ABSTRACT
Innate γδ T cells play critical roles in mucosal immunity such as regulating intestinal epithelial homeostasis. In addition, γδ T cells are significantly increased in the inflamed mucosa of patients with ulcerative colitis. However, γδ T cells are a heterogeneous population. IL-17-producing versus IFNγ-producing γδ T cells play differential roles in different disease settings. Therefore, dissecting the exact role of different subsets of γδ T cells in colitis is essential for understanding colitis immunopathogenesis. In the current study, we found that TCR δ-deficient mice had a more severe dextran sodium sulfate (DSS)-induced colitis that was reduced upon reconstitution of γδT17 cells but not IFNγ-producing γδ T cells. Immunophenotyping of the cellular infiltrate upon DSS-induced colitis showed a reduced infiltration of Gr-1+CD11b+ myeloid cells into the sites of inflammation in mice lacking γδT17 cells. Further experiments demonstrated that IL-17, IL-18, and chemokine CXCL5 were critical in Gr-1+CD11b+ myeloid cell recruitment. In vitro T cell suppressive assay indicated that this Gr-1+CD11b+ population was immunosuppressive. Depletion of Gr-1+CD11b+ myeloid cells resulted in an increase severity of DSS-induced colitis. Our study elucidates a new immune pathway involving γδT17-dependent recruitment of Gr-1+CD11b+ myeloid cells to the site of colitis inflammation important in the protection of colitis initiation and progression.
KEYWORDS:
Introduction
Inflammatory Bowel Disease (IBD) is a complex, multifactorial disorder affecting over a million Americans each year.Citation1 IBD ensues from a disruption of the epithelial cell barrier lining the gastrointestinal (GI) tract resulting in a gut microbiota activated immune system leading to chronic inflammation.Citation2 Two of the most common IBD are Crohn's disease and ulcerative colitis (UC). Due to the bacterial load of the colon compared with the small intestine, colitis is especially susceptible to immune activation and dysregulation due to microbial antigen overload from epithelial barrier integrity failure. One immune cell constitutively located and functional at the epithelial surface with the primary responsibility of up keeping the barrier against antigens of the gut lumen is innate γδ T cell. Previous studies show that in inflammatory diseases driven by cytokines such as IL-17, γδ T cells play a major role directing the immune response in addition to more conventional αβ T cells.Citation3,4 Investigation into the role of IL-17-producing γδ T cells (γδT17) in the immunopathogenesis of colitis was necessary to determine whether these cells help direct immune responses to gut inflammation possibly leading to the discovery of new targets for therapeutic purposes.
The mucosal barrier separating billions of bacteria from the vulnerable interstitial tissues and blood system surrounding the lumen of the gut is protected by various factors. Perhaps the most important component for a healthy colon is the symbiotic relationship created between the gut immune system and the healthy microflora.Citation5 IL-17-producing CD4+ T cells (Th17) regulate and direct this system by stimulating epithelial cells through IL-17 as well as other factors including acetylcholine to secrete antimicrobial factors capable of shaping the microflora population at the surface.Citation5,6 The role of γδ T cells in protecting the colon from inflammation development leading to colitis has similar parallels to Th17 cells.
γδ T cells at epithelial sites such as the intestinal lamina, lungs, and skin make up a large portion of intraepithelial lymphocyte populations.Citation3,7,8 γδT17 play pivotal roles in the regulation and resolution of different inflammatory conditions at these important interfaces, especially under acute settings.Citation9,10 However, under chronic inflammatory processes γδT17 promote the progression of inflammatory diseases.Citation3,11,12 Studies so far trying to ascertain the role γδ T cells in acute colitis have used dextran sodium sulfate (DSS) induced intestinal inflammation in TCR δ-deficient mice. The lack of γδ T cells have shown in multiple studies more severity in DSS-induced colitis. They have shown γδ T cells support epithelial integrity through induction of protective IgA responses, directly secreting keratinocyte growth factor (KGF) promoting epithelial cell turnover and KC (GRO-1) leading to granulocyte infiltration necessary for inflammation resolution.Citation13-15
One caveat of these studies using complete γδ T cell deficient mice is the open question as to whether γδT17 cells or some other subset of γδ T such as IFNγ-producing γδ T cells are protective and if so by what mechanism. Whether γδT17 cells rely upon direct interaction with intestinal epithelial cells as mentioned previouslyCitation14 or whether they interact with other immune cells present in the colon during colitis inflammation has yet to be determined. Tsuchiya et al. showed total γδ T protection in colitis requires the recruitment of granulocytes through GRO-1 secretion.Citation15 However, further investigation is needed in determining whether these are neutrophils necessary for bacterial clearance or whether they are immunosuppressive granulocytes recruited by γδ T cells specifically to quail the overactive inflammatory responses during colitis.
In the current study, we show that γδT17 cells are the major resident γδ T population in the gut lamina propria. TCR δ-deficient mice have a more severe DSS-induced colitis that is reduced upon reconstitution of γδT17 cells and not IFNγ-producing γδ T cells. Immunophenotyping of the cellular infiltrate upon DSS-induced colitis shows a reduced infiltration of Gr-1+CD11b+ myeloid cells into the sites of inflammation in mice lacking γδT17 cells. Further experiments demonstrate that IL-17, IL-18, and chemokine CXCL5 are critical in Gr-1+CD11b+ myeloid cell recruitment. In vitro T cell suppressive assay indicates that this Gr-1+CD11b+ population is immunosuppressive. Interestingly, γδ T cells from inflamed colon also show immunosuppressive activity. Depletion of Gr-1+CD11b+ myeloid cells leads to an increase severity of DSS-induced mucosal ulceration. Our study shown here elucidates a new immune pathway involving γδT17-dependent recruitment of Gr-1+CD11b+ myeloid cells to the site of colitis inflammation important in the protection of colitis initiation and progression.
Results
Innate γδ T cells in LPL predominantly secrete IL-17 and are significantly increased in DSS-induced colon
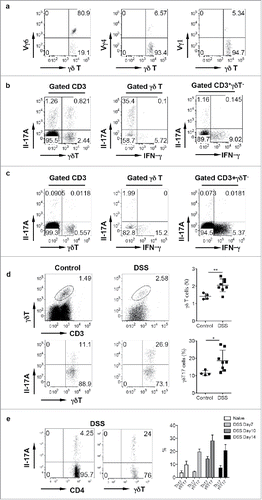
Innate γδ T cells constitute approximately 3–5% of total CD3+ T cells in the colon LPL. The LPL γδ T cells preferentially expressed Vγ6 TCR. The total percentage of Vγ6 was as high as 80% of the total γδ T cells in LPL () whereas Vγ4 and Vγ1 γδ T cells took up approximately 5% of total γδ T cells, respectively. Interestingly, αβ T cells were primarily IFNγ producers rather than IL-17. In contrast, γδ T cells in LPL produced large amounts of IL-17 with low level of IFNγ (). Vγ6 (80%) and Vγ4 (20%) are the main IL-17 producer while Vγ1 did not secrete IL-17 (data not shown). However, in the mesenteric lymph nodes (mLN), γδ T cells constituted a small fraction of total T cells and they predominately expressed IFNγ with minimal IL-17 production, similar as αβ T cells (). Upon DSS treatment, γδ T cells were significantly expanded in LPL (). This is consistent with findings from human UC.Citation16,17 In addition, IL-17-producing γδ T cells (γδT17) were also significantly increased (). We further examined time kinetics of γδT17/Th17 cells in this model. As shown in , γδT17 cells were significantly increased over the time, peaking at day 10, whereas Th17 cells were only transiently increased at Day10. Taken together, we show that innate γδ T cells in LPL predominately produce IL-17. In the acute inflammatory condition, both γδ T cells and γδT17 cells are significantly increased.
Figure 1. γδ T cells in the LPL predominantly express Vγ6 and secrete IL-17 and are significantly increased in DSS-induced colon. (A) γδT cells in the LPL were stained with Vγ1, Vγ4, and Vγ6 mAbs and representative dot plots are shown. (B) LPLs were stimulated with PMA+ionomycin and intracellular IL-17 and IFNγ staining was performed. (C) Single cell suspensions from mLNs were stimulated with PMA+ionomycin and intracellular IL-17 and IFNγ staining was performed. Cells were gated on differential populations as indicated. (D) LPL from control and DSS-treated mice were stained with CD3, pan γδTCR, and intracellular IL-17. Total γδT cells and γδT17 cells were summarized. Each dot represents one mouse. (E) Groups of mice (n = 5) were treated with or without DSS water for indicated time and then killed. LPLs were stimulated with PMA+ionomycin and then stained with CD4 and γδ TCR mAbs and intracellular IL-17. Representative dot plots and summarized percent of Th17 and γδT17 cells are shown. *p < 0.05, **p < 0.01.
Protective role of γδ T cells in DSS-induced colitis is associated with Gr-1+CD11b+ myeloid suppressor cells
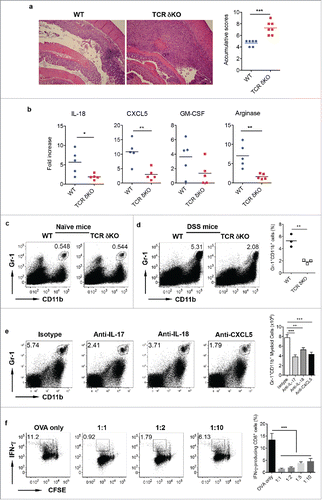
We next examined the role of γδ T cells in DSS-induced colitis using complete TCR δ KO mice. Histological examination of the colon from DSS-treated mice revealed that inflammation characterized by inflammatory cellular infiltration and severe mucosal erosion was more severe in TCR δ KO mice as compared with WT mice (). Real-time (RT)–PCR analysis indicated that chemokines IL-18 and CXCL5 were significantly lower in the colon of TCR δ KO mice compared with those from WT mice. GM-CSF mRNA level was also trending low in TCR δ KO mice. Furthermore, the mRNA level of Arginase was also significantly decreased in TCR δ KO mice compared with WT mice (). Since these chemokines are related to myeloid cell migration and trafficking, we stained LPL preparations with Gr-1 and CD11b mAbs. The frequency of Gr-1+CD11b+ myeloid cells was comparable in naïve WT and TCR δ KO mice (). Upon DSS treatment, Gr-1+CD11b+ cells were significantly increased. However, the frequency of Gr-1+CD11b+ cells was significantly less in the colon LPL of TCR δ KO mice compared with WT mice (). To further examine the effector molecules and their cellular sources during this process, we sorted CD45 negative cells, CD45+Gr1+CD11b+, CD45+ CD11b +Gr-1−, and CD45+CD11b−Gr-1− (see the gating strategy in the Fig. S1a) from DSS-treated mice. In addition, neutralizing mAbs against IL-17, IL-18, and CXCL5 were injected into DSS-treated WT mice. Isotype control mAb was used as control. As shown in the Fig. S1a, IL-18 could be produced by all four cellular populations, while CXCL5 was predominately produced by CD45 negative cells and CD45+CD11b−Gr-1− cells. To examine whether neutralizing IL-18 impacts on IL-17 and CXCL5 production, we evaluated γδT17 and Th17 cells in these mice as well as CXCL5 expression. As shown in the Fig. S1b, neutralizing IL-18 did not significantly influence Th17 and γδT17 cells. The CXCL5 mRNA level was not significantly impacted either. In contrast, neutralizing IL-17 did significantly decrease IL-18 mRNA expression in Gr-1+CD11b+ cells and CD45-negative cells while CXCL5 mRNA expression level was not significantly altered. In addition, neutralizing IL-17, IL-18, and CXCL−5 significantly reduced Gr-1+CD11b+ myeloid cell percentage and absolute number (), suggesting that these molecules play important roles in Gr-1+CD11b+ myeloid cell recruitment in the gut.
Figure 2. γδ T cells play a protective role in DSS-induced colitis. (A) WT and TCR δKO mice were treated with DSS water for 7 d. Colon tissues were collected for histological examination. Representative slides and accumulative scores are shown. (B) Colon tissues were put into Trizol and RNAs were extracted. The mRNA expression levels of IL-18, CXCL5, GM-CSF, and Arginase were measured by real-time PCR analysis. (C) Single cell suspensions from LPL of naïve WT and TCR δ KO mice were stained with CD11b and Gr-1 mAbs. (D) Single cell suspensions from LPLs of WT and TCR δ KO mice treated with DSS for 7 d were stained with CD11b and Gr-1 mAbs. Representative dot plots and summarized frequencies of Gr-1+CD11b+ cells are shown. (E) Groups of WT mice (n = 5) were treated with anti-IL-17, anti-IL-18, anti-CXCL5 mAb or isotype control mAb at days −2, 0, 2, and 5. Mice were fed with DSS water on day 0 for 7 d and then killed. LPLs were stained with Gr-1 and CD11b mAbs with viability dye. Representative dot plots and absolute numbers from each group are shown. (F) Splenocytes from OT-1 mice were labeled with CFSE and then co-cultured with Gr-1+CD11b+ cells sorted from LPL of DSS-treated WT mice at indicated ratios in the presence of OVA for 3 d. Cells were stimulated with PMA+ionomycin and intracellular IFNγ staining was performed. Cells were gated on CD8+ cells. Representative dot plots and summarized IFNγ-producing CD8 T cells are shown. *p < 0.05, **p < 0.01, ***p < 0.001.
Gr-1+CD11b+ myeloid-derived cells in tumors are potent immunosuppressive cells on effector T cells.Citation18,19 To examine whether these Gr-1+CD11b+ myeloid cells have similar immunosuppressive function, we sorted Gr-1+CD11b+ cells from the colon LPL of DSS-treated mice and then co-cultured with CFSE-labeled splenotyes from CD8+ OVA Tg mice. Although CD8+ T cell proliferation was not altered by the addition of Gr-1+CD11b+ myeloid cells, IFNγ production by CD8+ T cells was significantly decreased in the presence of Gr-1+CD11b+ myeloid cells (). In addition, Gr-1+CD11b+ myeloid cells from WT mice and TCR δ KO mice had comparable inhibitory effect on IFNγ production by CD8+ T cells (data not shown). To examine whether Gr-1+CD11b+ myeloid cells are bone-fide suppressive cells in the gut, we sorted these cells from naïve mice and performed T cell suppressive assay. As shown in the Fig. S2, Gr-1+CD11b+ myeloid cells from naïve mice did not show any suppressive activity, implying these cells are educated by the microenvironment and become suppressive myeloid cells. Taken together, these findings suggest that innate γδ T cells have a protective role in DSS-induced colitis. This effect may be related to Gr-1+CD11b+ suppressive myeloid cells.
IFNγ-producing γδ T cells are not essential in DSS-induced colitis
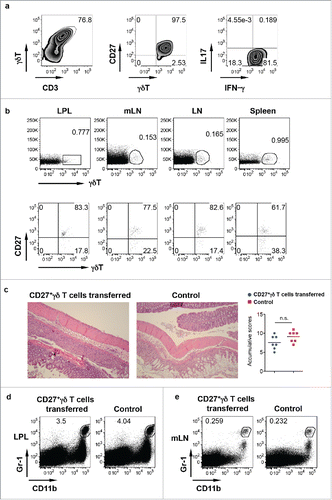
Although γδ T cells in the LPL predominately produce IL-17, a small fraction of γδ T cells (approximately 5%) did produce IFNγ. To examine whether IFNγ-producing γδ T cells are protective, we ex vivo expanded these CD27+ IFNγ-producing γδ T cells () and then adoptively transferred into TCR δ KO mice. As indicated in , transferred γδ T cells were readily seen in the LPL, mLN, body LN, and spleens and predominately expressed CD27. However, upon DSS treatment, histological examination of the colon in IFNγ-producing γδ T cell-transferred mice showed comparable inflammation severity as compared with non-transferred mice. In addition, the frequency of Gr-1+CD11b+ myeloid cells was not altered in the LPL () and in the mLN () in mice transferred with or without IFNγ-producing γδ T cells. These findings suggest that IFNγ-producing γδ T cells do not appear to have a protective role in DSS-induced colitis.
Figure 3. IFNγ-producing γδ T cells do not have a protective role in DSS-induced colitis. (A) CD27+ γδ T cells sorted from spleen and LNs were expanded ex vivo for 6 d. They predominately express CD27 and produce IFNγ but not IL-17. (B) Ex vivo expanded CD27+ γδ T cells were adoptively transferred into TCR δ KO mice. γδ T cell and CD27 staining in LPL, mLN, body LN, and spleen was shown. (C) Mice transferred with or without CD27+ γδ T cells were fed with DSS for 7 d. Representative histological slides and accumulative scores are shown. (D) Representative dot plots of Gr-1 and CD11b in LPL are shown. (E) Representative dot plots of Gr-1 and CD11b in mLN are shown.
IL-17-producing γδ T cells play a protective role in DSS-induced colitis
We next examined whether IL-17-producing γδ T cells have a protective role in DSS-induced colitis. To achieve this goal, we used our previously reported neonatal thymocyte/bone marrow (BM) chimeric mouse model,Citation20 in which IL-17-producing γδ T cells are predominately reconstituted. We used neonatal thymocytes from WT mice and adoptively transferred these cells into TCR δ KO mice as recipient mice followed by administration of BM cells from δ KO mice. In thymocytes/BM chimeric mice, γδ T cells were fully reconstituted and predominately produced IL-17 in both mLN and LPL ( and ). As expected, TCR δ KO recipient mice reconstituted with BM from TCR δ KO mice did not have γδ T cells. We then treated these mice with DSS for 7 d to induce inflammatory colitis. As shown in , mice reconstituted with BM cells alone had much severe mucosal erosion and inflammatory cellular infiltration as compared with mice reconstituted with IL-17-producing γδ T cells. This was also associated with significantly decreased frequency of Gr-1+CD11b+ myeloid cells in mice reconstituted with BM alone ().
Figure 4. Mice reconstituted with γδT17 cells have milder colitis. (A and B) Neonatal thymocytes from WT mice were transferred into lethally irradiated TCR δKO mice following BM cell transfer from TCR δ KO mice. Mice were reconstituted for at least eight weeks. Mice with BM cell transfer alone were used as control. γδT cell and intracellular IL-17 and IFNγ staining was performed in mLN (A) and LPL (B), respectively. (C) Reconstituted mice were fed with DSS water for 7 d. Representative histological slides and accumulative scores are shown. (D) Representative dot plots of Gr-1 and CD11b staining in LPL are shown. (E) Splenocytes from OT-1 mice were labeled with CFSE and then co-cultured with γδ T cells sorted from LPL of DSS-treated WT mice (day 7) in the presence of OVA for 3 d. Cells were stimulated with PMA+ionomycin and intracellular IFNγ staining was performed. Cells were gated on CD8+ cells. Representative dot plots and summarized IFNγ-producing CD8 T cells are shown. (F) LPLs from DSS-treated mice were stained with CD3, CD4, γδ TCR, and intracellular galectin-1 and galectin-9. Summarized percentages of galctin-1 or galectin-9-positive CD4 or γδ T cells are shown. *p < 0.05, **p < 0.01.
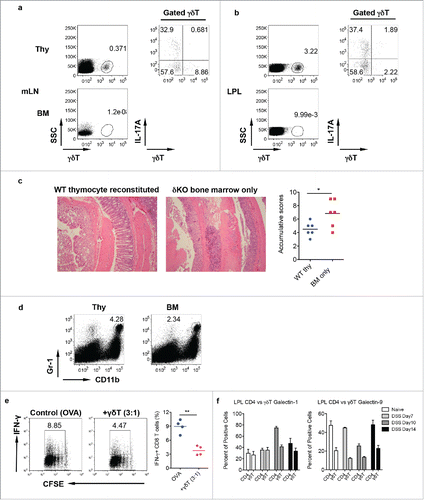
Previous studies have shown that there is the reciprocal effect between myeloid-derived suppressor cells (MDSC) and γδ T cells.Citation21 Exposure of γδ T cells to MDSC is sufficient to drive γδ T cells to become immunosuppressive cells. To examine this possibility, γδ T cells were sorted from inflamed gut to performed effector T cell suppressive assay. Indeed, γδ T cells from DSS-treated mice (day 7) showed suppressive activity (). However, intracellular galectin-1 and galectin-9 levels were not significantly altered in γδ T cells and CD4+ T cells upon DSS treatment (). Taken together, these data suggest that γδT17 cells in LPL exhibit a protective role in DSS-induced colitis associated with increased Gr-1+CD11b+ myeloid suppressor cells. In addition, these γδ T cells also show immunosuppressive activity.
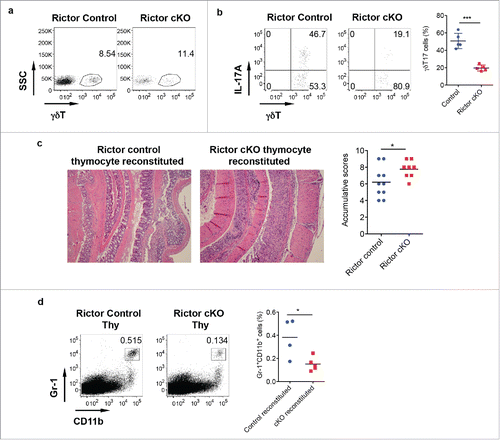
Decreased IL-17 production from γδ T cells of Rictor cKO mice ameliorates protective effect of γδT17 cells
To examine whether IL-17 production from γδ T cells is critical in γδT17-mediated protective role in DSS-induced colitis, we used Rictor control and cKO mice. In CD2-cre Rictor cKO mice, we found that although total γδ T cells in the LPL were not changed in Rictor cKO compared with control mice (), γδT17 cells were significantly decreased in Rictor cKO mice (). We then used neonatal thymocytes from Rictor control and Rictor cKO mice and BM cells from TCR δ KO mice to establish chimeric mice with TCR δ KO mice as recipient mice. In this model, only γδT17 cells were from Rictor control or cKO mice, all other cell components were the same. Analysis of chimeric mice indeed showed significantly decreased γδT17 cells (data not shown). These chimeric mice were treated with DSS for 7 d to induce colitis. As shown in , TCR δKO mice reconstituted with Rictor control neonatal thymocytes had significantly less pathology (mucosal erosion and thickened muscular layer) as compared with mice reconstituted with Rictor cKO neonatal thymocytes. The severity of pathology in Rictor cKO neonatal thymocyte reconstituted mice was correlated with decreased frequency of Gr-1+CD11b+ myeloid cells (), further suggesting that γδT17 cells play a critical role in DSS-induced colitis.
Figure 5. Rictor-deficiency significantly reduces γδT17 cells and abrogates γδT-cell mediated protective activity in DSS-induced colitis. (A) γδT cell staining in LPL of control and Rictor cKO mice. (B) Single cell suspensions from LP were stimulated with PMA+ionomycin and intracellular IL-17 staining was performed. Representative dot plots and summarized γδT17 percentages are shown. (C) Mice reconstituted with neonatal thymocytes from Rictor control or cKO mice were fed with DSS water for 7 d. Representative histological slides and accumulative scores are shown. (D) Representative dot plots of Gr-1 and CD11b staining and summarized Gr-1+CD11b+ myeloid cells in LPL of DSS-treated mice are shown. *p < 0.05, ***p < 0.001.
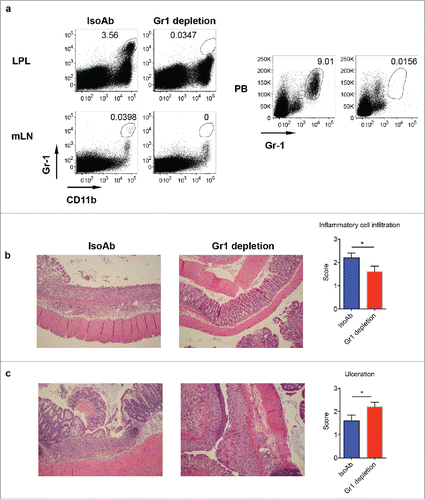
Depletion of Gr-1+CD11b+ myeloid cells promotes DSS-induced colitis
It appears that Gr-1+CD11b+ myeloid cells play a critical role in γδT17 cells-mediated protective roles in DSS-induced colitis. We reasoned that depletion of Gr-1+CD11b+ myeloid cells might abrogate this protective role. To this end, we injected anti-Gr-1 to deplete Gr-1+CD11b+ myeloid cells. As shown in , Gr-1 mAb effectively depleted this subset from PB, LPL, and mLN (). We then treated these mice with DSS to induce colitis. As expected, depletion of Gr-1+CD11b+ cells decreased cellular infiltrates as indicated by histological examination (). However, colonic ulceration was significantly enhanced in these mice (), suggesting that Gr-1+CD11b+ myeloid cells suppress DSS-induced colonic inflammation.
Figure 6. Depletion of Gr-1+CD11b+ myeloid cells induces severe disease in DSS-induced colitis. (A) WT mice were treated with anti-Gr-1 or isotype control mAb. Representative dot plots show that anti-Gr-1 effectively depleted this subset from LPL, mLN and periphery. (B) Mice injected with Gr-1 or isotype mAb were fed with DSS water for 7 d. Representative histological slides show less inflammatory cell infiltration in Gr-1 depleted mice. Summarized inflammatory cell infiltration scores are shown. (C) Representative histological slides show severe mucosal erosion and ulceration in Gr-1 depleted mice. Summarized ulceration scores are shown. *p < 0.05.
Discussion
γδ T cells in the colon LPL have previously been shown to play a direct, unique role in regulating the epithelial integrity and initiating repair mechanisms after DSS-induced colitis.Citation22 As cells of the innate immune response, γδ T cells are often studied and cited for their direct effector responses such as the secretion of growth factors or the direct killing of tumor cells.Citation23 In the last few years, more studies are showing that γδ T cells even innate like, professional IL-17 producers (γδT17) can regulate the immune response and direct other effector cells in complex inter-cellular immune networks.Citation12,24 In colitis, γδ T cells have been shown to help regulate the IgA protective responses against oral antigens which play a role in colitis development.Citation13 During colitis inflammation, γδ T cells communicate with epithelial cells and regulate their release of chemokines such as GRO-1 necessary for the influx of granulocytes responsible for helping to repair damaged tissue.Citation15 Determining whether in colitis, γδ T cells and specifically the predominant γδT17 population helps regulate and directs other immune cell components is essential in understanding the disease's pathophysiology and resolution.
Our results show that γδT17 cells are a major γδ T cell component of the lamina propria where immune infiltration during colitis inflammation is a hallmark of disease development and progression. In the absence of γδ T cells, DSS-induced colitis is more severe with a reduction in tissue mRNA levels for Arginase-1, IL-18, CXCL5, and GM-CSF. These molecules are associated with protection against DSS-induced colitis progression.Citation25-28 After ex vivo expansion and adoptive transfer of IFNγ producing γδ T cells into DSS-induced colitis model in TCR δ KO mice, we show that the IFNγ subset of γδ T cells does not play a protective role in acute colitis development. However, in the absence of γδT17 cells, as shown in our thymocyte plus BM or BM alone reconstitution models treated with DSS, acute colitis development is more severe with a drastic drop of Gr-1+CD11b+ suppressive myeloid cells. It is worth noting that IL-17-producing γδ T cells are reconstituted from neonatal thymocytes whereas IFNγ-producing γδ T cells are ex vivo expanded. Thus, the functional difference could be due to different reconstitution approaches although it is unlikely. Our previous studies have shown that Rictor deficiency specifically in T cells and B cells has a dramatic effect on the ability of γδT17 cells to produce IL-17. Using control and Rictor deficient thymocytes plus BM for reconstitution, we specifically show that γδT17 cells are essential for the protective effect of γδ T cells against DSS-induced colitis. These studies also show a specific correlation between the presence of γδT17 cells and Gr-1+CD11b+ suppressive myeloid cells suggesting that γδT17 are necessary for the infiltration of the suppressive myeloid cell population into the DSS induced inflammatory site. This notion is further supported by the data showing that depletion of Gr-1+CD11b+ myeloid cells results in more severe disease.
Our finding proposes a new regulatory pathway involving the activation and effector function of γδT17 cells in producing factors associated with the recruitment of Gr-1+CD11b+ myeloid suppressor cells. MDSC are capable of inhibiting inflammatory cells responsible for driving colitis development, including IFNγ-producing CD8+ T cells.Citation29 Gr-1+CD11b+ myeloid cells can suppress inflammatory T cell responses through the production of Arginase 1, which is important in breaking down arginine, a critical amino acid for effector T cell energy.Citation30 Reduction in IL-18 found in TCR δ KO mice and the correlation with increase disease severity supports previous studies showing the protective role of inflammasomes and IL-18 responses against colitis and gut homeostasis.Citation31,32 The mechanism of this protection could be related to how IL-18 signaling on MDSCs enhances the MDSC's suppression of T effector cells.Citation33 IL-18 signaling on epithelial cells directly, however, produces an opposite phenotypic effect by promoting intestinal inflammation and colitis development.Citation34 GM-CSF is a key cytokine needed for the development and regulation of granulocytes.Citation35 In the human colon, it has been shown γδT17 cells are co-producers of GM-CSF at very high levels.Citation12 Deficiency of γδ T cells in the colon in this study shows a strong correlation with reduction in Arginase 1, GM-CSF, CXCL5 and IL-18 suggesting that γδT17 cells are capable of directly regulating Gr-1+CD11b+ myeloid cells in both numbers as well as their suppressive function. In addition, some of these cytokines/chemokines are critical in Gr-1+CD11b+ myeloid cell recruitment as neutralizing mAbs against IL-17/IL-18/CXCL5 significantly decrease MDSC accumulation in the inflamed gut. By looking at suppressive activity in vitro using Gr-1+CD11b+ myeloid cells sorted from WT and TCR δ KO mice after DSS treatment, the data shows no different in suppressive activity on a cellular basis; however, the reduced numbers of the myeloid cell population correlates with decrease in transcript levels for Arginase 1.
Previous studies have shown that MDSC and γδ T cells are reciprocally regulated.Citation21 γδ T cells within the tumor microenvironment are also reported to have Treg-like function.Citation36-38 We show here that γδ T cells from inflamed gut gain an immunosuppressive function. Previous studies showed that granulocytic MDSC drive immunosuppressive γδ T cell phenotype via production of galectin-1 by γδ T cells.Citation21 However, the intracellular levels of galectin-1 and galectin-9 from γδ T cells are not significantly different between naïve mice and DSS-treated mice. γδ T cells in colon can be activated and polarized toward IL-17 production by inflammatory DCs via secretion of IL-1β and IL-23.Citation12 Indeed the mRNA levels of IL-1β and IL-23 are significantly increased upon DSS treatment although only IL-1β mRNA level is significantly decreased in TCR δKO mice as compared with WT mice (data not shown). As we reported previously, IL-1β is essential for γδT17 cell proliferation and expansion.Citation20 In addition, bacterial products and microbiota have already been shown to be important in regulating γδ T17 responses in human colorectal and lung cancer.Citation12,39 In addition, the gut microbiota play a critical role in the pathophysiology of IBD.Citation40 Thus, the relationship among the gut microbiota, γδ T17 cell activation, and colitis immunopathogenesis needs to be determined in the future.
More and more studies suggest that the inflammation associated with colitis is directly linked to intestinal carcinoma development and progression.Citation41,42 Suppressive myeloid cells such as the Gr-1+CD11b+ cells studied here appear to be protective against acute, severe colitis inflammation; however, these cells might be important in neoplasia development in more chronic colitis models.Citation43 In addition, γδ T cells interact with MDSC become immunosuppressive, further amplifying this immunosuppressive loop. Our model suggests that suppressive myeloid cell populations that are important in resolving inflammation associated with colitis in chronic cases could actually lead to the suppression of effector T cells needed to kill off neoplastic cancer cell formation. More investigation is needed to determine whether in chronic inflammatory conditions whether these Gr-1+CD11b+ cells are precursor cells to MDSCs important in cancer development and progression. Our studies show that γδT17 cells, directly through IL-17 and other factors, are important in the recruitment of suppressive Gr-1+CD11b+ myeloid cells to the site of inflammation in colitis. Therefore, γδT17 cells need to be studied as a potential therapeutic target with potent abilities to promote inflammation resolution under acute settings. However, a fine line must be recognized as acute colitis inflammation approaches chronic considering γδT17 cells have extraordinary abilities to promote immunosuppressive responses responsible for driving colon cancer development and progression.Citation12,44
Materials and methods
Mice
For the comparison experiments, WT and TCR δ-deficient (δ KO) mice on C57BL/6 background were home bred in the same animal facility. For other experiments, C57BL/6 mice were from the Jackson laboratory. CD2-cre mice and Rictor flox/flox mice were also from the Jackson laboratory and bred in our facility to generate CD2-cre+Rictorflox/flox conditional KO (cKO) mice. OVA TCR Tg OT-I mice were from Taconic. All animals were housed and treated with autoclaved food and water in the animal facility of University of Louisville, according to institutional guidelines and approved by the IACUC of University of Louisville.
Colonic lamina proprial lymphocyte (LPL) preparation
Large intestine between cecum and anal verge was cut out and open longitudinally. Tissues were washed with cold PBS to remove the fecal and then were cut cross-sectionally into 0.5–1 cm long pieces and then mixed with 15 mL pre-warmed PBS/FCS/EDTA in the shaker at 37 °C for 15 min. The supernatants were discarded and pellets were washed with RPMI complete medium. The colon pieces were then transferred to a new tube and digested with collagenase type IV for 40 min. All the contents were passed through a cell strainer. LPLs were obtained using the 40/80 Percoll centrifugation.
CD27+ γδ T cells in vitro culture and adoptive transfer
The whole spleen and lymph nodes of C57BL/6 mice were processed for a single cell suspension and CD27+ γδ T cells were sorted by MoFlo high-speed sorter and then added into 24-well plate pre-coated with CD3 mAb in the presence of IL-2 (10 ng/mL) and IL-7 (10 ng/mL). Cells were harvested on day 7 and CD27+ γδ T cells (4 × 106/mouse) were adoptively transferred to Tcrd−/− mice.
Flow cytometry analysis and intracellular staining
Fluorochrome-labeled mAbs, including mouse γδ TCR (clone GL3), Vγ4 (clone UC3–10A6), Vγ1 (clone 2.11), CD27 (clone LG.3A10), Gr-1 (clone RB6–8C5), CD11b (clone M1/70), CD8+α (clone 53–6.7), IL-17A (clone TC11–18H10.1), IFNγ (clone XMG1.2), GM-CSF (clone MPI-22E9), galectin-9 (clone 108A2) were obtained from Biolegend. Anti-galectin-1 was purchased from R&D. Anti-mouse Vγ6 (clone 17D1) was provided by Dr. Tigelaar (Department of Dermatology, Yale University). For intracellular cytokine staining, cells were first blocked with anti-CD16/32 (clone 2.4G2) and then stained with different cell surface antibodies (Abs). Cells were then fixed, permeabilized and stained intracellularly for IL-17, IFNγ, GM-CSF, galectin-1, and galectin-9. The relevant isotype control mAbs and viability dye were also used. Samples were harvested with BD FACS Canto (Becton Dickinson, San Jose, CA, USA) and analyzed with FlowJo software (TreeStar).
Establishment of DSS-induced colitis mouse model
Mice were fed with autoclaved drinking water with 3% DSS, 36,000–50,000 M.Wt. MP Biomedicals) for 7 d or different days as indicated. Mice were then killed and tissues were collected for analysis. In some experiments, mice were treated with anti-IL-17 (MM17F3, purified from serum-free culture medium, 100 μg/mouse each time), anti-CXCL5 (Leinco Technology, 30 μg/mouse each time), anti-IL-18 (BioX Cell, 100 μg/mouse each time), and isotype control mAb (BioX Cell, 100 μg/mouse each time) on days −2, 0, 2, and 5. Mice were fed with DSS water on day 0 for 7 d.
Neonatal thymocytes/bone marrow (BM) reconstitution
The detailed protocol was described previously.Citation20 In brief, recipient TCR δ KO mice were lethally irradiated with 950 cGy and then were intravenously transferred with 1.5 × 107 neonatal thymocytes from WT, Rictor control, or Rictor cKO mice. After 24 h, the recipient mice received 6 × 106 BM cells from TCR δ KO mice. Neonatal thymocytes used in all experiments were taken from pups born within 48 h. All chimeric mice were allowed to reconstitute for at least eight weeks before experiments.
In vitro T cell suppressive assay
Gr-1+CD11b+ cells and γδ T cells were sorted from LP of mice treated with DSS water for one week or from naïve mice and then co-cultured with CFSE-labeled splenocytes from OT-I mice in the presence of OVA for 3 d. Cells were restimulated with PMA plus ionomycin for 6 h and then surface stained with CD8+ followed by intracellular IFNγ staining.
RNA extraction and real-time quantitative PCR analysis
RNAs were isolated using a Qiagen RNeasy kit. After reverse transcription into cDNA, qPCR was performed on Bio-Rad MyiQ single color RT–PCR detection system using SYBR Green Supermix (Bio-Rad) and gene-specific primers were listed as follows:
β-MG: 5′–CTTTCTGGTGCTTGTCTC–3′; 5′–TCAGTATGTTCGGCTTCC–3′
Arg1: 5′–TTTTAGGGTTACGGCCGGTG–3′; 5′–CCTCGAGGCTGTCCTTTTGA–3′
IL-18: 5′–GGAGACCTGGAATCAGACAAC–3′; 5′–GGGTTCACTGGCACTTTG–3′
CXCL5: 5′–TGCCCTACGGTGGAAGTCAT–3′; 5′–AGCTTTCTTTTTGTCACTGCCC–3′
GM-CSF: 5′–CCTGTCACGTTGAATGAAGAG–3′; 5′–GGCAGTATGTCTGGTAGTAGC–3′
IL-1β: 5′–GCCACCTTTTGACAGTGATGAG–3′; 5′–GACAGCCCAGGTCAAAGGTT–3′
IL-22: 5′–ATACATCGTCAACCGCACCTTT–3′; 5′–AGCCGGACATCTGTGTTGTTAT–3′
IL-23p19: 5′–TATCCAGTGTGAAGATGGTTGTG–3′; 5′–CACTAAGGGCTCAGTCAGAGTTG–3′
Targeted gene expression level was normalized to β−2 microglobulin (β-MG) housekeeping gene and data were shown as fold changes by the 2−ΔΔCt method, where ΔCt = Ct target gene−Ct β-MG and ΔΔCt = ΔCt induced−ΔCt reference.
Colon histological staining and pathology scoring
For histology analysis, colon samples were fixed in 10% formalin. Paraffin embedded blocks were sectioned and stained with hematoxylin and eosin (H&E). H&E stained colonic tissue slides were scored by a blinded pathologist using a previously published systemCitation45 for the following measures: crypt distortion (normal, 0 – severe crypt distortion with loss of entire crypts, 3), degree of inflammatory cell infiltration (normal, 0 – dense inflammatory infiltrate, 3), muscle thickening (base of crypt sits on the muscularis mucosae, 0 – marked muscle thickening present, 3), goblet cell depletion (absent, 0 – present, 1) crypt abscess (absent, 0 – present, 1) and ulceration (0 – severe ulceration, 3). The total histological damage score was the sum of each individual score.
Gr-1+ cell depletion in vivo
Depletion of Gr-1+ cells was performed by i.p. injection of the anti-Gr-1 mAb (RB6–8C5, BioXCell) at a dose of 100 μg in 100 μL PBS on days −1, 2, 4, 6 of the 7 d of DSS treatment. Control mice received corresponding isotype mAb.
Statistical analysis
All quantitative data were shown as mean ± s.e.m. unless otherwise indicated. All samples were compared using unpaired Student's T-test. A p value <0.05 was considered significant. Statistical analysis was performed with GraphPad Prism software.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
X.S. participated in the design and coordination of the research project, collected, performed, and analyzed data, and contributed to manuscript writing. Y.H.C, C.F., Z.T., and C.D. participated in the design and coordination of the research project, collected, performed, and analyzed data. M.Y.Q participated in some supporting experiments for manuscript revision. C.F. contributed to manuscript writing. Z.W. reviewed and scored all histological slides. H.G.Z. participated in experimental design. J.S. and J.Y. participated in the design and coordination of the research project, supervised whole project, analyzed data, revised the manuscript and approved the final version of the manuscript.
Supplementary_figures.pdf
Download PDF (355.6 KB)Funding
This work was partly supported by the NIH grant and the Kentucky Research Challenge Trust Fund. Huang-Ge Zhang is supported by a Research Career Scientist (RCS) Award.
References
- Colombel JF, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology 2017; 152:351-61; PMID:27720840; https://doi.org/10.1053/j.gastro.2016.09.046
- Ahmed I, Roy BC, Khan SA, Septer S, Umar S. Microbiome, metabolome and inflammatory bowel disease. Microorganisms 2016; 4:pii: E20; PMID:27681914; https://doi.org/10.3390/microorganisms4020020
- Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity 2011; 35:596-610; PMID:21982596; https://doi.org/10.1016/j.immuni.2011.08.001
- Becher B, Pantelyushin S. Hiding under the skin: Interleukin-17-producing gammadelta T cells go under the skin? Nat Med 2012; 18:1748-50; PMID:23223063; https://doi.org/10.1038/nm.3016
- Pagliari D, Urgesi R, Frosali S, Riccioni ME, Newton EE, Landolfi R, Pandolfi F, Cianci R. The interaction among microbiota, immunity, and genetic and dietary factors is the Condicio Sine Qua Non Celiac disease can develop. J Immunol Res 2015; 2015:123653; PMID:26090475; https://doi.org/10.1155/2015/123653
- Dhawan S, De Palma G, Willemze RA, Hilbers FW, Verseijden C, Luyer MD, Nuding S, Wehkamp J, Souwer Y, de Jong EC et al. Acetylcholine-producing T cells in the intestine regulate antimicrobial peptide expression and microbial diversity. Am J Physiol Gastrointest Liver Physiol 2016; 311:G920-33; PMID:27514477; https://doi.org/10.1152/ajpgi.00114.2016
- Ishikawa H, Li Y, Abeliovich A, Yamamoto S, Kaufmann SH, Tonegawa S. Cytotoxic and interferon gamma-producing activities of gamma delta T cells in the mouse intestinal epithelium are strain dependent. Proc Natl Acad Sci USA 1993; 90:8204-8; PMID:8367483; https://doi.org/10.1073/pnas.90.17.8204
- Lahn M, Kanehiro A, Takeda K, Joetham A, Schwarze J, Köhler G, O'Brien R, Gelfand EW, Born W. Negative regulation of airway responsiveness that is dependent on gammadelta T cells and independent of alphabeta T cells. Nat Med 1999; 5:1150-6; PMID:10502818; https://doi.org/10.1038/13476
- Carding SR, Egan PJ. The importance of gamma delta T cells in the resolution of pathogen-induced inflammatory immune responses. Immunol Rev 2000; 173:98-108; PMID:10719671; https://doi.org/10.1034/j.1600-065X.2000.917302.x
- Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan TK et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 2015; 43:727-38; PMID:26431948; https://doi.org/10.1016/j.immuni.2015.09.003
- Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, Becher B. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J Clin Invest 2012; 122:2252-6; PMID:22546855; https://doi.org/10.1172/JCI61862
- Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang Z, Wang C, Zhang Z, Xia W et al. gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 2014; 40:785-800; PMID:24816404; https://doi.org/10.1016/j.immuni.2014.03.013
- Fujihashi K, McGhee JR, Kweon MN, Cooper MD, Tonegawa S, Takahashi I, Hiroi T, Mestecky J, Kiyono H. gamma/delta T cell-deficient mice have impaired mucosal immunoglobulin A responses. J Exp Med 1996; 183:1929-35; PMID:8666951; https://doi.org/10.1084/jem.183.4.1929
- Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci USA 2002; 99:14338-43; PMID:12376619; https://doi.org/10.1073/pnas.212290499
- Tsuchiya T, Fukuda S, Hamada H, Nakamura A, Kohama Y, Ishikawa H, Tsujikawa K, Yamamoto H. Role of gamma delta T cells in the inflammatory response of experimental colitis mice. J Immunol 2003; 171:5507-13; PMID:14607957; https://doi.org/10.4049/jimmunol.171.10.5507
- Yeung MM, Melgar S, Baranov V, Oberg A, Danielsson A, Hammarström S, Hammarström ML. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TcR-gammadelta expression. Gut 2000; 47:215-27; PMID:10896913; https://doi.org/10.1136/gut.47.2.215
- McVay LD, Li B, Biancaniello R, Creighton MA, Bachwich D, Lichtenstein G, Rombeau JL, Carding SR. Changes in human mucosal gamma delta T cell repertoire and function associated with the disease process in inflammatory bowel disease. Mol Med 1997; 3:183-203; PMID:9100225
- Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol 2016; 37:208-20; PMID:26858199; https://doi.org/10.1016/j.it.2016.01.004
- Ma G, Pan PY, Eisenstein S, Divino CM, Lowell CA, Takai T, Chen SH. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity 2011; 34:385-95; PMID:21376641; https://doi.org/10.1016/j.immuni.2011.02.004
- Cai Y, Xue F, Fleming C, Yang J, Ding C, Ma Y, Liu M, Zhang HG, Zheng J, Xiong N et al. Differential developmental requirement and peripheral regulation for dermal Vgamma4 and Vgamma6T17 cells in health and inflammation. Nat Commun 2014; 5:3986; PMID:24909159; https://doi.org/10.1038/ncomms4986
- Rutkowski MR, Stephen TL, Svoronos N, Allegrezza MJ, Tesone AJ, Perales-Puchalt A, Brencicova E, Escovar-Fadul X, Nguyen JM, Cadungog MG et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell 2015; 27:27-40; PMID:25533336; https://doi.org/10.1016/j.ccell.2014.11.009
- Meehan TF, Witherden DA, Kim CH, Sendaydiego K, Ye I, Garijo O, Komori HK, Kumanogoh A, Kikutani H, Eckmann L et al. Protection against colitis by CD100-dependent modulation of intraepithelial gammadelta T lymphocyte function. Mucosal Immunol 2014; 7:134-42; PMID:23695512; https://doi.org/10.1038/mi.2013.32
- Chien YH, Meyer C, Bonneville M. Gammadelta T cells: first line of defense and beyond. Annu Rev Immunol 2014; 32:121-55; PMID:24387714; https://doi.org/10.1146/annurev-immunol-032713-120216
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol 2013; 13:88-100; PMID:23348415; https://doi.org/10.1038/nri3384
- Wang Y, Tian J, Tang X, Rui K, Tian X, Ma J, Ma B, Xu H, Lu L, Wang S. Exosomes released by granulocytic myeloid-derived suppressor cells attenuate DSS-induced colitis in mice. Oncotarget 2016; 7:15356-68; PMID:26885611; https://doi.org/10.18632/oncotarget.7324
- Ratsimandresy RA, Indramohan M, Dorfleutner A, Stehlik C. The AIM2 inflammasome is a central regulator of intestinal homeostasis through the IL-18/IL-22/STAT3 pathway. Cell Mol Immunol 2016; 14:127-142; PMID:27524110; https://doi.org/10.1038/cmi.2016.35
- Kryczek I, Wang L, Wu K, Li W, Zhao E, Cui T, Wei S, Liu Y, Wang Y, Vatan L et al. Inflammatory regulatory T cells in the microenvironments of ulcerative colitis and colon carcinoma. Oncoimmunology 2016; 5:e1105430; PMID:27622054; https://doi.org/10.1080/2162402X.2015.1105430
- Xu Y, Hunt NH, Bao S. The role of granulocyte macrophage-colony-stimulating factor in acute intestinal inflammation. Cell Res 2008; 18:1220-9; PMID:19030026; https://doi.org/10.1038/cr.2008.310
- Funderburg NT, Stubblefield Park SR, Sung HC, Hardy G, Clagett B, Ignatz-Hoover J, Harding CV, Fu P, Katz JA, Lederman MM et al. Circulating CD4(+) and CD8(+) T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology 2013; 140:87-97; PMID:23600521; https://doi.org/10.1111/imm.12114
- Zhao Y, Wu T, Shao S, Shi B, Zhao Y. Phenotype, development, and biological function of myeloid-derived suppressor cells. Oncoimmunology 2016; 5:e1004983; PMID:27057424; https://doi.org/10.1080/2162402X.2015.1004983
- Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 2010; 32:367-78; PMID:20226691; https://doi.org/10.1016/j.immuni.2010.02.012
- Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 2010; 32:379-91; PMID:20303296; https://doi.org/10.1016/j.immuni.2010.03.003
- Lim HX, Hong HJ, Cho D, Kim TS. IL-18 enhances immunosuppressive responses by promoting differentiation into monocytic myeloid-derived suppressor cells. J Immunol 2014; 193:5453-60; PMID:25362180; https://doi.org/10.4049/jimmunol.1401282
- Nowarski R, Jackson R, Gagliani N, de Zoete MR, Palm NW, Bailis W, Low JS, Harman CC, Graham M, Elinav E et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 2015; 163:1444-56; PMID:26638073; https://doi.org/10.1016/j.cell.2015.10.072
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182:4499-506; PMID:19342621; https://doi.org/10.4049/jimmunol.0802740
- Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 2007; 27:334-48; PMID:17656116; https://doi.org/10.1016/j.immuni.2007.05.020
- Ye J, Ma C, Hsueh EC, Eickhoff CS, Zhang Y, Varvares MA, Hoft DF, Peng G. Tumor-derived gammadelta regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J Immunol 2013; 190:2403-14; PMID:23355732; https://doi.org/10.4049/jimmunol.1202369
- Daley D, Zambirinis CP, Seifert L, Akkad N, Mohan N, Werba G, Barilla R, Torres-Hernandez A, Hundeyin M, Mani VR et al. gammadelta T cells support pancreatic oncogenesis by restraining alphabeta T Cell activation. Cell 2016; 166:1485-99 e15; PMID:27569912; https://doi.org/10.1016/j.cell.2016.07.046
- Cheng M, Qian L, Shen G, Bian G, Xu T, Xu W, Shen G, Hu S. Microbiota modulate tumoral immune surveillance in lung through a gammadeltaT17 immune cell-dependent mechanism. Cancer Res 2014; 74:4030-41; PMID:24947042; https://doi.org/10.1158/0008-5472.CAN-13-2462
- Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol 2015; 37:47-55; PMID:25420450; https://doi.org/10.1007/s00281-014-0454-4
- Colman RJ, Rubin DT. Histological inflammation increases the risk of colorectal neoplasia in ulcerative colitis: a systematic review. Intest Res 2016; 14:202-10; PMID:27433141; https://doi.org/10.5217/ir.2016.14.3.202
- Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol 2016; 22:4794-801; PMID:27239106; https://doi.org/10.3748/wjg.v22.i20.4794
- Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 2013; 24:631-44; PMID:24229710; https://doi.org/10.1016/j.ccr.2013.10.009
- Yan J, Huang J. Innate gammadeltaT17 cells convert cancer-elicited inflammation into immunosuppression through myeloid-derived suppressor cells. Oncoimmunology 2014; 3:e953423; PMID:25610744; https://doi.org/10.4161/21624011.2014.953423
- Kim JJ, Shajib MS, Manocha MM, Khan WI. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp 2012;Feb 1; (60). pii:3678; PMID:22331082; https://doi.org/10.3791/3678
