ABSTRACT
Rehmannia glutinosa polysaccharide (RGP) has shown an activation of immune cells in vitro. However, the immune stimulatory effect of RGP in a mouse in vivo is not well studied. In this study, we examined the effect of RGP on dendritic cell (DC) activation and anticancer immunity in vivo. Treatments of RGP in C56BL/6 mice induced increased levels of co-stimulatory molecule expression and pro-inflammatory cytokine production in spleen DCs dependent on toll-like receptor 4 (TLR4), and those DCs promoted interferon-gamma (IFNγ) production in CD4+ and CD8+ T cells. RGP also enhanced ovalbumin (OVA) antigen (Ag)-specific immune activation in tumor-bearing mice, including Ag presentation in DCs, OT-I and OT-II T-cell proliferation, migration of OT-I and OT-II T cells into the B16-OVA tumor, OVA-specific IFNγ production, and the specific killing of OVA-coated splenocytes, which consequently inhibited B16-OVA tumor growth dependent on TLR4 and CD8+ T cells. Finally, the combination of RGP and self-Ag treatment efficiently inhibited CT26 carcinoma and B16 melanoma tumor growth in BLAB/c and C57BL/6 mice, respectively. These data demonstrate that RGP could be a useful adjuvant molecule for immunotherapy against cancer.
Abbreviations
| Ag | = | antigen |
| APC | = | Ag-presenting cell |
| CTL | = | cytotoxic T lymphocyte |
| DC | = | dendritic cell |
| IFN | = | interferon |
| IL | = | interleukin |
| MHC | = | major histocompatibility complex |
| OVA | = | ovalbumin |
| RGP | = | Rehmannia glutinosa polysaccharide |
| TLR | = | toll-like receptor |
| Th | = | T helper |
| TNF | = | tumor necrosis factor |
| TRP2 | = | tyrosinase-related protein 2 |
Introduction
It is well known that natural polysaccharides such as fucoidan, ascophyllan, and carrageenan present immunostimulatory and antitumor effects.Citation1-3 Rehmannia glutinosa polysaccharide (RGP) has also been shown to have an immunostimulatory effect in mouse bone marrow-derived dendritic cells (BMDCs). RGP treatment promotes the upregulation of co-stimulatory molecules as well as IL-12p70 and TNF-α production in the BMDCs.Citation4,5 RGP-stimulated BMDCs also show enhanced allogeneic lymphocyte proliferation.Citation4 On the other hand, RGP is also used for an antigen (Ag) delivery system after liposome synthesis formation, in which the liposome induces immune activation as an adjuvant.Citation6 Although RGP has already provided reliable effects in immune activation in vitro, the effects of DC activation in the spleen and draining lymph node (drLN) dendritic cells (DCs) have not been well investigated in vivo. Nor has antitumor immunity by RGP, which mediates DC-induced Ag-specific immune responses, been studied.
DCs are Ag-presenting cells (APCs) in mammalian immune systems. The main function of DCs is the processing and presenting of Ag to the T cells.Citation7 Immature DCs phagocytose Ag, and the DCs undergo maturation, which increases the expression levels of co-stimulatory molecules and the production of pro-inflammatory cytokines and which presents Ag on MHC class I and II.Citation7,8 The matured DCs promote T-cell proliferation and differentiation.Citation9 The activated and stimulated T cells matured by DCs will find Ag-expressing pathogens and eliminate the pathogens.Citation8,10
For effective immunotherapy against cancer, cancer Ag-specific immune responses are required, which lead to the efficient killing of cancer cells without undesired side effects.Citation8,11-13 However, the tumor microenvironment-produced anti-inflammatory molecules and the interactions between cancer cells and immune cells interrupt the activation of immune cells and induce immune suppression, allowing cancer cells to escape immune attack, potentially promoting tumor growth.Citation10,12 In addition, cancer Ag is not able to induce the Ag-specific T and B cell activation, since cancer Ag promotes low levels of DC activation, including low levels of co-stimulatory molecule expression and cancer Ag presentation. Therefore, for effective immunotherapy against cancer, adjuvant (or immune activator) components are required.Citation8,11,14,15
In this study, we hypothesized that RGP can function as an adjuvant for inducing tumor Ag-specific immune responses together with DC maturation, and the current study was undertaken to examine this hypothesis, using ovalbumin (OVA) and self-Ags for the treatment of CT26 carcinoma and B16 melanoma, respectively.
Results
Rehmannia glutinosa polysaccharide (RGP) induced activation of DCs in vitro and in vivo
Since RGP promotes BMDC activation in vitro,Citation5 we examined the dose-dependent effect of RGP in the activation of BMDC in vitro and that of spleen DCs in vivo. Bone marrow single cells were prepared from C57BL/6 mice, and the cells were cultured with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) for 6 d. CD11c+ immature BMDCs were further treated with 10, 50, and 100 μg/mL RGP, with lipopolysaccharide (LPS) as a positive control. The dendritic morphology of the BMDCs was dramatically changed after 24 h of RGP treatment at 50–100 μg/mL concentrations (). The expression of co-stimulatory molecules in the BMDCs was also significantly increased by 50–100 μg/mL RGP treatments (). In spleen DC activation, C57BL/6 mice were injected intravenously (i.v.) with 12.5, 25, and 50 mg/kg RGP, with 1 mg/kg LPS as a control. The spleen DCs were defined as lineage−CD11c+ cells in live leukocytes (Fig. S1). Twenty-four hours after RGP treatment, the expression levels of co-stimulatory molecules and MHC class I and II were substantially increased at 25 and 50 mg/kg RGP treatment in the spleen DCs, and 50 mg/kg RGP-induced expression levels of co-stimulatory molecules were almost similar to those induced by LPS (). Furthermore, the treatment of 100 μg/mL RGP in the BMDCs and of 50 mg/kg RGP in the mice promoted marked increases in IL-6, IL-12p40, and TNF-α production in the cultured medium and serum, respectively ( and ).
Figure 1. RGP-induced activation of BMDCs and spleen DCs. BMDCs were treated with 10, 50, and 100 μg/mL RGP and with 2 μg/mL LPS for 24 h. (A) Morphological changes of BMDCs. (B) Mean fluorescence intensity levels of CD40, CD80, CD86, MHC class I, and MHC class II in the BMDCs. (C) C57BL/6 mice were injected intravenously (i.v.) with 12.5, 25, and 50 mg/kg RGP and with 1 mg/kg LPS. The flow cytometric analyses of co-stimulatory molecules and MHC class I and II in gated lineage-CD11c+ cells from the spleen are shown. (D) Concentrations of IL-6, IL-12p40, and TNF-α in BMDC-cultured medium. (E) The serum concentration of IL-6, IL-12p40, and TNF-α in RGP-treated mouse is shown. (F) C57BL/6 mice were injected i.v. with 50 mg/kg RGP. Three days later, the mice were injected again with the same amount of RGP, and then 3 d later, intracellular IFNγ, IL-4, and IL-17 in CD4+ and CD8+ T cells in the spleen were analyzed with flow cytometry. All data are representative of the average of analyses of six independent samples (two mice per experiment, total three independent experiments). *p < 0.05, **p < 0.01.
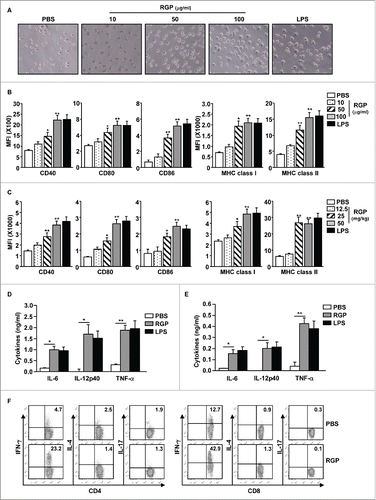
Since activated DCs promote T-cell stimulation,Citation9 we next examined whether RGP can induce T-cell activation in vivo. C57BL/6 mice were injected i.v. with 50 mg/kg RGP, and 3 d later, the same concentration of RGP was injected into the mice again; analysis occurred 3 d after the second injection. The RGP treatment induced a marked increase in the IFNγ-producing CD4+ and CD8+ T cells, whereas IL-4- and IL-17-producing CD4+ and CD8+ T cells were not increased by the treatment (). In addition, mRNA levels of IFNγ and T-bet, a transcription factor of T helper 1 (Th1) cells, were substantially increased by RGP, while IL-4, IL-17A, and transcription factors of those cytokines were not changed by RGP (Fig. S2). These data indicate that RGP induced the activation of spleen DCs, including the upregulation of co-stimulatory molecule expression and pro-inflammatory cytokine production as well as T-cell activation in the mouse in vivo.
RGP-induced activation of spleen DCs was dependent on toll-like receptor 4 (TLR4)
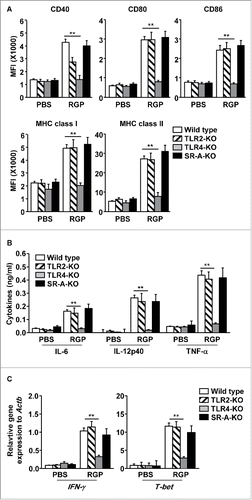
DCs can be stimulated by the activation of pattern recognition receptors (PPRs), such as toll-like receptors (TLRs), scavenger receptors (SRs), C-type lectins, mannose receptors, and complement receptors.Citation16 Therefore, using the receptor knockout mice, we examined whether RGP-induced activation of spleen DCs requires TLR2, TLR4, or scavenger receptors-A (SR-A). Consistent with C57BL/6 wild-type mice, the spleen DCs in the TLR2-knockout (TLR2-KO) and SR-A-KO mice expressed significantly increased levels of co-stimulatory molecules and MHC class I and II by RGP, whereas the spleen DCs in TLR4-KO mice did not upregulate the expression levels of co-stimulatory molecules and MHC class I and II by the RGP treatment (). Nor were the production of pro-inflammatory cytokines in the serum of TLR4-KO mice or the mRNA levels of IFNγ and T-bet in the splenocytes of TLR4-KO mice elevated by RGP compared with phosphate buffered saline (PBS)-treated controls ( and ). In contrast to the effects of RGP in the TLR4-KO mice, RGP induced substantially increased levels of the cytokine production and mRNA expression in the TLR2-KO and SR-A-KO mice ( and ). These data suggest that RGP-induced spleen DC activation in vivo was dependent on TLR4 stimulation.
Figure 2. RGP-induced activation spleen DCs were dependent on TLR4. C56BL/6 wild-type, TLR2-KO, TLR4-KO, and SR-A-KO mice were treated i.v. with PBS and 50 mg/kg RGP for 24 h. (A) Co-stimulatory molecules and MHC class I and II were measured in the spleen DCs. (B) The serum concentrations of IL-6, IL-12p40, and TNF-α after RGP treatment are shown. (C) The mRNA levels of IFNγ and T-bet in the spleen are shown. All data are the average of analyses of six independent samples (two mice per experiment, total three independent experiments). **p < 0.01.
RGP enhanced antigen (Ag)-specific immune activation
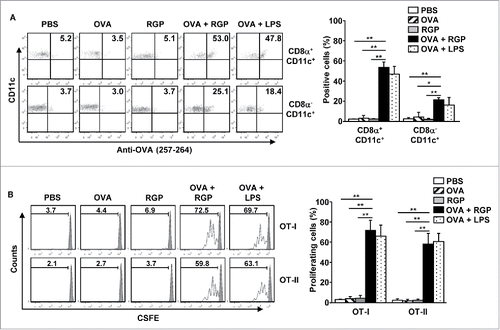
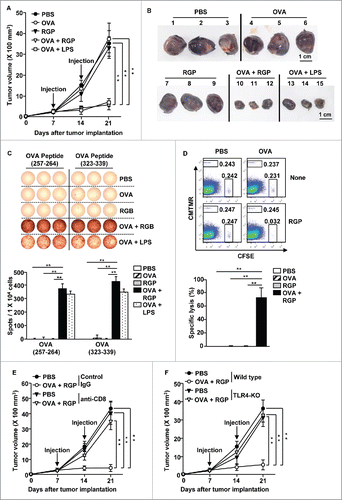
Our data showing that RGP induced the activation of spleen DCs prompted us to examine whether RGP can induce Ag-specific immune responses. We injected C57BL/6 mice i.v. with 2.5 mg/kg ovalbumin (OVA), 50 mg/kg RGP and a combination of RGP and OVA, with a combination of OVA and 1 mg/kg LPS as a positive control, and we analyzed the presentation of OVA peptide on the surface of the spleen DC subsets. The subsets of spleen DCs were divided into CD8α+CD11c+ cells and CD8α−CD11c+ DCs in live lineage− cells (Fig. S3). As shown in , treatment with the combination of RGP and OVA led to significant increases in the surface presentation percentages of the OVA peptide (257–264) in CD8α+ and CD8α− cDCs, whereas treatment with either OVA or RGP alone did not increase the presentation of the OVA peptide on the surface of these DCs. To further determine Ag-specific immune response with RGP, we examined an OVA-specific T-cell proliferation assay by transferring CFSE-labeled OT-I and OT-II T cells into CD45.1 congenic mice. Twenty-four hours after the cell transfer, the mice were treated for 3 d with PBS, 2.5 mg/kg OVA, 50 mg/kg RGP, and the combination of RGP and OVA, with the combination of OVA and LPS as a positive control. The combined treatment with RGP and OVA promoted substantial increases in the proliferation of OT-I and OT-II T cells, whereas OVA or RGP alone did not promote the proliferation, as indicated the division of 5(6)-Carboxyfluorescein diacetate N-succinimidyl ester (CFSE) levels (). Moreover, the levels of OVA peptide presentation and OT cell proliferation induced by the combination of RGP and OVA were similar to those induced by the combination of LPS and OVA treatment (). These data suggest that RGP enhanced the Ag presentation in DCs and promoted Ag-specific T-cell proliferation in vivo.
Figure 3. RGP-enhanced OVA presentation in DCs and OVA-specific T cell proliferation. C57BL/6 mice were injected i.v. with PBS, 2.5 mg/kg OVA, 50 mg/kg RGP, and the combination of RGP and OVA. The combination of LPS and OVA also injected to mice as a positive control. (A) The surface presentation of OVA Ag on the spleen CD8α+ and CD8α− DCs was measured by anti-OVA (257–264) antibodies 24 h after RGP treatment (left panel). Mean positive cells of OVA peptide on the surface of the spleen CD8α+ and CD8α− DCs are shown (right panel). (B) The proliferation of adaptive transferred CSFE-labeled OT-I and OT-II cells in the CD45.1 congenic mice was analyzed with flow cytometry (left panel). Mean proliferating cells in OT-I and OT-II are shown (right panel). All data are representative of the average of analyses of six independent samples (three mice per experiment, total two independent experiments). *p < 0.05, **p < 0.01.
RGP induced DC maturation in tumor-bearing mice
Since the tumor microenvironment promotes immune suppression,Citation12,17 we further evaluated the DC maturation effect of RGP in tumor-bearing mice. However, before we examined the DC activation in tumor-bearing mice with RGP, we studied the injection routes of RGP for spleen and drLN DC activation, because i.v. injection of therapeutic vaccine molecules will cause them to spread to every organ and promote inflammation in the tissues.Citation18,19 C57BL/6 mice were treated orally, subcutaneously (s.c.), through the foot pad, intraperitoneally (i.p.), and through i.v. with 50 mg/kg RGP. Twenty-four hours after injection, the spleen and inguinal drLN were harvested, and co-stimulatory molecule expression in the DCs was measured. Injection of RGP by s.c., foot pad, i.p., and i.v. induced substantial increases in the co-stimulatory molecule expression, whereas oral administration of RGP did not induce this upregulation (Fig. S4). Since s.c. injection of RGP could induce DC activation in the spleen and inguinal drLN, we evaluated the DC activation capacity of RGP in tumor-bearing mice by s.c. injection. C57BL/6 mice were injected s.c. with 1 × 106 B16 melanoma cells. Once a tumor was well established on day 15, the mice were treated s.c. with 50 mg/kg RGP and 1 mg/kg LPS for 24 h. The treatment with RGP induced significant upregulation of CD40, 80, 86, MHC class I, and MHC class II expression in the tumor drLN and spleen DCs (). RGP also promoted the enhancement of IL-6, IL-12p40, and TNF-α production in the serum of the tumor-bearing mice ().
Figure 4. RGP-promoted DC maturation in the tumor microenvironment. C57BL/6 mice were injected subcutaneously (s.c.) with 1 × 106 B16 melanoma cells or B16-OVA cells. Fifteen days after tumor injection, the mice were treated with PBS, 50 mg/kg RGP and 1 mg/kg LPS for 24 h. (A) MFI of CD40, CD80, CD86, and MHC class I and II levels was measured in the spleen and tumor drLN DCs. (B) Concentrations of IL-6, IL-12p40, and TNF-α in serum. (C) Surface OVA peptide (257–264) presentation was measured in the tumor drLN DCs after treatment of PBS, 50 mg/mL RGP, 1 mg/kg LPS with or without 2.5 mg/kg OVA (left panel). Mean positive cells of OVA peptide presenting DCs are shown (right panel). (D) CFSE-labeled OT-I and OT-II T-cell proliferation in B16 tumor-bearing CD45.1 congenic mice were analyzed with flow cytometry. (E) Percentage of IFNγ+ and TNF-α+ cells in B16-OVA tumor-infiltrated OT-I and OT-II cells. (F) OT-I and OT-II T-cell proliferation in wild-type and TLR4-KO mice. (G) Intracellular IFNγ- and TNF-α-producing OT-I and OT-II cells in B16-OVA tumor in the wild-type and TLR4-KO mice. All data are representative of the average of analyses of six independent samples (three mice per experiment, total two independent experiments). **p < 0.01, *p < 0.05.
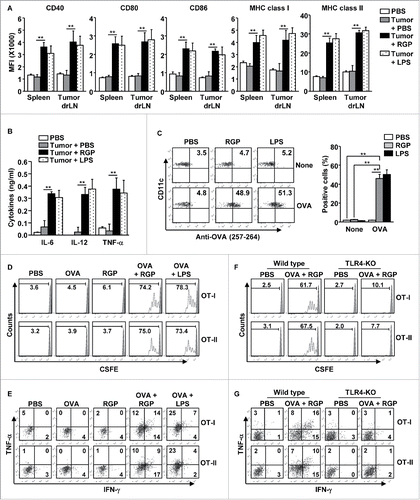
We further examined whether RGP can induce Ag-specific immune responses in a tumor microenvironment. Treatment with the combination of OVA and RGP induced marked increases in the presentation of OVA peptide in the tumor drLN DCs compared with the control treated with PBS, OVA, and RGP alone (). The combined RGP and OVA treatment in the B16-OVA tumor-bearing CD45.1 congenic mice that transferred CSFE-labeled OT-I and OT-II T cells promoted dramatic increases in the OT-I and OT-II T-cell proliferation (), and the tumor-infiltrated OT-I and OT-II cells treated with the combination of OVA and RGP produced substantially increased levels of IFNγ and TNF-α, while treatment with PBS, OVA, and RGP alone did not induce the cytokine production (). Consistent with wild-type mice data, the RGP-induced maturation of DCs in tumor-bearing mice were similar to those induced by LPS. Thus, these data suggest that RGP induced the maturation of DCs and Ag-specific immune activation in the tumor environment.
Since the RGP-induced DC activation was dependent on TLR4, we also examine the DC activation effect of RGP in tumor-bearing TLR4-KO mice. The treatment of RGP did not promote increased activation marker expression in the spleen and tumor-drLN DCs in the TLR4-KO mice (Fig. S5A). Moreover, serum levels of pro-inflammatory cytokines were also not elevated in the tumor-bearing TLR4-KO mice by RGP treatment (Fig. S5B). In addition, the tumor-drLN DCs did not increase the presentation of OVA peptide in TLR4-KO mice by RGP (Fig. S5C), which consequently did not promote OT-I and OT-II T cell proliferation in the tumor-bearing TLR4-KO mice (). The tumor-infiltrated OT-I and OT-II cells did not produce IFNγ and TNF-α in tumor-bearing TLR4-KO mice by RGP treatment (). Therefore, these data suggested that RGP induced Ag-specific immune activation was dependent on TLR4 stimulation.
The combination of RGP and OVA reduced B16-OVA tumor growth
Since the combination of RGP and OVA can induce OVA-specific immune activation in tumor-bearing mice, we next examined the antitumor effect of the combination of RGP and OVA in the B16-OVA tumor-bearing mice. Once B16-OVA tumors were well established on day 7 in C57BL6 mice, the mice were treated s.c. with PBS, 2.5 mg/kg OVA, 50 mg/kg RGP, and the combination of RGP and OVA. Seven days later, the same amount of RGP was injected again, and the tumor sizes were monitored during treatment. The combination of LPS and OVA also treated the mice as a positive control. The treatment with the combination of RGP and OVA dramatically suppressed the B16-OVA tumor growth, whereas treatment with PBS, OVA, and RGP alone did not significantly inhibit tumor growth (). On day 21 of the tumor injection, the tumor mass was also much smaller in the mice treated with the combination of RGP and OVA compared with the control treated with PBS, OVA, and RGP alone (). To determine the Ag-specific immune activation-mediated antitumor effect, we next examined OVA peptide-specific IFNγ production in splenocytes using ELISPOT analysis. The combination of RGP and OVA-treated splenocytes in the tumor-bearing mice induced greatly increased numbers of plots, whereas treatment with PBS, OVA, and RGP alone did not, which indicates that a large amount of IFNγ was produced in the mice splenocytes treated with the combination of RGP and OVA, in response to the OVA peptides (257–264) and (323–339) (). The inhibitory effect of tumor growth and OVA-specific IFNγ production caused by the combination of RGP and OVA was similar to those treated with the combination of LPS and OVA (). In addition, the treatment of combined RGP and OVA promoted strong Ag-specific cytotoxic T lymphocyte (CTL) activities, indicated by a 70% OVA-pulsed target cell lysis, compared with treatment with PBS, OVA, and RGP alone (). To further examine the OVA-specific CTL activation by RGP, we measured the antitumor effect in the CD8+ T-cell-depleted mice and found that the treatment of RGP and OVA failed to reduce B16-OVA tumor growth (), which indicated that the antitumor effect of the combination of RGP and OVA was dependent on CD8+ T-cell activation. Furthermore, the antitumor effect of RGP and OVA was abrogated in the TLR4-KO mice, consistent with RGP's failure to activate DCs in TLR4-KO mice (). Thus, these data suggested that the treatment with combined RGP and OVA induced an antitumor effect in the mouse in vivo through Ag-specific immune activation dependent on TLR4 and CTL activation.
Figure 5. The combination of RGP and OVA treatment prevented B16-OVA tumor growth. C57BL/6 mice were injected s.c. on the right side with 1 × 106 B16-OVA cells. Once tumors were well established on day 7, the mice received s.c. injections of PBS, 2.5 mg/kg OVA, 50 mg/kg RGP, the combination of OVA and RGP and the combination of OVA and LPS as a positive control. On day 14, the mice received the same treatment again. (A) The curves of B16-OVA tumor growth in mice are shown. (B) The size of the tumor masses on day 21 after B16-OVA tumor cell challenge. (C) OVA peptide (257–264)- and (323–339)-specific IFNγ production was analyzed by ELISPOT analysis (Upper panel). The mean number of spots is shown (lower panel). (D) Cytotoxic T lymphocyte (CTL) activity was assessed in vivo on day 21 by adoptive transfer of CFSE-labeled and SIINFEK-loaded splenocytes, and also of a control splenocyte population without peptide labeled with CMTMR. Dot plots show the percentage of SIINFEK-loaded CFSE+ cells and non-peptide-loaded CMTMR+ cells (upper panel). Mean percentages of Ag-specific lysis (lower panel). (E, F) Antitumor effect of the combination of OVA and RGP were measured in (E) CD8+ T cell-depleted and (F) TLR4-KO mice. Data are from analyses of six individual mice (three mice per experiment, total two independent experiments). **p < 0.01.
The combination of RGP and Ag treatment inhibited carcinoma and melanoma growth
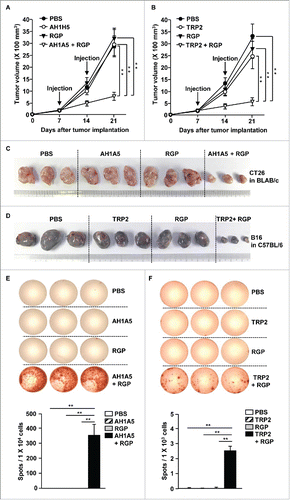
We next evaluated the anticancer effect of RGP with the self-Ag of carcinoma and melanoma in the BLAB/c and C57BL/6 mice, respectively. We chose the AH1A5 peptide for the carcinoma self Ag and the tyrosinase-related protein 2 (TRP2) for melanoma Ag.Citation20,21 BLAB/c mice were injected s.c. with 1 × 106 CT26 cells, and C57BL/6 mice were inoculated s.c. with 1 × 106 B16 cells. On day 7 after the tumor cell injection, the BLAB/c mice received PBS, 2.5 mg/kg AH1A5, 50 mg/kg RGP, and a combination of RGP and AH1A5. In the case of B16 melanoma, the mice were treated with PBS, 2.5 mg/kg TRP2, 50 mg/kg RGP, and a combination of RGP and TRP2. The carcinoma and melanoma tumor growth was much slower ( and ), and on day 21 after the injection, the tumor mass was also much smaller ( and ) with the combined RGP and self-Ag treatment compared with tumor mass in the mice treated with PBS, self-Ag, and RGP alone. We also examined Ag-specific IFNγ production in the splenocytes and found that the combination of RGP and self-Ag treatment induced substantially increased levels of IFNγ production in response to the Ag peptide ( and ). These data suggest that RGP can induce self-Ag-specific immune activation and inhibit tumor growth through self-Ag-specific immune responses.
Figure 6. RGP promoted self-antigen (Ag)-specific immune activation and antitumor immunity. BLAB/c mice were injected s.c. with 1 × 106 CT26 carcinoma cells. The mice were treated with PBS, 2.5 mg/kg AH1A5, 50 mg/kg RGP, and the combination of AH1A5 and RGP on days 7 and 14 of tumor injection. C57BL/6 mice were inoculated s.c. with 1 × 106 B16 melanoma cells. Once tumors were well established, the mice received PBS, 2.5 mg/kg TRP2, 50 mg/kg RGP, and the combination of RGP and TRP2 on days 7 and 14 of tumor injection. The growth curves of (A) CT26 tumor in BALB/c and (B) B16 tumor in C57BL/c are shown. Data are the average of analyses of six independent samples (two mice per experiment, total three independent experiments). **p < 0.01. (C) Tumor masses of CT26 carcinoma and (D) B16 melanoma are shown. (E) AH1A5-specific IFNγ production in splenocyte of BALB/c mice and (F) TRP2-specific IFNγ production in the splenocytes of C57BL/c mice were analyzed by ELISPOT analysis (upper panel). The mean number of spots is shown (lower panel). All data are representative of the average of analyses of six independent samples (three mice per experiment, total two independent experiments). **p < 0.01.
Discussion
DCs are professional APCs and promote T-cell activation.Citation7,11 Humans and mice have different subsets of DCs that exhibit different abilities in processing and presenting Ags.Citation22-25 Mouse CD8α+ DCs can cross-present extracellular Ags with MHC class I molecules to CD8+ T cells.Citation23,24,26 In contrast to CD8α+ DCs, CD8α− DCs present extracellular and exogenous Ags through MHC class II and direct presentation to CD4+ T cells.Citation23,24 In this study, we demonstrated that RGP treatment induced the enhancement of Ag presentation in CD8α+ and CD8α− DCs in the spleen and tumor drLN. These data suggest that RGP has the potential ability to enhance not only CD8α− DC-mediated direct presentation but also CD8α+ DC-induced cross-presentation. We also further observed an interesting discrepancy in the action of RGP in the CD4+ and CD8+ T-cell immune responses. It is known that stimulated APCs can induce the proliferation and differentiation of T cells, in which T cells produce specific cytokines such as IFNγ, IL-4, and IL-17.Citation9,27 Here, we defined a crucial function of RGP in promoting T-cell activation and identified RGP as an important new player in enhancing IFNγ-producing CD4+ and CD8+ T-cell immune responses. Moreover, combined treatment with RGP and Ag promoted Ag-specific T-cell proliferation. According to our observations, RGP promotes the generation of IFNγ-producing T cells through enhancing DC maturation in the mouse in vivo.
A cancer microenvironment promotes immune suppression, which causes the immune escape of cancer cells.Citation12 The main process of immune suppression in the cancer microenvironment involves DCs not being fully activated and presenting low levels of cancer Ag, which consequently interrupt the development of cancer Ag-specific Th and CTL activation.Citation28,29 Therefore, the treatment of cancer by immunotherapy requires the enhancement of immune activities against cancer Ag by an effective adjuvant, which can induce cancer Ag-specific immune responses with the maturation of DCs in the cancer microenvironment.Citation8,11,30 The findings of the present study indicated that RGP induced the maturation of DCs, particularly DCs in the tumor drLN. We also found that treatment with combined RGP and Ag promoted Ag-specific T-cell proliferation and the infiltration of those T cells into a tumor; the infiltrating T cells also produced high levels of IFNγ and TNF-α. Since RGP induced the maturation of DCs and the activation of Ag-specific immune responses in tumor-bearing mice, RGP may be able to be used for cancer immunotherapy in humans as an effective adjuvant.
An effective adjuvant should boost adaptive immune activation, including humoral and cellular immunity, to efficiently eliminate pathogens.Citation8,30 Natural polysaccharides, including fucoidan, ascophyllan, laminarin, pullulan, and λ-carrageenan, have shown an activation of immune cells in mice in vivo, and these molecules especially promote the maturation of DCs and the activation of CTLs as an adjuvant.Citation1,2,31,32 These polysaccharides have also shown different receptor activation; as shown in a previous study, fucoidan binds to SR-A,Citation33 and laminarin induces dectin-1 activation.Citation34,35 In this study, we also found that RGP, a polysaccharide purified from Rehmannia glutinosa, promoted humoral and cellular immunity via the maturation of DCs in mice in vivo. The RGP is composed of l-arabinose:d-galactose:l-rhamnose:d-galacturonic acid in molar ratios of 10:10:1:1.Citation36,37 The difference between RGP and other polysaccharides is that the RGP is mainly made up of arabino-3,6 galactan-type structural units.Citation37 Interestingly, RGP treatment did not induce the activation of spleen DCs in the TLR4-KO mice, which indicates that TLR4 is required for DC activation by RGP and the RGP may be included in the binding site to TLR4. Since the RGP contained 4 types of monosaccharides, the functional groups of RGP for binding to TLR4 and DC activation will be evaluated by those monosaccharides in the spleen DCs in a subsequent study. In addition, different immune modulatory effects were shown in the mice, depending on the injection routes of the polysaccharides. Oral administration of fucoidan promotes anti-inflammatory responses in LPS-induced endotoxemia,Citation38 but i.v. and i.p. injection of fucoidan induces pro-inflammatory immune responses.Citation39 Also, the oral administration of LPS, the strongest immune activator, induced intestinal-only B-1 cell activation and prevented intestinal bacterial infection,Citation40,41 but it could not promote a systemic immune response.Citation40,42 Similarly, the oral administration of RGP did not promote DC activation in the spleen and drLN, while i.v. and i.p. injection strongly induced the activation of DCs. Our future studies will examine the effect of RGP in intestinal immunity and bacterial infection through oral administration.
In conclusion, our results demonstrate that RGP is a novel DC maturation reagent as an adjuvant that can induce Ag-specific Th1 and CTL activation, which consequently promote the inhibition of As-expressing tumor growth, including B16 melanoma and CT26 carcinoma cells in vivo. Thus, the RGP will be a promising candidate and will be potentially useful for developing an immunotherapy reagent in clinical research for human uses against cancer.
Materials and methods
Mice and cell lines
C57BL/6 (6 weeks old), BALB/c, OT-I and OT-II TCR transgenic mice and C57BL/6-Ly5.1 (CD45.1) congenic mice were obtained from Shanghai Public Health Clinical Center. TLR2, TLR4 and SR-A-KO mice were received from Nanjing Animal Center. The mice were kept under pathogen-free conditions at Shanghai Public Health Clinical Center. The mice were maintained in a room with controlled temperature (20–22 °C), humidity (50–60%) and light (12 h:12 h) with free access to standard rodent chow and water. All experiments were performed under the guidelines of the Institutional Animal Care and Use committee at the Shanghai Public Health Clinical Center. The protocol was approved by the committee on the Ethics of Animal Experiments of the Shanghai Public Health Clinical Center (Mouse Protocol Number: SYXK-2010-0098). Mice were killed by CO2 inhalation euthanasia, and all efforts were made to minimize suffering. The murine melanoma cell line B16F10 (ATCC, CRL-6475) expressing OVA (B16-OVA) and murine carcinoma cell line CT26 (ATCC, CRL-2639) were cultured in RPMI 1640 (Sigma Aldrich, 10% FBS, 2 mM glutamine, 1 M HEPES, 100 μg/mL streptomycin and 100 U/mL penicillin and 2 mM 2-mercaptoethanol). All cell lines were cultured at 37 °C in a humidified atmosphere of 5% CO2 and air.
Chemicals and Ags
RGP was purchased from Shangxi Ciyuan Biotech, and chicken OVA was obtained from Sigma-Aldrich. RGP and OVA solution was passed through an endotoxin-removal column (Detoxi-gel), and subsequently filtered through an endotoxin-removal filter (Zetapor Dispo). OVA peptide 257–264 (SIINFEKL), OVA 323–339 (ISQAVHAAHAEINEAGR), TRP2 peptide 180–188 (SVYDFFVWL), and AH1A5 peptide (SPSYAYHQF) were purchased from Phtdpeptides Co. Ltd.
Antibodies
Isotype control antibodies (Abs) (IgG1, IgG2a or IgG2b), CD11c (HL3), CD4+ (GK1.5), CD8α (YTS169.4), CD40 (3/23), CD80 (16-10A1), CD86 (GL-1), anti-IL-4 (11B11), and anti-IL-12p40 (C17.8) were from BioLegend; anti-MHC class I (AF6-88.5.3), anti-MHC class II (M5/114.15.2), anti-IFNγ (XMG1.2), anti-IL-17 (TCC11-18H10.1) and anti-TNF-α (MP6-XT22) were from eBioscience.
Flow cytometry analysis
Cells were washed with phosphate buffered saline (PBS) containing 0.5% BSA, pre-incubated for 15 min with unlabeled isotype control Abs, and then labeled with fluorescence-conjugated Abs by incubation on ice for 30 min followed by washing with PBS. Cells were analyzed on a FACS Aria II (Becton Dickinson) and FlowJo 8.6 software (Tree Star). Cellular debris was excluded from the analysis by forward- and side-scatter gating. Dead cells were further excluded by 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) staining and gating on the DAPI-negative population. As a control for nonspecific staining, isotype-matched irrelevant mAbs were used.
In vitro BMDC generation
The initial cultures were prepared as described previously.Citation3,43 Bone-marrow nucleated cells (1 × 106 cells/mL) were cultured in 5 mL modified RPMI 1640 medium containing 10% FBS in 6 well plates; 50 ng/mL rmGM-CSF plus 50 ng/mL rmIL-4 were added to the medium to support the generation of BMDCs. Unless otherwise stated, the cells were cultured for 6 d at 37 °C under 10% CO2. The cultured cells were washed twice in fresh medium before the additional experiments were conducted.
DC analysis
Spleen and drLN DCs were analyzed as described previously.Citation1,31 Briefly, the tissues were cut into small fragments and digested by adding 2% fetal bovine serum (FBS) with collagenase for 20 min at room temperature. The cells from the digest were centrifuged to pellets, which were re-suspended in 5 mL of a 1.077 histopaque (Sigma-Aldrich). An additional 5 mL of histopaque and the culture medium were layered below and above the cell suspension, respectively, which was then centrifuged at 1,700g for 10 min. The light density fraction (< 1.077 g/cm3) was collected and incubated for 30 min with the following FITC-conjugated monoclonal antibodies (mAbs): anti-CD3 (17A2), anti-Thy1.1 (OX-7), anti-B220 (RA3–6B2), anti-Gr1 (RB68C5), anti-CD49b (DX5), and anti-TER-119 (TER-119). The lineage−CD11c+ cells were defined as cDCs, which were further divided into CD8α+ and CD8α− cDCs. The analysis was performed using FACS Aria II (Becton Dickinson).
Ex vivo T-cell stimulation and intracellular cytokine staining
As described in detail previously,Citation44 the single cell suspension prepared from spleen and tumor were stimulated in vitro for 4 h with phorbol 12-myristate 13-acetate (50 ng/mL) and ionomycin (1 μM; both from Calbiochem); Monensin Solution (BioLegend) was added during the final 2 h. In the intracellular cytokine staining, the cells first were stained for surface molecules first, fixed, and permeabilized with Cytofix/Cytoperm buffer (eBioscience) and subsequently incubated with anti-cytokine antibodies in Perm/Wash buffer (eBioscience) for 30 min. Control staining with isotype control IgGs was performed in all experiments.
ELISA
IL-6, IL-12p70, and TNF-α concentrations in the sera were measured in triplicate using standard ELISA kits (BioLegend, San Diego, CA).
Real-time PCR
The total RNA was reverse-transcribed into cDNA using Oligo (dT) and M-MLV reverse transcriptase (Promega). The cDNA was subjected to real-time PCR amplification (Qiagen) for 40 cycles with annealing and extension temperature at 60 °C using a LightCycler 480 Real-Time PCR System (Roche, Basel, Switzerland). The primer sequences were as follows: mouse β-actin forward, 5′-TGGATGACGATATCGCTGCG-3′; reverse, 5′-AGGGTCAGGATACCTCTCTT-3′, T-bet forward, 5′-CAACAACCCCTTTGCCAAAG-3′; reverse, 5′-TCCCCCAAGCATTGACAGT-3′, GATA3 forward, 5′-CGGGTTCGGATGTAAGTCGAGG-3′; reverse, 5′-GATGTCCCTGCTCTCCTTGCTG-3′, RORγt forward, 5′-CCGCTGAGAGGGCTTCAC-3′; reverse 5′-TGCAGGAGTAGGCCACATTACA-3′, IFNγ forward, 5′-GGATGCATTCATGAGTATTGC-3′; reverse, 5′-CTTTTCCGCTTCCTGAGG-3′, IL-4 forward, 5′-ACAGGAGAAGGGACGCCAT-3′; reverse 5′-GAAGCCCTACAGACGAGCTCA-3′, IL-17A forward, 5′-GCGCAAAAGTGAGCTCCAGA-3′; reverse 5′-ACAGAGGGATATCTATCAGGG-3′.
Tumor treatment
C57BL/6 mice were treated s.c. with PBS alone, 2.5 mg/kg Ag in PBS, 50 mg/kg RGP and Ag mixed with RGP in PBS on days 7 and 14 after tumor challenge. On day 21, mice were killed, and splenocytes were harvested for further analysis.
OT-I and OT-II T-cell proliferation
CD4+ T cells from OT-II mice or CD8+ T cells from OT-I mice were isolated from spleens using CD4+ T cell or CD8+ T-cell isolation kit (Miltenyi Biotec), respectively. The cells were suspended in PBS/0.1% BSA containing 10 μM CFSE (Invitrogen) and incubated for 10 min. CFSE-labeled cells (1 × 106) were i.v. transferred into CD45.1 congenic mice, and 24 h later, the mice were injected with PBS alone, 2.5 mg/kg of OVA in PBS, 50 mg/kg RGP and the combination of RGP and OVA in PBS. At 72 h after treatment, splenocytes were harvested and OT-I or OT-II T-cell proliferation was determined by analyzing the CFSE fluorescence intensity through flow cytometry.
Analysis of tumor infiltrated T cells
OT-I or OT-II cells were transferred into B16-OVA tumor-bearing CD45.1 congenic mice; 24 h later, the mice were injected with PBS, OVA, RGP, and the combination of OVA and RGP. Three days after the injection, the tumors were harvested and their weight was measured. The tumors were cut into small fragments and then digested with a 2% fetal bovine serum (FBS) containing collagenase for 20 min at room temperature. The cells from the digest were centrifuged to pellets, which were re-suspended in 4 mL of 1.077 histopaque (Sigma-Aldrich), layered below by an additional 4 mL of histopaque, and then centrifuged at 1,700g for 10 min. The light density fraction (< 1.077 g/cm3) was collected and stained with anti-CD45.2, anti-CD3, anti-CD4+, and anti-CD8+. The infiltrated OT-1 and OT-II cells were defined by CD45.2+CD8+ or CD45.2+CD4+ cells in the CD3+ cells.
In vivo cytotoxicity assay
Mice were injected i.v. with a mixture of splenocytes differentially labeled with CFSE (200 nM) and loaded with 100 nM SIINFEKL peptide, and spleen cells labeled with 10 mM 5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine CellTracker™ Orange CMTMR (Life Technologies) and not loaded with peptide. A total of 10 × 106 cells per mouse were injected, consisting of a mixture containing each target cell population. Splenocytes were collected 24 h after injection of target cells. Percentage killing was calculated using the formula as described.Citation45
ELISPOT assay
Mouse IFNγ ELISPOTs were performed according to the manufacturer's protocol (BioLegend). In short, spleens were harvested from treated mice and isolated mononuclear cells by density cut. The cells were seeded at 50 × 103 cells/well in a pre-coated plate. The cells then were stimulated with 2 μg/mL of the OVA peptide 257–264 (SIINFEKL) and OVA 323–339 (ISQAVHAAHAEINEAGR), or a negative control peptide at 37 °C for 24 h. ELISPOT plates were counted automatically using a CTL ELISPOT reader (CTL Europe GmbH), and the number of spots observed with a control peptide subtracted from the number of spots with specific peptides for each mouse.
Statistical analysis
Results are expressed as the mean ± standard error of the mean (SEM). Data sets were analyzed by one-way ANOVA using the Tukey multiple comparison test with GraphPad Prism 4. p values smaller than 0.05 were considered to be statistically significant.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
J.O.J. and P.C.L. designed and wrote the paper. L.X., M.K., W.Z., and Z.L. performed experiments and analyzed data.
KONI_A_1325981_supplementary_Figures.docx
Download MS Word (283 KB)Acknowledgments
We thank the Shanghai Public Health Clinical Center animal facility for maintaining the animals in this study.
Funding
J.O. Jin was supported by Research fund for International Young Scientists from National Natural Science Foundation of China (81550110507). L. Xu was supported by General Project of Shanghai Public Health Clinical Center (KY-GW-2017-15). M. Kwak was supported by a grant from Marine Biotechnology Program (20150220) funded by the Ministry of Oceans and Fisheries, Republic of Korea. P.C.-W. Lee was supported by grants from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2016R1A2B4006313).
References
- Jin JO, Zhang W, Du JY, Wong KW, Oda T, Yu Q. Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific T cell immune responses. PloS One 2014; 9:e99396; PMID:24911024; https://doi.org/10.1371/journal.pone.0099396
- Luo M, Shao B, Nie W, Wei XW, Li YL, Wang BL, He ZY, Liang X, Ye TH, Wei YQ. Antitumor and adjuvant activity of lambda-carrageenan by stimulating immune response in cancer immunotherapy. Sci Rep 2015; 5:11062; PMID:26098663; https://doi.org/10.1038/srep11062
- Zhang W, Du JY, Jiang Z, Okimura T, Oda T, Yu Q, Jin JO. Ascophyllan purified from Ascophyllum nodosum induces Th1 and Tc1 immune responses by promoting dendritic cell maturation. Mar Drugs 2014; 12:4148-64; PMID:25026264; https://doi.org/10.3390/md12074148
- Huang Y, Jiang C, Hu Y, Zhao X, Shi C, Yu Y, Liu C, Tao Y, Pan H, Feng Y et al. Immunoenhancement effect of rehmannia glutinosa polysaccharide on lymphocyte proliferation and dendritic cell. Carbohydr Polym 2013; 96:516-21; PMID:23768595; https://doi.org/10.1016/j.carbpol.2013.04.018
- Zhang Z, Meng Y, Guo Y, He X, Liu Q, Wang X, Shan F. Rehmannia glutinosa polysaccharide induces maturation of murine bone marrow derived Dendritic cells (BMDCs). Int J Biol Macromol 2013; 54:136-43; PMID:23246902; https://doi.org/10.1016/j.ijbiomac.2012.12.005
- Huang Y, Liu Z, Bo R, Xing J, Luo L, Zhen S, Niu Y, Hu Y, Liu J, Wu Y et al. The enhanced immune response of PCV-2 vaccine using Rehmannia glutinosa polysaccharide liposome as an adjuvant. Int J Biol Macromol 2016; 86:929-36; PMID:26851361; https://doi.org/10.1016/j.ijbiomac.2016.02.003
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392:245-52; PMID:9521319; https://doi.org/10.1038/32588
- Dubensky TW, Jr, Reed SG. Adjuvants for cancer vaccines. Semin Immunol 2010; 22:155-61; PMID:20488726; https://doi.org/10.1016/j.smim.2010.04.007
- Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol 2000; 1:199-205; PMID:10973276; https://doi.org/10.1038/79734
- Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol 2005; 5:296-306; PMID:15803149; https://doi.org/10.1038/nri1592
- Cohn L, Delamarre L. Dendritic cell-targeted vaccines. Front Immunol 2014; 5:255; PMID:24910635; https://doi.org/10.3389/fimmu.2014.00255
- Finn OJ. Cancer immunology. N Eng J Med 2008; 358:2704-15; PMID:18565863; https://doi.org/10.1056/NEJMra072739
- Lim S, Koo JH, Choi JM. Use of cell-penetrating peptides in dendritic cell-based vaccination. Immune Netw 2016; 16:33-43; PMID:26937230; https://doi.org/10.4110/in.2016.16.1.33
- Miconnet I, Coste I, Beermann F, Haeuw JF, Cerottini JC, Bonnefoy JY, Romero P, Renno T. Cancer vaccine design: a novel bacterial adjuvant for peptide-specific CTL induction. J Immunol 2001; 166:4612-9; https://doi.org/10.4049/jimmunol.166.7.4612
- Perret R, Sierro SR, Botelho NK, Corgnac S, Donda A, Romero P. Adjuvants that improve the ratio of antigen-specific effector to regulatory T cells enhance tumor immunity. Cancer Res 2013; 73:6597-608; PMID:24048821; https://doi.org/10.1158/0008-5472.CAN-13-0875
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010; 11:373-84; PMID:20404851; https://doi.org/10.1038/ni.1863
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14:1014-22; PMID:24048123; https://doi.org/10.1038/ni.2703
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ 2000; 321:1237-8; PMID:11082069; https://doi.org/10.1136/bmj.321.7271.1237
- Dresser DW. Specific inhibition of antibody production. II. Paralysis induced in adult mice by small quantities of protein antigen. Immunology 1962; 5:378-88; PMID:13887798
- Parkhurst MR, Fitzgerald EB, Southwood S, Sette A, Rosenberg SA, Kawakami Y. Identification of a shared HLA-A*0201-restricted T-cell epitope from the melanoma antigen tyrosinase-related protein 2 (TRP2). Cancer Res 1998; 58:4895-901; PMID:9809996
- Toda M, Martuza RL, Kojima H, Rabkin SD. In situ cancer vaccination: an IL-12 defective vector/replication-competent herpes simplex virus combination induces local and systemic antitumor activity. J Immunol 1998; 160:4457-64; PMID:9574551
- Jin JO, Zhang W, Du JY, Yu Q. BDCA1-positive dendritic cells (DCs) represent a unique human myeloid DC subset that induces innate and adaptive immune responses to Staphylococcus aureus infection. Infect Immunity 2014; 82:4466-76; PMID:25114114; https://doi.org/10.1128/IAI.01851-14
- Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O'Keeffe M, Shao QX, Chen WF et al. Cutting edge: generation of splenic CD8+ and CD8− dendritic cell equivalents in FMS-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol 2005; 174:6592-7; PMID:15905497; https://doi.org/10.4049/jimmunol.174.11.6592
- Pooley JL, Heath WR, Shortman K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8− dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J Immunol 2001; 166:5327-30; PMID:11313367; https://doi.org/10.4049/jimmunol.166.9.5327
- Schnorrer P, Behrens GM, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, Davey G, Kupresanin F, Li M, Maraskovsky E et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci USA 2006; 103:10729-34; PMID:16807294; https://doi.org/10.1073/pnas.0601956103
- Salio M, Shepherd D, Dunbar PR, Palmowski M, Murphy K, Wu L, Cerundolo V. Mature dendritic cells prime functionally superior melan-A-specific CD8+ lymphocytes as compared with nonprofessional APC. J Immunol 2001; 167:1188-97; PMID:11466333; https://doi.org/10.4049/jimmunol.167.3.1188
- Grodeland G, Fossum E, Bogen B. Polarizing T and B cell responses by APC-targeted subunit vaccines. Front Immunol 2015; 6:367; PMID:26257735; https://doi.org/10.3389/fimmu.2015.00367
- Kaufman HL, Disis ML. Immune system versus tumor: shifting the balance in favor of DCs and effective immunity. J Clin Invest 2004; 113:664-7; PMID:14991063; https://doi.org/10.1172/JCI21148
- Ma Y, Shurin GV, Gutkin DW, Shurin MR. Tumor associated regulatory dendritic cells. Semin Cancer Biol 2012; 22:298-306; https://doi.org/10.1016/j.semcancer.2012.02.010
- Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity 2010; 33:492-503; PMID:21029960; https://doi.org/10.1016/j.immuni.2010.10.002
- Zhang W, Okimura T, Xu L, Zhang L, Oda T, Kwak M, Yu Q, Jin JO. Ascophyllan functions as an adjuvant to promote anti-cancer effect by dendritic cell activation. Oncotarget 2016; 7:19284-98; PMID:27008707; https://doi.org/10.18632/oncotarget.8200
- Zhang W, Yu X, Kwak M, Xu L, Zhang L, Yu Q, Jin JO. Maturation of dendritic cells by pullulan promotes anti-cancer effect. Oncotarget 2016; 7:44644-59; PMID:27341129; https://doi.org/10.18632/oncotarget.10183
- Jin JO, Park HY, Xu Q, Park JI, Zvyagintseva T, Stonik VA, Kwak JY. Ligand of scavenger receptor class A indirectly induces maturation of human blood dendritic cells via production of tumor necrosis factor-alpha. Blood 2009; 113:5839-47; PMID:19351958; https://doi.org/10.1182/blood-2008-10-184796
- Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med 2002; 196:407-12; PMID:12163569; https://doi.org/10.1084/jem.20020470
- Gersuk GM, Underhill DM, Zhu L, Marr KA. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J Immunol 2006; 176:3717-24; PMID:16517740; https://doi.org/10.4049/jimmunol.176.6.3717
- Tan W, Yu KQ, Liu YY, Ouyang MZ, Yan MH, Luo R, Zhao XS. Anti-fatigue activity of polysaccharides extract from Radix Rehmanniae Preparata. Int J Biol Macromol 2012; 50:59-62; PMID:21983027; https://doi.org/10.1016/j.ijbiomac.2011.09.019
- Tomoda M, Miyamoto H, Shimizu N. Structural features and anti-complementary activity of rehmannan SA, a polysaccharide from the root of Rehmannia glutinosa. Chem Pharma Bull 1994; 42:1666-8; PMID:7954919; https://doi.org/10.1248/cpb.42.625 10.1248/cpb.42.1666
- Kuznetsova TA, Besednova NN, Somova LM, Plekhova NG. Fucoidan extracted from Fucus evanescens prevents endotoxin-induced damage in a mouse model of endotoxemia. Mar Drugs 2014; 12:886-98; PMID:24492521; https://doi.org/10.3390/md12020886
- Zhang W, Oda T, Yu Q, Jin J-O. Fucoidan from Macrocystis pyrifera has powerful immune-modulatory effects compared to three other fucoidans. Mar Drugs 2015; 13:1084-104; PMID:25706632; https://doi.org/10.3390/md13031084
- Inagawa H, Kohchi C, Soma G. Oral administration of lipopolysaccharides for the prevention of various diseases: benefit and usefulness. Anticancer Res 2011; 31:2431-6; PMID:21873155
- Murakami M, Tsubata T, Shinkura R, Nisitani S, Okamoto M, Yoshioka H, Usui T, Miyawaki S, Honjo T. Oral administration of lipopolysaccharides activates B-1 cells in the peritoneal cavity and lamina propria of the gut and induces autoimmune symptoms in an autoantibody transgenic mouse. J Exp Med 1994; 180:111-21; PMID:8006578; https://doi.org/10.1084/jem.180.1.111
- Oketani K, Inoue T, Murakami M. Effect of E3040, an inhibitor of 5-lipoxygenase and thromboxane synthase, on rat bowel damage induced by lipopolysaccharide. Eur J Pharmacol 2001; 427:159-66; PMID:11557269; https://doi.org/10.1016/S0014-2999(01)01234-1
- Zhang W, Cho SY, Xiang G, Min KJ, Yu Q, Jin JO. Ginseng berry extract promotes maturation of mouse dendritic cells. PloS One 2015; 10:e0130926; PMID:26090808; https://doi.org/10.1371/journal.pone.0130926
- Jin JO, Han X, Yu Q. Interleukin-6 induces the generation of IL-10-producing Tr1 cells and suppresses autoimmune tissue inflammation. J Auto Immunity 2013; 40:28-44; PMID:22921334; https://doi.org/10.1016/j.jaut.2012.07.009
- Hermans IF, Silk JD, Yang J, Palmowski MJ, Gileadi U, McCarthy C, Salio M, Ronchese F, Cerundolo V. The VITAL assay: a versatile fluorometric technique for assessing CTL- and NKT-mediated cytotoxicity against multiple targets in vitro and in vivo. J Immunol Methods 2004; 285:25-40; PMID:14871532; https://doi.org/10.1016/j.jim.2003.10.017
