ABSTRACT
Head and neck squamous cell carcinoma (HNSCC) is one of the most diffused cancer types, characterized by a high reoccurrence rate, mainly due to the inability of current therapeutic approaches to completely eradicate cancer cells. HNSCC patients often have defective immune functions, thus allowing cancer immune escape and cancer spreading. Particularly important in driving immune escape during HNSCC progression are T-helper and T-regulatory cells. New insights into their mechanisms of action might support the development of effective and long-lasting immunotherapy.
KEYWORDS:
Abbreviations
| AE | = | adverse effect |
| DCR | = | disease control rate |
| five-FU | = | 5 fluoruracil |
| MTD | = | maximum tolerated dose |
| ORR | = | overall response rate |
| OS | = | overall survival |
| PFS | = | progressive-free survival |
| RT | = | radio therapy |
Introduction
Cancer is still a global health problem, as the number of deaths due to cancer is growing rapidly due to population aging and increased cancer risk behavior, especially in low-developed countries.Citation1
Head and neck squamous cell carcinoma (HNSCC) is an aggressive epithelial malignancy and the most common neoplasm arising in the upper aerodigestive tract. It is the sixth most diffused cancer worldwide, with an estimated incidence of almost 50,000 new cases per year in United States only.Citation2
Despite therapeutic improvements that have brought 5 y survival rate over 60% in Western Countries, such as United States,Citation3 HNSCC treatment is characterized by high failure rates. The currently applied treatments in fact often fail in completely eradicating the primary tumor, thus allowing local or distant reoccurrence within short time.
HNSCC and immune escape
In recent years, considerable attention has been focused on the involvement of immune system in the development of a long-lasting anticancer immunity and consequently on how cancer cells may escape such a surveillance. Multiple studies have indicated that HNSCC is associated with immune suppression and this could explain the high reoccurrence rate of this type of cancer.Citation4
Malignant HNSCC cells may escape immune surveillance by different strategies: they can avoid immune recognition or they may affect immune system efficiency. For instance, recognition of tumor cell is hampered by the significant decrease of antigen-presenting cells and their effectiveness.Citation5-7 In addition, tumor cells may avoid immune surveillance by downregulating histocompatibility molecules, such as HLA class I gene, required to proper antigen presentation.Citation7 Besides that, many cancer cells release cytokines and chemotactic factors in an unregulated way that might result in an ineffective and more tumor favorable immune response.
Several pieces of evidence have demonstrated that immune functions are usually compromised or at least defective in HNSCC patients. Some reports have shown a decrease of tumor-infiltrating lymphocytes (TIL) in HNSCC,Citation9 but the clinical outcome seems to depend more on the type of TIL rather than on their total number. For instance, cytotoxic CD8+ T cells are significantly decreased in HNSCC patients if compared with healthy controls,Citation9-15 probably due to an increase apoptotic rate among this cell population in HNSCC.Citation13 On the top of that T cells isolated from HNSCC patients are significantly less responsive to in vitro stimulation than those isolated from healthy controls.Citation9
T-helper cells and HNSCC development
T-helper (Th) lymphocytes, characterized by CD4+ glycoprotein expression, are key players in directing the immune responses. They include many cell subtypes, classified as Th1, Th2 or Th17, and their differentiation relies on acquisition of differential cytokine production. Th1 cells are characterized by the production of INFγ and IL-2. Th2 cells instead mainly release IL-4, IL-6 and IL-10. Finally, Th17 cells are characterized by the production and release of IL-17, a pleiotropic inflammatory cytokine.Citation16
As in many other cancer types,Citation17 a progressive increase in Th2 response has been described in HNSCC;Citation18-20 nevertheless, the shift toward Th2 cells seems incomplete. Indeed, a concomitant increase of both Th1 and Th2 cells with a minimal variation of Th1 over Th2 cells ratio has been often reported in HNSCC.Citation18,21
Similarly, a shift toward Th2-related cytokines, with an increase of IL-4, IL-10 and TGFβ, and a subsequent decrease of the most important Th1 mediator, INFγ, is often observed in HNSCC.Citation9,22-28 The increase of IL-10, known as one of the strongest immune suppressive factors, is correlated with tumor staging, nodal involvement and to a worst prognostic factor.Citation25 Moreover, IL-10 levels markedly decrease after chemotherapeutic treatment, thus suggesting that cancer cells are the main source of this cytokine in HNSCC.Citation27 These results demonstrate that HNSCC induces a powerful change in Th1 and Th2 cytokines, producing an environment that is no more effective in promoting a proper cell-mediated antitumor response. Nevertheless, the shift toward Th2 response is characterized by an incomplete decline of Th1 cytokines, as the level of Th1-associated cytokine IL-2 remains high during HNSCC progression, while the levels of IL-12 and IL-22, two important mediators of Th1 differentiation and function, are not modulated during HNSCC progression, as they are the same in HNSCC patients and healthy controls.Citation28
HNSCC development is accompanied by a significant increase of both circulating and tumor-infiltrating Th17 cells with a concomitant decrease of Th1/Th17 ratio.Citation29,30 Furthermore, enhanced levels of Th17 cells strongly correlated with metastasis occurrence.Citation30 Th17 cells proliferation is probably fostered by the high level of IL-6 and IL-23 released by cancer cells. These data are sustained by studies in a murine model of 4-nitroquinoline-1-oxide induced oral carcinogenesis, where the progression from premalignant lesion to oral squamous cell carcinoma (OSCC) was characterized by a progressive increase of Th17 cells and IL-17.Citation4,31 How Th17 cells increase may be a favorable factor in tumor progression is still a matter of debate; nevertheless, one convincing hypothesis is that Th17 cells might have a significant role in promoting intra-tumor angiogenesis.Citation29
IL-17 steadily increases during HNSCC progression.Citation30 Indeed, although HNSCC patients have lower IL-17 plasma levels than patients harbouring premalignant lesions, their levels are higher than those in healthy controls.Citation31,32 Above reported data demonstrate that IL-17 increases during HNSCC progression, although a slight decrease is often observed in the latest phases, probably due to the high levels of TGFβ, which may inhibit Th17 differentiation while promoting regulatory T (Treg) cells differentiation.Citation32
On the other hand, Punt and coworkers did not find any significant variation in T cells frequency between OSCC and healthy controls.Citation33 Their results showed that Th17 cells infiltrate more easily HPV+ than HPV− tumors. Interestingly, they found high levels of IL-17+ but non-Th17 cells in HPV− tumors, and this is correlated with a worst prognostic value. The authors concluded that Th17 cells are associated with better prognosis, while elevated levels of IL-17+ cells, whose only 6% are Th17 cells, are related to a poor prognosis.Citation33 These data also suggest that many previously reported studies could be biased by the high levels of IL-17 releasing non-Th17 cells, such as macrophage and granulocytes, that might have been counted as Th17 cells.
The mechanisms of HNSCC immune escape may be briefly reassumed as follows: in premalignant lesions, there are high levels of inflammatory cytokines, such as IL-2, IL-6 and IL-17, released mainly by IL-17+ Th17 cells, whose proliferation is sustained by the elevated levels of IL-23. However, immune response may fail to counteract tumor growth with a subsequent decrease of many pro-inflammatory cytokines and with a switch toward Th2 cytokines, probably driven by mediators, i.e., TGFβ, released by cancer cells themselves.Citation32 Therefore, in the latest phases of HNSCC progression, the elevated levels of TGFβ seem to unbalance the ratio between Th17 and Treg cells, promoting Treg differentiation with a consequent increase of anti-inflammatory cytokine IL-10 (). As a result, the original antitumor response is converted into a more tumor favorable response.
Figure 1. Cytokines modulation during HNSCC progression. HNSCC development is characterized by the progressive decline of Th1 related cytokine INFγ. Conversely, IL-23 levels increase steadily, probably as the result of an inflammatory response against cancer. Nevertheless, as HNSCC progresses, a significant rise of TGFβ and a concomitant decrease of IL-23 levels promote Treg differentiation with a consequent decline of Th17 cells. As a result, in the latest phase of HNSCC progression, anti-inflammatory and anti-immune cytokines such as IL-4 and IL-10 prevail.
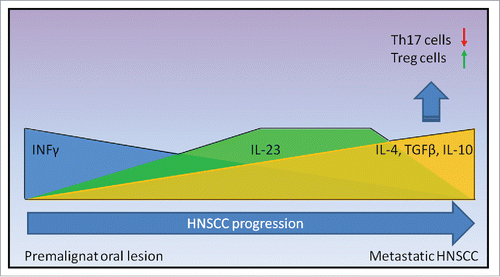
All available studies regarding Th cells and Th-related cytokines modulation in HNSCC are summarized in and , respectively.
Table 1. Studies evaluating T-helper cells modulation in HNSCC.
Table 2. Studies analyzing T-helper-related cytokines in HNSCC.
Regulatory T cells in HNSCC development
Treg cells represent a minor heterogenic subset of CD4+ Th lymphocytes, accounting for less than 5% of them in peripheral blood. Treg are committed to regulate immune response to prevent an excessive immune reactivity and their activity is mainly toward other immune cells such as effector T cells.Citation34 These cells are often involved in cancer; indeed, unfortunately, the mechanisms that prevent autoimmunity are the same that limit the immune system to recognize tumor cells since the majority of tumor-associated antigens are self-antigens or only minimal modified self-antigens harboring genetic modifications.Citation35
A definitive Treg marker has not been discovered yet. Recent approaches have used multiple markers to delineate Tregs, including the IL-2 receptor CD25, the α chain of IL-7 receptor CD127, the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and forkhead/winged-helix transcription factor box P3 (FOXP3). The levels of expression and the intracellular localization of such markers are important factors for Treg identification. For instance, high expression level of CD25 is a peculiar sign of activated Treg. Conversely, CD127 expression on Treg inversely correlates to Foxp3 expression; therefore, low CD127 expression level is considered a reliable marker of activated Treg.Citation36 Thus, a general now accepted panel for Treg identification is CTLA-4+, CD25high, CD127low and nuclear expression of Foxp3. Nevertheless, Foxp3 expression has been reported also in HNSCC cells; thus, it is hard to surely state if studies that evaluated Treg infiltrate analyzing Foxp3 expression have identify proper Treg and not Foxp3+ cancer cells.Citation37
Elevated levels of Treg have been found in several cancer types, including lung, breast and pancreatic cancer.Citation38 Multiple pieces of evidence show a general increase of both circulating and infiltrating Treg during HNSCC development.Citation12,20,27,29,35,37,39-42 Furthermore, Treg levels increase accordingly with tumor staging and they are particularly elevated in patients with active disease.Citation20,35,39
A murine model of oral carcinogenesis demonstrated a cancer cells promoted skew toward Treg, probably due to the decreased levels of IL-23, also observed in the late phase of HNSCC development in humans.Citation32 Accordingly, Treg would increase in advanced stage HNSCC as a result of the unbalance of Th17-promoting cytokines.Citation32 Th17 and Treg cells share a common lineage and a high plasticity degree, even though their mediator effects are opposing. Thus, a shift in cytokine milieu form the Th17 sustaining pro-inflammatory IL-23 toward TGFβ may easily result in a significant skew toward Treg as premalignant lesions progress to oral cancer.Citation31
Treg significance in HNSCC prognosis
An agreement regarding the prognostic value of Treg in HNSCC has yet to be found. Several data strongly sustained a negative prognostic index associated to high levels of Tregs,Citation10,39,41 but other studies described a completely different situation, with high numbers of Treg associated to an improved loco-regional control and better overall survival.Citation12,42,45,46 These apparent contradictions might be explained, in part by the difficulties to proper identify Treg cells and in part by the tissue dependent modulation of Treg levels; indeed, tumors arising in the rich lymphoid tissue oropharynx have higher level of infiltrating Treg than cancer types arising in larynx.Citation42,46 Furthermore, synchronous multifocal cancers, the cancers arising at the same time in different oral cavity area, have more elevated numbers of Treg.Citation44 This aspect sustains a model where Treg increase is a promoting factor rather than a consequence of cancer development.
HPV infection is an important etiologic factor for oropharyngeal SCC. HPV+ HNSCC have a significantly better prognosis than HPV− tumors.Citation47 The role of immune cells in this aspect has been investigated by Punt and colleagues in a set of oropharyngeal SCC. They found a higher infiltration of Foxp3+ Treg cells both in stroma and in the epithelium of HPV+ tumors, which significantly correlated with improved survival.Citation33 These data are in agreement with the findings from Mandal and coworkers, reporting high levels of both CD8+ T cells and Treg in a large set of HPV+ HNSCC.Citation46 Interestingly, HPV+ tumors had almost twice ratio of total T cells over Treg cells if compared with HPV− tumors, thus suggesting that the better prognosis of HPV+ tumors might not be directly related to Treg infiltration but rather to the higher number of immune cells infiltrated. Consistently, Mandal and coworkers emphasized that CD8+/Treg cells ratio was higher in patients having a relative low immune infiltrate if compared with patients with high levels of T cells infiltration.Citation46 This suggests that immune-high HNSCC are subjected to a higher degree of immunoregulation and only the relationship between the different players of immune response could give significant information about prognosis and potential immunotherapy effectiveness.
Mechanism of Treg accumulation in HNSCC
The mechanisms underlying the potential Treg driven pro-tumor effect remain elusive. Four independent studies have studied the immunolocalized Treg cells in HNSCC and they all highlighted a marked presence of cells in the tumor microenvironment.Citation20,37,39,42 Two studies prevalently found Tregs at the tumor margins,Citation39,42 thus sustaining the hypothesis that these cells might oversee the interactions between SCC cells and effector immune cells at the border between tumor and normal tissue.
The accumulation of Treg in tumor might be the result of CD4+CD25+ T-cells recruitment from the periphery or of the activation of resting naive CD4+ T cells directly into the tumor tissue. The proportion of Foxp3+ cells is significantly more elevated in TIL than in peripheral blood mononuclear cells (PBMC).Citation43 Moreover, immune-staining data show high Treg frequency in the peripheral area of the tumor, sometimes in the stroma, but never in the tumor nest,Citation11 in such a way supporting the hypothesis that these cells are recruited from peripheral circulation into the tumor by chemotactic factors released by cancer cells. Consistently with this hypothesis the increased expression of Monocyte Chemotactic Protein-1 (MCP-1), C-C motif Chemokine Ligand 22 (CCL22), C-C chemokine receptors type 4 and 7 (CCR4 and CCR7), that are associated to a migratory phenotype, has been observed on HNSCC isolated Treg and in HNSCC tissue.Citation10,11,35,48 In addition, ligands for CCR4 and CCR7 were more expressed in HNSCC if compared with the adjacent healthy tissue.Citation10 In partial disagreement with the above-reported data, Sun and coworkers found high level of CCR7+ cells in the peripheral blood of HNSCC, but CCR7 expression was elevated in all T lymphocytes, irrespective of the subsets.Citation48 Only the increased expression of CCR4 is a peculiar characteristic of Treg isolated from HNSCC patients. CCR4 expression is a marker of Treg activation as CCR4+ Tregs are readily suppressive in vitro, while CCR4− Treg needs further activation before becoming suppressive.Citation49 The fundamental role of CCR4 in driving Treg migration toward tumor tissue has been further confirmed by experiments in mice, where blocking of MCP-1/CCR4 signaling came out in a lower frequency of Treg infiltrating the tumor and a significant inhibition of tumor growth.Citation48
Tumor-isolated Treg have a more immunosuppressive phenotype, with elevated expression of ectonucleotidase CD39, TGFβ and CTLA-4. These cells are also more effective in inhibiting T cells proliferation in vitro than those isolated from peripheral blood, hence sustaining the activator effect of tumor microenvironment.Citation43 Importantly, a murine model unveiled that peripheral CD4+CD25−FoxP3− T cells convert extrathymically into FoxP3+ Treg cells under conditions found in tumor environment.Citation50 Therefore, it is likely that peripheral Treg are recruited into the tumor tissue where they are induced toward a highly immunosuppressive phenotype by tumor-associated factors, such as TGFβ.
On the other hand, some reports have also shown the increased frequency of activated Treg in peripheral blood of HNSCC patients.Citation43 Moreover, activated CD4+CD25+Foxp3+ T cells in peripheral blood of HNSCC patients are significantly more effective in inhibiting effector T cells than those from healthy controls.Citation51 Therefore, it is conceivable that cancer-released cytokines might affect Treg cells development outside the tumor tissue, although at a lesser degree than in tumor stroma.
Mechanisms of Treg-induced immunosuppression in HNSCC
Treg-mediated immunosuppression may occur by cell-to-cell contact or by cytokines secretion.
Many pieces of evidence sustain a soluble factor mediated suppressive action of Treg. A significant increase of IL-10 and TGFβ levels has been reported in tumor, probably promoted by Tregs since Treg isolated from OSCC significantly induced IL-10 and TGFβ release from co-cultured allogenic PBMC.Citation51 Nevertheless, Strauss and colleagues demonstrated that, at least for circulating Treg, immune suppression is mainly due to direct cell-to-cell interaction. In fact, co-incubation of peripheral blood isolated Treg with autologous T cells separated by permeable transwell insert resulted in no suppression, thus indicating that soluble factors diffusion is not enough to mediate immune suppression and that it rather relies on direct cell-to-cell contact.Citation10
The ectonucleotidases CD39 and CD73 and the Immune-Checkpoint Receptors (ICRs) have been proposed as key molecules for Treg immunosuppression. ICRs include CTLA-4, the programmed cell death-1 (PD-1), expressed on activated lymphocytes and glucocorticoid-induced tumor necrosis factor receptor (GITR), a T-cell stimulatory molecule.
CTLA-4 prevents the activation of the co-stimulatory molecule CD28 on T cells, hence significantly reducing T-cell proliferation. CTLA-4 blockade inhibits immunosuppressive activity of Treg.Citation52 CD39 and CD73 sequentially convert ATP and ADP into adenosine, which is a very effective suppressive factor, since it inhibits dendritic cells and macrophages functions.Citation53 Anti-CD39 blocking antibodies almost completely abrogate Treg immunosuppressive function while restoring effector T cells activity.Citation54 Jie et al. evaluated the expression of ICRs on Treg.Citation43 They observed a higher percentage of CTLA-4 and PD-1-positive Treg cells in TIL compared with circulating paired. Similarly, CD39 and CD73 expression levels were higher in TIL than in peripheral blood. In particular, the majority of tumor-infiltrated Treg co-expressed both CTLA-4 and CD39, suggesting that these two molecules are fundamental in mediating immunosuppression in the tumor microenvironment.Citation43
Another molecule potentially involved in cell-to-cell mediated immune suppression is β-galactoside binding protein (β-GBP). β-GBP is overexpressed in both OSCC cells and activated Treg cells and its blockade significantly attenuate the inhibitory effects of Treg cells.Citation49 The surface bound form of β-GBP mediates T-cell suppression by interacting with glycoproteins on T-cell surface and by inducing growth arrest and apoptosis on activated T cells. β-GBP plays a double role since it promotes cancer cell proliferation and at the same time it impairs T-cell effectiveness by promoting IL-10 and IL-35 release. Intriguingly, using a specific β-GBP inhibitor, thiodigalactoside, a decrease of IL-10+ and IL-35+ Tregs and an impairment of cancer cells growth were observed.Citation55
The data reported above, although far from definitely depicting the Treg way of action in HNSCC, suggest that cytokine mediators released by cancer cells activate and recruit circulating Treg. As a result, Treg migrate into the tumor tissue, where they are further activated by the cytokine milieu found in tumor stroma. Finally, Treg suppress the activity of cytotoxic and effector cells via both cell-to-cell contact and humoral mechanisms, thanks to the expression of CTLA-4, β-GBP and CD39 and the release of immune modulating factors such as IL-10, IL-35 and TGFβ.
Immune therapy: A new promising strategy for HNSCC treatment
Immune-based therapy has recently gained consideration and it is now considered the new frontier in anticancer strategies. The aim of immune therapy is to modulate immune response, shifting the balance toward an antitumor response, to achieve long-lasting tumor suppression.
Current immunotherapies adopt different strategies to sustain immune response by adoptive T-cell transfer, dendritic cells vaccine or by infusion with adjuvant cytokines, such as IL-1β, IL-2, INFγ and TGFα.Citation56-58 Recent approaches tried to modulate immune response by directly targeting molecules involved in the interactions between cancer and the immune system. In particular, several experimental pieces of evidence have demonstrated that Treg eradication by chemical drugs or specific antibodies is a promising strategy to induce a significant anticancer immune response.Citation38 Low doses of anti-proliferative drugs such as Indomethacin or Cyclophosphamide and human monoclonal antibodies against CTLA-4, PD-1 and GITR have been used to selectively target Treg.Citation3,38 Nevertheless, these approaches are often hampered by the occurrence of autoimmune diseases; therefore, it is necessary to refine these strategies allowing to selectively target Treg inside tumor tissue, without depleting them systemically.
Some of the above-mentioned therapies have proved promising in preclinical studies and they are now in clinical trials; indeed, a recent query in the ClinicalTrials.gov database for “HNSCC and immunotherapy” revealed 54 ongoing studies, the most of them being phase I and II.Citation59
One of the most promising approaches to prevent suppression of anticancer immunity is the blockade of immune checkpoint receptors (ICRs) by blocking antibodies. Thus, new monoclonal antibodies against PD-1 and CTLA-4, such as Pembrolizumab, Nivolumab, Durvalumab, Tremelimumab and Ipilimumab, have been developed and they are now in clinical trials. Ongoing trials for antibodies targeting ICR are summarized in .
Table 3. Ongoing trials for anti-ICRs antibodies in HNSCC immunotherapy.
Pembrolizumab is a humanized antibody targeting PD-1 receptor; it has been approved for melanoma treatment by Food and Drug Administration (FDA) and is in clinical trials for HNSCC therapy. Although all the studies are ongoing, preliminary results confirmed that Pembrolizumab is better tolerated than traditional chemotherapy and it has achieved encouraging results as 25% of the patients responded to treatment.Citation60 Three phase III studies have been recently set up to evaluate Pembrolizumab efficacy in increasing overall survival in metastatic HNSCC patients in comparison with 5-fluorouracil or cetuximab plus a platinum-based drug therapy (NCT03040999, NCT02252042 and NCT02358031).
Nivolumab is another humanized antibody targeting PD-1, approved by FDA for the treatment of melanoma and renal carcinoma. Interestingly, a phase III study aiming to unveil whether Nivolumab would improve overall survival in comparison to cetuximab, carboplatin or methotrexate in patients with recurrent or metastatic head and neck carcinoma completed the results analysis. Nivolumab showed a significant superior performance of the understudied item (NCT02105636).
Durvalumab instead targets the ligand of PD-1, PD-L1, and it has been recently approved for use in bladder cancer by FDA.
CTLA-4 is another hotspot of immune therapy in HNSCC; indeed, there are several clinical trials for antibodies targeting this receptor. Ipilimumab is a monoclonal human antibody that targets CTLA-4; it was approved by FDA in 2011 for the treatment of advanced melanoma and it is now being evaluated for HNSCC. In particular, a large phase III trials has been recently set up to analyze its efficacy in combination with Nivolumab in patients with recurrent or metastatic HNSCC (NCT02741570).
Safety and tolerability of Tremelimumab, a human monoclonal antibody against CTLA-4, is being evaluated in eight studies, in combination therapy with Durvalumab and standards of care such as cisplatin, carboplatin, azacitidine, 5-fluorouracil (please see for references). Two phase III trials are now recruiting participants to determine the efficacy and safety of Tremelimumab plus Durvalumab combination therapy versus standards of care in HNSCC.
Conclusion
The above-reported data strongly confirmed the role of immune system in regulating HNSCC development. In particular, Treg cells play a pivotal role in modulating immune system response in a tumor favorable way. New insights coming from preclinical studies are mandatory since they will further unveil the mechanisms underlying this process, thus allowing the optimization of new strategies to let the immune system re-gain control over tumor development.
Given the extremely complex relations between immune system and cancer cells, it is necessary to deeply analyze the immune scenario characterizing each patient and each tumor. The role of Th and Treg cells in cancer is context dependent and it should always be examined in relation to other effector T cells.
The fragile balance between the different Th subtypes and effector T cells seems the true determinant of the immune response; hence, the success of any potential therapy aiming to restore cancer immune surveillance would rely on a slight modulation of such a balance. Successful therapy should target a precise molecular target in a tailored fit treatment, where the ability to hit a chosen mechanism on a wanted cell subtype would be more important than the quantity of the modulation achieved.
In this context, immunotherapy is a very promising strategy for the treatment of HNSCC, as sustained by preliminary data from clinical trials. Intriguingly, immunotherapy would permit to obtain long-lasting effects and a better disease control over time, thus preventing re-occurrence and metastasization. It is likely that a combination of traditional standards of care and immunotherapy would be particularly effective. Nevertheless, the preliminary results obtained so far need to be confirmed in enlarged phase III and IV trials. Moreover, the optimization of knowledge about Treg cell interactions with both cancer cells and other Th cells will surely provide better tool to selectively target cancer driven immune suppression, thus avoiding undesirable non-selective unbalance of immune regulation that could come out in autoimmunity.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We are grateful to Dr. Susan Green for language assistance.
References
- Torre LA, Siege R, Ward E, Jemal A. Global cancer incidence and mortality rates and trends – an update. Cancer Epidemiol Biomarkers Prev 2016; 25(1):16-27; PMID:26667886; https://doi.org/10.1158/1055-9965.EPI-15-0578
- Siegel R, Miller K, Jemal A. Cancer statistics, 2016. Cancer J Clin 2016; 66:7-30; PMID:26742998; https://doi.org/10.3322/caac.21332
- Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis0ìì. Oncologist 2010; 15(9):994-1001; PMID:20798198; https://doi.org/10.1634/theoncologist.2009-0289
- De Costa AM, Schuyler CA, Walker DD, Young MR. Characterization of the evolution of immune phenotype during the development and progression of squamous cell carcinoma of the head and neck. Cancer Immunol Immunother 2012; 61(6):927-39; PMID:22116344; https://doi.org/10.1007/s00262-011-1154-8
- Hartmann E1, Wollenberg B, Rothenfusser S, Wagner M, Wellisch D, Mack B, Giese T, Gires O, Endres S, Hartmann G. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res 2003; 63(19):6478-87; PMID:14559840
- Schuler PJ, Börger V, Bölke E, Habermehl D, Matuschek C, Wild CA, Greve J, Bas M, Schilling B, Bergmann C et al. Dendritic cell generation and CD4+ CD25high FOXP3+ regulatory t cells in human head and neck carcinoma during radio-chemotherapy. Eur J Med Res 2011; 16(2):57-62; PMID:21463982; https://doi.org/10.1186/2047-783X-16-2-57
- Bron L, Jandus C, Andrejevic-Blant S, Speiser DE, Monnier P, Romero P, Rivals JP. Prognostic value of arginase-II expression and regulatory T-cell infiltration in head and neck squamous cell carcinoma. Int J Cancer 2013; 132(3):E85-93; PMID:22815199; https://doi.org/10.1002/ijc.27728
- Grandis JR, Falkner DM, Melhem MF, Gooding WE, Drenning SD, Morel PA. Human leukocyte antigen class I allelic and haplotype loss in squamous cell carcinoma of the head and neck: clinical and immunogenetic consequences. Clin Cancer Res 2000; 6:2794-802; PMID:10914726
- Bose A, Chakraborty T, Chakraborty K, Pal S, Baral R. Dysregulation in immune functions is reflected in tumor cell cytotoxicity by peripheral blood mononuclear cells from head and neck squamous cell carcinoma patients. Cancer Immun 2008; 12:8-10; PMID:18547033
- Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res 2007; 13(21):6301-11; PMID:17975141; https://doi.org/10.1158/1078-0432.CCR-07-1403
- Watanabe Y, Katou F, Ohtani H, Nakayama T, Yoshie O, Hashimoto K. Tumor-infiltrating lymphocytes, particularly the balance between CD8(+) T cells and CCR4(+) regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109(5):744-52; PMID:20303300; https://doi.org/10.1016/j.tripleo.2009.12.015
- Lim KP, Chun NA, Ismail SM, Abraham MT, Yusoff MN, Zain RB, Ngeow WC, Ponniah S, Cheong SC. CD4+CD25hiCD127low regulatory T cells are increased in oral squamous cell carcinoma patients. PLoS One 2014; 9(8):e103975; PMID:25153698; https://doi.org/10.1371/journal.pone.0103975
- Grimm M, Feyen O, Hofmann H, Teriete P, Biegner T, Munz A, Reinert S. Immunophenotyping of patients with oral squamous cell carcinoma in peripheral blood and associated tumor tissue. Tumour Biol 2016; 37(3):3807-16; PMID:26474587; https://doi.org/10.1007/s13277-015-4224-2
- Wolf GT, Chepeha DB, Bellile E, Nguyen A, Thomas D, McHugh J. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: A preliminary study. Oral Oncol 2015; 51(1):90-5; PMID:25283344; https://doi.org/10.1016/j.oraloncology.2014.09.006
- Partlová S, Bouček J, Kloudová K, Lukešová E, Zábrodský M, Grega M, Fučíková J, Truxová I, Tachezy R, Špíšek R et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 2015; 4(1):e965570; PMID:25949860; https://doi.org/10.4161/21624011.2014.965570
- Wan YY. Multi-tasking of helper T cells. Immunology 2010; 130(2):166-71; PMID:20557575; https://doi.org/10.1111/j.1365-2567.2010.03289.x
- Becker Y. Molecular immunological approaches to biotherapy of human cancers – a review, hypothesis and implications. Anticancer Res 2006; 26(2A):1113-34; PMID:16619514
- Chikamatsu K, Sakakura K, Takahashi G, Okamoto A, Furuya N, Whiteside TL, DeLeo AB, Masuyama K. CD4+ T cell responses to HLA-DP5-restricted wild-type sequence p53 peptides in patients with head and neck cancer. Cancer Immunol Immunother 2009; 58(9):1441-8; PMID:19184003; https://doi.org/10.1007/s00262-009-0661-3
- Kesselring R, Thiel A, Pries R, Wollenberg B. The number of CD161 positive Th17 cells are decreased in head and neck cancer patients. Cell Immunol 2011; 269(2):74-7; PMID:21570678; https://doi.org/10.1016/j.cellimm.2011.03.026
- Sun W, Li WJ, Fu QL, Wu CY, Lin JZ, Zhu XL. Functionally distinct subsets of CD4+ regulatory T cells in patients with laryngeal squamous cell carcinoma are indicative of immune deregulation and disease progression. Oncol Rep 2015; 33(1):354-62; PMID:25333227; https://doi.org/10.3892/or.2014.3553
- Sakakura K, Chikamatsu K, Takahashi K, Whiteside TL, Furuya N. Maturation of circulating dendritic cells and imbalance of T-cell subsets in patients with squamous cell carcinoma of the head and neck. Cancer Immunol Immunother 2006; 55(2):151-9; PMID:15889251; https://doi.org/10.1007/s00262-005-0697-y
- Eyigor M, Eyigor H, Osma U, Yilmaz MD, Erin N, Selcuk OT, Sezer C, Gultekin M, Koksoy S. Analysis of serum cytokine levels in larynx squamous cell carcinoma and dysplasia patients. Iran J Immunol 2014; 11(4):259-68; PMID:25549593; https://doi.org/ IJIv11i4A4
- Gaur P, Singh AK, Shukla NK, Das SN. Inter-relation of Th1, Th2, Th17 and Treg cytokines in oral cancer patients and their clinical significance. Hum Immunol 2014; 75(4):330-7; PMID:24486578; https://doi.org/10.1016/j.humimm.2014.01.011
- Kaskas NM, Moore-Medlin T, McClure GB, Ekshyyan O, Vanchiere JA, Nathan CA. Serum biomarkers in head and neck squamous cell cancer. Otolaryngol Head Neck Surg 2014; 140(1):5-11; PMID:24232368; https://doi.org/10.1001/jamaoto.2013.5688
- Sparano A, Lathers DM, Achille N, Petruzzelli GJ, Young MR. Modulation of Th1 and Th2 cytokine profiles and their association with advanced head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg 2004; 131(5):573-6; PMID:15523428; https://doi.org/10.1016/j.otohns.2004.03.016
- Jebreel A1, Mistry D, Loke D, Dunn G, Hough V, Oliver K, Stafford N, Greenman J. Investigation of interleukin 10, 12 and 18 levels in patients with head and neck cancer. J Laryngol Otol 2007; 121(3):246-52; PMID:17040593; https://doi.org/10.1017/S0022215106002428
- Alhamarneh O, Agada F, Madden L, Stafford N, Greenman J. Serum IL10 and circulating CD4(+) CD25(high) regulatory T cell numbers as predictors of clinical outcome and survival in patients with head and neck squamous cell carcinoma. Head Neck 2011; 33(3):415-23; PMID:20645289; https://doi.org/10.1002/hed.21464
- Lathers DM1, Achille NJ, Young MR. Incomplete Th2 skewing of cytokines in plasma of patients with squamous cell carcinoma of the head and neck. Hum Immunol 2003; 64(12):1160-6; PMID:14630398; https://doi.org/10.1016/j.humimm.2003.08.024
- Kesselring R, Thiel A, Pries R, Trenkle T, Wollenberg B. Human Th17 cells can be induced through head and neck cancer and have a functional impact on HNSCC development. Br J Cancer 2010; 103(8):1245-54; PMID:20877351; https://doi.org/10.1038/sj.bjc.6605891
- Li C, Zhao Y, Zhang W, Zhang W. Increased prevalence of T(H)17 cells in the peripheral blood of patients with head and neck squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011; 112(1):81-9; PMID:21458322; https://doi.org/10.1016/j.tripleo.2010.11.032
- Young MR, Levingston CA, Johnson SD. Treatment to sustain a Th17-type phenotype to prevent skewing toward Treg and to limit premalignant lesion progression to cancer. Int J Cancer 2016; 138(10):2487-98; PMID:26756968; https://doi.org/10.1002/ijc.29989
- Woodford D, Johnson SD, De Costa AM, Young MR. An inflammatory cytokine milieu is prominent in premalignant oral lesions, but subsides when lesions progress to squamous cell carcinoma. J Clin Cell Immunol 2014; 5(3):230; PMID:25419481; https://doi.org/10.4172/2155-9899.1000230
- Punt S, Dronkers EA, Welters MJ, Goedemans R, Koljenović S, Bloemena E, Snijders PJ, Gorter A, van der Burg SH, Baatenburg de Jong RJ, et al. A beneficial tumor microenvironment in oropharyngeal squamous cell carcinoma is characterized by a high T cell and low IL-17(+) cell frequency. Cancer Immunol Immunother 2016; 65(4):393-403; PMID:26899388; https://doi.org/10.1007/s00262-016-1805-x
- Whiteside TL, Jackson EK. Adenosine and prostaglandin e2 production by human inducible regulatory T cells in health and disease. Front Immunol 2013; 4:212; PMID:23898333; https://doi.org/10.3389/fimmu.2013.00212
- Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer 2005; 92(5):913-20; PMID:15714205; https://doi.org/10.1038/sj.bjc.6602407
- Yu N, Li X, Song W, Li D, Yu D, Zeng X, Li M, Leng X, Li X. CD4(+)CD25 (+)CD127 (low) T cells: a more specific Treg population in human peripheral blood. Inflammation 2012; 35(6):1773-80; PMID:22752562; https://doi.org/10.1007/s10753-012-9496-8
- Schipmann S, Wermker K, Schulze HJ, Kleinheinz J, Brunner G. Cutaneous and oral squamous cell carcinoma-dual immunosuppression via recruitment of FOXP3+ regulatory T cells and endogenous tumour FOXP3 expression? J Craniomaxillofac Surg 2014; 42(8):1827-33; PMID:25087653; https://doi.org/10.1016/j.jcms.2014.06.022
- Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res 2017; 27(1):109-118; PMID:27995907; https://doi.org/10.1038/cr.2016.151
- Al-Qahtani D1, Anil S, Rajendran R. Tumour infiltrating CD25+ FoxP3+ regulatory T cells (Tregs) relate to tumour grade and stromal inflammation in oral squamous cell carcinoma. J Oral Pathol Med 2011; 40(8):636-42; PMID:21352381; https://doi.org/10.1111/j.1600-0714.2011.01020.x
- Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res 2003; 9(12):4404-8; PMID:14555512
- Liang YJ1, Liu HC, Su YX, Zhang TH, Chu M, Liang LZ, Liao GQ. Foxp3 expressed by tongue squamous cell carcinoma cells correlates with clinicopathologic features and overall survival in tongue squamous cell carcinoma patients. Oral Oncol 2011; 47(7):566-70; PMID:21641272; https://doi.org/10.1016/j.oraloncology.2011.04.017
- Bron L, Jandus C, Andrejevic-Blant S, Speiser DE, Monnier P, Romero P, Rivals JP. Prognostic value of arginase-II expression and regulatory T-cell infiltration in head and neck squamous cell carcinoma. Int J Cancer 2013; 132(3):E85-93; PMID:22815199; https://doi.org/10.1002/ijc.27728
- Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, Ferris RL. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer 2013; 109(10):2629-35; PMID:24169351; https://doi.org/10.1038/bjc.2013.645
- Wang WL, Chang WL, Yang HB, Chang IW, Lee CT, Chang CY, Lin JT, Sheu BS. Quantification of tumor infiltrating Foxp3+ regulatory T cells enables the identification of high-risk patients for developing synchronous cancers over upper aerodigestive tract. Oral Oncol 2015; 51(7):698-703; PMID:25958829; https://doi.org/10.1016/j.oraloncology.2015.04.015
- Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, Mosseri V, Laccourreye O, Bruneval P, Fridman WH, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res 2006; 12(2):465-72; PMID:16428488; https://doi.org/10.1158/1078-0432.CCR-05-1886
- Mandal R, Şenbabaoğlu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, Lee KW, Ganly I, Hakimi AA, Chan TA et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016; 1(17):e89829; PMID:27777979; https://doi.org/10.1172/jci.insight.89829
- Wansom D, Light E, Worden F, Prince M, Urba S, Chepeha DB, Cordell K, Eisbruch A, Taylor J, D'Silva N et al. Correlation of cellular immunity with human papillomavirus 16 status and outcome in patients with advanced oropharyngeal cancer. Arch Otolaryngol Head Neck Surg 2010; 136:1267-73; PMID:21173378; https://doi.org/10.1001/archoto.2010.211
- Sun W, Li WJ, Wei FQ, Wong TS, Lei WB, Zhu XL, Li J, Wen WP. Blockade of MCP-1/CCR4 signaling-induced recruitment of activated regulatory cells evokes an antitumor immune response in head and neck squamous cell carcinoma. Oncotarget 2016; 7(25):37714-27; PMID:27177223; https://doi.org/10.18632/oncotarget.9265
- Baatar D, Olkhanud P, Sumitomo K, Taub D, Gress R, Biragyn A. Human peripheral blood T regulatory cells (Tregs), functionally primed CCR4+ Tregs and unprimed CCR4− Tregs, regulate effector T cells using FasL. J Immunol 2007; 178:4891-900; PMID:17404270; https://doi.org/10.4049/jimmunol.178.8.4891
- Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25- lymphocytes is thymus and proliferation independent. Cancer Res 2006; 66:4488-95; PMID:16618776; https://doi.org/10.1158/0008-5472.CAN-05-4217
- Gasparoto TH, de Souza Malaspina TS, Benevides L, de Melo EJ Jr, Costa MR, Damante JH, Ikoma MR, Garlet GP, Cavassani KA, da Silva JS et al. Patients with oral squamous cell carcinoma are characterized by increased frequency of suppressive regulatory T cells in the blood and tumor microenvironment. Cancer Immunol Immunother 2010; 59(6):819-28; PMID:20012605; https://doi.org/10.1007/s00262-009-0803-7
- Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH. Powrie f blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol 2006; 177(7):4376-83; PMID:16982872; https://doi.org/10.4049/jimmunol.177.7.4376
- de Oliveira Bravo M, Carvalho JL, Saldanha-Araujo F. Adenosine production: a common path for mesenchymal stem-cell and regulatory T-cell-mediated immunosuppression. Purinergic Signal 2016; 12(4):595-609; PMID:27557887; https://doi.org/10.1007/s11302-016-9529-0
- Nikolova M1, Carriere M, Jenabian MA, Limou S, Younas M, Kök A, Huë S, Seddiki N, Hulin A, Delaneau O et al. CD39/adenosine pathway is involved in AIDS progression. PLoS Pathog 2011; 7(7):e1002110; PMID:21750674; https://doi.org/10.1371/journal.ppat.1002110
- Aggarwal S, Das SN. Thiodigalactoside shows antitumour activity by beta-galactoside-binding protein and regulatory T cells inhibition in oral squamous cell carcinoma. Oral Dis 2016; 22(5):445-53; PMID:27004748; https://doi.org/10.1111/odi.12479
- Economopoulou P, Perisanidis C, Giotakis EI, Psyrri A. The emerging role of immunotherapy in head and neck squamous cell carcinoma (HNSCC): Anti-tumor immunity and clinical applications. Ann Transl Med 2016; 4(9):173; PMID:27275486; https://doi.org/10.21037/atm.2016.03.34
- Wolf GT, Fee WE, Dolan RW, Moyer JS, Kaplan MJ, Spring PM, Suen J, Kenady DE, Newman JG, Carroll WR et al. Novel neoadjuvant immunotherapy regimen safety and survival in head and neck squamous cell cancer. Head Neck 2011; 33(12):1666-74; PMID:21284052; https://doi.org/10.1002/hed.21660
- Egan JE, Quadrini KJ, Santiago-Schwarz F, Hadden JW, Brandwein HJ, Signorelli KL. IRX-2, a novel in vivo immunotherapeutic, induces maturation and activation of human dendritic cells in vitro. J Immunother 2007; 30(6):624-33; PMID:17667526; https://doi.org/10.1097/CJI.0b013e3180691593
- ClinicalTrials.gov. ClinicalTrials.gov database, a service of the U.S. National Institutes of Health. https://clinicaltrials.gov/
- Starr P. Encouraging results for pembrolizumab in head and neck cancer. Am Health Drug Benefits 2015; 8:16; PMID:26380607
- Chiakamatsu K, Sakakura K, Whiteside TL, Furuya N, Relationships between regulatory T cells and CD8+ effector populations in patients with squamous cell carcinoma of the head and neck. Head Neck 2017; 29(2):120–7; https://doi.org/10.1002/hed.20490
