ABSTRACT
Pancreatic ductal adenocarcinoma (PDAC) is the third leading cause of cancer-related death in the United States, exhibiting a five-year overall survival (OS) of only 7% despite aggressive standard of care. Recent advances in immunotherapy suggest potential application of immune-based treatment approaches to PDAC. To explore this concept further, we treated orthotopically established K-rasG12D/p53−/− PDAC tumors with gemcitabine and a cell-based vaccine previously shown to generate durable cell-mediated (TH1) immunity. Tumor progression was monitored by IVIS. The results indicated that the combination of chemotherapy and dendritic cell (DC) vaccination was effective in eliminating tumor, preventing metastasis and recurrence, and significantly enhancing OS. No animal that received the combination therapy relapsed, while mice that received gemcitabine-only or vaccine-only regimens relapsed and progressed. Analysis of circulating PBMC demonstrated that mice receiving the combination therapy exhibited significantly elevated levels of CD8+IFNγ+CCR7+NK1.1+ T-cells with significantly reduced levels of exhausted GITR+CD8+ T-cells after the cessation of treatment. Retro-orbital tumor re-challenge of surviving animals at six-months post-treatment demonstrated durable antitumor immunity only among mice that had received the combination therapy. CD8+ splenocytes derived from surviving mice that had received the combination therapy were sorted into NK1.1pos and NK1.1neg populations and adoptively transferred into naive recipients. Transfer of only 1,500 CD8+NK1.1pos T-cells was sufficient to mediate tumor rejection whereas transfer of 1,500 CD8+NK1.1neg T-cells imparted only minimal effects. The data suggest that addition of a TH1 DC vaccine regimen as an adjuvant to existing therapies can mediate eradication of tumors and offer durable protection against PDAC.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the tenth most common cancer worldwide, yet the third most common cause of neoplastic death in the United States. Modern chemotherapeutic regimens including FOLFIRINOX (folininic acid, 5′-fluorouracil, irinotecan, oxaliplatin) and gemcitabine/nab-paclitaxel can increase median survival to 11 mo in selected patients with good performance status. Pancreatectomy with curative intent is possible in fewer than 20% of cases and increases median survival to only 20–23 mo in conjunction with adjuvant or neoadjuvant therapy; five-year overall survival (OS) remains a stubborn 7%.Citation1 In the United States in 2016, there will be an estimated 53,000 pancreatic cancer diagnoses (all types) and nearly 42,000 deaths. Every year since 2000, the incidence rate of PDAC has increased by 1.2%, and the death rate by 0.4%.Citation2,3 Given these grim statistics, it is clear that there remains an unmet medical need for better treatment regimens that can enhance survival in PDAC.
The use of immunotherapy for the treatment of PDAC is an area of active investigation, and attempts at targeting PDAC by vaccine immunotherapy have shown some success. Of note, Lepisto et al. reported 33% four-year survival among 12 PDAC patients treated with MUC1 peptide-loaded dendritic cells (DC),Citation4,5 and Morse et al. reported that 3 of 3 PDAC patients treated with CEA mRNA-transfected DC were alive with NED at 30 mo post-vaccination.Citation4,6 Additionally, several animal models have also established the utility of using total tumor antigens (mRNA or lysate) for DC immunotherapy,Citation7,8 a critical point given the significant contribution of desmoplasia to the total antigenic content of the pancreatic tumor. Old vaccination concepts for PDAC have also been given new life with the advent of immune checkpoint inhibitor drugs. In a recent randomized trial in which the anti-CTLA-4 inhibitor drug ipilimumab was compared to ipilimumab + GVAX (allogeneic irradiated cancer cells transduced with GM-CSF) in heavily pretreated patients with advanced PDAC, patients assigned to the ipilimumab + GVAX arm exhibited significantly greater median OS (5.7 mo vs. 3.6 mo) and 1 y OS (27% vs. 7%). Most importantly, 2 of 15 patients in the ipilimumab + GVAX arm experienced long-term disease stabilization and were alive at 30 mo whereas all 15 patients receiving ipilimumab-only had died by 17 mo post-treatment.Citation9 In addition, investigators have demonstrated that effective anticancer immune responses can specifically target the neoplastic support stroma of a variety of different tumor types. Hence the presence of desmoplasia commonly associated with PDAC does not appear to be a barrier to immunotherapy.Citation10
DC are the most important of the professional antigen presenting cells (APCs) that initiate and direct adaptive immune responses. Upon detection of a danger signal DC migrate to local lymph nodes where they engage T cells and, depending upon a broad host of variables, induce a variety of immunogenic or tolerogenic responses. For the past decade our group has been interrogating a basic regulatory mechanism that adds to the paradigm by which regulation of cellular immunity is understood. We have previously demonstrated that the loading of DC with antigenically similar (homologous) MHC class I and II antigens leads to a cell-intrinsic upregulation of TH1 polarization. This polarization consists of a differential ability to secrete IL-12, IL-23, CTLA-4,Citation11 and the novel TH1 polarizing cytokine AIMp1,Citation12-15 to upregulate surface CD83 and CD40, and to induce the preferential generation of IFNγ-secreting CD8+ cytolytic T-cells. DC loaded in this manner also demonstrate a substantial reorganization of the transcriptome, exhibiting a transcriptional profile significantly different from alternatively loaded DC by over 1,700 transcripts in areas such as interferon signaling, antigen-presentation, antiviral responses, protein ubiquitination, and DC licensing.Citation16-18 Using nearly 40 different independent antigenic systems, previous work demonstrated that loading of DC MHC class I and II with antigens of similar or identical content stimulates release of the TH1-promoting cytokine AIMp1/p43 with concomitant reduction in the release of CTLA-4+ regulatory microvesicles, and that these events are not dependent on innate pattern recognition or TLR ligands.Citation18 This concept has subsequently been utilized for the generation of cell-based vaccine platforms that specifically target cancer with highly cytotoxic T-cell responses of durable memory potential. Here, we have generated such a platform for experimental treatment of orthotopically established K-rasG12D/p53−/− PDAC tumors in mice and report that a combination therapy of TH1 DC vaccination and standard of care gemcitabine results in durable elimination of tumor through the generation of long-lived memory cells that prevent relapse. Additional data suggest that antitumor immunity may be dependent upon a subpopulation of CD8+NK1.1+ T-cells not previously described in the context of cancer.
Materials and methods
Reagents
Antibodies: αHuman/mouse CTLA-4 (WB) (Abcam; Cambridge, MA); αHuman/mouse AIMP1 (Lifespan Biosciences Inc., Seattle, WA); αHuman/mouse β-actin was purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Flow cytometry antibodies for staining against CD3, CD25, CD8, CD11c, CD80, CD83, CD86, IFNγ, NK1.1, CCR7, Lag3, GITR, and CD152 were purchased from BD Biosciences (San Jose, CA). Six to eight-week-old female C57BL/6 mice were obtained from Baylor College of Medicine's Center for Comparative Medicine. All mice were maintained in accordance with the specific IACUC requirements of Baylor College of Medicine.
Orthotopic tumor implantation
Tumor cells were orthotopically implanted in mice. Briefly, mice were anesthetized with isoflurane and a 1 cm incision in the left subcostal region was made. Murine PDAC cells (KrasG12Dp53−/−luc2+) were injected into the caudal pancreas at 0.5–1.0 × 106 cells per mouse. The peritoneum and skin were closed with the sutures. Buprenorphine (0.5 mg/kg) was administered postoperatively to minimize pain. Each experimental cohort consisted of five mice per group unless otherwise indicated. The KrasG12Dp53−/−luc2+ cell line was obtained from Dr. Steven Ullrich at the University of Texas MD Anderson Cancer Center in August, 2014. This cell line was authenticated by the University of Texas MD Anderson Cancer Center Characterized Cell Line Core in April, 2013 by short tandem repeat (STR) DNA fingerprinting using the AmpFlSTR Identifiler Kit according to the manufacturer's instructions (Applied Biosystems, Foster City, CA).
Dendritic cell manipulations
DC were derived from the long bones of C57BL/6 mice. Bone marrow leukocytes were flushed from mouse tibia and femur and cultured in AIM-V containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 1% antibiotic/antimycotic (Invitrogen) and supplemented with 50 ng/mL mGM-CSF (R&D Systems, Minneapolis, MN) and 10 ng/mL mIL-4 (R&D Systems) for 3 d. Cells were cultured in a humidified chamber at 37°C and 5% atmospheric CO2. The culture medium was removed and replenished with an equal volume of fresh medium and cytokines on days three and five. The immature DC were harvested on day six and pulsed with mRNA and lysate derived from the original luc2-negative KrasG12Dp53−/− parental cell line as described previously.Citation17 Briefly, lysate was prepared by resuspension of tumor cells at 2 × 107/mL in PBS followed by three rapid freeze/thaw cycles in liquid nitrogen and a 37°C water bath. Freeze/thawed lysate was stored at −20°C until use. Antigen-pulsed DC were matured using a cocktail of 50 ng/mL GM-CSF, 10 ng/mL IL-4, 10 ng/mL IL-1β (R&D Systems), 10 ng/mL TNF-α (R&D Systems), 15 ng/mL IL-6 (R&D Systems), and 1 μg/mL PGE2 (Sigma-Aldrich, St. Louis, MO). Unused mature DC were cryopreserved for future use.
Vaccination and chemotherapy
Three days post tumor implantation, mice received primary vaccination with 200,000 DC per mouse i.p. The TLR-7 agonist Imiquimod was given as an adjuvant during primary vaccination. A booster injection of DC was given 10 d later. The mice in the combination therapy and drug monotherapy groups received 40 mg/kg gemcitabine i.p. for two weeks every alternate day starting on day 13 post-implantation.
IVIS imaging
Post tumor implantation, mice were injected with 100 μL of 10 mg/mL D-Luciferin (Regis Technologies, Morton Grove, IL), incubated for 10 min and bioluminescence was measured with the IVIS imaging system (Caliper Life Sciences, Waltham, MA) after a 30 sec exposure. Imaging was done at weekly intervals. On post-inoculation day 181, surviving mice that received combination therapy (4/4) or drug monotherapy (3/5) were retro-orbitally re-challenged with 500,000 KrasG12Dp53−/− cells. Tumor growth was monitored by IVIS imaging. Five naive mice were included as controls.
Analysis of circulating lymphocytes
Mice were bled retro-orbitally on days 49 and 101 post tumor implantation. Red blood cells were lysed by treatment with ammonium chloride (Sigma-Aldrich) as recommended by the manufacturer's instructions. The white blood cell pellet was washed once with PBS and resuspended in AIM-V medium + 10% mouse serum. Cryopreserved, loaded DC were thawed and washed and co-cultured overnight with the fresh PBMC in a 96 well plate at a ratio of 1:10. The following day, cells were stained with anti-CD3, CD8, CD25, IFNγ, NK1.1, CCR7, Lag3, and GITR for analysis by flow cytometry. All flow cytometric analysis was performed using an LSR II flow cytometer (BD Biosciences) and analyzed with FlowJo version 10.0.00003 (Tree Star Inc., Ashland, OR) for OS-X.
Histology and immunohistochemistry
Intact tissues were fixed in 10% formalin for 24 h, rinsed with 70% ethanol, embedded in paraffin, and sectioned in 5 μm increments. Paraffin sections were stained with hematoxylin and eosin (H&E) for gross histological analysis by light microscopy using an Olympus CX41 microscope (Olympus Corporation, Center Valley, PA) with an Olympus DP70 digital camera (Olympus Corporation).
Splenocyte adoptive transfer
On post-inoculation day 259, splenocytes were harvested from the mice that received the combination therapy and survived both the primary challenge and the secondary retro-orbital re-challenge. Non-adherent lymphocytes were separated from the monocytic fraction by plastic adherence, and CD8+ cells were negatively selected by magnetic separation (Miltenyi-Biotec, San Diego, CA). Purified CD8+ cells were activated by overnight culture with PDAC antigen loaded DC at the ratio of 1 DC per 10 T-cells. The following day, NK1.1pos and NK1.1neg populations were separated on a FACSAria IIu, (BD Biosciences, San Jose, CA), and 1,500 sorted cells were adoptively transferred by i.p. administration into naive mice. 250,000 KrasG12Dp53−/− tumor cells were injected intraperitoneally the following day.
Western blotting and analysis
All gel electrophoreses was performed under denaturing, reducing conditions on a 12% polyacrylamide gel with subsequent transfer to a 0.45 μm nitrocellulose membrane for antibody probing. All blocking and antibody staining steps were carried out in 5% milk, and primary antibodies were applied overnight at 4°C. Western blot chemiluminescent signal was detected using a ChemiDoc XRS digital imaging system supported by Image Lab software Version 2.0.1 (Bio-Rad Laboratories, Hercules, CA). All Western blots were quantitated by densitometry of Ponceau S (Sigma-Aldrich) stained membranes. Contamination of supernatants with residual cell lysate or debris from cell death was controlled for by immunostaining with anti-β-actin (Santa Cruz) and additional densitometry. Densitometry was performed using ImageJ software (NIH, Bethesda, MD). All western blots are representative of at least three independent experiments.
RT-PCR
Total RNA was extracted from the DC by means of the Trizol method and cDNA synthesis was performed using RT PCR using High-Capacity cDNA Reverse Transcription Kit (Life Technologies) according to the manufacturer's instructions. Real time PCR was performed with the 7500 Real-time PCR system (Applied Biosystems, Foster City, CA) using the Taqman Realtime PCR assay (ThermoFisher Scientific, Waltham MA) according to the manufacturer's instructions. The primers used were IL1-β (Mm00434228_m1, FAM), IL-6 (Mm00446190_m1, FAM), IL-12a (Mm00434165_m1, FAM), Ctla4 (Mm01253995_m1, FAM), and 18s rRNA (4319413E, VIC).
Statistical analysis
Statistical significance was determined by the two-tailed Student's t test or ANOVA using Prism Software (GraphPad Software, La Jolla, CA). Survival was analyzed according to the Kaplan–Meier method. Bonferoni correction was applied when necessary to control for type I errors during multiple comparisons. Data are presented as the mean ± SEM unless stated otherwise. Statistical significance was defined as p ≤ 0.05.
Results
Dendritic cells loaded in a homologous fashion with PDAC antigens exhibit hallmarks of the TH1 DC phenotype
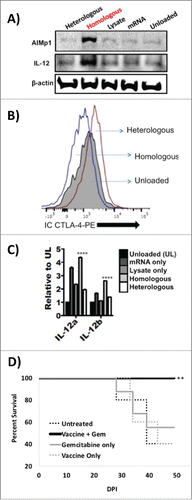
As a first step in the development of a TH1 DC vaccine, DC were loaded with mRNA and lysate derived from KrasG12Dp53−/−luc2neg cells, matured, and analyzed for previously described hallmarks Citation16-18 of homologous antigenic loading. Also as described previously,Citation16-18 DC were loaded singly with PDAC mRNA, singly with PDAC cell lysate, and doubly in a heterologous fashion with PDAC mRNA and irrelevant B16 tumor lysate to serve as comparative controls. Western blot of the DC lysates probed with AIMp1 antibody indicated that only homologous antigenic loading resulted in significantly elevated levels of AIMp1 production () while intracellular flow cytometry demonstrated a concomitant decrease in the levels of CTLA-4 protein among homologously loaded (TH1) DC (). Total RNA was isolated from all differentially loaded DC, and RT-PCR was performed to characterize expression levels of IL-12 transcript subunits IL-12α and IL-12β. As indicated in , there was a significant increase in the transcript levels of both IL-12 subunits among DC loaded in a homologous fashion. This transcriptional difference was particularly pronounced for the IL-12β subunit (IL-12 p40), the subunit previously described to regulate IL-12 p70 stability.Citation19 Western blot analysis confirmed a highly significant increase in stable IL-12 protein subunit levels only among DC loaded in a homologous fashion (). All analyses were performed on purified DC that had no previous or subsequent contact with T-cell populations as described previously.Citation16 These results confirmed that homologous antigenic loading of DC with PDAC antigens could recapitulate the previously characterized TH1 polarizing phenotype.
Figure 1. Homologous antigenic loading of Dendritic cells with PDAC antigens recapitulates the TH1 DC phenotype. (A) Western blot analysis of cell lysates derived from differentially loaded DC and probed with anti-AIMp1 and anti-IL-12 antibodies demonstrated that only homologously loaded DC exhibited significant upregulation of AIMp1 and IL-12 p35 protein levels. Heterologous: DC doubly loaded with PDAC KrasG12Dp53−/− mRNA and heterologous B16 melanoma lysate. Homologous: DC doubly loaded with PDAC KrasG12Dp53−/− mRNA and homologous PDAC KrasG12Dp53−/− lysate. Lysate: DC loaded with only PDAC KrasG12Dp53−/− lysate. mRNA: DC loaded with only PDAC KrasG12Dp53−/− mRNA. Unloaded: unloaded DC. (B) Intracellular flow cytometry analysis of the differentially loaded dendritic cells revealed that only homologous antigenic loading resulted in concomitant downregulation of intracellular CTLA-4. DC loaded with homologous PDAC antigens, DC loaded with heterologous PDAC and B16 antigens, or unloaded DC are shown. X-axis: IC CTLA-4 PE MFI. (C) Total RNA was isolated from all differentially loaded DC, and RT-PCR was performed to characterize expression levels of IL-12 transcript subunits IL-12α and IL-12β. Error bars = ± SEM. ****p < 0.001. (D) Combination gemcitabine and TH1 DC vaccination enhances OS following orthotopic PDAC implantation. One million KrasG12Dp53−/− PDAC tumor cells were orthotopically implanted into the pancreata of wild type C57BL/6 mice after which mice were treated therapeutically with gemcitabine alone, TH1 DC vaccination alone, or gemcitabine in combination with a TH1 DC vaccine. Untreated controls were also included. Differences in survival were assessed by Kaplan–Meier survival analysis. X-axis: day post injection. Y-axis: percent survival. **p < 0.01.
Gemcitabine treatment in combination with TH1 DC vaccination mediates tumor eradication
Using an aggressive therapeutic model system, 1.0 × 106 KrasG12Dp53−/− PDAC tumor cells were orthotopically implanted into the pancreata of wild type C57BL/6 mice after which mice were treated therapeutically with gemcitabine (40 mg/kg) alone, TH1 DC vaccination alone, or gemcitabine in combination with a TH1 DC vaccine. Though median survival was identical for untreated controls, mice treated with gemcitabine alone, and mice treated with vaccination alone, 100% of mice treated with a combination of gemcitabine and vaccination remained alive through study day 53 (, p = 0.003).
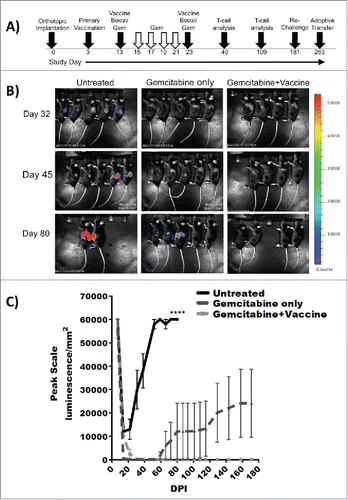
Given these unexpected results, a second therapeutic model system was constructed to comprehensively characterize this phenomenon. In this model system, 0.5 × 106 KrasG12Dp53−/−luc2 PDAC tumor cells were orthotopically implanted into the pancreata of wild type C57BL/6 mice after which mice were treated therapeutically with gemcitabine (40 mg/kg) alone or gemcitabine in combination with TH1 DC vaccination (treatment schema outlined in ). Tumor growth was monitored weekly by IVIS imaging, and by day 32 post-implantation, all treated mice were in remission. However, by day 85, 80% of mice treated with gemcitabine alone had relapsed whereas all animals treated with gemcitabine + vaccine remained in complete remission. All the mice that received the combination therapy survived in complete remission through study day 180 whereas 40% of mice treated with gemcitabine alone had died and 40% were alive with progressive disease ().
Figure 2. Combination gemcitabine and TH1 DC vaccination results in eradication of orthotopic PDAC. (A) Experimental treatment schema. (B) Longitudinal IVIS imaging analysis of treatment groups and (C) tumor burden as assessed by IVIS imaging indicate eradication of orthotopic PDAC following combined gemcitabine chemotherapy and TH1 DC immunotherapy. X-axis: Post-inoculation day. Y-axis: Peak scale luminescence/mm2. Error bars ± SEM. ****p < 0.0001.
Combination therapy results in higher levels of activated, antigen-specific CD8+ T-cells
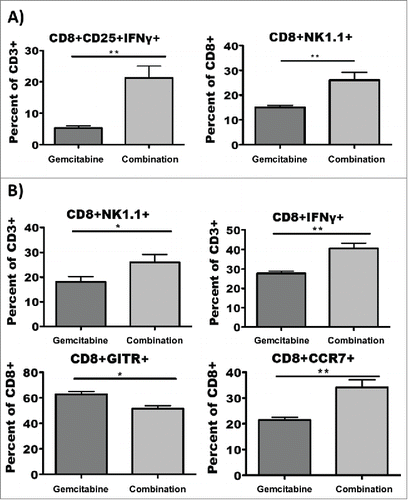
To determine if the durable remissions imparted by combination therapy could be correlated with identifiable immunologic parameters, peripheral blood was collected retro-orbitally from mice on post-inoculation days 49 and 109, and T-cells were analyzed by flow cytometry after overnight culture with either PDAC antigen-loaded or PDAC antigen-naive DC stimulators. When day 49 T-cells were cultured with PDAC antigen-loaded DC, those derived from mice treated with combination therapy exhibited exceptionally elevated levels of activated IFNγ producing CD8+ T-cells as well elevated levels of atypical CD3+CD8+NK1.1+ T-cellsCitation20 (). These same phenomena were observed among day 109 T-cells as well as a marked upregulation of (presumably central memory, ref Citation21) CD8+CCR7+ T-cells and marked downregulation of regulatory CD8+GITR+ T-cellsCitation22 (). No statistically significant differences were observed among populations of T-cells co-cultured with antigen-naive DC (not shown) with the exception of CCR7, a marker not induced by activation.
Figure 3. Combination therapy results in higher levels of activated, antigen-specific CD8+ T-cells. Peripheral blood was collected retro-oribitally on post-treatment days 49 and 109, and lymphocytes were cultured overnight with PDAC antigen loaded DC. (A) Mice that received combination therapy exhibited elevated levels of IFNγ producing CD8+ T-cells as well as elevated levels of atypical CD3+CD8+NK1.1+ T-cells on post-treatment day 49. (B) Among post-treatment day 109 T-cells, in addition to the upregulation of CD3+CD8+NK1.1+ T-cells seen on post-treatment day 49, there was a marked upregulation of CD8+CCR7+ T-cells and marked downregulation of regulatory CD8+GITR+ T-cells in the mice that received combination therapy. *p < 0.05. **p < 0.01. Error bars ± SD.
Combination therapy provides ongoing and durable protection against tumor
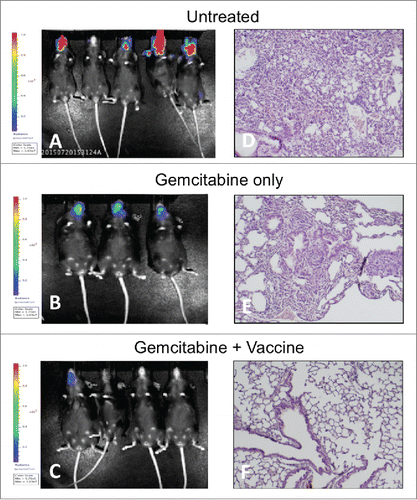
To determine the durability of the antitumor immune response imparted by various treatment regimens, mice that remained alive on day 181 post-inoculation were re-challenged retro-orbitally with 500,000 KrasG12Dp53−/−luc2+ cells. A group of five naive mice were also retro-orbitally challenged as a control. All mice in the drug monotherapy group that had survived their original PDAC inoculation (including a mouse that was NED) as well as all naive mice rapidly developed significant cranial tumor burden and died whereas only 25% of mice that had received combination therapy exhibited any evidence of cranial tumor burden () and none died (). Postmortem histopathology showed extensive lung metastases in all naive mice and mice treated with gemcitabine monotherapy as well as sporadic metastases to other organs including spleen and heart. In contrast, no metastases were observed among any mice that had received combination therapy (). Overall, the results indicated that TH1 DC vaccine in combination with gemcitabine could not only eradicate tumor but also provide durable protection against relapse and metastasis.
Figure 4. Combination therapy provides durable protection against tumor relapse. Mice still alive on day 181 after initial tumor inoculation were retro-orbitally rechallenged with 500,000 KrasG12Dp53−/−luc2 cells and imaged by IVIS to monitor tumor progression. Five naive mice were included as untreated controls. (A) Significant tumor burden was immediately observed in treatment naive mice and mice that received gemcitabine monotherapy whereas only 25% of mice that had received combination therapy developed cranial tumor. X-axis: Day post-re-challenge. Y-axis: Average radiance p/s/cm2/sr. Error bars ± SEM. (B) Kaplan–Meier survival curve of mice challenged retro-orbitally with tumor bolus indicates rapid death of control-treated and gemcitabine-treated mice by post-re-challenge day 43. Animals treated with both chemotherapy and immunotherapy remained alive with NED or MRD.
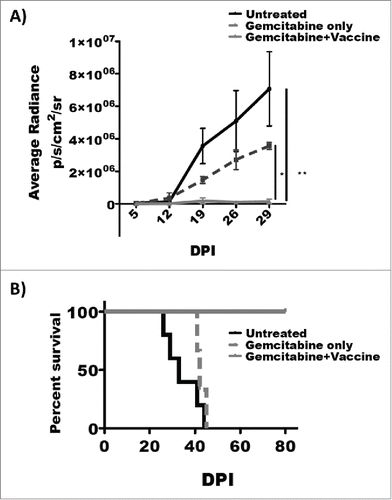
Figure 5. Combination therapy provides durable protection against tumor relapse and prevents metastasis to lung. IVIS imaging demonstrates significant cranial tumor burden in treatment-naive and gemcitabine-treated mice whereas only one of four mice that had received combination therapy exhibited any tumor burden. Histopathological analysis (H&E) revealed lung metastasis as well as sporadic metastases to other organs including liver in all treatment-naive and gemcitabine-treated mice. In contrast, no metastases were observed among any mice that had received combination therapy. (A/D) Treatment naive. (B/E) Treatment with gemcitabine only. (C/F) Treatment with combination therapy. Lung histopathology shown at 200×.
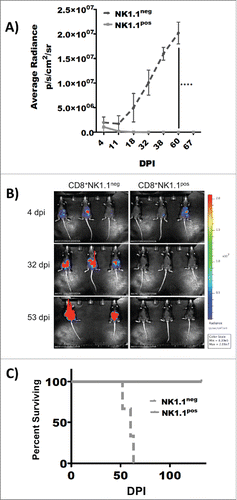
CD8+NK1.1+ splenocytes mediate robust and durable immunological memory
Analysis of peripheral blood lymphocytes revealed a significant correlation between levels of CD3+CD8+NK1.1+ cells and survival (i.e., ). To determine if durable immunologic memory could be transferred along with this cell type, non-adherent CD8+ splenocytes were negatively selected from the spleens of mice treated with combination therapy 260 days prior. These cells were activated overnight with PDAC-loaded DC stimulators after which responding NK1.1pos and NK1.1neg cells were separated by flow sorting. Scant populations of 1,500 NK1.1pos and NK1.1neg cells were adoptively transferred into each of three naive recipient mice in conjunction with i.p. PDAC challenge using 250,000 KrasG12Dp53−/−luc2+ tumor cells. Tumor growth was subsequently measured and quantified by IVIS imaging. The data () indicated that antitumor immunity could be transferred from vaccinated host to naive recipient by very small numbers of CD8+NK1.1+ cells, indicating that this cell type can mediate durable antitumor protection and memory responses in this model of pancreatic cancer. Mice that received CD8+NK1.1+ cells remained in permanent remission () with NED (no evidence of disease).
Figure 6. Atypical CD8+NK1.1+ splenocytes mediate robust and durable immunological memory. Elevated levels of atypical CD8+NK1.1+ in treated mice were correlated with survival in this model system. To determine if tumor immunity could be transferred along with this cell type, non-adherent CD8+ splenocytes were negatively selected from the spleens of mice treated with combination therapy 260 days prior. prior. These cells were activated overnight with PDAC-loaded DC stimulators after which responding NK1.1pos and NK1.1neg cells were separated by flow sorting. Scant populations of 1,500 NK1.1pos and NK1.1neg cells were adoptively transferred into each of three naive recipient mice in conjunction with i.p. PDAC challenge using 250,000 KrasG12Dp53−/−luc2+ tumor cells. Tumor growth was subsequently measured and quantified by IVIS imaging. (A) Quantitation of tumor burden by IVIS imaging indicates rapid tumor rejection among mice adoptively transferred with CD8+NK1.1+ splenocytes. X-axis: Post-transfer day. Y-axis: Average radiance p/s/cm2/sr. ****p < 0.001. Error bars ± SEM. (B) Longitudinal IVIS imaging demonstrates disappearance of i.p. tumor burden in recipients that received 1,500 CD8+CNK1.1pos splenocytes from mice vaccinated 260 days prior. Recipients that received 1,500 CD8+CNK1.1neg splenocytes from mice vaccinated 260 days prior progressed and died. (C) Kaplan–Meir survival analysis shows long-term survival with NED of animals adoptively transferred with CD8+NK1.1pos splenocytes. X-axis: Post-transfer day. Y-axis: Percent survival.
Discussion
Despite decades of research, PDAC remains the malignancy with the most abysmal of prognoses. Vaccine immunotherapy has long been anticipated to eradicate cancer in a targeted, non-toxic fashion but meaningful success up to now has been elusive in clinical studies. DC-based vaccination has previously been shown to be effective in some murine models of pancreatic cancer. Several studies have reported that DC vaccination of tumor bearing mice has led to increased tumor specific lysis by CD8+ T cells, expansion of IFNγ secreting T cells, and tumor regression.Citation23-25 Targeting primary or metastatic lesions with intra-tumoral DC was effective in murine pancreatic cancer models and could be beneficial in human trials since a majority of patients are unresectable at the time of diagnosis. In one open label clinical trial, intra-tumoral injection of DC led to enhanced immunity and regression of tumor in several patients with pancreatic cancer.Citation26 Although clinical trials have demonstrated that tumor-specific immunity can be regularly established by DC-based vaccines, it has also become evident that clinical responses to immunotherapy occur only rarely in patients with gastrointestinal malignancies,Citation27 and the field in recent years has tried to identify strategies that, not only overcome tumor-induced immunosuppression, but also do not interfere with the activation of a tumor-directed immune response. Increasing evidence suggests that well established treatment strategies such as radiation, surgical debulking, or chemotherapy can be successfully combined with immunotherapeutic approaches.Citation28-30 Here, we show that gemcitabine chemotherapy augments the therapeutic efficacy of TH1 DC vaccination in a murine model of PDAC. Gemcitabine has paradoxically been reported to enhance immunocompetence in certain PDAC patient populations via various mechanisms including selective deletion of myeloid-derived suppressor cells (MDSC) that can inhibit antitumor immunity.Citation31-33 A previous study also found that gemcitabine can induce the proliferation of CD14+ monocytes and CD11c+ DC, findings that could provide a rationale for the combination of gemcitabine and specific immunotherapy.Citation34 When used in combination with the standard of care chemotherapy drug gemcitabine, DC vaccination was previously shown to increase tumor free survival in a murine model of pancreatic cancer.Citation35 In the present study, the combination of TH1 DC vaccination and gemcitabine chemotherapy resulted in significant improvement in tumor-free survival in the p53−/−KrasG12D murine tumor model of PDAC. This is in accordance with previous findings in which gemcitabine was shown to augment the effectiveness of immune stimulation by in vivo CD40 ligation.Citation36 In a human in vitro model, gemcitabine was shown to sensitize pancreatic carcinoma cells to CTL responses.Citation37 Apoptosis induced by gemcitabine was also shown to increase cross-presentation of tumor antigens to CTLs by intra-tumoral DCs.Citation38 Although inhibition of B cell-mediated immune responses has been described in animal models, gemcitabine can be administered to patients with pancreatic cancer without relevant loss of T cell and DC function.Citation39 Moreover, in cancer patients, gemcitabine may even inhibit TH2- and specifically augment TH1-type immune responses.Citation39 Studies by Beatty et al. Citation36 have also shown therapeutic effects of gemcitabine treatment with CD40 agonist, CP-870,893 in patients with metastatic PDAC.
We have recently characterized novel and critical biological parameters that govern cell-mediated immune responses and have applied these concepts to PDAC immunotherapy. Our substantial preliminary dataset indicates that orthotopically injected KrasG12Dp53−/− PDAC tumors can be completely eradicated through a combination of TH1 DC vaccine and conventional gemcitabine whereas gemcitabine alone could not prevent tumor recurrence. These results were due in part to enhanced induction of non-classical PDAC-specific CD8+NK1.1+ T-cells among vaccinated animals. NKR-P1 is a family of disulfide-linked homodimers of which NK1.1 (CD161 in humans) is a member.Citation40 NKRP1A and C are activating molecules that can trigger cytokine production and cytolytic activity by NK cells,Citation41,42 while NKRP1B appears to be inhibitory.Citation43,44 The PK136 antibody recognizes the NKR-P1C receptor (NK1.1) in the mouse. This antibody depletes NK cells in vivo Citation45 and induces activation and proliferation of both NK and NKT cells in vitro.Citation46,47 Assarsson et al. Citation48 first described the presence of NK1.1+TCRαβ+ cells within lymphokine-activated killer cell cultures derived from CD1d1−/− and Ja281−/− C57BL/6 mice that lack classical NKT cells. Unlike classical NKT cells, 50–60% of these NK1.1+TCRαβ+ cells expressed CD8+ with a diverse TCR repertoire. Upon in vitro stimulation with IL-2, IL-4, or IL-15, purified NK1.1neg CD8+ T-cells rapidly acquired surface expression of NK1.1. The induction of NK1.1 on CD8+ T cells was not just an in vitro phenomenon as Assarsson also observed a five-fold increase of NK1.1+CD8+ T cells in the lungs of influenza virus-infected mice. These data suggest that CD8+ T cells can acquire NK1.1 and other NK cell-associated molecules upon appropriate stimulation in vitro and in vivo. T-cell responses to influenza were reported to be deficient in mice treated with the anti-NK1.1 monoclonal antibody.Citation49 In the interceding years, the analogous population of CD3+CD8+CD161+ T-cells has been described in human populations, though never in the context of cancer or tumor immunity. These cells are described in the infectious disease literature as highly cytotoxic memory T-cells, often with antiviral specificity.Citation50-54 Given that TH1 DC vaccination mimics an antigenic micro-environment present only in the context of viral infection,Citation16-18 the appearance of such T-cell responses might be anticipated.
In summary, we demonstrate that adjuvant TH1 DC vaccination can lead to durable cure of KrasG12Dp53−/− PDAC when applied in conjunction with standard of care gemcitabine chemotherapy. Durable tumor immunity was correlated with the appearance of atypical CD8+NK1.1+ cells, a memory cell previously described in the context of viral infection but not in cancer. Additional work will be necessary to delineate the role of TH1 vaccination in the development of these novel anticancer responses.
Disclosure of potential conflicts of interest
WKD and MMH hold ownership stakes in Diakonos Research, Ltd. All other authors declare no competing financial interests.
Author contributions
VK, QCY, and WKD conceived of and designed the study. VK, DLi, MMH, DLiang, ZL, and YC acquired data. VK, DLi, MMH, WEF, JML, 490 SP, QCY, and WKD analyzed and interpreted data. VK and WKD drafted the manuscript. MMH, WEF, JML, and QCY critically revised the manuscript for important intellectual content. VK and WKD provided statistical analysis. QCY and WKD obtained funding, provided material support, and supervised the study. JML provided technical support.
Acknowledgments
The authors are deeply indebted to the unwavering support and dedicated commitment from the Faust Family – Don, Dan, Tyson, and Tina – in remembrance of Robert Anthony Faust.
Funding
This work was supported in part by grant funding from Alex's Lemonade Stand Childhood Cancer Foundation and Cancer Cures 4 Kids Foundation (both to WKD) as well as the Barry Stephen Smith Memorial Pancreatic Cancer Research Award (to QCY) and NIH R01 CA183984 (also to QCY). This project was also supported by the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the NIH (NIAID P30AI036211, NCI P30CA125123, and NCRR S10RR024574) and the assistance of Joel M. Sederstrom.
References
- Ansari D, Gustafsson A, Anderson R. Update on the management of pancreatic cancer: surgery is not enough. World J Gastroenterol 2015; 21:3157-65; PMID:25805920; http://dx.doi.org/10.3748/wjg.v21.i11.3157
- Fesinmeyer MD, Austin MA, Li CI, De Roos AJ, Bowen DJ. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2005; 14:1766-73; PMID:16030115; http://dx.doi.org/10.1158/1055-9965.EPI-05-0120
- American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016
- Niccolai E, Prisco D, D'Elios MM, Amedei A. What is recent in pancreatic cancer immunotherapy? Biomed Res Int 2013; 2013:492372; PMID:23509731; http://dx.doi.org/10.1155/2013/492372
- Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, McKolanis JR, Geller BA, Schmotzer A, Potter DP, Whiteside T. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther 2008; 6:955-64; PMID:19129927
- Morse MA, Nair SK, Boczkowski D, Tyler D, Hurwitz HI, Proia A, Clay TM, Schlom J, Gilboa E, Lyerly HK. The feasibility and safety of immunotherapy with dendritic cells loaded with CEA mRNA following neoadjuvant chemoradiotherapy and resection of pancreatic cancer. Int J Gastrointest Cancer 2002; 32:1-6; PMID:12630764; http://dx.doi.org/10.1385/IJGC:32:1:1
- Kalady MF, Onaitis MW, Emani S, Abdul-Wahab Z, Pruitt SK, Tyler DS. Dendritic cells pulsed with pancreatic cancer total tumor RNA generate specific antipancreatic cancer T cells. J Gastrointest Surg 2004; 8:175-81; PMID:15036193; http://dx.doi.org/10.1016/j.gassur.2003.11.003
- Akiyama Y, Maruyama K, Nara N, Hojo T, Cheng JY, Mori T, Wiltrout RH, Yamaguchi K. Antitumor effects induced by dendritic cell-based immunotherapy against established pancreatic cancer in hamsters. Cancer Lett 2002; 84:37-47; PMID:12104046; http://dx.doi.org/10.1016/S0304-3835(02)00189-1
- Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA Jr, Donehower RC, Jaffee EM et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 2013; 36:382-9; PMID:23924790; http://dx.doi.org/10.1097/CJI.0b013e31829fb7a2
- Kammertoens T, Schuler T, Blankenstein T. Immunotherapy: target the stroma to hit the tumor. Trends Mol Med 2005; 11:225-31; PMID:15882610; http://dx.doi.org/10.1016/j.molmed.2005.03.002
- Halpert MM, Konduri V, Liang D, Chen Y, Wing JB, Paust S, Levitt JM, Decker WK. Dendritic cell secreted CTLA-4 regulates the T-cell response by downmodulating bystander surface B7. Stem Cells Dev 2016; 15:774-87; PMID:26979751; http://dx.doi.org/10.1089/scd.2016.0009
- Cheng W, Ren X, Zhang C, Cai J, Liu Y, Han S, Wu A. Bioinformatic profiling identifies an immune-related risk signature for glioblastoma. Neurology 2016; 86:2226-34; PMID:27225222; http://dx.doi.org/10.1212/WNL.0000000000002770
- Liang D, Halpert MM, Konduri V, Decker WK. Stepping out of the cytosol: AIMp1/p43 potentiates the link between innate and adaptive immunity. Int Rev Immunol 2015; 34:367-81; PMID:26325028; http://dx.doi.org/10.3109/08830185.2015.1077829
- Hong HJ, Kim E, Jung MY, Kim S, Kim TS. AIMP1 deficiency enhances airway hyperreactivity in mice via increased TH2 immune responses. Clin Immunol 2012; 143:256-65; PMID:22472603; http://dx.doi.org/10.1016/j.clim.2012.02.004
- Kim E, Kim SH, Kim S, Cho D, Kim TS. AIMP1/p43 protein induces the maturation of bone marrow-derived dendritic cells with T helper type 1-polarizing ability. J Immunol 2008; 180:2894-902; PMID:18292511; http://dx.doi.org/10.4049/jimmunol.180.5.2894
- Decker WK, Xing D, Li S, Robinson SN, Yang H, Steiner D, Komanduri KV, Shpall EJ. Th-1 polarization is regulated by dendritic cell comparison of MHC class I and class II antigens. Blood 2009; 113:4213-23; PMID:19171878; http://dx.doi.org/10.1182/blood-2008-10-185470
- Decker WK, Xing D, Li S, Robinson SN, Yang H, Yao X, Segall H, McMannis JD, Komanduri KV, Champlin RE et al. Double loading of dendritic cell MHC class I and class II with an AML antigen repertoire enhances correlates of T-cell immunity in vitro via amplification of T-cell help. Vaccine 2006; 24:3203-16; PMID:16480795; http://dx.doi.org/10.1016/j.vaccine.2006.01.029
- Halpert MM, Carstens JL, Schissler P et al. Homologous antigenic loading of dendritic cell MHC class I and II initiates AIMp1-mediated TH1 immunity. Submitted.
- Jalah R, Rosati M, Ganneru B, Pilkington GR, Valentin A, Kulkarni V, Bergamaschi C, Chowdhury B, Zhang GM, Beach RK et al. The p40 subunit of interleukin (IL)-12 promotes stabilization and export of the p35 subunit. J Biol Chem 2013; 288:6763-76; PMID:23297419; http://dx.doi.org/10.1074/jbc.M112.436675
- Ruiz AL, Soudja SM, Deceneux C, Lauvau G, Marie JC. NK1.1+CD8+ T cells escape TGF-β control and contribute to early microbial pathogen response. Nat Commun 2014; 5:5150; PMID:25284210; http://dx.doi.org/10.1038/ncomms6150
- Krupnick AS, Lin X, Li W, Higashikubo R, Zinselmeyer BH, Hartzler H, Toth K, Ritter JH, Berezin MY, Wang ST et al. Central memory CD8+ T lymphocytes mediate lung allograft acceptance. J Clin Invest 2014; 124:1130-43; PMID:24569377; http://dx.doi.org/10.1172/JCI71359
- Zou Q, Wu B, Xue J, Fan X, Feng C, Geng S, Wang M, Wang B. CD8+ Treg cells suppress CD8+ T cell-responses by IL-10 dependent mechanism during H5N1 influenza virus infection. Eur J Immunol 2014; 44:103-14; PMID:24114149; http://dx.doi.org/10.1002/eji.201343583
- Dauer M, Herten J, Bauer C, Renner F, Schad K, Schnurr M, Endres S, Eigler A. Chemosensitization of pancreatic carcinoma cells to enhance T cell-mediated cytotoxicity induced by tumor lysate-pulsed dendritic cells. J Immunother 2005; 28:332-42; PMID:16000951; http://dx.doi.org/10.1097/01.cji.0000164038.41104.f5
- Kim HS, Choo YS, Koo T, Bang S, Oh TY, Wen J, Song SY. Enhancement of antitumor immunity of dendritic cells pulsed with heat-treated tumor lysate in murine pancreatic cancer. Immunol Lett 2006; 103:142-8; PMID:16313973; http://dx.doi.org/10.1016/j.imlet.2005.10.021
- Schmidt T, Ziske C, Marten A, Endres S, Tiemann K, Schmitz V, Gorschlüter M, Schneider C, Sauerbruch T, Schmidt-Wolf IG. Intratumoral immunization with tumor RNA-pulsed dendritic cells confers antitumor immunity in a C57BL/6 pancreatic murine tumor model. Cancer Res 2003; 63:8962-8967; PMID:14695214
- Nair SK, Hull S, Coleman D, Gilboa E, Lyerly HK, Morse MA. Induction of carcinoembryonic antigen (CEA)-specific cytotoxic T-lymphocyte responses in vitro using autologous dendritic cells loaded with CEA peptide or CEA RNA in patients with metastatic malignancies expressing CEA. Int J Cancer 1999; 82:121-124; PMID:10360830; http://dx.doi.org/10.1002/(SICI)1097-0215(19990702)82:1%3c121::AID-IJC20%3e3.0.CO;2-X
- Mule JJ. Dendritic cell-based vaccines for pancreatic cancer and melanoma. Ann NY Acad Sci 2009; 1174:33-40; PMID:19769734; http://dx.doi.org/10.1111/j.1749-6632.2009.04936.x
- Mosolits S, Ullenhag G, Mellstedt H. Therapeutic vaccination in patients with gastrointestinal malignancies. A review of immunological and clinical results. Ann Oncol 2005; 16:847-62; PMID:15829493; http://dx.doi.org/10.1093/annonc/mdi192
- Broomfield S, Currie A, van der Most RG, Brown M, van Bruggen I, Robinson BW, Lake RA. Partial, but not complete, tumor-debulking surgery promotes protective antitumor memory when combined with chemotherapy and adjuvant immunotherapy. Cancer Res 2005; 65:7580-4; PMID:16140921
- Correale P, Cusi MG, Tsang KY, Del Vecchio MT, Marsili S, Placa ML, Intrivici C, Aquino A, Micheli L, Nencini C et al. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer patients. J Clin Oncol 2005; 23:8950-8; PMID:16061910; http://dx.doi.org/10.1200/JCO.2005.12.147
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 2005; 11:6713-21; PMID:16166452; http://dx.doi.org/10.1158/1078-0432.CCR-05-0883
- Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol 2007; 179:977-83; PMID:17617589; http://dx.doi.org/10.4049/jimmunol.179.2.977
- Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol 2009; 9:900-9; PMID:19336265; http://dx.doi.org/10.1016/j.intimp.2009.03.015
- Soeda A, Morita-Hoshi Y, Makiyama H, Morizane C, Ueno H, Ikeda M, Okusaka T, Yamagata S, Takahashi N, Hyodo I et al. Regular dose of gemcitabine induces an increase in CD14+ monocytes and CD11c+ dendritic cells in patients with advanced pancreatic cancer. Jpn J Clin Oncol 2009; 39:797-806; PMID:19797418; http://dx.doi.org/10.1093/jjco/hyp112
- Bauer C, Bauernfeind F, Sterzik A, Orban M, Schnurr M, Lehr HA, Endres S, Eigler A, Dauer M. Dendritic cell-based vaccination combined with gemcitabine increases survival in a murine pancreatic carcinoma model. Gut 2007; 56:1275-82; PMID:17395611; http://dx.doi.org/10.1136/gut.2006.108621
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011; 331:1612-6; PMID:21436454; http://dx.doi.org/10.1126/science.1198443
- Nowak AK, Robinson BW, Lake RA. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res 2002; 62:2353-8; PMID:11956096
- Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, Frelinger JA, Robinson BW. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross tolerizing host tumor-specific CD8 T cells. J Immunol 2003; 170:4905-13; PMID:12734333; http://dx.doi.org/10.4049/jimmunol.170.10.4905
- Plate JM, Plate AE, Shott S, Bograd S, Harris JE. Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother 2005; 54:915-25; PMID:15782312; http://dx.doi.org/10.1007/s00262-004-0638-1
- Yokoyama WM, Seaman W. The Ly-49 and NKR-P1 gene families encoding lectin-like receptors on natural killer cells: the NK gene complex. Annu Rev Immunol 1993; 11:613; PMID:8476574; http://dx.doi.org/10.1146/annurev.iy.11.040193.003145
- Ryan JC, Turck J, Niemi EC, Yokoyama WM, Seaman WE. Molecular cloning of the NK1.1 antigen, a member of the NKR-P1 family of natural killer cell activation molecules. J Immunol 1992; 149:1631; PMID:1506685
- Ryan JC, Seaman WE. Divergent functions of lectin-like receptors on NK cells. Immunol Rev 1997; 155:79; PMID:9059884; http://dx.doi.org/10.1111/j.1600-065X.1997.tb00941.x
- Carlyle JR, Martin A, Mehra A, Attisano L, Tsui FW, Zúñiga-Pflücker JC. Mouse NKR-P1B, a novel NK1.1 antigen with inhibitory function. J Immunol 1999; 162:5917; PMID:10229828
- Kung SK, Su RC, Shannon J, Miller RG. The NKR-P1B gene product is an inhibitory receptor on SJL/J NK cells. J Immunol 1999; 162:5876; PMID:10229823
- Koo GC, Dumont FJ, Tutt M, Hackett J Jr, Kumar V. The NK-1.12 mouse: a model to study differentiation of murine NK cells. J Immunol 1986; 137:3742; PMID:3782794
- Arase H, Arase N, Saito T. Interferon-g production by natural killer (NK) cells and NK1.11 T cells upon NKR-P1 cross-linking. J Exp Med 1996; 183:2391; PMID:8642351; http://dx.doi.org/10.1084/jem.183.5.2391
- Reichlin A, Yokoyama WM. Natural killer cell proliferation induced by anti-NK1.1 and IL-2. Immunol Cell Biol 1998; 76:143; PMID:9619484; http://dx.doi.org/10.1046/j.1440-1711.1998.00726.x
- Assarsson E, Kambayashi T, Sandberg JK, Hong S, Taniguchi M, Van Kaer L, Ljunggren HG, Chambers BJ. CD8+ T cells rapidly acquire NK1.1 and NK cell-associated molecules upon stimulation in vitro and in vivo. J Immunol 2000; 165:3673-9; PMID:11034371; http://dx.doi.org/10.4049/jimmunol.165.7.3673
- Kos FJ, Engleman EG. Role of natural killer cells in the generation of influenza virus-specific cytotoxic T cells. Cell Immunol 1996; 173:1; PMID:8871595; http://dx.doi.org/10.1006/cimm.1996.0245
- Fergusson JR, Huhn MH, Swadling L, Walker LJ, Kurioka A, Llibre A, Bertoletti A, Holländer G, Newell EW, Davis MM et al. CD161intCD8+ T cells: a novel population of highly functional, memory CD8+ T cells enriched within the gut. Mucosal Immunol 2016; 9:401-13; PMID:26220166; http://dx.doi.org/10.1038/mi.2015.69
- Billerbeck E, Kang Y-H, Walker L, Lockstone H, Grafmueller S, Fleming V, Flint J, Willberg CB, Bengsch B, Seigel B et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci USA 2010; 107:3006-11; PMID:20133607; http://dx.doi.org/10.1073/pnas.0914839107
- Fergusson JR, Fleming VM, Klenerman P. CD161-expressing human T-cells. Front Immunol 2011; 2:1-7; PMID:22566792; http://dx.doi.org/10.3389/fimmu.2011.00036
- Northfield JW, Kasprowicz V, Lucas M, Kersting N, Bengsch B, Kim A, Phillips RE, Walker BD, Thimme R, Lauer G et al. CD161 expression on hepatitis C virus-specific CD8+ T cells suggests a distinct pathway of T cell differentiation. Hepatology 2008; 47:396-406; PMID:18219672; http://dx.doi.org/10.1002/hep.22040
- Poon K, Montamat-Sicotte D, Cumberbatch N, McMichael AJ, Callan MF. Expression of leukocyte immunoglobulin-like receptors and natural killer receptors on virus-specific CD8+ T cells during the evolution of Epstein-Barr virus-specific immune responses in vivo. Viral Immunol 2005; 18:513-22; PMID:16212530; http://dx.doi.org/10.1089/vim.2005.18.513
