ABSTRACT
Programmed Death 1 (PD-1) and T cell Ig and mucin domain-3 protein (Tim-3) are immune checkpoint receptors highly expressed on tumor infiltrating T lymphocytes (TIL). PD-1 inhibits T cell activation and type-1 T cell responses, while Tim-3 is proposed to mark more extensively exhausted cells, although the mechanisms underlying Tim-3 function are not clear. Trials of anti-PD-1 therapy have identified a large subset of non-responder patients, likely due to expression of alternative checkpoint molecules like Tim-3. We investigated the phenotypic and functional characteristics of T cells with differential expression of PD-1 (high/low) and Tim-3 (positive/negative), using TIL directly isolated from head and neck squamous cell carcinomas (HNSCC). Unexpectedly, we found that expression of Tim-3 alone does not necessarily mark TIL as dysfunctional/exhausted. In Tim-3-TIL, PD-1 levels correlate with T cell dysfunction, with a PD-1low/intermed phenotype identifying recently activated and still functional cells, whereas PD-1hiTim-3− T cells are actually exhausted. Nonetheless, PD-1intermed cells are still potently suppressed by PD-L1. PD-1 expression was associated with reduced phosphorylation of ribosomal protein S6 (pS6), whereas Tim-3 expression was associated with increased pS6. Using a novel mouse model for inducible Tim-3 expression, we confirmed that expression of Tim-3 does not necessarily render T cells refractory to further activation. These results suggest the existence of PD-1 and Tim-3 crosstalk in regulating antitumor T cell responses, with important implications for anti-PD-1 immunotherapy.
KEYWORDS:
Abbreviations
| HNSCC | = | head and neck squamous cell carcinomas |
| mAb | = | monoclonal antibodies |
| PD-1 | = | Programmed Death 1 |
| PD-L1 | = | Programmed Death-Ligand 1 |
| TCM | = | central memory T cells |
| TEM | = | effector memory T cells |
| TEMRA | = | CD45RA+ effector memory T cells |
| TIL | = | tumor-infiltrating T lymphocytes |
| Tim-3 | = | T cell Ig and mucin domain-3 protein |
| TN | = | naïve T cells |
| Treg | = | regulatory T cells |
Introduction
HNSCC is associated with a highly immunosuppressive tumor microenvironmentCitation1,2 that may limit the efficacy of cancer therapies. Immunosuppression in the HNSCC microenvironment is mediated by regulatory T cells (Treg), myeloid derived suppressor cells (MDSC)Citation3-6 and dysfunctional or exhausted antitumor effector T cells. During chronic viral infection, exhausted T cells undergo an altered transcriptional program with upregulation of several transcription factors, including Eomes, Blimp-1 and BATF, and downregulation of T-bet.Citation7 Another feature of T cell exhaustion is the increased expression of immune checkpoint receptors (ICR) like PD-1, CTLA-4 and Tim-3Citation8. Since these ICRs and their ligands are often overexpressed in tumors,Citation9,10 they have been recently targeted for therapeutic approach to reverse immunosuppression in cancer patients.Citation11 Indeed, blockade of cytotoxic T lymphocyte antigen 4 (CTLA-4) and/or PD-1 with monoclonal antibodies (mAb) ipilimumab or nivolumab/pembrolizumab, respectively, has shown clinical efficacy for some patients with melanoma, renal cell carcinoma, non-small cell lung cancer and, more recently HNSCC.Citation12-14
Despite recent progress, improved success of mAbs targeting PD-1 has been hindered by a relatively poor understanding of mechanisms of resistance to these mAbs, which occurs in the majority of patients, as well as a lack of biomarkers of clinical response. One explanation for variable responses to anti-PD-1 therapy is the heterogeneous expression of alternative checkpoint inhibitory receptors on TIL, such as Tim-3, reflecting greater complexity to the phenotypic and functional attributes of T cell “exhaustion” than was initially appreciated. In preliminary studies, dual blockade of PD-1 and Tim-3 has been shown to enhance antitumor T cell responses, compared to PD-1/PD-L1 blockade alone.Citation15,16 In addition, PD-1 expression on TIL has actually been identified as a favorable prognostic biomarker for HPV+ head and neck cancer,Citation17 which has raised questions about using PD-1 as the sole defining marker of “exhausted” T cells in the tumor microenvironment. Therefore, we reasoned that TIL from human cancer patients would provide direct evidence for phenotypic and functional consequences of PD-1 and/or Tim-3 expression, in order to verify activation versus exhaustion of these important TIL subsets. Finally, whether blockade of Tim-3 on single positive T cells would be deleterious or beneficial has not been determined.
We segregated subsets of CD8+ and CD4+ effector TIL from HNSCC patients, based on expression of PD-1 and Tim-3, to compare their phenotypic and functional characteristics. Our findings first confirm that expression of high levels of PD-1 are associated with T cell exhaustion, with increased expression of the exhaustion-related transcription factors Blimp-1, BATF and Eomes, deficient production of Th1 cytokines and decreased clonal expansion. By contrast, T cells with intermediate levels of PD-1 appear activated rather than exhausted. Surprisingly, expression of Tim-3 by T cells is also associated with functional competence, at least at the level of proliferation, although this can still be overridden by high levels or ligation of PD-1. In addition, PD-1 appears to interfere with activation events (such as pS6) downstream of TCR and Tim-3 signaling, representing a potential mechanism of crosstalk between PD-1 and Tim-3. Finally, we show that expression of Tim-3 alone augments the activation of events downstream of TCR signaling. Our findings are consistent with those of others who have suggested that the expression of one or more “checkpoint” receptors (e.g. PD-1 and Tim-3) is not necessarily indicative of functional exhaustion.Citation18,19 As such, our findings also have implications for reversing immune escape from anti-PD-1 therapy currently in the clinic, and for the development of new therapies targeting Tim-3 itself.
Results
Definition of HNSCC TIL by expression of PD-1 and Tim-3
Levels of PD-1 and Tim-3 are upregulated on TIL in tumor-bearing miceCitation16 and on antigen-specific CD8+ T cells in melanoma patients,Citation15 and PD-1/Tim-3 co-expression has been associated with T cell exhaustion in chronic viral infection.Citation15,16,20 In order to establish the expression pattern of PD-1 and Tim-3 on TIL from a cohort of stage III–IV HNSCC patients, and to facilitate analysis of phenotypic and functional characteristics of the TIL subsets, we assessed PD-1 and Tim-3 expression on these TIL by flow cytometry. Consistent with previous reports,Citation21 we observed high expression of PD-1 or Tim-3 individually on CD8+ TIL, as well as co-expression of PD-1 on a subset of Tim-3+ CD8+ TIL. Interestingly, PD-1+Tim-3+ CD8+ TIL had the highest expression level of PD-1 (). Among CD4+ TIL, Tim-3 was mainly expressed on Treg (CD25hiFoxp3+CD4+) but not on Foxp3−CD4+ effector TIL, whereas a distinct population of PD-1hiTim-3− cells was sometimes seen in these Foxp3−CD4+ effector T cells. By contrast, most of the PD-1 single positive Treg had an intermediate level of PD-1 expression ().
Figure 1. Definition of TIL subsets, based on PD-1 and Tim-3 expression. Expression of PD-1, Tim-3, CCR7 and CD45RA on CD8+ and CD4+ peripheral blood T lymphocytes (PBL) and tumor infiltrating T lymphocytes (TIL) from HNSCC patients (n = 7) was analyzed by flow cytometry. Representative figures showing expression patterns of PD-1 and Tim-3 on CD8+, Foxp3−CD4+ and regulatory T cells (CD25hiFoxp3+CD4+ T) in the tumor sites of HNSCC patients. Statistical significance was determined by Wilcoxon–Mann–Whitney test. *p < 0.05.
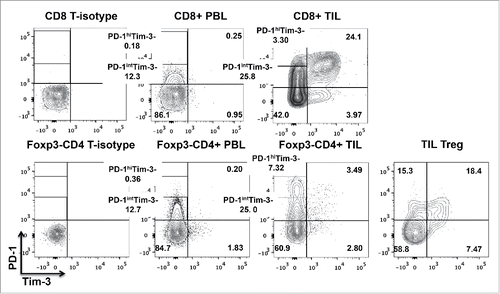
Having defined the various subsets of TIL based on PD-1 and Tim-3 expression, we further characterized these cells (gating strategies described in Materials and Methods) using classical phenotypic markers of naïve (TN), central memory (TCM) versus effector memory (TEM) T cell populations. Most of the CD8+ and Foxp3−CD4+ effector T lymphocytes at tumor sites were TCM (CCR7+CD45RA−) or TEM (CCR7−CD45RA−), compared to those in the peripheral circulation (Fig. S1A). PD-1hiTim-3− and PD-1+Tim-3+ CD8+ and Foxp3−CD4+ TIL comprised a significantly higher proportion of effector memory cells than other subsets (Fig. S1B–C), suggesting that most tumor antigen-experienced T cells in the tumor microenvironment are PD-1hiTim-3− or PD-1+Tim-3+. In order to study the PD-1-expressing CD8+, as well as the Foxp3− or CD25lo/−CD4+ effector, T cell subsets more precisely, we segregated PD-1hi and PD-1int by flow sorting among PD-1+Tim-3− TIL to investigate their biological or functional properties. The proportions of PD-1hiTim-3− and PD-1intTim-3− in CD8+ and CD4+ effector TIL are shown in .
Table 1. Proportion of PD-1hiTim-3− and PD-1intTim-3− cells in CD8+ and CD4+ effector TIL.
Table 2. Clinicopathological features of the HNSCC patients in this study.
PD-1hiTim-3− and PD-1+Tim-3+ CD8+ TIL display molecular signatures associated with T cell exhaustion
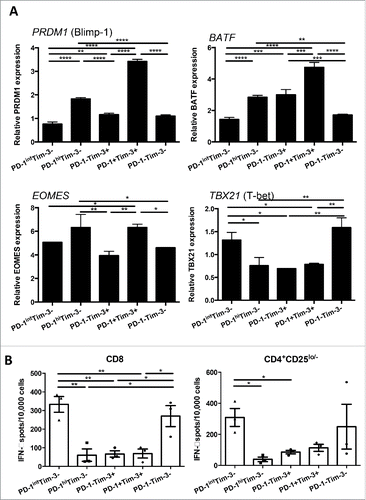
Exhaustion of T cells in chronic viral infection is associated with a transcriptional program distinct from that of functional effector or memory T cells,Citation22 including upregulation of Blimp-1 and BATF.Citation7 However, the expression of these exhaustion-associated transcription factors in freshly isolated TIL from human cancer have not yet been well characterized. Therefore, we sought to define the T cell exhaustion signatures of TIL expressing PD-1 and or Tim-3 isolated from HNSCC patients. Interestingly, PD-1hiTim-3− and PD-1+Tim-3+ CD8+ TIL had higher levels of transcripts for Blimp-1 (encoded by PRDM1) and BATF, transcription factors previously shown to impair T cell proliferation and cytokine secretion and thereby foster T cell exhaustion.Citation23,24 Moreover, higher expression of Eomes, which is associated with exhausted terminal progeny T cells, and lower levels of T-bet, a marker of robust and functional progenitors,Citation25 were observed primarily in PD-1hiTim-3− and PD-1+Tim-3+ CD8+ TIL (). However, PD-1−Tim-3+ CD8+ TIL expressed higher BATF and lower TBX21, but similar levels of PRDM1 and EOMES transcripts, compared with PD-1intTim-3− and PD-1−Tim-3− CD8+ TIL. Thus, PD-1hiTim-3− and PD-1+Tim-3+ CD8+ TIL possessed molecular signatures associated with exhausted T cells found in chronic viral infection, and are most likely to be dysfunctional.
Figure 2. PD-1hiTim-3− and PD-1+Tim-3+ CD8+ TIL expressed higher amount of Blimp-1, BATF, Eomes and lower amount of T-bet transcripts. (A) PD-1hiTim-3−, PD-1intTim-3−, PD-1−Tim-3+, PD-1+Tim-3+ and PD-1−Tim-3− CD8+ T cells were sorted from TIL. Each TIL subset from four different HNSCC patients was subjected to RNA purification and then analyzed by real time quantitative PCR. Summarized graphs of relative expression of PRDM1 (Blimp-1), BATF, EOMES and TBX21 (T-bet) transcripts in each subset are shown. The quantity of each cDNA sample was normalized by GUSB. All analyses were performed in triplicate. The graphs present the mean ± SD from replicates. Statistical significance was determined by RM One-way ANOVA analysis followed by multiple comparisons. *p < 0.05, **p < 0.01, ***p < 0.001. (B) PD-1hiTim-3− and PD-1+Tim-3+ effector TIL display defective Th1 cytokine production. Summary data of IFNγ production by each sorted TIL subset (n = 3), measured by IFNγ ELISPOT after anti-CD3/CD28 stimulation. Experiments were performed in duplicate. Statistical significance was determined by RM One-way ANOVA analysis followed by multiple comparisons. *p < 0.05, **p < 0.01, ***p < 0.001.
High levels of PD-1 expression are associated with defective T cell function, while Tim-3 expression alone is associated with a “split” exhaustion phenotype
In order to investigate the functional characteristics of tumor-infiltrating T cells with differential PD-1 and Tim-3 expression, we sorted TIL from HNSCC patients into the same T cell subsets described above, to facilitate detailed analysis of their responses to TCR stimulation. First, we examined the ability of these subsets to produce IFNγ and TNF-α, which are hallmark Th1 cytokines critical for effective antitumor T cell responses. IFNγ and TNF-α mRNA in sorted TIL subsets were measured after anti-CD3/CD28 bead stimulation (6 h), and production of secreted IFNγ cytokines were also tested by IFNγ ELISPOT after stimulation (18 h). Interestingly, PD-1hiTim-3− PD-1−Tim-3+ and PD-1+Tim-3+ CD8+ TIL displayed defective production of Th1 cytokines compared with the other two subsets, expressing lower IFNγ and TNF-α transcripts (Fig. S2) and less secreted IFNγ () upon TCR stimulation. PD-1hiTim-3− PD-1−Tim-3+ and PD-1+Tim-3+ CD4+CD25lo/− effector TIL also showed a similar deficiency in Th1 cytokine production. However, PD-1intTim-3− TIL showed comparable, sometimes even higher, expression of IFNγ and TNF-α transcripts (Fig. S2) and higher secreted IFNγ compared to PD-1−Tim-3− TIL (), indicating that PD-1intTim-3− cells are in fact activated instead of exhausted, at least in terms of Th1 cytokine production.
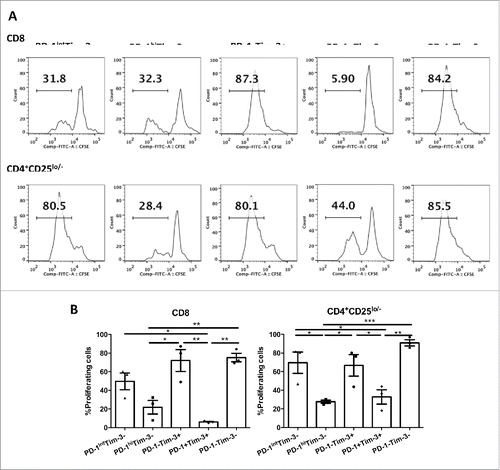
We next compared the proliferative capacity of the sorted TIL subsets after anti-CD3/CD28 stimulation. Different TIL subsets from HNSCC patients were sorted based on expression of PD-1 and Tim-3 as described above, labeled with CFSE and stimulated with anti-CD3/CD28 beads for 4 d. The dilution of CFSE was measured using flow cytometry (). Of note, PD-1hiTim-3− and PD-1+Tim-3+ effector TIL appeared to be less proliferative in response to TCR stimulation (). By contrast, PD-1intTim-3− and PD-1−Tim-3+ TIL subsets showed similarly robust proliferation compared to PD-1−Tim-3− T cells (). Thus, PD-1hiTim-3− and PD-1+Tim-3+ T cells in the tumor microenvironment, which are likely to be tumor antigen-specific,Citation21 are extremely dysfunctional, losing both the capacity to produce Th1 cytokines (Fig. S2 and ) and to expand clonally (). However, PD-1−Tim-3+ TIL are highly proliferative but defective in Th1 cytokine production.
Figure 3. PD-1intTim-3− and PD-1−Tim-3+ effector TIL retain significant proliferative potential. Sorted TIL subsets from HNSCC patients (n = 3) were rested overnight, labeled with CFSE and then stimulated with anti-CD3/CD28 microbeads (bead:cell = 5:1) for 4 d. Dilution of CFSE was then measured by flow cytometry. (A) Representative figures showing dilution of CFSE of each subset after 4-d stimulation. (B) Summary data showing percentage of proliferating cells (CFSElow cells) in each TIL subset. Statistical significance was determined by RM One-way ANOVA analysis followed by multiple comparisons. *p < 0.05, **p < 0.01, ***p < 0.001.
High levels of PD-1 are associated with reduced S6 phosphorylation, while Tim-3 expression itself drives increased S6 phosphorylation
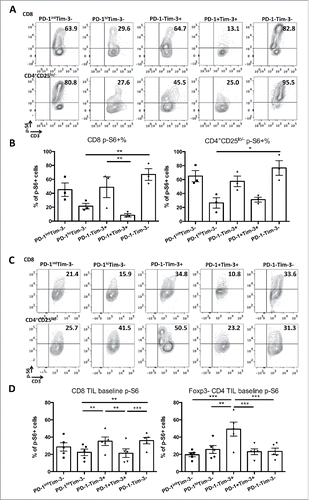
Tim-3 has been shown to augment TCR downstream signaling, including increased downstream phosphorylation of ribosomal protein S6 (pS6),Citation26 which promotes proliferation of T cells. In contrast, PD-1:PD-L1 ligation has been shown to suppress TCR signaling, and to inhibit activation of pS6 in T cells.Citation27 We hypothesized that high PD-1 expression and the concomitant high expression of SH2 domain-containing tyrosine phosphatase-2 (SHP-2)Citation27 could interfere with the putative enhancement of TCR signaling by Tim-3, leading to suppression of T cell activation, as a mechanism of crosstalk between PD-1 and Tim-3. To test this hypothesis, we stimulated sorted TIL subsets from HNSCC patients with anti-CD3/CD28 beads for 48 h and performed intracellular flow cytometry to quantify pS6, as a readout of TCR and Tim-3 signaling. As expected, PD-1hiTim-3− and PD-1+Tim-3+ effector TIL subsets showed dampened pS6 induction upon anti-CD3/CD28 stimulation (), consistent with their impaired proliferation (). Moreover, among Tim-3+ cells, PD-1+Tim-3+ TIL expressed lower pS6 than PD-1−Tim-3+ cells (). Among Tim-3− cells, the levels of TCR-stimulated pS6 followed a trend of PD-1hiTim-3−<PD-1intTim-3−<PD-1−Tim-3− (). To further explore a potential positive role for Tim-3, we also examined the state of S6 phosphorylation under basal (no additional stimulation) conditions. Thus, as shown in , expression of Tim-3 alone on TIL was associated with a higher basal level of pS6, a phenotype also observed in T cells from HIV-infected individualsCitation28 and consistent with our previous studies in T cell lines with ectopic expression of Tim-326.
Figure 4. High levels of PD-1 are associated with reduced S6 phosphorylation, while Tim-3 expression drives increased S6 phosphorylation. T cells were purified from isolated TIL from HNSCC patients (n = 3). Then, PD-1hiTim-3−, PD-1intTim-3−, PD-1−Tim-3+, PD-1+Tim-3+ and PD-1−Tim-3− CD8+ and CD4+CD25lo/− T cells were sorted from purified T cells. After sorting, cells were rested overnight and stimulated with anti-CD3/CD28 microbeads (bead: cell = 5:1) for 48 h. Cells were harvested and pS6 levels were analyzed by intracellular flow cytometry. (A) Representative figures showing expression of pS6 in each subset after 48 h stimulation. (B) Summary data showing frequency of pS6+ (S235/236) cells in each TIL subset after TCR stimulation. (C) Representative figures showing expression of pS6 in each subset without stimulation. (D) Summary data showing frequency of pS6+ cells in each TIL subset without stimulation. Statistical significance was determined by RM one-way ANOVA analysis followed by multiple comparisons. *p < 0.05, **p < 0.01.
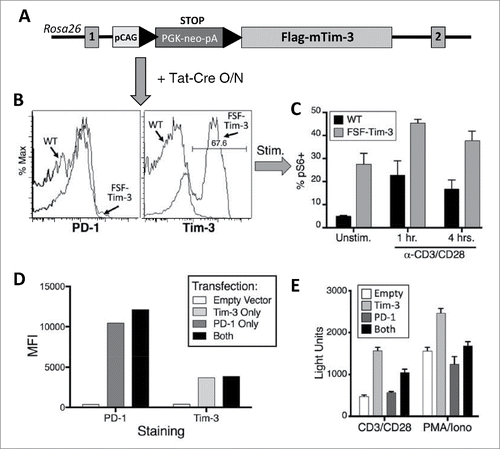
Our studies thus far suggest that, contrary to many current models, expression of Tim-3 by T cells among TIL is not necessarily indicative of a dysfunctional state, since Tim-3 itself seems to result in a “split” exhaustion phenotype, with enhanced TCR signaling and proliferation, while cytokine production is still suppressed. In order to obtain a better mechanistic understanding of the intrinsic role of Tim-3 on T cells, we generated a novel knock-in mouse model, in which we can induce Tim-3 expression in a Cre-dependent fashion (). We purified T cells from the spleens and LN of these mice and cultured them overnight with purified Tat-Cre fusion protein. As shown in , this resulted in robust expression of Tim-3 on the knock-in (but not WT) T cells, without upregulating PD-1. When these cells were subsequently stimulated with anti-CD3/CD28 antibodies, we observed that the induction of pS6 was significantly greater on T cells expressing Tim-3 (). This finding is consistent with results shown above, in which we observed that TIL expressing Tim-3 (but not PD-1) were still capable of proliferating upon re-stimulation. In order to better define possible cross-talk between PD-1 and Tim-3, we transfected a T cell line (D10.G41)Citation26,29 with cDNAs for human PD-1 and/or Tim-3 ( and Fig. S3), along with an NFAT/AP1-luciferase reporter, as a read-out for TCR signaling. Consistent with data presented in , and above, expression of Tim-3 alone enhanced reporter activation. However, D10.G41 T cells co-transfected with PD-1 and Tim-3 displayed less upregulation of TCR signaling than cells transfected with Tim-3 alone (). Taken together, our data indicate that while Tim-3 has an intrinsic ability to augment T cell activation, high expression of PD-1 interferes with both TCR signaling and the apparently positive effects of Tim-3. Nonetheless, PD-1 activity is known to be regulated at least in part by binding to its ligands. We next sought to examine this aspect of PD-1 function in the TIL samples.
Figure 5. Direct enhancement of pS6 by Tim-3 can be overcome by PD-1. (A–C) Intrinsic ability of Tim-3 to enhance T cell activation. (A) Strategy for generation of the Cre-inducible FSF-Tim-3 mouse model. Shown is the status of the Rosa26 locus before homologous recombination. LoxP sites are depicted with solid black triangles. (B) Flow cytometry analysis of bulk T cells after overnight treatment with Tat-Cre fusion protein, showing robust expression of Tim-3 (but not PD-1) on only the FSF-Tim-3 T cells (right panel). (C) Relative levels of pS6 in CD8+ T cells shown in panel F, after stimulation with anti-CD3/CD28 mAb. Results shown are from duplicate samples of a single experiment, and representative of four independent experiments. (D-E) Tim-3 enhances TCR signaling, which is countered by PD-1. (D) Quantitation of flow cytometry analysis of the relative levels of PD-1 and Tim-3 after transient transfection of D10 T cells. (E) Cells from panel C were stimulated for 6 h as indicated, then analyzed for luciferase activity. Results shown are from quadruplicate samples of a single experiment, and representative of three independent experiments.
TIL with intermediate levels of PD-1 can still be suppressed by PD-L1
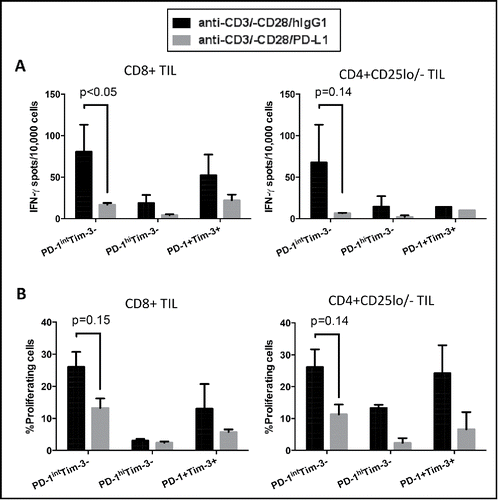
The in vivo function of exhaustion markers like PD-1 is critically regulated by interaction with specific ligands. PD-L1, one of the ligands for PD-1, is often expressed on tumor cells, which is thought to facilitate immune escape of tumors.Citation10 We therefore investigated how the various populations of PD-1 and/or Tim-3-expressing effector TIL respond to TCR stimulation in the presence of PD-L1. Sorted PD-1hiTim-3−, PD-1intTim-3− and PD-1+Tim-3+ effector TIL from HNSCC patients were stimulated with anti-CD3/-CD28/hIgG1 or anti-CD3/–CD28/PD-L1 beads, followed by assessment of IFNγ and proliferation. PD-1intTim-3− effector TIL showed the largest decrease in TCR-stimulated production of IFNγ () and proliferative capacity () in the presence of bead-coated PD-L1. TIL expressing high levels of PD-1 (with or without Tim-3) were already severely impaired when stimulated with anti-CD3/-CD28/hIgG1 beads, with little further suppression when stimulated with anti-CD3/-CD28/PD-L1 beads (). These results suggest that PD-1intTim-3− TIL are the cells most likely to be suppressed by PD-L1 expressed on tumor cells in the tumor microenvironment and to be reinvigorated by anti-PD-1 targeted immunotherapy.
Figure 6. Ligation of PD-1 with PD-L1 diminished production of IFNγ and proliferation in PD-1+ TIL, with greatest efficacy on PD-1intTim-3− cells. Sorted PD-1hiTim-3−, PD-1intTim-3− and PD-1+Tim-3+ CD8+ and CD4+CD25lo/− effector TIL were stimulated with anti-CD3/CD28/hIgG1 or anti-CD3/-CD28/PD-L1 beads (bead: cell = 30:1) for 18 h, followed by ELISPOT measurement of IFNγ (A). Summary data show secretion of IFNγ by each subset after indicated stimulations for 18 h. (B) Sorted PD-1hiTim-3−, PD-1intTim-3− and PD-1+Tim-3+ CD8+ and CD4+CD25lo/− effector TIL were labeled with CFSE and stimulated with anti-CD3/CD28/hIgG1 or anti-CD3/-CD28/PD-L1 beads (bead: cell = 30:1) for 4 d. Dilution of CFSE was then measured by flow cytometry. Summary graphs show the percentage of proliferating cells (CFSElow cells) in each TIL subset. Statistical significance was determined by RM One-way ANOVA analysis followed by multiple comparisons. *p < 0.05.
Discussion
In the present study, we set out to provide a more precise and thorough phenotypic and functional profile of TIL from HNSCC patients with heterogeneous expression of PD-1 and Tim-3. Our data demonstrate that PD-1hiTim-3− and PD-1+Tim-3+ TIL are likely to be effector memory cells, which is consistent with the prior chronic TCR-stimulation induced expression of these two receptors on T cells in the microenvironment.Citation30,31 Gene expression analysis of sorted CD8+ TIL subsets showed that Blimp-1, BATF and Eomes, transcription factors previously implicated in CD8+ T cell exhaustion, were upregulated, while T-bet, which is important for sustained T cell responses, was reduced in PD-1hiTim-3− and PD-1+Tim-3+ CD8+ TIL. In vitro stimulation of the sorted TIL subsets also revealed that PD-1hiTim-3− and PD-1+Tim-3+ effector TIL are the most dysfunctional in terms of Th1 cytokine production and proliferation after TCR stimulation, whereas PD-1intTim-3− TIL remained competent to produce Th1 cytokines and proliferate after stimulation. Nonetheless, PD-1intTim-3− effector TIL were the most susceptible to suppression by PD-L1 treatment, indicating that PD-1intTim-3− TIL is the major subset to be targeted for anti-PD-1 therapy. Furthermore, the mTOR/pS6 pathway, which is activated by TCR/CD28 signaling and is further enhanced by Tim-326, was suppressed by high expression levels of PD-1. These findings suggest that low expression of PD-1 in the absence of Tim-3 correlates with the activation status of T cells, while high levels of PD-1, or co-expression of PD-1 and Tim-326, are markers of T cell dysfunction in the tumor microenvironment.
T cell exhaustion was first defined, and has been most extensively studied, in the mouse model of chronic lymphocytic choriomeningitis virus (LCMV) infection.Citation32,33 As defined in that system, exhaustion is a special state of T cell dysfunction with gradual loss of effector function, starting with IL-2, cytolytic activity and proliferative potential at early stages and IFNγ production at later stages.Citation22 Exhaustion of CD8+ and CD4+ T cells during chronic viral infection is associated with—and possibly driven by—co-expression of multiple inhibitory receptors (including PD-1, LAG3, 2B4 and CD160), in addition to those that we have studied here. This dysfunctional state is also associated with a pattern of transcription factor expression distinct from those of functional effector or memory T cells.Citation7,8,23,24,34
Functional exhaustion of CD8+ T cells is also observed in cancers and is associated with co-expression of PD-1 and Tim-3Citation15,16 However, exhaustion of CD4+ effector T cells in cancer is still not well defined. In the cohort of HNSCC patients, we have studied sorted PD-1hiTim-3− and PD-1+Tim-3+ CD8+ TIL manifested exhausted phenotypes with defective Th1 cytokine production and proliferation (), concomitant with increased exhaustion-associated molecular signatures such as high Blimp-1, BATF, Eomes and low T-bet (). Among CD4+ effector TIL, PD-1hiTim-3− and PD-1+Tim-3+ cells also showed a similar exhausted phenotype, but we were not able to analyze the changes in their transcriptional programs by real time qPCR because of the scarcity of these cells in the tumor microenvironment. The exhausted PD-1hiTim-3− subpopulation of tumor-infiltrating T cells has been overlooked in past studies, since it has not been appreciated that the PD-1+Tim-3− T cell subset is actually a mixed population with heterogeneous PD-1 expression. Indeed, cells with high versus low levels of PD-1 appear phenotypically and functionally distinct. Thus, according to a previous report,Citation35 two subpopulations of exhausted CD8+ T cells with high or intermediate expression of PD-1 were identified during chronic LCMV infection in mice. Although both subsets were functionally exhausted compared to memory CD8+ T cells in acute infection, the PD-1int subset had higher proliferative potential and was preferentially reinvigorated by PD-L1 blockade than were the PD-1hi cells, indicating that the exhaustion status of T cells can be tuned by the relative expression level of PD-1. In our study, the PD-1intTim-3− CD8+ and CD4+ effector TIL from HNSCC patients were clearly activated, manifesting high TCR-stimulated production of Th1 cytokines and proliferation. Their function was dramatically impaired in the presence of PD-L1 (), suggesting a potential therapeutic benefit of using PD-1 blockade to reinvigorate the PD-1intTim-3− subset, which represents a large proportion of TIL in our studies (24 ± 7% in CD8+ and 31 ± 9% in CD4+ effector TIL). We propose the responsiveness of this particular subset as a biomarker for patients treated with anti-PD-1 based immunotherapy. PD-1+Tim-3+ cells may represent a negative prognostic biomarker of anti-PD-1 therapy, unless the function of these cells can be restored with Tim-3 blockade.
PD-1 drives T cell exhaustion by recruiting the tyrosine phosphatase SHP-2 to inhibit TCR-proximal phosphorylation eventsCitation36,37 and downstream pSTAT1/T-bet driven Tc1/Th1 differentiation.Citation27 Nonetheless, how Tim-3 mediates suppression of T cell activation or effector function is poorly understood, even though Tim-3 has been recognized as an additional marker of exhaustion on PD-1+ T cells in cancers and chronic viral infection. Recently, Tim-3 was proposed to help drive T cell exhaustion by augmenting TCR/CD28 signaling,Citation26,38 since excessive and constitutive TCR signaling, especially mTORC1, has been suggested to be critical for the development of T cell exhaustion. Another non-exclusive possibility is that the expression of Tim-3 observed in settings of high levels of PD-1 (including in HNSCC TIL) may represent a pro-survival mechanism in such cells. Thus, high levels of PD-1 would be expected to more severely depress signaling pathways that regulate both activation and survival of effector T cells. Possible signaling crosstalk between the phosphatase(s) downstream of PD-1 and the kinases activated by Tim-3 needs to be further investigated.
Due to the paucity of TIL that could be isolated from most HNSCC tumor specimens, replicates in our study required larger tumors, which could skew the results and our interpretation of earlier stage disease. However, the differences of these TIL subsets were consistent among the patients studied, in spite of some variation. In our ex vivo analyses, we first stimulated sorted TIL subsets for cytokine expression and proliferation in the absence of ligands of PD-1 and Tim-3 expressed on antigen-presenting cells or tumor cells. We introduced PD-1 ligand by coating PD-L1 together with anti-CD3 and anti-CD28 on beads to see which subset is most susceptible to suppression by PD-1 signaling. However, since there is still not a unique, specific, ligand defined for Tim-3, we were not able to test how PD-1+Tim-3+ TIL respond to simultaneous engagement by ligand.
Anti-PD-1 immunotherapy is clinically effective in some patients with solid tumors in early clinical trials.Citation13 However, this promising cancer immunotherapy still lacks complete response (objective response rate: ∼30%) despite the high frequency of PD-1+ tumor-reactive T cellsCitation21 in the treated tumors. The limited efficacy of PD-1 blockade to reinvigorate exhausted T cells in the tumor microenvironment could be partly explained by the presence of Tim-3 on a subset of the PD-1+ cells. Dual blockade of PD-1 and Tim-3 might be needed to generate improved clinical responses. Moreover, since PD-1intTim-3− TIL were the most susceptible to suppression by PD-L1 (), clinical responses of anti-PD-1 immunotherapy should not only be evaluated in the context of PD-L1 expression in the tumor microenvironment, but also the frequency of PD-1intTim-3− TIL, in order to improve the efficacy of anti-PD-1 therapies, and to predict those most likely to benefit from the strategies.
Materials and methods
Patients and specimens
Peripheral blood samples and fresh tumor specimens were obtained from 26 HNSCC patients. All patients were treated in the Department of Otolaryngology at the University of Pittsburgh Medical Center, and all subjects signed written informed consent approved by the Institutional Review Board of the University of Pittsburgh (UPCI# 99-069). The clinical-pathological features of the HNSCC patients in this study are shown in . The patient cohort included six females and 20 males with a mean age of 62.5 y (range: 39–89 y).
Peripheral blood mononuclear cells (PBMC) and tumor infiltrating lymphocytes (TIL)
Venous blood from HNSCC patients was drawn into heparinized tubes and centrifuged on Ficoll-Hypaque gradients (GE Healthcare Life Sciences 17-1440-03). PBMC were recovered, washed in RPMI-1640 medium (Sigma R8758), and either used immediately for experiments or resuspended in freezing media containing 10% DMSO, transferred to Mr. Frosty containers (Thermo Scientific, 5100-0001), and stored at −80°C until flow cytometry analysis. For TIL isolation, fresh tumors from HNSCC patients were minced into small pieces manually or using a gentleMACS Dissociator (Miltenyi Biotec 130-093-235), then transferred to 70 μm cell strainers (BD) and mechanically separated using the plunger of a 5-mL syringe. The cells passing through the cell strainer were collected and subjected to Ficoll-Hypaque gradient centrifugation. After centrifugation, mononuclear cells were recovered and stored at −80°C until flow cytometry analysis or immediately used for experiments.
Antibodies and flow cytometry
The following anti-human antibodies were used for staining of PBMC or TIL: CD3-Alexa Fluor 700 (BD 557917), CD8+-PE (BD 555367), CD4+-PercP/Cy5.5 (BD 560650), FOXP3-PerCP/Cy5.5 (BD 561493), Tim-3-BV421 (Biolegend 345007), CD25-PE-Cy7 (Biolegend 302612), CD4+-PE-TR (Life Technologies MHCD0417), PD-1-APC (eBioscience 17-9969-42) and CD45RA-PE-Cy7 (eBioscience 25-0458-71), CCR7-FITC (R&D Systems FAB197F) and phospho-S6 (Ser235/236)-Alexa Fluor 488 (Cell Signaling Technology 4803). Antibodies to mouse proteins were as follows: Tim-3-PE (R&D, 215008); PD1-APC (Biolegend 109111); CD3-biotin (BD 553060); CD28-biotin (BD 553296). Streptavidin was from Calbiochem. In D10 transfection experiments, human Tim-3 and PD-1 were stained with APC-conjugated clone 344823 (R&D FAB2365A) and BV421-conjugated clone EH12.1 (BD 562516), respectively. Intracellular staining for FOXP3 and pS6 was performed as follows: PBMC or TIL were stained with surface marker antibodies, fixed with fixation/permeabilization buffer (eBioscience 00-5521), washed, and stained for intracellular antigens in 1X permeabilization buffer. Cells were analyzed on an LSRFortessa (BD Biosciences) flow cytometer, and data analyzed using Flow Jo. The acquisition and analysis gates were restricted to the lymphocyte gate based on characteristic properties of the cells in the forward and side scatter. Dead cells were excluded based on viability dye staining (Zombie Aqua Fixable Viability Dye, Biolegend 423101).
Gating strategies for TIL subsets
PD-1 or Tim-3 positivity was determined by isotype control. PD-1+ Tim-3− CD8+ or Foxp3− CD4+ TIL with similar fluorescence intensity of PD-1 with CD8+ or Foxp3− CD4+ T cells from matched PBMC were recognized as PD-1intTim-3−, while those with higher expression of PD-1 than peripheral blood T cells were PD-1hiTim-3−. To set gates consistently across different patients, we used the same amount of antibodies for staining and the same voltage on the cytometer.
Anti-CD3/-CD28/hIgG1 and anti-CD3/-CD28/PD-L1 beads
LEAF purified anti-human CD3 (clone UCHT1, Biolegend, 300414), LEAF purified anti-human CD28 (clone CD28.2, Biolegend 302914) plus PD-L1-hIgG1 Fc fusion protein (R&D Systems, Q9NZQ7) or control human IgG1 (Southern Biotech, Birmingham, AL) were covalently coupled to Dynabeads M-450 Epoxy beads according to the manufacturer's protocol (Life Technologies 14011). The total amount of protein was kept constant at 5 μg per 107 beads as previously described.Citation39 Generally, 107 beads were coated with 1 μg of anti-CD3 (20% of total protein), 1 μg of anti-CD28 and 60% of either PD-L1-hIGg1 Fc fusion protein or control human IgG1. Covalent coupling of the proteins to the beads was performed in 0.1 M sodium phosphate buffer for 24 h at room temperature with gentle tilting and rotation.
Sorting of TIL subsets
TIL were isolated from tumor specimens as described above and T cells were purified using EasySep™ Human T Cell Enrichment Kit (Stemcell technologies 19051). Then, the T cells were stained with CD4+-PercP-Cy5.5, CD8+-PE, CD25-PE-Cy7, PD-1-APC (eBioscience 17-9969-42) and Tim-3-BV421 (Biolegend 345007) antibodies. PD-1hiTim-3−, PD-1intTim-3−, PD-1-Tim-3+, PD-1+Tim-3+ and PD-1-Tim-3− CD8+ and CD4+CD25lo/− cells were sorted using Beckman Coulter MoFlo Astrios. Post-sort purity of the sorted populations was ∼94% (Fig. S4).
Re-stimulation of TIL using anti-CD3/CD28 beads, anti-CD3/-CD28/hIgG1 or anti-CD3/-CD28/PD-L1 beads
T lymphocyte subsets were sorted based on PD-1 and Tim-3 expression from freshly isolated TIL from HNSCC patients, rested in 30% Human AB serum overnight and then subjected to re-stimulation experiments. Sorted TIL were cultured with Dynabeads® Human T-Activator CD3/CD28 beads at a fixed cell: bead ratio of 1:5 or anti-CD3/-CD28/hIgG1 and anti-CD3/-CD28/PD-L1 at a cell: bead ratio of 1:30. The cultures were incubated at 37°C with 5% CO2 for indicated time periods. Magnetic beads were removed before analysis.
Quantitative Real-Time RT-PCR
Sorted TIL subsets were either subjected to RNA extraction or stimulated with anti-CD3/CD28 beads for 6 h as described above and harvested before RNA extraction. Total RNA was extracted using Trizol reagent (Invitrogen 15596018) and purified using RNA cleanup (Qiagen), followed by Purelink on-column DNase digestion (Invitrogen 12185-010) according to manufacturer's instructions. The concentration and purity of RNA was determined by measuring absorbance at 260 and 280 nm. RNA was used for first strand cDNA synthesis using random hexamers and MultiScribe reverse transcriptase (Applied Biosystems) according to manufacturer's instructions. PCR probes for PRDM1 (Hs00153357_m1), BATF (Hs00232390_m1), EOMES (Hs00172872_m1), TBX21 (Hs00203436_m1), IFNG (Hs00989291_m1), TNF (Hs99999043_m1) and GUSB (Hs99999908_m1) were purchased from Applied Biosystem for TaqMan® Gene Expression Assay. Real-time PCR cycling was performed using StepOne™ Real-Time PCR Systems (Applied Biosystems). GUSB was amplified as an internal control. All of the experiments were performed in triplicate. Relative expression of the target genes to endogenous control gene (GUSB) was calculated using the ΔCT method: relative expression = 2−ΔCT, where ΔCT = CT (target gene) − CT (GUSB).
IFNγ ELISPOT
ELISPOT assays were performed as previously described.Citation40 Briefly, MultiScreen-IP filter plates (Millipore MAIPS4510) were coated with anti-human IFNγ mAb 1-D1K (Mabtech 3420-2H) (10 μg/mL in PBS) overnight at 4°C. Plates were washed with PBS and then blocked for 1 h at 37°C with RPMI supplemented with 10% human serum. Sorted cells were added to wells in duplicate (5 × 103) and then stimulated with anti-CD3/CD28 beads (bead: cell = 5:1), anti-CD3/-CD28/hIgG1 or anti-CD3/-CD28/PD-L1 beads (bead: cell = 30:1). Following an 18 h incubation at 37°C, plates were washed with PBS/0.5% Tween 20 (PBS-T), and incubated with biotinylated anti-IFNγ mAb (2 μg/mL in PBS/0.5% BSA) for 2 h at 37°C. Plates were washed with PBS-T and incubated with Streptavidin-HRP (1:500 in PBS/0.5% BSA) for 1 h at 37°C. Plates were washed with PBS-T and TMB substrate solution (Vector Laboratories SK-4400) was added. After distinct spots emerged, color development was stopped by washing extensively in tap water. Plates were dried and spots were counted using CTL ImmunoSpot® Analyzers (CTL) and analyzed by CTL Professional Double Color Software.
Proliferation assay
Sorted cells were labeled with CFSE using CellTrace CFSE Cell Proliferation Kit (Life Technologies C34554). Briefly, cells were incubated with 2 uM CellTrace CFSE in 1 mL PBS/0.1% BSA for 10 min at 37°C and then washed twice with complete medium. Then, the labeled cells were cultured anti-CD3/CD28 beads at a fixed cell: bead ratio of 1:5, anti-CD3/-CD28/hIgG1 or anti-CD3/-CD28/PD-L1 beads at a cell: bead ratio of 1:30 for 4 d. Dilution of CFSE was measured by flow cytometry.
Cre-inducible FSF-Tim-3 mouse model
A cDNA construct containing the BL/6 allele of murine Tim-3 (gene name Havcr2), with an N-terminal Flag tag,Citation26 was inserted into a targeting vector containing part of the Rosa26 locus (genOway S.A.). The resulting targeting construct contained floxed PGK-neo-PA “STOP” cassette upstream of the Flag-Tim-3 sequence. The STOP cassette was preceded by a hybrid CAG promoter to enhance transcription of the Flag-Tim-3 sequence after removal of the STOP cassette by Cre-mediated recombination. The targeting construct was transfected into BL/6 ES cells and clones with targeted recombination were selected (genOway S.A.). The resulting clones were then injected into BALB/C embryos for the generation of chimeric animals, which were then bred to BL/6 mice to generate F1 offspring (U.C. Davis Mouse Biology Program). These animals were then maintained on a BL/6 background by breeding to C57BL/6 mice (Jackson).
Cre-mediated induction of Tim-3 and T cell activation
Naïve T cells from FSF-Tim-3 mice, or littermate controls, were treated overnight with 1.5 uM Tat-Cre (Excellgen). The next day, cells were stimulated with anti-CD3/CD28 antibodies for 1–4 h. Cells were then fixed and stained with an Alexa647-conjugated antibody to phospho-S6 (pS6(Ser235/236); Cell Signaling 5548). Flow cytometry was performed with a Becton Dickinson LSRFortessa flow cytometer, and data were analyzed with FlowJo (Treestar).
T cell transfection and luciferase assays
A fast-growing variant of the D10.G41 murine T cell clone was maintained and transfected as previously described.Citation26 Cells were transfected with cDNA's encoding the human allele of Tim-3 and/or PD-1, along with an NFAT/AP1-luciferase reporter construct. The next day, cells were analyzed for protein expression by flow cytometry or stimulated as indicated for 6 h. Luciferase activity was then determined on a 96-well plate luminometer (Promega).
Statistical analysis
Averages were calculated as means. p-values were calculated by Wilcoxon–Mann–Whitney tests or RM One-way ANOVA analysis followed by multiple comparisons using GraphPad Prism (GraphPad, La Jolla, CA). p-values < 0.05 were considered to be significant. *p < 0.05, **p < 0.01, ***p < 0.001.
Disclosure of potential confllicts of interest
Dr. Ferris reports research funding and advisory board service for Bristol Myers Squibb, Merck, Astra-Zeneca/Medimmune and Celgene.
Author contributions
JL was involved in concept, design and performing of experiments, and wrote the manuscript. GS was involved in performing experiments and revising the manuscript. LA performed experiments and provided feedback on the manuscript. HJ was involved in processing patient samples. NGL and NS helped collect patient samples from the clinic. BL and LPK were involved in experimental design and manuscript revision. RLF supervised the study, was involved in concept and design of experiments, critically involved in writing and editing of the manuscript. All authors read and approved the final manuscript.
KONI_A_1200778_Supplementary_Materials.zip
Download Zip (729.6 KB)Funding
This study was supported by NIH grant R01 DE019727 and P50 CA097190 (R.L.F.); R03 AI109605 (L.P.K.). This project used the University of Pittsburgh Cancer Institute Flow Cytometry Facility that is supported in part by award P30 CA047904. Jing Li and Gulidanna Shayan are supported by the China Scholarship Council. This work was funded by NIH grant R01 DE019727 (R.L.F.), P50 CA097190 (R.L.F.); CA206517 (R.L.F and L.P.K), and R03 AI109605 (L.P.K.).
References
- Ferris RL. Immunology and Immunotherapy of Head and Neck Cancer. J Clin Oncol 2015; 33:3293-304; PMID:26351330; http://dx.doi.org/10.1200/JCO.2015.61.1509
- Li J, Jie HB, Lei Y, Gildener-Leapman N, Trivedi S, Green T, Kane LP, Ferris RL. PD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironment. Cancer Res 2015; 75:508-18; PMID:25480946; http://dx.doi.org/10.1158/0008-5472.CAN-14-1215
- Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res 2007; 13:6301-11; PMID:17975141; http://dx.doi.org/10.1158/1078-0432.CCR-07-1403
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253-68; PMID:22437938; http://dx.doi.org/10.1038/nri3175
- Li J, Srivastava RM, Ettyreddy A, Ferris RL. Cetuximab ameliorates suppressive phenotypes of myeloid antigen presenting cells in head and neck cancer patients. J Immunother Cancer 2015; 3:54; PMID:26579227; http://dx.doi.org/10.1186/s40425-015-0097-6
- Jie HB, Schuler PJ, Lee SC, Srivastava RM, Argiris A, Ferrone S, Whiteside TL, Ferris RL. CTLA-4(+) Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res 2015; 75:2200-10; PMID:25832655; http://dx.doi.org/10.1158/0008-5472.CAN-14-2788
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007; 27:670-84; PMID:17950003; http://dx.doi.org/10.1016/j.immuni.2007.09.006
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8(+) T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009; 10:29-37; PMID:19043418; http://dx.doi.org/10.1038/ni.1679
- Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, Ferris RL. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer 2013; 109:2629-35; PMID:24169351; http://dx.doi.org/10.1038/bjc.2013.645
- Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 2013; 73:1733-41; PMID:23288508; http://dx.doi.org/10.1158/0008-5472.CAN-12-2384
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:22437870; http://dx.doi.org/10.1038/nrc3239
- Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res 2011; 17:6958-62; PMID:21900389; http://dx.doi.org/10.1158/1078-0432.CCR-11-1595
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200690
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122-33; PMID:23724867; http://dx.doi.org/10.1056/NEJMoa1302369
- Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 2010; 207:2175-86; PMID:20819923; http://dx.doi.org/10.1084/jem.20100637
- Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 2010; 207:2187-94; PMID:20819927; http://dx.doi.org/10.1084/jem.20100643
- Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 2013; 73:128-38; PMID:23135914; http://dx.doi.org/10.1158/0008-5472.CAN-12-2606
- Legat A, Speiser DE, Pircher H, Zehn D, Fuertes Marraco SA. Inhibitory Receptor Expression Depends More Dominantly on Differentiation and Activation than “Exhaustion” of Human CD8 T Cells. Front Immunol 2013; 4:455; PMID:24391639; http://dx.doi.org/10.3389/fimmu.2013.00455
- Utzschneider DT, Legat A, Fuertes Marraco SA, Carrie L, Luescher I, Speiser DE, Zehn D. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat Immunol 2013; 14:603-10; PMID:23644506; http://dx.doi.org/10.1038/ni.2606
- Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A 2010; 107:14733-8; PMID:20679213; http://dx.doi.org/10.1073/pnas.1009731107
- Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest 2014; 124:2246-59; PMID:24667641; http://dx.doi.org/10.1172/JCI73639
- Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12:492-9; PMID:21739672; http://dx.doi.org/10.1038/ni.2035
- Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, Wherry EJ. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity 2009; 31:309-20; PMID:19664943; http://dx.doi.org/10.1016/j.immuni.2009.06.019
- Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med 2010; 16:1147-51; PMID:20890291; http://dx.doi.org/10.1038/nm.2232
- Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 2012; 338:1220-5; PMID:23197535; http://dx.doi.org/10.1126/science.1229620
- Lee J, Su EW, Zhu C, Hainline S, Phuah J, Moroco JA, Smithgall TE, Kuchroo VK, Kane LP. Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways. Mol Cell Biol 2011; 31:3963-74; PMID:21807895; http://dx.doi.org/10.1128/MCB.05297-11
- Li J, Jie HB, Lei Y, Gildener-Leapman N, Trivedi S, Green T, Kane LP, Ferris RL. PD-1/SHP-2 negatively regulate Tc1/Th1 phenotypic responses and activation of T cells in the tumor microenvironment. Cancer Res 2015; 75(3):508-518; PMID:25480946; http://dx.doi.org/10.1158/0008-5472.CAN-14-1215
- Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med 2008; 205:2763-79; PMID:19001139; http://dx.doi.org/10.1084/jem.20081398
- Kaye J, Porcelli S, Tite J, Jones B, Janeway CA, Jr. Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med 1983; 158:836-56; PMID:6193236; http://dx.doi.org/10.1084/jem.158.3.836
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996; 8:765-72; PMID:8671665; http://dx.doi.org/10.1093/intimm/8.5.765
- Mujib S, Jones RB, Lo C, Aidarus N, Clayton K, Sakhdari A, Benko E, Kovacs C, Ostrowski MA. Antigen-Independent Induction of Tim-3 Expression on Human T Cells by the Common gamma-Chain Cytokines IL-2, IL-7, IL-15, and IL-21 Is Associated with Proliferation and Is Dependent on the Phosphoinositide 3-Kinase Pathway. J Immunol 2012; 188:3745-56; PMID:22422881; http://dx.doi.org/10.4049/jimmunol.1102609
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJD, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 1998; 188:2205-13; PMID:9858507; http://dx.doi.org/10.1084/jem.188.12.2205
- Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I peptide complexes. J Exp Med 1998; 187:1383-93; PMID:9565631; http://dx.doi.org/10.1084/jem.187.9.1383
- Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, Wherry EJ. Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity 2014; 40:289-302; PMID:24530057; http://dx.doi.org/10.1016/j.immuni.2014.01.005
- Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A 2008; 105:15016-21; PMID:18809920; http://dx.doi.org/10.1073/pnas.0801497105
- Wang SF, Fouquet S, Chapon M, Salmon H, Regnier F, Labroquere K, Badoual C, Damotte D, Validire P, Maubec E et al. Early T Cell Signalling Is Reversibly Altered in PD-1(+) T Lymphocytes Infiltrating Human Tumors. Plos One 2011; 6:e17621; PMID:21408177; http://dx.doi.org/10.1371/journal.pone.0017621
- Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med 2012; 209:1201-17; PMID:22641383; http://dx.doi.org/10.1084/jem.20112741
- Ferris RL, Lu B, Kane LP. Too Much of a Good Thing? Tim-3 and TCR Signaling in T Cell Exhaustion. J Immunol 2014; 193:1525-30; PMID:25086175; http://dx.doi.org/10.4049/jimmunol.1400557
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009; 206:3015-29; PMID:20008522; http://dx.doi.org/10.1084/jem.20090847
- Andrade Filho PA, Lopez-Albaitero A, Gooding W, Ferris RL. Novel immunogenic HLA-A*0201-restricted epidermal growth factor receptor-specific T-cell epitope in head and neck cancer patients. J Immunother 2010; 33:83-91; PMID:19952953; http://dx.doi.org/10.1097/CJI.0b013e3181b8f421
