ABSTRACT
The introduction of immune checkpoint blockade into the clinical practice resulted in improvement of survival of a significant portion of melanoma patients. Consequently, predictive biomarkers of response are needed to optimize patient's stratification and the development of combination therapies. The aim of this study was to determine whether levels of soluble NKG2D ligands (MICA, MICB, ULBP1, 2 and 3; sNKG2DLs) in the serum of melanoma patients can serve as useful predictors of response to the treatment with immune checkpoint blockade. sNKG2DLs were measured by ELISA in baseline and post-treatment serum and these results were correlated with the clinical outcome of melanoma patients (N = 194). The same determinations were performed also in a cohort of patients (N = 65) treated with either chemotherapy, radiotherapy, or mutated BRAF inhibitors (BRAFi). Absence of soluble MICB and ULBP-1 in baseline serum correlated with improved survival (OS = 21.6 and 25.3 mo and p = 0.02 and 0.01, respectively) of patients treated with immunological therapies while detectable levels of these molecules were found in poor survivors (OS = 8.8 and 12.1 mo, respectively). Multivariate analysis showed that LDH (p <0.0001), sULBP-1 (p = 0.02), and sULBP-2 (p = 0.02) were independent predictors of clinical outcome for the cohort of melanoma patients treated with immune checkpoint blockade. Only LDH but not sNKG2DLs was significantly associated with the clinical outcome of patients treated with standard or BRAFi regimens. These findings highlight the relevance of sNKG2DLs in the serum of melanoma patients as biomarkers for patients' stratification and optimization of immune checkpoint inhibition regimens.
Abbreviations
| Ab | = | antibody |
| CTLA-4 | = | cytotoxic T-lymphocyte antigen-4 |
| DCR | = | disease control rate |
| FBS | = | fetal bovine serum |
| HS | = | human serum |
| mAb | = | monoclonal antibody |
| MICA | = | MHC (HLA) class I chain-related gene A |
| MICB | = | MHC (HLA) class I chain-related gene B |
| NKG2D | = | the activating receptor NK cell group 2 member D (NKG2D) |
| OS | = | overall survival |
| PD-1 | = | programmed cell death – 1 |
| PD-L1 | = | programmed cell death ligand 1 |
| ULBP-1 or -2 | = | UL16-binding protein-1 or -2 |
Introduction
In tumors with different histological origin, the adaptive immune response can influence recurrence, metastatic spread, and the overall survival (OS).Citation1-3 This concept has been further characterized by the demonstration that the nature, location, and density of tumor-infiltrating lymphocytes (TILs) is associated with the prognosis of cancer patients, allowing better staging of disease and consisting in a more reliable prognostic marker compared with traditional TNM staging.Citation4-7 Nevertheless, the immunesurveillance of tumors is often impaired by immunomodulatory mechanisms occurring at tumor site, such as regulatory immunological cell populations (T regulatory cells, Tregs and myeloid-derived suppressor cells, MDSCs), the pro-tumor cross-talk between cancer cells and tumor microenvironment (TME), and the presence of negative regulatory factors in the TME (Indoleamine 2,3-dioxygenase; IDO, IL-10, IL-13, TGF-β, etc.).Citation8-10 Immunotherapy aims at circumventing negative immunomodulatory pathways to induce potent systemic immunological responses against tumors.Citation10 Antibodies (Abs) that block immune checkpoints, such as the anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) and the anti-programmed cell death-1 (PD-1) or its ligand (PD-L1), can potentiate or rescue the effector functions of the antitumor cell-mediated immune responses.Citation11,12 The clinical activity of immune checkpoints agents has been conclusively demonstrated for different types of tumors.Citation13-18 The combination treatment with anti-CTLA-4 and anti-PD-1 mAbs for metastatic melanoma yielded striking clinical results, leading to the FDA approval.Citation19,20 The efficacy of this combination is also under evaluation in other solid tumors such as lung cancer.Citation21 Nevertheless, similarly to single-agent therapies, a proportion of patients do not respond to this combination, thus the optimization of these strategies is still under investigation.Citation22-24
In this context, it is desirable to identify biomarkers to be used prospectively for the selection of patients more likely to respond to single agent or the combination therapy and for the optimization of treatment schedules. This information might also provide insights about how to prevent immune-related adverse events (iAEs), in particular high-grade toxicities (≥ 3) observed in about 10–20% of patients treated with single agents and in up to 50% of cases treated with the combination.Citation24
We recently showed that the baseline serum levels of soluble NKG2D ligands (sNKG2DLs) can discriminate melanoma patients treated with the combination of ipilimumab plus chemotherapy who experience poor clinical outcome from those with long-term survival.Citation25
The aim of this study was to assess the value of serum levels of sNKG2DLs as predictors of responsiveness in melanoma patients undergoing immunotherapy regimens. We determined sNKG2DLs levels in pre- and post-treatment sera of melanoma patients treated with immune checkpoint blockade (anti-CTLA-4 or anti-PD-1 mAb monotherapy or their combinations) and the results were correlated with patients' (N = 194) clinical outcome. The same determinations were performed in a control group of melanoma patients treated with standard therapy or mutated BRAF inhibitors (N = 65; BRAFi).
Results
Detection of sNKG2DLs in the serum of patients
sNKG2DLs levels were recorded pre- and post-treatment (12 weeks following the first drug administration) in the sera of 162 melanoma patients undergoing treatment with immune checkpoint blockade. The clinical and treatment characteristics of the patients are summarized in . MICA, MICB, and ULBP-2 were detectable in a minority of patients at baseline (N = 18, 42, and 38, respectively), A, B, and D. In some patients (N = 11, 19, and 10, respectively), these factors were detectable only in post-treatment samples. Few patients (N = 4, 8, and 2, respectively) showed ≥ 50% reduction in the concentration of these factors 12 weeks' post-treatment. Similarly, increased levels of sMICA, sMICB, and ULBP-2 after treatment were observed in few cases (N = 6, 11, and 17, respectively). Higher levels of sULBP-1 and -3, were observed as compared with the others NKG2DLs, in the sera of patients with peak concentrations of 1 × 105–1 × 106 pg/mL ( C and E). Moreover, these molecules were most frequently found in the serum of patients both at pre- (N = 83, 62.5% and N = 65, 40%, respectively) and post-treatment (N = 85, 65% and N = 62, 38%) time points ( D and E). The presence of sULBP-1, due to the limiting amount of serum for some patients, was determined in N = 131 patients. Modulation of the concentration of sULBPs according to treatment followed a trend like MICA and MICB ().
Table 1. Clinical-pathological features of melanoma patients treated with immune checkpoint blockade agents or with standard/targeted therapies.
Figure 1. sNKG2DLs in the serum of melanoma patients treated with immune checkpoint blockade agents. The presence of sMICA (Panel A), sMICB (Panel B), and sULBP-2, 3 (Panels D and E) in the serum at baseline (W1; black circle) and post-treatment (W12; black square) of melanoma patients (N = 162) treated with immune checkpoint blockade agents was measured by ELISA assay (see Material and methods). ULBP-1 determinations at pre- and post-treatment were performed in N = 131 and 128 patients, respectively (Panel C). Mean and error bars are shown in the graphs. As negative control the serum of N = 10 HD was used in ELISA assays (data not shown; see Ref. Citation[25]).
![Figure 1. sNKG2DLs in the serum of melanoma patients treated with immune checkpoint blockade agents. The presence of sMICA (Panel A), sMICB (Panel B), and sULBP-2, 3 (Panels D and E) in the serum at baseline (W1; black circle) and post-treatment (W12; black square) of melanoma patients (N = 162) treated with immune checkpoint blockade agents was measured by ELISA assay (see Material and methods). ULBP-1 determinations at pre- and post-treatment were performed in N = 131 and 128 patients, respectively (Panel C). Mean and error bars are shown in the graphs. As negative control the serum of N = 10 HD was used in ELISA assays (data not shown; see Ref. Citation[25]).](/cms/asset/7a240c02-91d1-4d36-a48d-f5e2ffb48a33/koni_a_1323618_f0001_b.gif)
Baseline levels of sNKG2DLs were also analyzed in melanoma patients not undergoing any immunotherapy and rather treated either with standard therapy or BRAFi (see for clinical details). As shown in , all sNKG2DLs could be detected in the serum of these patients (MICA in N = 19, MICB in N = 25, ULBP-1 in N = 32, ULBP-2 in N = 20, and ULBP-3 in N = 15 patients). The levels of sNKG2DLs were heterogeneous with peak of concentration, except for ULBP-2, lower as compared with patients treated with immunotherapy. Serum levels of sNKG2DLs from patients treated with ipilimumab plus chemotherapy (NIBIT-M1 study; N = 37) that have been described previously,Citation25 were also included in the subsequent analyses.
Figure 2. Detection of sNKG2DLs in the serum of control group melanoma patients. The presence of soluble NKG2DLs (MICA, Panel A; MICB, Panel B; ULBP-1, Panel C; ULBP-2, Panel D; ULBP-3, Panel E) was measured by ELISA assay (see Material and methods) in the baseline serum (W1; black circle) of melanoma patients (N = 65) treated with either standard or BRAFi therapies. Mean and error bars are shown in the graphs. As negative control the serum of N = 10 HD was used in ELISA assays (data not shown; see Ref. Citation[25]).
![Figure 2. Detection of sNKG2DLs in the serum of control group melanoma patients. The presence of soluble NKG2DLs (MICA, Panel A; MICB, Panel B; ULBP-1, Panel C; ULBP-2, Panel D; ULBP-3, Panel E) was measured by ELISA assay (see Material and methods) in the baseline serum (W1; black circle) of melanoma patients (N = 65) treated with either standard or BRAFi therapies. Mean and error bars are shown in the graphs. As negative control the serum of N = 10 HD was used in ELISA assays (data not shown; see Ref. Citation[25]).](/cms/asset/f821851d-964e-4bde-b295-3b309b846d85/koni_a_1323618_f0002_b.gif)
Soluble MICA and MICB were most commonly detected in stage III melanoma patients (p = 0.05 and 0.001, respectively); conversely the detection of sULBP-1 was most frequently associated (p = 0.02) with stage IV. No associations between stage of the disease and detection of soluble levels of ULBP-2 and -3 were observed (data not shown).
Identification of biomarkers of clinical outcome in melanoma patients treated with immune checkpoint blockade
Association between the presence or absence of sNKG2DLs in baseline or post-treatment serum of melanoma patients receiving either anti-CTLA-4 or -PD-1 mAbs as monotherapy or their combination and clinical outcome was determined for N = 194 melanoma patients ( and ). This analysis included also melanoma patients treated with the combination of anti-CTLA-4 and fotemustine (N = 37, see ) for which the levels of sNKG2DLs and the modulation during treatment have been previously reported.Citation25 Disease control (DC) and OS information were available for N = 193 and 194 patients, respectively.
Table 2. Association between the levels at baseline serum of sNKG2DLs and the disease control of melanoma patients.
Table 3. Association between the levels at baseline serum of sNKG2DLs and the OS of melanoma patients.
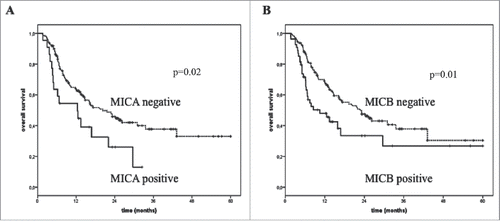
Absence of sULBP-1 in baseline serum of 77/162 evaluated patients correlated with better clinical outcome in terms of DC (DC rate 57.1%, p = 0.002; ). No relationship was found between the serum levels of sNKG2DLs and the clinical outcome of patients not treated with immunotherapy (control group; ). Absence or presence at baseline of detectable sULBP1, respectively discriminated patients with improved (N = 78 patients; median OS = 25.3 mo; p = 0.01) from poor OS (N = 85 patients; median OS = 12.1 mo; ). Similarly, lack of sMICB in pre-treatment serum identified patients experiencing long-term survival (N = 151 patients; median OS = 21.6 mo; p = 0.02) compared with those with detectable soluble molecules (N = 42 patients; median OS = 8.8 mo; ). No relationship between serum levels of sNKG2DLs and OS was observed in the control group was ().
Absent detection of sMICA and sMICB in post-treatment serum of melanoma patients undergoing immune checkpoint blockade correlated with improved survival (median OS = 20.2 and 22.8 mo; p = 0.02 and 0.01, respectively; ) compared with cases in which the two factors could be detected (median OS = 12.4 and 10.4 mo, respectively).
No significant association between post-treatment serum levels of sNKG2D and OS of melanoma patients treated with BRAFi was found (). represents the Kaplan–Meier analysis of OS for melanoma patients treated either with immunotherapy (Panels A and B), standard therapy or BRAFi (Panels C and D) according to detection in baseline serum of sMICB (Panels A and C) and sULBP-1 (Panels B and D). These findings highlight an inverse association between levels of sNKG2DLs and the OS specifically in patients treated with immunotherapy. No significant association was detected between levels of sMICB (p = 0.40) and sULBP-1 (p = 0.84) and OS of patients who did not undergo immunotherapy ( Panels C and D). An inverse significant association (p = 0.02 and 0.01, respectively) between levels of sMICA and sMICB in post-treatment serum and OS of melanoma patients treated with immunotherapy are portrayed by the Kaplan–Meier curves in (Panels A and B).
Figure 3. Overall survival of melanoma patients treated with immunotherapy in relation with the presence or not of sNKG2DLs in serum. Kaplan–Meier plots of overall survival of melanoma patients treated with immune checkpoint blockade agents (Panels A and B) or with standard or BRAFi therapies (Panels C and D) in relation with the detection (black line) or not (dotted line) at baseline of sMICB (Panels A and C) and sULBP-1 (Panels B and D). The baseline serum levels of sMICB (Panel A) and ULBP-1 (Panel B) could discriminate melanoma patients with long-term survival (median OS = 21.6 and 25.3 mo, p = 0.02 and 0.01, respectively) from poor survivors (median OS = 8.8 and 12.1 mo, respectively) for the cohort of patients treated with immune checkpoint blockade agents. Panels C and D show the absence of association between the serum levels of these ligands and OS in the control group of patients (median OS = 12.0 vs. 13.1 and 8.5 vs.15.6 and p = 0.4 and 0.85, respectively).
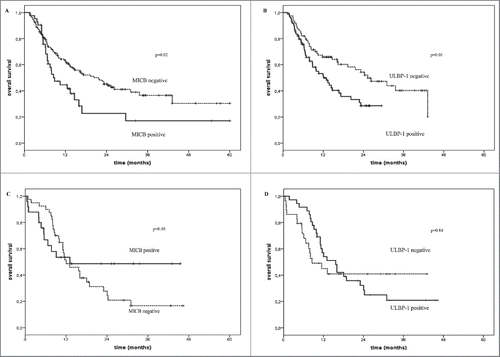
Figure 4. Overall survival of melanoma patients treated with immunotherapy in association with the levels of sNKG2DLs in post-treatment serum. The absence (dotted line) in the post-treatment serum of sMICA (Panel A) and sMICB (Panel B) correlated with improved OS (median OS = 20.2 and 22.8 vs. 10.4 mo, p = 0.02 and 0.01, respectively) while detectable levels of these molecules were found in the serum of poor survivor patients (median OS = 12.4 and 10.4 mo, respectively) (black line).
The available cancer sets in the Cancer Genome Atlas (TCGA) were used to assess any relationship between the expression of NKG2DLs, that we found as candidate predictors for immunotherapy treatment of melanoma patients, and the clinical outcome of 30 cohorts of patients with different types of primary tumors. The hazard ratio through COX analysis of the highest versus the lowest tertile of expression of these molecules was calculated (S). The lowest expression of MICB was significantly associated with favorable clinical outcome in lower grade glioma (LGG), pancreatic adenocarcinoma (PADD), and thymoma patients (THYM) (p = 0.000369, 0.012, and 0.0399, respectively, S). Low or negative expression of ULBP-1 inversely correlated with the risk of death for eight different cohorts of patients with tumors with different histological origins, including LGG (p = 0.000131), glioblastoma (GBM; p = 0.014), breast cancer (BRCA; p = 0.0166), mesothelioma (MESO; p = 0.00963) (S). Scant information is available in these data sets regarding metastatic melanoma patients and the therapeutic regimens administered to cancer patients. Although our observations could not be confirmed through TCGA in the same setting of cancer patients, the data represented in S corroborate the role of NKG2DLs as prognostic candidate biomarkers for the clinical outcome of cancer patients.
Multivariate analysis of biomarkers
The impact of individual and clinical parameters, such as age, LDH, PS, stage, etc. () and the levels of sNKG2DLs in the serum of melanoma patients was evaluated by Cox regression analysis (). LDH was heterogeneously detected in melanoma patients with a range of 124–2190 IU/L and median = 302 IU/L. This molecule was reported as increment of 10 IU/L revealing to be the strongest (HR = 1.01, p <0.0001) prognostic markers for OS survival for melanoma patients treated either with immune checkpoint blockade or with standard or BRAFi therapies (). PS and disease stage are associated with clinical benefit only for the control group (HR = 2 and 6.2 and p = 0.03 and 0.01, respectively; ). The latest two markers were not significantly associated (p = 0.18 and 0.10, respectively) with clinical responses to immunotherapy. Interestingly, MICB, ULBP-1, and ULBP-2 significantly predicted clinical outcome of patients undergoing immunotherapy strategies (HR = 1.67 and 1.78 and p = 0.02 and 0.01, respectively; ). Multivariate COX regression analysis confirmed the role of LDH and ULBP-1 as independent prognostic biomarkers of the clinical outcome (HR = 1.02 and 1.72 and p = 0.02 and 0.0001, respectively; ) in melanoma patients treated with immune checkpoint blockade. Of note, ULBP-2 also resulted as a marker associated with the clinical responses of this cohort of melanoma patients (HR = 1.91 and p = 0.02; ). The same multivariate analysis applied to the melanoma patients in the control group demonstrated that only LDH and age were associated with prognosis (HR = 1.03 and p <0.0001 and p = 0.02, respectively; ).
Table 4. COX regression analysis to identify biomarkers influencing the clinical outcome of melanoma patients.
Discussion
In the present study, we assessed the levels of sNKG2DLs in the serum of melanoma patients to test whether they could represent baseline predictors of clinical outcome in response to treatment with immune checkpoint blockade. Absence of sMICB and sULBP-1 in patient's serum at baseline distinguished long-term from poor survivors (OS 21.6 and 25.3 vs. 8.8 and 12.1 mo and p = 0.02 and 0.01, respectively). In univariate analysis, the HR for patients with detectable levels of sMICB and/or sULBP-1 in baseline serum was 1.67 and 1.78, respectively (p = 0.02 and 0.01, respectively). LDH was confirmed as a predictive marker (HR = 1.01 and p <0.0001) for the clinical outcome of melanoma patients independent on the type of therapeutic treatment.Citation26,27 sULBP-1 and ULBP-2 were identified through multivariate analysis as candidate independent predictive markers (HR = 1.72 and 1.91, respectively, and p = 0.02) of clinical response in patients treated with immune checkpoint inhibitors. Thus, the level of these biomarkers in baseline serum enables the distinction of melanoma patients with favorable clinical outcome from poor survivors to the treatment with immune checkpoint blockade.
NKG2D-mediated signaling plays a relevant role in tumor immunosurveillance.Citation28-30 This receptor is expressed by NK, T, NKT, and γδT cells, providing activating signal to NK and co-stimulation to T cells.Citation30 Both in vitro and in vivo studies have demonstrated that the expression of NKG2DLs by tumor cells can lead to the efficient development of antitumor immune responses.Citation30-29 NKG2DLs are expressed by tumor cells of different histological origin, although the surface expression of these ligands is strictly regulated by different mechanisms and by the interaction with the TME.Citation31,30 The presence of sNKG2DLs in the serum of cancer patients has been widely documented in association with tumor progression.Citation28,32-35 sULBP-2 was identified as a prognostic factor, stronger than S100B, in early-stage (I–III) melanoma patients.Citation29 NKG2DLs can either promote anticancer immune responses or mediate immune evasion of cancer cells, depending upon their pattern of expression, e.g., membrane localization or proteolytic shedding in soluble form by tumor cells.Citation30,36 sNKG2DLs can suppress antitumor immune responses through multiple mechanisms. The most common is binding of soluble ligands to the NKG2D receptors on T and NK cells facilitating their endocytosis and degradation thus impairing the antitumor activity of the lymphocyte populations.Citation28,37,38 The suppression of antitumor immune responses by tumor cells secreting sMICB was clearly shown in a prostate cancer model of humanized transgenic mice.Citation39 sMICA can interfere with NK homeostatic maintenance in the peripheral bloodCitation39 and can also promote the expansion of MDSCs.Citation40 On the other hand, high expression of membrane ULBP-1 positively correlated with OS of pancreatic cancer patients while sULBP-2 was found as an independent marker of poor clinical outcome for these patients indicating that the molecular nature of NKG2DLs can affect positively or negatively the clinical outcome.Citation35 The phenomenon described above might explain our observation that the presence of sNKG2DLs in baseline serum is associated with poor clinical outcome of melanoma patients treated with immunotherapy strategies. sNKG2DLs could impair antitumor T-cell-mediated responses thus counterbalancing the unleashing of immune responses by immune checkpoint blockade, such as anti-CTLA-4 or PD-1 mAbs. Similarly, sNKG2DLs can abolish the unlocking activity of anti-PD-1 mAbs on tumor-reacting NK cells.
Our preliminary analysis of cancer sets in TCGA indicated that the expression of either MICB or ULBP-1 was significantly associated with favorable clinical outcome of cancer patients with different type of tumors (e.g., LGG, PADD, THYM, GBM, BRCA, HNSC MESO, SARC, KIRC, KIRP, DLBC). Moreover, the highest expression of the activatory/co-stimulatory receptor, NKG2D, was associated with a reduced risk of death for cancer patients with LGG (p = 1.59 × 10−6), HNSC (p = 0.00029), BRCA (p = 0.011), THYM (p = 0.0064), bladder carcinoma (BLCA; p = 2.02 × 10−8), cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC; p = 0.00094), lung squamous cell carcinoma (LUSC; p = 0.0073), stomach adenocarcinoma (STAD; p = 0.039), uterine corpus endometrial carcinoma (UCEC; p = 0.012) (S). Interestingly, for some type of tumors, such as LGG, GBM, HNSC, BRCA, SARC, THYM, highest expression of NKG2D and lowest expression of either ULBP-1 or MICB were detected in patients with reduced risk of death (S). Limited information regarding either metastatic melanoma patients or the therapeutic regimens received by cancer patients are included in the TCGA data sets, preventing us to perform any association analysis between gene expression of NKG2D and OS of patients treated with immune checkpoint blockade. Nevertheless, this exploratory analysis can corroborate the role of NKG2DLs as candidate predictors for the prognosis of cancer patients and the need to further investigate their role as biomarkers for the clinical responses to immunotherapy.
We have described previously the role of ULBP-1 and -2 as candidate predictive markers for the clinical outcome of melanoma patients with metastatic disease treated with ipilimumab and fotemustine (NIBIT-M1 study).Citation25 These findings are substantiated by the present study that evaluated patients treated with immune checkpoint blockade both as monotherapy and in combination. In this study, we also evaluated a cohort of melanoma patients treated with standard therapies or BRAFi. In this context, the levels of sNKG2DLs did not affect clinical outcome, indicating that these molecules represent useful tools predicting the clinical activity of immune checkpoint blockade (see ). We have also evaluated the relationship between the presence of sNKG2DLs in the serum after treatment and clinical outcome. Indeed, the absence of sMICA and sMICB in post-treatment serum was significantly associated (p = 0.02 and 0.01, respectively) with improved OS (20.2 and 22.8 mo, respectively) of melanoma patients undergoing anti-CTLA-4 or anti-PD-1 mAb therapy. These results confirm the relevance of levels of sNKG2DLs in pre- and post-treatment sera in predicting clinical responses in melanoma patient receiving immunotherapy.
The clinical activity of ipilimumab in combination with a vaccine composed by tumor cells secreting GM-CSF was observed in patients with high levels of autoantibodies directed to MICA.Citation41 The impairment mediated by sNKG2DLs on T and NK-cell-mediated antitumor responses could be rescued by treatment with neutralizing antibodies.Citation42 Taken together these and our observations suggest a rationale to explore the therapeutic efficacy of the combination of immune checkpoint blockade with sNKG2DL neutralizing mAbs.Citation43 In addition, high sMICA levels in the serum were found to be associated with less frequency of immune-related adverse events in a cohort of melanoma patients treated with ipilimumabCitation44 suggesting that sNKG2DLs can indeed play a relevant role in determining the fate of antitumor immune responses unleashed by immune checkpoint blocking agents.
Our findings demonstrate that sNKG2DLs can play a role as predictive biomarkers for OS of melanoma patients treated with immune checkpoint blocking mAbs (including ipilimumab, nivolumab, pembrolizumab, and their combinations) and broaden the list of parameters that can be worthy of monitoring in melanoma patients. Of note, these findings identify candidate biomarkers determinable in the serum of patients through assays easily accessible in different clinical centers. Further prospective investigation of the role of these molecules as baseline biomarkers of clinical outcome of cancer patients treated with immune checkpoint blockade agents and their combinations are warranted. It might also be interesting to elucidate the relationship that exists among gene expression in tumor tissues and the soluble protein levels in the serum for NKG2DLs in cancer patients with different type of histology for which immunotherapy either represents a promising strategy or is currently under investigation. It will be worthy to assess these determinations in association with the extent of NKG2D expression, as a marker of lymphocyte infiltration, at tumor site to establish the most accurate possible biomarker immune signature(s) for patients undergoing immune-based therapies.
Material and methods
Melanoma patients
Patients (N = 162) with measurable unresectable stage III or stage IV melanoma were included in this study; see for detailed patient's characteristics and treatments. These melanoma patients have been treated with (i) ipilimumab at 3 or 10 mg/kg in the context of expanded access programs (EAP) or, more recently, as “on-label usage”; (N = 132); (ii) pembrolizumab for patients previously treated with ipilimumab (N = 15); (iii) monotherapy with ipilimumab or nivolumab or their combination (N = 15). Moreover, patients treated with ipilimumab plus chemotherapy (NIBIT-M1 study; N = 37) that have been described previously,Citation25 were included in this study to carry out a more extensive evaluation. A control group of melanoma patients included subjects with metastatic disease treated either with standard chemotherapy or radiotherapy regimens (N = 31) or with BRAF inhibitor (BRAFi; vemurafenib; or dabrafenib) -based targeted therapies (N = 34). These patients did not ever receive any immunotherapy regimen. The therapeutic treatment of melanoma patients that were performed in the context of clinical studies were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice and have been approved by the Ethics Committee of the University Hospital of Siena. An informed consent for bio-banking and use of biologic samples and clinical data for scientific research was obtained from all the patients enrolled in this study.
Response criteria were assessed according the proposed immune-related response criteria for immunotherapy treatments, where objective response included immune-related complete or partial response while DC included immune-related confirmed complete, partial, or stable disease.Citation45,46 For patients not treated with immunotherapy, response criteria were assessed according WHO. Clinical responses evaluated as DC and OS were available for from N = 193 and 194 patients, respectively.
Biological samples
Serum from melanoma patients was collected at pre-treatment (baseline; W1) and (W12), post-treatment and then isolated by centrifugation and cryopreserved.
Detection of sNKG2DLs in the serum of melanoma patients
The concentration in the serum of melanoma patients (N = 162) of sNKG2DL (MICA, MICB, ULBP-2, ULBP-1), was assessed by the usage of ELISA kits (R&D Systems). Commercially available pair antibodies and related reagents (R&D Systems) were used to set up the ELISA assay to determine the concentration in the serum of sULBP-3. A standard curve with determined titrations of the recombinant human proteins allowed to measure sNKG2DL concentrations in the experimental samples. Data are means of duplicates and are represented as pg/mL. In some cases, N = 100 patients, the amount of available serum allowed to repeat twice the Elisa assays; the inter-assay coefficient of variation has been calculated and was in the range of 2–8%. Statistical analysis of differences between means of concentration of NKG2DLs at different time points was performed using two-tailed t-test (p < 0.05). The concentration of sULBP-1 was determined in the baseline and W12 time points serum of N = 131 and 128 patients, respectively. The serum of N = 10 HD was used as negative control as reported elsewhere.Citation25
Gene expression analysis
Expression analysis for NKG2D, MICB, and ULBP-1 were obtained from available RNA sequencing (RNA-Seq) cancer sets in TCGA research network (http://cancergenome.nih.gov/) for 30 cohorts of cancer patients with different type of tumors.
Statistical analysis
Data were analyzed in a descriptive way using mean and standard deviations. This study was aimed at the identification of variations in candidate biomarkers previously identified see Ref. Citation[25] associated with DC and OS. Association between sNKG2DLs and DC was assessed by chi-squared test. Survival curves were estimated by the Kaplan–Meier method and differences were evaluated with the log-rank test. Differences in OS according to sNKG2DLs (MICA, MICB, ULBP-1, 2, 3), gender, age, stage, LDH, PS were analyzed. A Cox regression analysis was implemented to investigate the role of each factor considered and of its relationship with the other variables in correlating with OS. A forward stepwise selection method was used based on Wald statistics, resulting models were confirmed by a backward procedure. Hazard ratio and their 95% confidence interval (95% CI) were reported. IBM SPSS v. 21 was used for statistical analysis. For TCGA data, hazard ratio was calculated using R (v3.3.1) and survival package (v2.39–5). The forest plot was generated using the forest plot package (v1.5.1). The Cox proportional hazards regression model was applied on the highest versus lowest tertiles of expression in each cancer cohort; p-values were calculated using pchisq function form the base stats package.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary_materials.zip
Download Zip (146.9 KB)Funding
Part of this study was funded by Guido Berlucchi Foundation (Brescia, Italy) awarded to M. Maio.
References
- Mihm MC, Jr., Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: A histopathologic prognostic indicator and an expression of local immune response. Lab Invest 1996; 74(1):43-47; PMID: 8569196
- Dalerba P, Maccalli C, Casati C, Castelli C, Parmiani G. Immunology and immunotherapy of colorectal cancer. Crit Rev Oncol Hematol 2003; 46(1):33-57; PMID: 12672517; https://doi.org/10.1016/S1040-8428(02)00159-2
- Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: A prognostic factor that should not be ignored. Oncogene 2010; 29(8):1093-102; PMID: 19946335; https://doi.org/10.1038/onc.2009.416
- Bindea G, Mlecnik B, Fridman WH, Galon J. The prognostic impact of anti-cancer immune response: A novel classification of cancer patients. Semin Immunopathol 2011; 33(4):335-40; PMID: 21461991; https://doi.org/10.1007/s00281-011-0264-x
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011; 71(4):1263-71; PMID: 21303976; https://doi.org/10.1158/0008-5472.CAN-10-2907
- Fridman WH, Dieu-Nosjean MC, Pages F, Cremer I, Damotte D, Sautès-Fridman C, Galon J. The immune microenvironment of human tumors: General significance and clinical impact. Cancer Microenviron 2013; 6(2):117-22; PMID: 23108700; https://doi.org/10.1007/s12307-012-0124-9
- Angell H, Galon J. From the immune contexture to the Immunoscore: The role of prognostic and predictive immune markers in cancer. Curr Opin Immunol 2013; 25(2):261-67; PMID: 23579076; https://doi.org/10.1016/j.coi.2013.03.004
- Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol 2014; 27:16-25; PMID: 24531241; https://doi.org/10.1016/j.coi.2014.01.004
- Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, Spranger S. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol 2013; 25(2):268-76; PMID: 23579075; https://doi.org/10.1016/j.coi.2013.02.009
- Nelson D, Fisher S, Robinson B. The “Trojan Horse” approach to tumor immunotherapy: Targeting the tumor microenvironment. J Immunol Res 2014; 2014:789069; PMID: 24955376; https://doi.org/10.1155/2014/789069
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12(4):252-64; PMID: 22437870; https://doi.org/10.1038/nrc3239
- Pardoll DM. Immunology beats cancer: A blueprint for successful translation. Nat Immunol 2012; 13(12):1129-32; PMID: 23160205; https://doi.org/10.1038/ni.2392
- Wilden SM, Lang BM, Mohr P, Grabbe S. Immune checkpoint inhibitors: A milestone in the treatment of melanoma. J Dtsch Dermatol Ges 2016; 14(7):685-95; PMID:27373243; https://doi.org/10.1111/ddg.13012
- Guennoun A, Sidahmed H, Maccalli C, Seliger B, Marincola FM, Bedognetti D. Harnessing the immune system for the treatment of melanoma: Current status and future prospects. Expert Rev Clin Immunol 2016; 12:879-93; PMID:27070898; https://doi.org/10.1080/1744666X.2016.1176529
- Rajan A, Kim C, Heery CR, Guha U, Gulley JL. Nivolumab, anti-programmed death-1 (PD-1) monoclonal antibody immunotherapy: Role in advanced cancers. Hum Vaccin Immunother 2016; 12(9):2219-31; PMID: 27135835; https://doi.org/10.1080/21645515.2016.1175694
- Xia Y, Medeiros LJ, Young KH. Immune checkpoint blockade: Releasing the brake towards hematological malignancies. Blood Rev 2016; 30(3):189-200; PMID: 26699946; https://doi.org/10.1016/j.blre.2015.11.003
- Haanen JB, Robert C. Immune Checkpoint Inhibitors. Prog Tumor Res 2015; 42:55-66; PMID: 26382943; https://doi.org/10.1159/000437178
- Luke JJ, Ott PA. PD-1 pathway inhibitors: The next generation of immunotherapy for advanced melanoma. Oncotarget 2015; 6(6):3479-92; PMID: 25682878; https://doi.org/10.18632/oncotarget.2980
- Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373(13):1270-71; PMID: 26398076; https://doi.org/10.1056/NEJMc1509660
- Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 2016; 13(8):473-86; PMID: 27141885; https://doi.org/10.1038/nrclinonc.2016.58
- Antonia SJ. Moving beyond monotherapy in the immunotherapeutic arena: Prospects for combination therapies in lung cancer. Clin Adv Hematol Oncol 2016; 14(8):616-18; PMID: 27487105; https://doi.org/10.1016/S1470-2045(16)30098-5
- Ascierto PA, Marincola FM, Atkins MB. What's new in melanoma? Combination!. J Transl Med 2015; 13:213; PMID: 26141621; https://doi.org/10.1186/s12967-015-0582-1
- Ascierto PA. Editorial: Combination strategies in the treatment of melanoma. Front Oncol 2016; 6:67; PMID: 27047798; https://doi.org/10.3389/fonc.2016.00067
- Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016; 39(1):98-106; PMID: 26558876; https://doi.org/10.1097/COC.0000000000000239
- Maccalli C, Giannarelli D, Capocefalo F, Pilla L, Fonsatti E, Di Giacomo AM, Parmiani G, Maio M. Immunological markers and clinical outcome of advanced melanoma patients receiving ipilimumab plus fotemustine in the NIBIT-M1 study. Oncoimmunology 2016; 5(2):e1071007; PMID: 27057436; https://doi.org/10.1080/2162402X.2015.1071007
- Deichmann M, Benner A, Bock M, Jäckel A, Uhl K, Waldmann V, Näher H. S100-Beta, melanoma-inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American Joint Committee on Cancer stage IV melanoma. J Clin Oncol 1999; 17(6):1891-96; PMID: 10561230; https://doi.org/10.1200/JCO.1999.17.6.1891
- Weide B, Elsasser M, Buttner P, Pflugfelder A, Leiter U, Eigentler TK, Bauer J, Witte M, Meier F, Garbe C. Serum markers lactate dehydrogenase and S100B predict independently disease outcome in melanoma patients with distant metastasis. Br J Cancer 2012; 107(3):422-28; PMID: 22782342; https://doi.org/10.1038/bjc.2012.306
- Maccalli C, Scaramuzza S, Parmiani G. TNK cells (NKG2D+ CD8+ or CD4+ T lymphocytes) in the control of human tumors. Cancer Immunol Immunother 2009; 58(5):801-08; PMID: 19089424; https://doi.org/10.1007/s00262-008-0635-x
- Paschen A, Sucker A, Hill B, Moll I, Zapatka M, Nguyen XD, Sim GC, Gutmann I, Hassel J, Becker JC et al. Differential clinical significance of individual NKG2D ligands in melanoma: Soluble ULBP2 as an indicator of poor prognosis superior to S100B. Clin Cancer Res 2009; 15(16):5208-15; PMID: 19671853; https://doi.org/10.1158/1078-0432.CCR-09-0886
- Zhang J, Basher F, Wu JD. NKG2D ligands in tumor immunity: Two sides of a coin. Front Immunol 2015; 6:97; PMID: 25788898; https://doi.org/10.3389/fimmu.2015.00097
- Chitadze G, Bhat J, Lettau M, Janssen O, Kabelitz D. Generation of soluble NKG2D ligands: Proteolytic cleavage, exosome secretion and functional implications. Scand J Immunol 2013; 78(2):120-29; PMID: 23679194; https://doi.org/10.1111/sji.12072
- Paschen A, Baingo J, Schadendorf D. Expression of stress ligands of the immunoreceptor NKG2D in melanoma: Regulation and clinical significance. Eur J Cell Biol 2014; 93(1-2):49-54; PMID: 24629838; https://doi.org/10.1016/j.ejcb.2014.01.009
- Baragano Raneros A, Suarez-Alvarez B, Lopez-Larrea C. Secretory pathways generating immunosuppressive NKG2D ligands: New targets for therapeutic intervention. Oncoimmunology 2014; 3:e28497; PMID: 25050215; https://doi.org/10.4161/onci.28497
- Yamaguchi K, Chikumi H, Shimizu A, Takata M, Kinoshita N, Hashimoto K, Nakamoto M, Matsunaga S, Kurai J, Miyake N et al. Diagnostic and prognostic impact of serum-soluble UL16-binding protein 2 in lung cancer patients. Cancer Sci 2012; 103(8):1405-13; PMID: 22587355; https://doi.org/10.1111/j.1349-7006.2012.02330.x
- Nuckel H, Switala M, Sellmann L, Horn PA, Dürig J, Dührsen U, Küppers R, Grosse-Wilde H, Rebmann V. The prognostic significance of soluble NKG2D ligands in B-cell chronic lymphocytic leukemia. Leukemia 2010; 24(6):1152-59; PMID: 20428196; https://doi.org/10.1038/leu.2010.74
- Chitadze G, Lettau M, Bhat J, Wesch D, Steinle A, Fürst D, Mytilineos J, Kalthoff H, Janssen O, Oberg HH et al. Shedding of endogenous MHC class I-related chain molecules A and B from different human tumor entities: Heterogeneous involvement of the “a disintegrin and metalloproteases” 10 and 17. Int J Cancer 2013; 133(7):1557-66; PMID: 23526433; https://doi.org/10.1002/ijc.28174
- Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002; 419(6908):734-38; PMID: 12384702; https://doi.org/10.1038/nature01112
- El-Gazzar A, Groh V, Spies T. Immunobiology and conflicting roles of the human NKG2D lymphocyte receptor and its ligands in cancer. J Immunol 2013; 191(4):1509-15; PMID: 23913973; https://doi.org/10.4049/jimmunol.1301071
- Liu G, Lu S, Wang X, Page ST, Higano CS, Plymate SR, Greenberg NM, Sun S, Li Z, Wu JD. Perturbation of NK cell peripheral homeostasis accelerates prostate carcinoma metastasis. J Clin Invest 2013; 123(10):4410-22; PMID: 24018560; https://doi.org/10.1172/JCI69369
- Xiao G, Wang X, Sheng J, Lu S, Yu X, Wu JD. Soluble NKG2D ligand promotes MDSC expansion and skews macrophage to the alternatively activated phenotype. J Hematol Oncol 2015; 8:13; PMID: 25887583; https://doi.org/10.1186/s13045-015-0110-z
- Jinushi M, Hodi FS, Dranoff G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc Natl Acad Sci U S A 2006; 103(24):9190-95; PMID: 16754847; https://doi.org/10.1073/pnas.0603503103
- Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O'Reilly RJ, Dupont B, Vyas YM. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J Immunol 2003; 171(12):6891-99; PMID: 14662896; https://doi.org/10.4049/jimmunol.171.12.6891
- Wu J. Antibody targeting soluble NKG2D ligand sMIC refuels and invigorates the endogenous immune system to fight cancer. Oncoimmunology 2016; 5(3):e1095434; PMID: 27141357; https://doi.org/10.1080/2162402X.2015.1095434
- Felix J, Cassinat B, Porcher R, Schlageter MH, Maubec E, Pages C, Baroudjian B, Homyrda L, Boukouaci W, Tamouza R et al. Relevance of serum biomarkers associated with melanoma during follow-up of anti-CTLA-4 immunotherapy. Int Immunopharmacol 2016; 40:466-73; PMID: 27728898; https://doi.org/10.1016/j.intimp.2016.09.030
- Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res 2009; 15(23):7412-20; PMID: 19934295; https://doi.org/10.1158/1078-0432.CCR-09-1624
- Di Giacomo AM, Ascierto PA, Pilla L, Santinami M, Ferrucci PF, Giannarelli D, Marasco A, Rivoltini L, Simeone E, Nicoletti SV et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): An open-label, single-arm phase 2 trial. Lancet Oncol 2012; 13(9):879-86; PMID: 22894884; https://doi.org/10.1016/S1470-2045(12)70324-8
