therapy.Citation9-11
Here, we discuss the role of MDSCs in the immunosuppressive TME and detail the role of Signal Transducers and Activators of Transcription (STAT) proteins in MDSC accumulation and suppressive mechanisms. We elaborate on the potential of several clinically available drugs and natural compounds to inhibit MDSCs as an unintended effect, often mediated by STAT inhibition. Ultimately, we present some interesting strategies for combination regimens of these drugs and natural compounds with immunotherapy. The insights we discuss in this review relieve immunosuppression by targeting MDSCs and likely result in enhancement of antitumor immune responses by immunotherapy.
Myeloid-derived suppressor cells
In healthy individuals, myeloid progenitor cells and immature myeloid cells arise in the bone marrow and mature into granulocytes, macrophages or DCs. However, during cancer progression, tumor-derived factors, like granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulate myelopoiesis, but disturb maturation.Citation12 This leads to the appearance of a heterogeneous population of immature myeloid cells in the blood that have the morphology of granulocytes or monocytes, but lack some of the markers expressed by these cells.Citation13 Based on their ability to efficiently inhibit T cell function, these cells are referred to as MDSCs. In mice, MDSCs can be identified by the expression of Gr-1 and CD11b and can be subdivided into granulocytic or monocytic MDSCs (G-MDSCs or M-MDSCs) based on the expression of Ly6G or Ly6C, respectively.Citation14 In humans, adequate characterization is challenging due to the lack of specific markers. As a consequence, MDSCs have been defined by different marker combinations in different studies.Citation15 Generally, MDSCs can be defined as CD33+CD11b+HLA-DR−/low cells that can be further subdivided into G-MDSCs or M-MDSCs by the co-expression of either CD15 or CD14, respectively.Citation16 The importance for clinical outcome of the frequency of either MDSC subtype differs across cancer types. For example, high numbers of M-MDSCs, but not G-MDSCs, are associated with negative response of non-small-cell lung cancer patients to platinum-based chemotherapy and the combination treatment of platinum with bevacizumab.Citation17 Furthermore, elevated frequencies of M-MDSCs are also associated with decreased survival of melanoma patients, regardless of previous therapy.Citation18
MDSC suppressive mechanisms inhibit T cell development and function
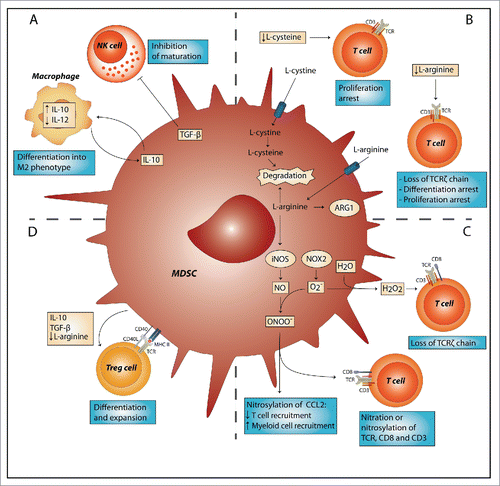
After activation, MDSCs can inhibit both innate and adaptive arms of the immune system. They affect the innate immune system mostly indirect by secretion of immune inhibitory cytokines like IL-10 and transforming growth factor (TGF)-β, driving macrophages to a suppressive M2 phenotype,Citation19 and negatively affecting natural killer cell maturation, respectively ().Citation20 The effect of MDSCs on adaptive immunity is more direct, involving the suppression of T cells, using several mechanisms. First, MDSCs inhibit T cell function and proliferation by depleting the essential amino acids L-arginine and L-cysteine from the TME (). L-arginine is a substrate for arginase-I and inducible nitric oxide synthase (iNOS), which are both highly expressed by MDSCs.Citation13 Depletion of L-arginine leads to loss of the T cell receptor (TCR)ζ chain, resulting in decreased growth and differentiation.Citation21 Similarly, MDSCs can deplete L-cysteine from the TME, resulting in decreased proliferation and activation of T cells.Citation22 Second, MDSCs produce reactive oxygen (ROS) and nitrogen species, like hydrogen peroxide (H2O2) and peroxynitrite (ONOO−) (). iNOS produces NO after T-cell-derived interferon (IFN)γ stimulation, which subsequently forms peroxynitrite after reacting with a superoxide anion (O2·−).Citation23 Superoxide anions are produced by NADPH oxidase (NOX) and can react with water to form H2O2. ONOO− causes nitration and nitrosylation of components of the TCR signaling complex and H2O2 causes loss of the TCRζ-chain, both thus decreasing T cell activation.Citation24,25 ONOO− release also leads to nitrosylation of chemokines like CCL2, resulting in decreased recruitment of tumor-infiltrating T cells and high infiltration of immunosuppressive myeloid cells, including tumor-associated macrophages and MDSCs.Citation26 Lastly, MDSCs can induce the development of regulatory T cells (Tregs), and expand the existing Treg population, both of these mechanisms requiring direct cell–cell contact ().Citation27,28 The secretion of several factors by MDSCs, including, TGF-β and IL-10 might be involved in this process, although the mechanism is still unclear.Citation29 Finally, L-arginine depletion by MDSCs also contributes to Treg expansion.Citation28
Figure 1. MDSC-suppressive mechanisms target innate and adaptive arms of the immune system. (A) Myeloid-derived suppressor cells (MDSCs) can inhibit the innate immune system by TGF-β-induced inhibition of NK cell function and induction of a M2 macrophage phenotype by secretion of IL-10. (B) MDSCs deprive T cells of amino acids L-cysteine and L-arginine, which are essential for proliferation and differentiation. (C) MDSCs release reactive oxygen species, such as hydrogen peroxide (H2O2) and peroxynitrite (ONOO−). H2O2 causes loss of the T cell receptor (TCR)ζ-chain and peroxynitrite causes nitration and nitrosylation of chemokines like CCL2 and components of the TCR signaling complex, thereby both inhibiting T cell activation and recruitment. (D) MDSCs induce the development of regulatory T cells (Tregs) or expand existing Treg cell populations; these effects are mediated by interaction of the TCR with MHC-II and CD40 with CD40L. Furthermore, secretion of factors like IL-10 and TGF-β, and deprivation of L-arginine by MDSCs induce Treg polarization. ARG1, arginase 1; CCL2, chemokine (C–C motif) ligand 2; iNOS, inducible nitric oxide synthase; NOX2, NADPH oxidase 2; NO, nitric oxide; NK, natural killer; TGF-β, transforming growth factor-β; IL, interleukin.
STAT protein signaling is important in regulation of MDSCs
MDSC expansion and suppressive mechanisms are mainly regulated by the STAT signaling pathway. This protein family consists of seven proteins that regulate many vital cellular functions, such as proliferation and cell survival. They are activated through binding of cytokines or growth factors to their receptors, leading to activation of Janus kinase (JAK) tyrosine kinases, which phosphorylate STAT proteins. The phosphorylated STATs then translocate to the nucleus and regulate the expression of STAT target genes.Citation30
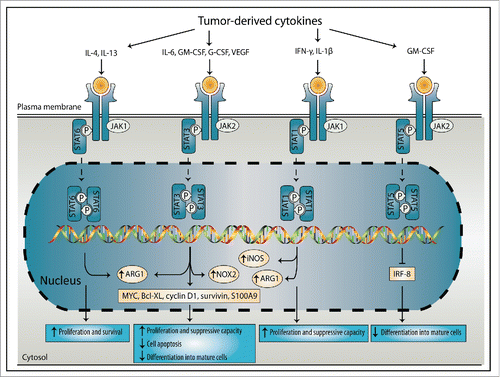
Many tumors exploit STAT signaling through the secretion of tumor-derived factors (). This hijacking of STAT signaling plays an important role during cancer initiation and progression and in maintaining an immunosuppressive TME, for instance by inducing accumulation of MDSCs or stimulation of their suppressive capacity.Citation12 Tumor-derived factors, like G-CSF, GM-CSF and VEGF, induce STAT3 signaling, resulting in increased expression of proliferation-inducing and anti-apoptotic proteins, including c-Myc, Bcl-XL, cyclin D1 and survivin. These proteins promote proliferation of immature myeloid cells, while preventing apoptosis and differentiation into mature cells, resulting in increased MDSC frequencies.Citation31 Additionally, STAT3 directly regulates MDSC suppressive mechanisms by inducing NOX2 expression,Citation32 and arginase production.Citation33 STAT3 also induces the gene expression and protein level of the pro-inflammatory protein S100A9 in myeloid progenitors. Overexpression of S100A9 prevents differentiation into mature myeloid cell types by directly facilitating ROS production, resulting in expansion of MDSCs.Citation34 Furthermore, S100A9 binds to CD33 on MDSCs and induces production of IL-10, TGF-β, arginase and ROS.Citation34,35 The presence of constitutively active STAT1 correlates with increased frequency of MDSCs in tumors of breast cancer patients Citation36 and induces proliferation and suppressive capacity by regulating iNOS and arginase-I activity.Citation31,37 A third STAT protein, STAT5 induces MDSC expansion by reducing differentiation into mature myeloid cells through inhibition of interferon regulatory factor (IRF)-8.Citation38 STAT6 induces MDSC proliferation and survival and enhances arginase-I activity in MDSCs.Citation39-41
Figure 2. Induction of MDSC expansion and suppressive functions by the STAT signaling proteins. Tumor-derived factors induce signal transducers of activators of transcription (STAT) signaling, which stimulated MDSC expansion and suppressive functions. IL-4 and IL-13 induce STAT6 that regulates ARG1, leading to enhanced MDSC proliferation and survival. IL-6, GM-CSF, G-CSF and VEGF induce STAT3 signaling, which regulates ARG1, NOX2 and the expression of factors like MYC, Bcl-XL, cyclin D1, survivin and S100A9. This leads to enhanced MDSC proliferation and suppressive capacity, reduced apoptosis and inhibition of differentiation into mature cells. IFNγ and IL-1β regulate STAT1 activation, which induces iNOS and ARG1 expression by MDSCs, leading to induced proliferation and suppressive capacity. STAT5 signaling is induced by GM-CSF and inhibits the differentiation of MDSCs into mature cells through inhibition of IRF-8. IL, interleukin; IRF, interferon regulatory factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; G-CSF, granulocyte-colony stimulating factor; VEGF, vascular endothelial growth factor; IFNγ, interferon-γ; JAK, Janus kinase; ARG1, arginase 1; NOX2, NADPH oxidase 2; iNOS, inducible nitric oxide synthase; Bcl-XL, B-cell lymphoma-extra-large.
The immunosuppressive capacity of MDSCs and their negative correlation with disease stage, treatment response and survival clearly suggest their importance in cancer progression and suboptimal outcomes of cancer immunotherapy. Eradication or reprogramming of MDSCs is a logical strategy to re-engineer the TME and improve immunotherapy efficacy. The important role of STATs in accumulation and function of MDSCs, make the STAT proteins interesting targets to achieve this goal.
MDSCs as a target for enhancing immunotherapy efficacy
Drugs are known to have off-target effects, which are the main source of unwanted drug-related side effects. However, increasing evidence shows that chemotherapeutics and other drugs also have unintended effects that are beneficial, such as stimulation of immune responses by reduction of inhibitory molecules on DCs Citation42 and inhibitory effects on MDSCs. Several chemotherapeutics, drugs that are currently not used in cancer treatment and natural compounds have unintended effects on MDSCs (). Generally, these effects can result in inhibition of expansion and recruitment of MDSCs, inhibition of suppressive functions, or induction of MDSC differentiation into mature myeloid cells (). Several of these drugs modulate STAT signaling pathways, further emphasizing the potential of this pathway as a MDSC-inhibitory target.
Figure 3. Mechanisms by which drugs and natural compounds inhibit MDSCs. Several drugs and natural compounds used in cancer treatment or for other indications have off-target effects that result in inhibition of myeloid-derived suppressive cells (MDSCs) through four distinct mechanisms. The off-target effects can inhibit expansion of MDSCs, inhibit their T cells suppressive capacity or induce the differentiation of MDSCs into mature APCs. Cimetidine induces the apoptosis of MDSCs. ARG1, arginase 1; iNOS, inducible nitric oxide synthase; APC, antigen-presenting cell; TCR, T cell receptor; ATRA, all-trans retinoid acid.
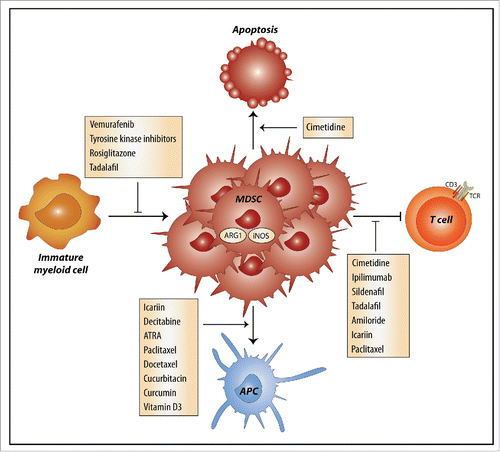
Table 1. Overview of drugs that target MDSCs.
Inhibition of MDSC expansion and recruitment
Targeted cancer therapies, like vemurafenib, affect MDSC expansion. Vemurafenib is a small molecule serine-threonine kinase inhibitor that is specific for V600 mutated, constitutively active, B-RAF and is used to treat melanoma patients. In a recent clinical trial, decreased M-MDSCs and G-MDSCs frequencies were observed in patients that achieved a clinical response. In addition, vemurafenib inhibited the production of cytokines, like IL-6, by melanoma cells, thereby inhibiting M-MDSC development.Citation43,44 Other kinase inhibitors also showed the potential to inhibit MSDC expansion, although this was mainly shown in preclinical models. Tyrosine kinase inhibitors axitinib, sorafenib and sunitinib inhibited tumor growth by inhibiting several growth factor receptors, including VEGF and platelet-derived growth factor receptor.Citation45 Axitinib treatment of mice significantly decreased the number of MDSCs in the spleen and tumor, by downregulating STAT3 expression.Citation46 Furthermore, the combination of axitinib with DC vaccination enhanced elimination of MDSCs compared to axitinib or vaccination alone.Citation47 Sorafenib similarly decreased MDSC frequency and their analogous mechanisms of action suggest that, like axitinib, sorafenib targets STAT3.Citation48 Sunitinib is used to treat metastatic renal cell carcinoma (RCC) patients, in whom it decreased the frequency of both G-MDSCs and M-MDSCs in peripheral blood, and partially restored IFNγ production by T cells.Citation49,50 However, intratumoral MDSCs are often less affected and can develop resistance to sunitinib. This was observed in RCC patients, where the majority of patients treated with sunitinib prior to primary tumor resection showed high intratumoral MDSC frequencies compared to non-treated primary tumors.Citation50 GM-CSF-induced STAT5 signaling is crucial in the development of sunitinib resistance, as MDSCs cultured in the presence of GM-CSF, developed sunitinib resistance via increased STAT5 signaling.Citation51 Additionally, sunitinib enhanced stromal cell-derived factor-1-dependent induction of MDSC frequency in mice bearing human RCC xenografts. Taken together, the overall effect of sunitinib on MDSCs remains unclear.Citation52
Other drugs that, similar to axitinib, sorafenib and sunitinib, inhibit VEGF can also induce MDSC eradication. The anti-VEGF antibody bevacizumab, reduced the frequency of immature myeloid cells in colorectal cancer patients.Citation53
Besides targeted cancer therapies, MDSC expansion can also be inhibited by drugs that are currently not used in cancer therapy. In a preclinical model, a significant reduction in early MDSC accumulation in the blood was obtained with the peroxisome proliferator-activated receptor (PPAR)-γ activator rosiglitazone, which is used in diabetes treatment.Citation54 Similarly, the histamine blocker cimetidine blocked the expansion of MDSCs in tumor-bearing mice by induction of apoptosis and by inhibition of NO and arginase production.Citation55
Inhibition of MDSC-suppressive activity
Investigating changes in MDSC-suppressive capacity in clinical settings is challenging and studies addressing this issue are limited. The most notable study reports that ipilimumab decreased the expression of arginase in melanoma patients, indicative for loss of MDSC suppressive capacity. Furthermore, treatment with ipilimumab for more than 3 weeks decreased the frequencies of G-MDSCs and M-MDSCs.Citation56,57 Phosphodiesterase-5 (PDE-5) inhibitors, like sildenafil, are generally used in erectile dysfunction and pulmonary hypertension and inhibit IL-4Rα signaling, which regulates suppressive pathways in MDSCs via STAT6.Citation58 Indeed, administration of sildenafil downregulated the activity of iNOS and arginase-I in MDSCs through a STAT6-mediated pathway, resulting in prolonged survival of melanoma-bearing mice.Citation59,60 Not much is known about the effects of sildenafil on human MDSCs, but intriguingly, sildenafil increased T cell proliferation of in-vitro-treated PBMCs obtained from patients with multiple myeloma and head and neck squamous cell carcinoma (HNSCC).Citation59 Recent clinical trials showed that tadalafil, another PDE-5 inhibitor, reduced the number of MDSCs as well as their production of arginase and iNOS in HNSCC and multiple myeloma patients, resulting in increased numbers of tumor-specific T cells.Citation61-63 Additionally, it was shown that NO release can activate cyclooxygenase enzymes.Citation64 Cyclooxygenase 2 is a key regulator of prostaglandin (PG)E2 synthesis, which can induce the expression of immunosuppressive factors, like IL-10 and IL-4Rα and inhibitory molecules, like programmed death-ligand (PD-L)1, by MDSCs.Citation65 Indirect inhibition of PGE2 release through inhibition of NO release by PDE-5 inhibitors could also contribute to the inhibitory effects of these drugs on MDSCs. These findings clearly illustrate the potential of PDE-5 inhibition as a way to inhibit MDSCs via STAT signaling regulation. Another interesting drug is amiloride, which is a diuretic drug used to treat high blood pressure. Amiloride inhibited MDSCs suppressive capacity by inhibiting the secretion of CSF-containing exosomes by the tumor and consequently inhibiting IL-6/STAT3 signaling. Patients with colorectal cancer receiving amiloride treatment indeed had decreased STAT3 activation and reduced MDSC suppressive capacity.Citation66
In addition to drugs, natural compounds can also have unintended effects on MDSC suppressive capacity. The natural compound icariin, the active ingredient of a herb used in Chinese medicine, inhibited ROS and NO production by MDSCs, through inhibition of STAT3 and AKT phosphorylation. Furthermore, it reduced MDSC frequency and promoted differentiation into macrophages and DCs.Citation67
In summary, the checkpoint inhibitor ipilimumab and several drugs that are currently not used as direct anticancer agents, like PDE-5 inhibitors, can inhibit MDSC suppressive mechanisms. Similar to the PDE-5 inhibitors, amiloride and icariin induce their effect by regulating STAT signaling pathways. The known mechanism of action and the fact that these drugs induce mild side effects compared to anticancer drugs make them the most promising candidates to use in combination strategies with immunotherapy.
Induction of MDSC differentiation into mature cells
Chemotherapeutic drugs, like the DNA methyltransferase inhibitor decitabine, can inhibit MDSCs by inducing their differentiation into mature antigen-presenting cells (APCs).Citation68 Furthermore, the metabolite of vitamin A, all-trans retinoic acid (ATRA), which is also used in the treatment of cancer, promoted the differentiation of MDSCs into mature myeloid cells in both tumor-bearing mice and metastatic RCC patients.Citation69,70 A clinical trial in patients with small cell lung cancer showed that combining ATRA with DC vaccination significantly reduced MDSC frequencies and enhanced IFNγ production by CD8+ cells compared to vaccination alone.Citation71 Additionally, by acting through ERK-dependent induction of glutathione, ATRA also reduced ROS production by MDSCs.Citation72 Paclitaxel, a chemotherapeutic of the taxane group, induced differentiation of MDSCs into DCs, via a Toll-like receptor 4-dependent mechanism.Citation73,74 Treatment of tumor-bearing mice with paclitaxel inhibited the number of tumor-infiltrating MDSCs and abrogated their T cell suppressive capacity.Citation75 Another taxane family member, docetaxel, decreased the frequency of MDSCs by promoting differentiation into M1-like macrophages.Citation76
Several natural compounds induce MDSC differentiation by direct inhibition of STAT signaling. In vitro, cucurbitacin I and B enhanced differentiation of spleen-derived immature myeloid cells into mature DCs by inhibition of the JAK2/STAT3 pathway. This coincided with a decrease of immature myeloid cells in spleens of treated tumor-bearing mice and in patients with advanced lung cancer.Citation77,78 However, tumor MDSCs were not sensitive to cucurbitacin I. Tumors have the ability to inhibit STAT3 activity in MDSCs by creating a state of hypoxia. These MDSCs then become functionally independent of STAT3, which diminishes the inhibitory effect of cucurbitacin. Interestingly, STAT3 expression in these tumor MDSCs could be restored by treatment with the CD45PTP-inhibitor sialidase, which blocked hypoxia-induced STAT3 downregulation. Combination treatment consisting of cucurbitacin I together with sialidase significantly decreased the frequencies of tumor MDSCs compared to cucurbitacin I alone.Citation79 Similarly, curcumin induced differentiation of MDSCs into M1-type macrophages through interaction with JAK2/STAT3.Citation80 Although an involvement of STAT signaling has not been reported, 1α,25-hydroxyvitamin D3 induced differentiation of CD34+ immature cells into mature DCs in both tumor-bearing mice and HNSCC patients.Citation81,82 Treatment of HNSCC patients with vitamin D3 before tumor resection resulted in higher levels of intratumoral CD8+ T cells and prolonged recurrence-free survival, which could be due to its effect on MDSCs.Citation83 Taken together, both chemotherapeutics and natural compounds are capable of inducing MDSC differentiation into mature cells, thereby preventing immune suppressive activity. However, due to possible severe side effects of chemotherapeutics like ATRA, and the known involvement of STAT3 in the mechanisms of action of cucurbitacin or curcumin, these compounds would be most promising to synergize with immunotherapy.
Discussion and future perspectives
Inhibition of the immunosuppressive TME and the presence of MDSCs in the TME is correlated with increased efficacy of immunotherapy. Targeting MDSCs by using clinically available drugs and natural compounds could improve antitumor immune responses induced by immunotherapy. The importance of STAT signaling pathways in the expansion and suppressive capacity provides a promising target to inhibit MDSCs. We discussed a number of drugs that can, as an unintended effect, inhibit the expansion of MDSCs, inhibit their suppressive functions, or promote their differentiation into mature APCs, often mediated by inhibition of STAT signaling pathways. Targeted cancer therapies, like tyrosine kinase inhibitors, and several chemotherapeutics reduce MDSC expansion or induce their differentiation into non-suppressive mature myeloid cells, but also have the potential for severe side effects.Citation84,85 Patients treated with immunotherapy can already experience severe side effects, which might be exacerbated when combined with these drugs.Citation86 We therefore propose to combine immunotherapy with drugs that have similar effects on MDSC expansion and function, but induce less severe side effects compared to conventional chemotherapy and some targeted therapies used in cancer treatment. We have highlighted several drugs and natural compounds used for diverse indications, which modulate MDSC function and differentiation. On a molecular level, most of these drugs exert their effect on MDSCs by interfering with the STAT signaling pathway. For instance, sildenafil and amiloride inhibit the suppressive mechanisms of MDSCs by interfering with STAT6 and STAT3 signaling, respectively. Natural compounds, like icariin, cucurbitacin and curcumin, inhibit the suppressive capacity of MDSCs or induce their maturation, by inhibiting STAT3. There is still a group of MDSC-inhibiting drugs, including rosiglitazone and cimetidine, for which the mechanism of action is unknown. However, the importance of STAT signaling in MDSC inhibition indicates that this pathway could be a potential mechanism for their effect. Furthermore, two specific JAK-inhibitors, tofacitinib and ruxolitinib, were FDA approved for the treatment of several auto-inflammatory diseases, including rheumatoid arthritis. Their specific targeting of JAK/STAT signaling makes them interesting candidates to target STAT signaling in MDSCs and to be used in combination with immunotherapy in anticancer regimes. However, the only study available on these drugs is in the context of rheumatoid arthritis and in that setting tofacitinib surprisingly resulted in the expansion of MDSCs.Citation87 On the other hand, in a melanoma mouse model, specific JAK inhibition with the experimental compound AZD1480 reduced MDSC frequencies, but it also enhanced their suppressive capacity.Citation88 These results could indicate that blocking of JAK signaling might not result in inhibition of STAT signaling and show that the effect of JAK signaling on MDSC expansion and suppressive capacity is still unclear and requires more research.
We propose that the drugs sildenafil and amiloride together with the natural compounds icariin, cucurbitacin I, cucurbitacin B and curcumin would be the prime candidates to test in combination with immunotherapy, as they were shown in experimental settings to inhibit the suppressive mechanisms of MDSCs or induce their maturation by targeting STAT6 or STAT3, with only mild side effects compared to chemotherapy and targeted cancer therapies. Clinical trials combining these possible candidate drugs with immunotherapy will have to prove their potential in the clinic.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank all researchers whose work contributed to advancing our understanding of MDSCs but could not be cited due to space constraints.
Funding
S. V. Hato and N. de Haas are supported by a grant from the Dutch Cancer Society and Alpe deHuZes foundation (KUN2013-5958). This work is also supported by KWO grant KUN2009-4402 from the Dutch Cancer Society. I. J. M. de Vries is recipient of NWO Vici grant no. 918.14.655. All authors declare no financial disclosures.
References
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372:2521-32; PMID:25891173; http://dx.doi.org/10.1056/NEJMoa1503093
- Rotte A, Bhandaru M, Zhou Y, McElwee KJ. Immunotherapy of melanoma: present options and future promises. Cancer Metastasis Rev 2015; 34:115-28; PMID:25589384; http://dx.doi.org/10.1007/s10555-014-9542-0
- Becker JC, Andersen MH, Schrama D, Thor Straten P. Immune-suppressive properties of the tumor microenvironment. Cancer Immunol Immunother 2013; 62:1137-48; PMID:23666510; http://dx.doi.org/10.1007/s00262-013-1434-6
- Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 2012; 18:1254-61; PMID:22842478; http://dx.doi.org/10.1038/nm.2883
- Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, Nakamoto Y, Kaneko S. Increase in CD14+HLA-DR −/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother 2013; 62:1421-30; PMID:23764929; http://dx.doi.org/10.1007/s00262-013-1447-1
- Laborde RR, Lin Y, Gustafson MP, Bulur PA, Dietz AB. Cancer vaccines in the world of immune suppressive monocytes (CD14(+)HLA-DR(lo/neg) Cells): the gateway to improved responses. Front Immunol 2014; 5:147; PMID:24772111; http://dx.doi.org/10.3389/fimmu.2014.00147
- Kodumudi KN, Weber A, Sarnaik AA, Pilon-Thomas S. Blockade of myeloid-derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma. J Immunol 2012; 189:5147-54; PMID:23100512; http://dx.doi.org/10.4049/jimmunol.1200274
- Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, Michielin O, Romano E, Speiser DE. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother 2014; 63:247-57; PMID:24357148; http://dx.doi.org/10.1007/s00262-013-1508-5
- Srivastava MK, Zhu L, Harris-White M, Kar UK, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, Dubinett S et al. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One 2012; 7:e40677; PMID:22815789; http://dx.doi.org/10.1371/journal.pone.0040677
- Morales JK, Kmieciak M, Graham L, Feldmesser M, Bear HD, Manjili MH. Adoptive transfer of HER2/neu-specific T cells expanded with alternating gamma chain cytokines mediate tumor regression when combined with the depletion of myeloid-derived suppressor cells. Cancer Immunol Immunother 2009; 58:941-53; PMID:18979098; http://dx.doi.org/10.1007/s00262-008-0609-z
- Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol 2007; 25:911-20; PMID:17664940; http://dx.doi.org/10.1038/nbt1323
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253-68; PMID:22437938; http://dx.doi.org/10.1038/nri3175
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:19197294; http://dx.doi.org/10.1038/nri2506
- Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 2008; 181:5791-802; PMID:18832739; http://dx.doi.org/10.4049/jimmunol.181.8.5791
- Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 2014; 1319:47-65; PMID:24965257; http://dx.doi.org/10.1111/nyas.12469
- Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother 2012; 61:1155-67; PMID:22692756; http://dx.doi.org/10.1007/s00262-012-1294-5
- Vetsika EK, Koinis F, Gioulbasani M, Aggouraki D, Koutoulaki A, Skalidaki E, Mavroudis D, Georgoulias V, Kotsakis A. A circulating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients. J Immunol Res 2014; 2014:659294; PMID:25436215; http://dx.doi.org/10.1155/2014/659294
- Martens A, Zelba H, Garbe C, Pawelec G, Weide B. Monocytic myeloid-derived suppressor cells in advanced melanoma patients: Indirect impact on prognosis through inhibition of tumor-specific T-cell responses? Oncoimmunology 2014; 3:e27845; PMID:24800171
- Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol 2007; 179:977-83; PMID:17617589; http://dx.doi.org/10.4049/jimmunol.179.2.977
- Marcoe JP, Lim JR, Schaubert KL, Fodil-Cornu N, Matka M, McCubbrey AL, Farr AR, Vidal SM, Laouar Y. TGF-β is responsible for NK cell immaturity during ontogeny and increased susceptibility to infection during mouse infancy. Nat Immunol 2012; 13:843-50; PMID:22863752; http://dx.doi.org/10.1038/ni.2388
- Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O'Neill A et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res 2005; 65:3044-8; PMID:15833831
- Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res 2010; 70:68-77; PMID:20028852; http://dx.doi.org/10.1158/0008-5472.CAN-09-2587
- Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol 2002; 168:689-95; PMID:11777962; http://dx.doi.org/10.4049/jimmunol.168.2.689
- Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res 2001; 61:4756-60; PMID:11406548
- Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med 2007; 13:828-35; PMID:17603493; http://dx.doi.org/10.1038/nm1609
- Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med 2011; 208:1949-62; PMID:21930770; http://dx.doi.org/10.1084/jem.20101956
- Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008; 135:234-43; PMID:18485901; http://dx.doi.org/10.1053/j.gastro.2008.03.020
- Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res 2008; 68:5439-49; PMID:18593947; http://dx.doi.org/10.1158/0008-5472.CAN-07-6621
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 2006; 66:1123-31; PMID:16424049; http://dx.doi.org/10.1158/0008-5472.CAN-05-1299
- O'Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med 2015; 66:311-28; PMID:25587654; http://dx.doi.org/10.1146/annurev-med-051113-024537
- Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol 2011; 32:19-25; PMID:21067974; http://dx.doi.org/10.1016/j.it.2010.10.002
- Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol 2009; 182:5693-701; PMID:19380816; http://dx.doi.org/10.4049/jimmunol.0900092
- Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, Blosser RL, Tam AJ, Bruno T, Zhang H et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest 2013; 123:1580-9; PMID:23454751; http://dx.doi.org/10.1172/JCI60083
- Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med 2008; 205:2235-49; PMID:18809714; http://dx.doi.org/10.1084/jem.20080132
- Chen X, Eksioglu EA, Zhou J, Zhang L, Djeu J, Fortenbery N, Epling-Burnette P, Van Bijnen S, Dolstra H, Cannon J et al. Induction of myelodysplasia by myeloid-derived suppressor cells. J Clin Invest 2013; 123:4595-611; PMID:24216507; http://dx.doi.org/10.1172/JCI67580
- Hix LM, Karavitis J, Khan MW, Shi YH, Khazaie K, Zhang M. Tumor STAT1 transcription factor activity enhances breast tumor growth and immune suppression mediated by myeloid-derived suppressor cells. J Biol Chem 2013; 288:11676-88; PMID:23486482; http://dx.doi.org/10.1074/jbc.M112.441402
- Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 2008; 111:4233-44; PMID:18272812; http://dx.doi.org/10.1182/blood-2007-07-099226
- Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S, Bogner PN, Farren MR, Lee KP, Liu K et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest 2013; 123:4464-78; PMID:24091328; http://dx.doi.org/10.1172/JCI68189
- Munera V, Popovic PJ, Bryk J, Pribis J, Caba D, Matta BM, Zenati M, Ochoa JB. Stat 6-dependent induction of myeloid derived suppressor cells after physical injury regulates nitric oxide response to endotoxin. Ann Surg 2010; 251:120-6; PMID:20032720; http://dx.doi.org/10.1097/SLA.0b013e3181bfda1c
- Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol 2005; 174:636-45; PMID:15634881; http://dx.doi.org/10.4049/jimmunol.174.2.636
- Roth F, De La Fuente AC, Vella JL, Zoso A, Inverardi L, Serafini P. Aptamer-mediated blockade of IL4Ralpha triggers apoptosis of MDSCs and limits tumor progression. Cancer Res 2012; 72:1373-83; PMID:22282665; http://dx.doi.org/10.1158/0008-5472.CAN-11-2772
- Hato SV, Khong A, de Vries IJ, Lesterhuis WJ. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res 2014; 20:2831-7; PMID:24879823; http://dx.doi.org/10.1158/1078-0432.CCR-13-3141
- Schilling B, Sucker A, Griewank K, Zhao F, Weide B, Gorgens A, Giebel B, Schadendorf D, Paschen A. Vemurafenib reverses immunosuppression by myeloid derived suppressor cells. Int J Cancer 2013; 133:1653-63; PMID:23526263; http://dx.doi.org/10.1002/ijc.28168
- Schilling B, Paschen A. Immunological consequences of selective BRAF inhibitors in malignant melanoma: neutralization of myeloid-derived suppressor cells. Oncoimmunology 2013; 2:e25218; PMID:24167759
- Bhargava P, Robinson MO. Development of second-generation VEGFR tyrosine kinase inhibitors: current status. Curr Oncol Rep 2011; 13:103-11; PMID:21318618; http://dx.doi.org/10.1007/s11912-011-0154-3
- Yuan H, Cai P, Li Q, Wang W, Sun Y, Xu Q, Gu Y. Axitinib augments antitumor activity in renal cell carcinoma via STAT3-dependent reversal of myeloid-derived suppressor cell accumulation. Biomed Pharmacother 2014; 68:751-6; PMID:25081318; http://dx.doi.org/10.1016/j.biopha.2014.07.002
- Bose A, Lowe DB, Rao A, Storkus WJ. Combined vaccine+axitinib therapy yields superior antitumor efficacy in a murine melanoma model. Melanoma Res 2012; 22:236-43; PMID:22504156; http://dx.doi.org/10.1097/CMR.0b013e3283538293
- Cao M, Xu Y, Youn JI, Cabrera R, Zhang X, Gabrilovich D, Nelson DR, Liu C. Kinase inhibitor Sorafenib modulates immunosuppressive cell populations in a murine liver cancer model. Lab Invest 2011; 91:598-608; PMID:21321535; http://dx.doi.org/10.1038/labinvest.2010.205
- Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 2009; 15:2148-57; PMID:19276286; http://dx.doi.org/10.1158/1078-0432.CCR-08-1332
- Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol 2011; 11:856-61; PMID:21315783; http://dx.doi.org/10.1016/j.intimp.2011.01.030
- Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, Rini B, Finke JH, Cohen PA. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res 2010; 70:3526-36; PMID:20406969; http://dx.doi.org/10.1158/0008-5472.CAN-09-3278
- Panka DJ, Liu Q, Geissler AK, Mier JW. Effects of HDM2 antagonism on sunitinib resistance, p53 activation, SDF-1 induction, and tumor infiltration by CD11b+/Gr-1+ myeloid derived suppressor cells. Mol Cancer 2013; 12:17; PMID:23497256; http://dx.doi.org/10.1186/1476-4598-12-17
- Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, Hurwitz HI, Dev I, Nixon AB, Lyerly HK et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother 2008; 57:1115-24; PMID:18193223; http://dx.doi.org/10.1007/s00262-007-0441-x
- Bunt SK, Mohr AM, Bailey JM, Grandgenett PM, Hollingsworth MA. Rosiglitazone and Gemcitabine in combination reduces immune suppression and modulates T cell populations in pancreatic cancer. Cancer Immunol Immunother 2013; 62:225-36; PMID:22864396; http://dx.doi.org/10.1007/s00262-012-1324-3
- Zheng Y, Xu M, Li X, Jia J, Fan K, Lai G. Cimetidine suppresses lung tumor growth in mice through proapoptosis of myeloid-derived suppressor cells. Mol Immunol 2013; 54:74-83; PMID:23220070; http://dx.doi.org/10.1016/j.molimm.2012.10.035
- Pico de Coana Y, Poschke I, Gentilcore G, Mao Y, Nystrom M, Hansson J, Masucci GV, Kiessling R. Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their Arginase1 production. Cancer Immunol Res 2013; 1:158-62; PMID:24777678; http://dx.doi.org/10.1158/2326-6066.CIR-13-0016
- Tarhini AA, Edington H, Butterfield LH, Lin Y, Shuai Y, Tawbi H, Sander C, Yin Y, Holtzman M, Johnson J et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One 2014; 9:e87705; PMID:24498358; http://dx.doi.org/10.1371/journal.pone.0087705
- Ugel S, Delpozzo F, Desantis G, Papalini F, Simonato F, Sonda N, Zilio S, Bronte V. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol 2009; 9:470-81; PMID:19616475; http://dx.doi.org/10.1016/j.coph.2009.06.014
- Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 2006; 203:2691-702; PMID:17101732; http://dx.doi.org/10.1084/jem.20061104
- Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, Borrello I, Kato M, Schadendorf D, Baniyash M et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci U S A 2011; 108:17111-6; PMID:21969559; http://dx.doi.org/10.1073/pnas.1108121108
- Weed DT, Vella JL, Reis IM, De la Fuente AC, Gomez C, Sargi Z, Nazarian R, Califano J, Borrello I, Serafini P. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res 2015; 21:39-48; PMID:25320361; http://dx.doi.org/10.1158/1078-0432.CCR-14-1711
- Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, Goodman S, Gourin CG, Ha PK, Fakhry C et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res 2015; 21:30-8; PMID:25564570; http://dx.doi.org/10.1158/1078-0432.CCR-14-1716
- Noonan KA, Ghosh N, Rudraraju L, Bui M, Borrello I. Targeting immune suppression with PDE5 inhibition in end-stage multiple myeloma. Cancer Immunol Res 2014; 2:725-31; PMID:24878583; http://dx.doi.org/10.1158/2326-6066.CIR-13-0213
- Salvemini D, Kim SF, Mollace V. Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: relevance and clinical implications. Am J Physiol Regul Integr Comp Physiol 2013; 304:R473-87; PMID:23389111; http://dx.doi.org/10.1152/ajpregu.00355.2012
- Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 2011; 118:5498-505; PMID:21972293; http://dx.doi.org/10.1182/blood-2011-07-365825
- Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 2010; 120:457-71; PMID:20093776; http://dx.doi.org/10.1172/JCI40483DS1
- Zhou J, Wu J, Chen X, Fortenbery N, Eksioglu E, Kodumudi KN, Pk EB, Dong J, Djeu JY, Wei S. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int Immunopharmacol 2011; 11:890-8; PMID:21244860; http://dx.doi.org/10.1016/j.intimp.2011.01.007
- Daurkin I, Eruslanov E, Vieweg J, Kusmartsev S. Generation of antigen-presenting cells from tumor-infiltrated CD11b myeloid cells with DNA demethylating agent 5-aza-2'-deoxycytidine. Cancer Immunol Immunother 2010; 59:697-706; PMID:19882154; http://dx.doi.org/10.1007/s00262-009-0786-4
- Lee JM, Seo JH, Kim YJ, Kim YS, Ko HJ, Kang CY. The restoration of myeloid-derived suppressor cells as functional antigen-presenting cells by NKT cell help and all-trans-retinoic acid treatment. Int J Cancer 2012; 131:741-51; PMID:21898392; http://dx.doi.org/10.1002/ijc.26411
- Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, Yancey D, Dahm P, Vieweg J. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res 2008; 14:8270-8; PMID:19088044; http://dx.doi.org/10.1158/1078-0432.CCR-08-0165
- Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother 2013; 62:909-18; PMID:23589106; http://dx.doi.org/10.1007/s00262-013-1396-8
- Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res 2007; 67:11021-8; PMID:18006848; http://dx.doi.org/10.1158/0008-5472.CAN-07-2593
- Michels T, Shurin GV, Naiditch H, Sevko A, Umansky V, Shurin MR. Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner. J Immunotoxicol 2012; 9:292-300; PMID:22283566; http://dx.doi.org/10.3109/1547691X.2011.642418
- Pfannenstiel LW, Lam SS, Emens LA, Jaffee EM, Armstrong TD. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell Immunol 2010; 263:79-87; PMID:20346445; http://dx.doi.org/10.1016/j.cellimm.2010.03.001
- Sevko A, Michels T, Vrohlings M, Umansky L, Beckhove P, Kato M, Shurin GV, Shurin MR, Umansky V. Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J Immunol 2013; 190:2464-71; PMID:23359505; http://dx.doi.org/10.4049/jimmunol.1202781
- Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res 2010; 16:4583-94; PMID:20702612; http://dx.doi.org/10.1158/1078-0432.CCR-10-0733
- Nefedova Y, Nagaraj S, Rosenbauer A, Muro-Cacho C, Sebti SM, Gabrilovich DI. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res 2005; 65:9525-35; PMID:16230418; http://dx.doi.org/10.1158/0008-5472.CAN-05-0529
- Lu P, Yu B, Xu J. Cucurbitacin B regulates immature myeloid cell differentiation and enhances antitumor immunity in patients with lung cancer. Cancer Biother Radiopharm 2012; 27:495-503; PMID:22746287; http://dx.doi.org/10.1089/cbr.2012.1219
- Kumar V, Cheng P, Condamine T, Mony S, Languino LR, McCaffrey JC, Hockstein N, Guarino M, Masters G, Penman E et al. CD45 Phosphatase inhibits STAT3 transcription factor activity in myeloid cells and promotes tumor-associated macrophage differentiation. Immunity 2016; 44:303-15; PMID:26885857; http://dx.doi.org/10.1016/j.immuni.2016.01.014
- Tu SP, Jin H, Shi JD, Zhu LM, Suo Y, Lu G, Liu A, Wang TC, Yang CS. Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev Res (Phila) 2012; 5:205-15; PMID:22030090; http://dx.doi.org/10.1158/1940-6207.CAPR-11-0247
- Young MR, Wright MA, Vellody K, Lathers DM. Skewed differentiation of bone marrow CD34+ cells of tumor bearers from dendritic toward monocytic cells, and the redirection of differentiation toward dendritic cells by 1alpha,25-dihydroxyvitamin D3. Int J Immunopharmacol 1999; 21:675-88; PMID:12609462; http://dx.doi.org/10.1016/S0192-0561(99)00044-2
- Kulbersh JS, Day TA, Gillespie MB, Young MR. 1alpha,25-Dihydroxyvitamin D(3) to skew intratumoral levels of immune inhibitory CD34(+) progenitor cells into dendritic cells. Otolaryngol Head Neck Surg 2009; 140:235-40; PMID:19201295; http://dx.doi.org/10.1016/j.otohns.2008.11.011
- Walsh JE, Clark AM, Day TA, Gillespie MB, Young MR. Use of α,25-dihydroxyvitamin D3 treatment to stimulate immune infiltration into head and neck squamous cell carcinoma. Hum Immunol 2010; 71:659-65; PMID:20438786; http://dx.doi.org/10.1016/j.humimm.2010.04.008
- Di Maio M, Gallo C, Leighl NB, Piccirillo MC, Daniele G, Nuzzo F, Gridelli C, Gebbia V, Ciardiello F, De Placido S et al. Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol 2015; 33:910-5; PMID:25624439; http://dx.doi.org/10.1200/JCO.2014.57.9334
- Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev 2014; 40:872-82; PMID:24830939; http://dx.doi.org/10.1016/j.ctrv.2014.04.004
- Gelao L, Criscitiello C, Esposito A, Goldhirsch A, Curigliano G. Immune checkpoint blockade in cancer treatment: a double-edged sword cross-targeting the host as an “innocent bystander”. Toxins (Basel) 2014; 6:914-33; PMID:24594636; http://dx.doi.org/10.3390/toxins6030914
- Nishimura K, Saegusa J, Matsuki F, Akashi K, Kageyama G, Morinobu A. Tofacitinib facilitates the expansion of myeloid-derived suppressor cells and ameliorates arthritis in SKG mice. Arthritis Rheumatol 2015; 67:893-902; PMID:25545152; http://dx.doi.org/10.1002/art.39007
- Maenhout SK, Du Four S, Corthals J, Neyns B, Thielemans K, Aerts JL. AZD1480 delays tumor growth in a melanoma model while enhancing the suppressive activity of myeloid-derived suppressor cells. Oncotarget 2014; 5:6801-15; PMID:25149535
