ABSTRACT
CEACAM1 is an extensively studied cell surface molecule with established functions in multiple cancer types, as well as in various compartments of the immune system. Due to its multi-faceted role as a recently appreciated immune checkpoint inhibitor and tumor marker, CEACAM1 is an attractive target for cancer immunotherapy. Herein, we highlight CEACAM1's function in various immune compartments and cancer types, including in the context of metastatic disease. This review outlines CEACAM1's role as a therapeutic target for cancer treatment in light of these properties.
Introduction
As our understanding of cancer biology and experience with therapeutics continues to evolve, the criteria to identify therapeutic targets are further refined. Ideal targets should have a tumor-specific role functioning in several hallmarks of cancer, thus providing an opportunity for a multi-pronged attack on the cancer with a single targeted agent.Citation1 Expression of the target should also be tumor-selective. If compartments of the immune system or the tumor stroma also express the target, its engagement within these compartments should coincide with an antitumor effect. Ideally, the target should be expressed at the cell surface, allowing specific targeting with a monoclonal antibody.Citation2
CEACAM1 is an extensively studied molecule that fits all of the aforementioned criteria and is currently being targeted in ongoing clinical trials.Citation3 CEACAM1 is a type-1 transmembrane protein containing an extra-cellular N-terminal variable domain followed by up to three constant C2-like immunoglobulin domains.Citation4 The extracellular domains of CEACAM1 are essential in its function, as they are required for homophilic (CEACAM1-CEACAM1) and heterophilic intercellular adhesion with CEA, as well as with the T cell-immunoglobulin and mucin-domain containing 3 (TIM-3) protein.Citation5 It is also a receptor for a variety of human and rodent pathogens.Citation6 CEACAM1 is the only member of the CEACAM family to possess an immunoreceptor tyrosine-based inhibitory motif (ITIM).Citation7 Phosphorylation of ITIMs in immune and epithelial cells inhibits signaling by binding a variety of effector proteins that downregulate cell signaling, in particular the tyrosine-phosphatase non-receptor type 6 (PTPN6; previously SHP-1) and PTPN11 (SHP-2) phosphatases.Citation8,9
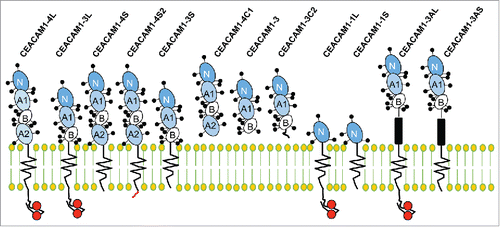
The CEACAM1 gene produces 12 different alternatively spliced isoforms (). One constant feature is the splicing of mRNAs into transcripts encoding two different cytoplasmic domains, either by inclusion (the long (-L) tail) or exclusion (the short (-S) tail) of the CEACAM1 exon 7.Citation10 In many cases, the presence of a particular tail isoform and the ratios between them impact the function of the protein. While the long isoform has ITIM motifs, the short isoform does not; it does, however, contain several Ser phosphorylation motifs.Citation11,12 Alternative splicing also leads to the incorporation of up to three C2-like domains generating isoforms differing in the length of the extracellular region, but each contains the membrane distal IgV-like N-domain involved in homophilic and heterophilic interactions.Citation6 In addition, CEACAM1 can be alternatively spliced to produce secreted variants. While the role of secreted variants of CEACAM1 is poorly understood, they are capable of inhibiting intercellular homophilic adhesion by acting as decoy receptors, and may be useful as serum or urine biomarkers for several malignancies.Citation13-16
Figure 1. Human CEACAM1 isoforms. CEACAM1 transcripts can be alternatively spliced to generate 12 different isoforms that have one variable (V)-like Ig domain, identified as the N domain (dark blue). The various isoforms have 1, 2 or 3 constant C2-like Ig domains, identified as A (light blue) or B (white), with the exception of CEACAM1-1L and CEACAM1-1S that lack C2-like Ig domains. According to standardized nomenclature, the number after CEACAM1 is indicative of the number of extracellular Ig-like domains. CEACAM1 isoforms are anchored to the cellular membrane via a transmembrane domain, with the exception of the secreted isoforms of CEACAM1 (CEACAM1-4C1, 3 and 3C2, respectively). CEACAM1 isoforms also possess 1 of 2 cytoplasmic domains, termed as long (L) and short (S) tails. The letter following the number in the standardized nomenclature points to the presence of either a long or short cytoplasmic tail, a unique terminus (C), or an Alu family repeat sequence (A) (black boxes). The CEACAM1-L cytoplasmic domain has ITIM motifs (red circles). All family members are highly glycosylated proteins, with glycosylation sites illustrated as the stick and balls on the extracellular domains.
T cells have been brought to the forefront of cancer immunotherapy due to the success of agents that block the cytotoxic T lymphocyte-associated protein 4 (CTLA4) and programmed cell death protein-1 (PD-1) pathways, which normally function as inhibitors of highly activated T cells. For both receptors, blocking their function serves to activate T cells so as to promote tumor killing and production of critical cytokines such as interferon-γ (IFNγ).Citation17 Activating T cells in the context of cancer is a rapidly growing avenue of investigation for novel cancer therapeutics, with many T cell activating agents in the clinical trial pipeline, including blocking antibodies of the checkpoints LAG3, TIM-3 and CEACAM1.Citation3,18
Herein, we describe CEACAM1's roles in tumor immunology and outline potential effects of CEACAM1 targeting on each compartment of the immune system in the context of cancer immunotherapy, as well as identify specific cancer types that should be targeted for the potential benefit of metastatic cancer patients in the context of clinical trials. To be effective and further prevent immune complications such as antibody-dependent cellular toxicity, future anti-CEACAM1 humanized antibodies used for immunotherapy will need to be an IgG4 isotype.Citation19
CEACAM1 in the immune compartment
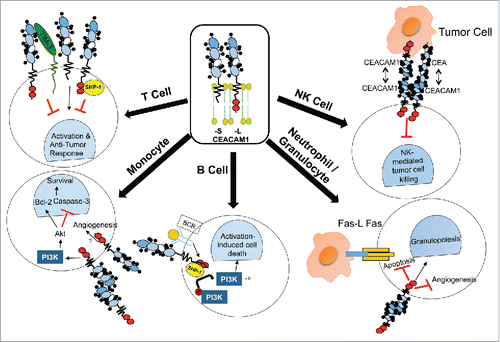
CEACAM1 has been studied in the immune system for its tumor-associated function, particularly in T and Natural Killer (NK) cells. While fewer studies have investigated the role of CEACAM1 in B cells, neutrophils and macrophages (), CEACAM1 also plays a functional role in these cells, so the effect of CEACAM1-directed therapies must be appreciated. We summarize the existing data on CEACAM1's function in various immune compartments, predict the effects of pharmacological inhibition of CEACAM1 and outline potential adverse events.
Figure 2. CEACAM1's function in immune cells. CEACAM1's function has been extensively characterized in many compartments of the immune system. T Cells: CEACAM1-L acts as an inhibitory receptor on T cells via recruitment of SHP-1 to CEACAM1-L's phosphorylated ITIMs, changing the activation threshold of T cell activation and therefore decreasing immunosurveillance in a cancer context. CEACAM1-S plays an opposing role and therefore can promote T cell activation leading to increased activation- induced cell death and distinct regulatory functions. CEACAM1 also interacts with TIM-3 on the surface of T cells, endowing TIM-3 with its inhibitory function so as to oppose T cell activation. NK Cells: CEACAM1-L expressed on the surface of NK cells interacts in trans with CEA or CEACAM1 on tumor cells, inhibiting NK-mediated tumor cell killing independently of MHC class I status. B Cells: Activation of the B cell receptor (BCR) leads to phosphorylation of CEACAM1-L's ITIM domain, leading to SHP-1 recruitment and dephosphorylation of PI3K, which promotes activation-induced cell death of B cells. Monocytes: CEACAM1 homophilic binding leads to PI3K activation, promoting AKT-mediated survival via activation of Bcl-2 and inhibition of caspase-3. Granulocytes: CEACAM1 on the surface of granulocytes promotes granulopoiesis while inhibiting granulocyte-mediated angiogenesis and apoptosis.
T cells
CEACAM1 plays an important role on activated T cells since it is itself a proliferative checkpoint similar to CTLA4 and PD-1. CEACAM1 expression is mostly excluded from resting (naïve) T cells and is expressed at high levels on T cells activated by stimulation with IL-2 or anti-CD3 antibodies via induced transcription and the recruitment of intracellular CEACAM1 to the cell surface.Citation20-22 It is notable that CEACAM1 is the only CEACAM family member expressed by activated T cells.Citation21,22 CEACAM1-L, the dominant isoform expressed in most T cells, then acts as an inhibitory receptor downregulating T cell activation and suppressing T cell functions,Citation23 while CEACAM1-S can act as a positive co-stimulant of T cell activation, and is linked to important regulatory activities.Citation24,25 The CEACAM1-L isoform expression typically dominates over the CEACAM1-S isoform in T and NK cells, except within intestinal tissues where CEACAM1-S overabundance is observed.Citation12,26 CEACAM1-L's inhibitory function is mediated by expression of two CEACAM1-L cytoplasmic domain ITIMs not present in CEACAM1-S.Citation27 Phosphorylation of these ITIMs by a variety of Src-related tyrosine kinases recruits the tyrosine phosphatases SHP-1 and SHP-2, which de-phosphorylate several tyrosine residues of either receptor tyrosine kinases or adaptor proteins at the cell surface rendering them inactive.Citation28 In T lymphocytes, CEACAM1-L is phosphorylated by p56lck resulting in association with SHP-1 in the vicinity of the T cell receptor (TCR) signaling complex.Citation28,29 This prevents activation through the TCR because SHP-1 dephosphorylates the TCR CD3-ζ chain and TCR-associated protein kinase 70 kDa (ZAP-70), halting signaling at its earliest steps. CEACAM1 in T cells can also suppress mitogen activated protein kinases ERK and JNK and their downstream pathways. These properties result in broad proximal suppression of TCR/CD3 complex signaling and inhibitory effects on a variety of effector functions, including T cell proliferation, Th1 and Th2 cytokine production and cytotoxicity associated with T cell activation ().Citation25 Importantly, with respect to antitumor immunity, when CEACAM1 is ligated by N-domain specific monoclonal antibodies in vitro under cross-linking conditions, differentiation of naïve T cells into IFNγ-producing Th1, but not Th2 cells, is observed via down-modulation of T-bet but not the STAT4 transcription factor.Citation30
Taken together, these studies show that CEACAM1-L is an inhibitor of T cell activation via its ITIM motifs and functions in a SHP-1-dependent manner. This suggests that CEACAM1 blockade will promote polarization toward the Th1 profile and restore IFNγ production in situations where CEACAM1 suppression is operative in highly active T cells. However, as CEACAM1-L and CEACAM1-S isoforms are co-expressed and regulate activated T cells in a tunable fashion,Citation12 it can be predicted that CEACAM1's behavior is highly dependent on the type of stimulus and the strength of the necessary response.Citation23,24,31 Thus, inhibition of CEACAM1-L signaling would relieve proximal inhibition of TCR/CD3 complex signaling and restore cytotoxicity and IFNγ secretion, whereas inhibition of CEACAM1-S signaling would reverse the expansion of unique regulatory populations such as CD4+LAP (latency associated peptide)+ T cells.Citation12,28
Consistent with these concepts, a CEACAM1-Fc construct encompassing the murine CEACAM1 N-domain as a Fc fusion protein was shown to inhibit inflammatory bowel disease in a murine colitis model through suppression of Th1 or Th2 cells, depending on the host genetic background.Citation5,30,32 In a similar manner, CEACAM1-L trans-rectal delivery via adenovirus in mouse models of ulcerative colitis (UC) provided similar beneficial results,Citation33 and a CEACAM1-Fc fusion protein inhibits experimental allergic encephalomyelitis.Citation34 This suggests that CEACAM1 inhibition might lead to intestinal inflammation as observed with other checkpoint inhibitors.Citation35,36 Although Ceacam1−/− mice do not develop spontaneous intestinal inflammation, adoptive transfer of naïve wild type T cells into Ceacam1−/− immunodeficient hosts (namely Rag2−/− mice) results in exacerbated colitis, suggesting that CEACAM1 deficiency predisposes to excessive intestinal inflammation upon exposure to inducing agents.Citation5 However, conflicting data exists in the literature as to whether CEACAM1 is overexpressed in UC and IBD patients.Citation37,38 Therefore, a clinical trial investigating a CEACAM1 inhibitory agent should consider excluding patients with a history of inflammatory bowel disease until these opposing issues are reconciled in future studies.
CEACAM1-L engagement also inhibits TCR-mediated cytotoxicity. This inhibition depends on the blocking of granule exocytosis consequent to ZAP-70 signaling and SHP-1 binding,Citation28 on inhibition of Fas-induced apoptosis through reduction of caspase 3/8 and by preventing β-catenin phosphorylation and destruction in the target cell.Citation39 This latter effect is dependent upon the cytoplasmic residue K470, as well as a critical residue located between the ITIMs (S508). CEACAM1-mediated inhibition of T cell cytotoxicity is enhanced by CEACAM1 cross-linking and blocked by CEACAM1 blockade.Citation22,28 Therefore, the inhibitory function of CEACAM1-L on T cells extends to cytotoxicity, which is highly important to antitumor immunity.
In T cells, CEACAM1 also cooperates with TIM-3, another well studied immunoglobulin molecule currently being targeted for cancer immunotherapy.Citation5 TIM-3 is an activation-induced inhibitory molecule involved in T cell tolerance, and is an inducer of T cell exhaustion.Citation40 However, TIM-3 can also be stimulatory under other experimental conditions.Citation41,42 TIM-3 is co-expressed with CEACAM1 on T cells during induction of tolerance, exhibits biochemical interactions with CEACAM1, and serves as a CEACAM1 heterophilic ligand.Citation5,43 CEACAM1 expression confers inhibitory functions onto TIM-3 by facilitating TIM-3 maturation and localization to the T cell surface and facilitates TIM-3-mediated signaling (). CEACAM1 can also ligate TIM-3 in trans suggesting that opposing cells, such as CEACAM1-expressing tumor cells, may provide an inhibitory signal via TIM-3 on a T cell. Treatment of murine colorectal cancer (CRC) CT26 tumors with TIM-3 and CEACAM1 monoclonal antibodies demonstrated synergistic antitumor effects in both preventative and therapeutic protocols. CEACAM1 inhibition also cooperates synergistically with PD-L1 inhibition.Citation5 Therefore, heterophilic engagement of CEACAM1 with TIM-3 mediates T cell inhibition, with both of these cell surface receptors regulating autoimmunity and antitumor immunity. Zhang et al. examined circulating and tumor-infiltrating CD8+ T lymphocytes of CRC patients and showed maximal T cell exhaustion upon TIM-3 and CEACAM1 co-expression, as was observed in mouse CRC models.Citation5,44 These studies indicate that CEACAM1 and TIM-3 mark highly exhausted T cells.
Taken together, CEACAM-L is an activation-induced inhibitory molecule on T cells due to its ITIM motifs. Akin to CTLA4 and PD-1, CEACAM1 shows promise in a preclinical setting as a target for cancer immunotherapy. There is significant need for new checkpoint inhibitors since, for example, only 20–40% of patients respond to currently approved checkpoint inhibitors in metastatic melanoma,Citation45 making further exploration of targets such as CEACAM1 and TIM-3 highly warranted.
NK cells
NK cells are lymphocytes critical to innate immunity, participating in early control of viral infection and immune-surveillance of tumors.Citation46 Many NK-regulating receptors can either stimulate (activating receptors such as natural cytotoxicity triggering receptors 1, 2 and 3, DNAX accessory molecule 1, NK group 2D or NKG2D and killer cell lectin-like receptor K1) or dampen (inhibitory receptors such as killer-cell immunoglobulin-like receptors or KIRs) NK cell reactivity.Citation47 Lack of inhibitory receptor signaling allows NK cells to detect and kill cells lacking major histocompatibility complex (MHC) class I, unlike cytotoxic T lymphocytes that recognize a foreign antigen presented on MHC class I to activate its cytotoxic function.Citation47
CEACAM1 expression robustly promotes evasion of NK-mediated killing of tumor cells. When CEACAM1 is present on the surface of both NK and melanoma cells, NK-mediated killing is inhibited independently of MHC class I expression.Citation48,49 In transporter associated with antigen processing (TAP-2)-deficient patients, which lack MHC class I-mediated inhibition of NK cells, CEACAM1 is upregulated and capable of compensating for this deficiency.Citation50 TAP-2 is an endoplasmic reticulum protein, responsible for loading peptides onto MHC class I. In the absence of TAP-2, MHC class I molecules are not displayed on the cell surface, which activates NK cell-mediated killing. CEACAM1 overexpression inhibits this effect and TAP-2-deficient patients are spared from excessive NK-mediated cytotoxicity and autoimmune disease early in life. However, soluble CEACAM1 can abrogate CEACAM1 homophilic interactions, and allow NK-mediated cytotoxicity in the context of TAP-2 deficiency.Citation13 Thus, the ratio of membrane-bound and soluble CEACAM1 binding to NK cells may play an important role in NK-mediated killing. In melanoma, this may also depend on alternative splicing since soluble CEACAM1 does not arise from surface cleavage, but instead requires active protein synthesis and vesicular transport.Citation14 NK cells have also recently been recognized to express TIM-3 upon activation, implicating a potential for cooperation between CEACAM1 and TIM-3 on NK cells as described for T cells.Citation51
In addition to CEACAM1, cancer cells frequently express other cell-adhesion molecules of the CEA family, including carcinoembryonic antigen (CEA, also known as CEACAM5) and CEACAM6.Citation7 CEA has its own important roles in many of the same processes as CEACAM1. Like CEACAM1, CEA can adhere to CEA, other CEA gene family members (including CEACAM1) and several other molecules on adjacent cells.Citation7 Depending upon which combination of CEA family members are expressed, their engagement may allow for cell survival, promote metastasis, initiate downstream signaling and/or engage immune cells. Homophilic (CEACAM1-CEACAM1) and heterophilic interactions between CEA and CEACAM1 expressed on melanoma cells with CEACAM1 expressed on the NK cell surface inhibits NK-mediated cytotoxicityCitation52 (). Deleting CEACAM1's ITIM motifs revealed their importance in this function. CEA and CEACAM1's N-domains as well as CEACAM1 N-domain residues (43R and 44Q) are also essential for either CEA-CEACAM1 adhesion or CEACAM1-mediated homophilic interactions and NK-mediated cytotoxicity.Citation53 These residues are also present in CEA but not CEACAM6, explaining why CEACAM1 and CEA adhere heterophilically, whereas CEACAM1 does not bind to CEACAM6. In addition, CEA is rapidly and specifically transferred to NK cells in an adhesion-dependent mechanism.Citation54 Together, these studies reveal the importance of adhesion between CEA family members on immune evasion by cancer cells.
Several models have been proposed to explain CEACAM1-mediated NK cell inhibition. CEACAM1 silencing in mouse and human cancer cells upregulated NK cell activating ligands on their surface, while overexpression of CEACAM1-3S and -3L in CRC cell lines caused sequestration of MICA/B intracellularly, preventing it from activating NK cells.Citation55 However, these mechanistic findings were contradicted by a recent study which demonstrated that while CEACAM1-4L expression downregulated cell surface expression of MICA and ULBP2 ligands by causing them to shed from the membrane, CEACAM1-3S upregulated NKG2D receptor ligands.Citation56 This discrepancy will require further investigation. Furthermore, CEACAM1 forms a complex with NKG2D whereupon CEACAM1 recruits SHP-1 involved in dephosphorylation of the guanine nucleotide exchange factor Vav1, subsequently blocking initiation of cytolysis.Citation57 Thus, CEACAM1 expression on tumor cells contributes to NK-mediated immune evasion by hiding away the ligands responsible for engaging NK cell surface receptors, and by engaging with NK-activating receptors to inhibit their downstream signaling.
Regardless of the mechanism by which CEACAM1-L inhibits NK cell activation, this effect is robust and coupled to CEACAM1-L-mediated inhibition of NKG2D signaling. While more preclinical and clinical data are still required, particularly to elucidate the CEACAM1 splice variant functions, inhibition of CEACAM1 on NK cells offers an additional advantage that is exploitable by CEACAM1 immunotherapy in advanced cancer treatments. The principle that modulating NK cell function in a cancer setting can lead to improved outcomes has been established in vivo.Citation58 Unlike currently approved T-cell targeted PD-1 and CTLA4 inhibitors, whose functions in NK cells are debated,Citation47 targeting CEACAM1 for cancer immunotherapy can offer a second mechanism of action to promote tumor killing by immune surveillance.
B cells
B cells are professional antigen-presenting cells that are part of the adaptive immune system. Their main function is in the secretion of antibodies, while their role in tumorigenesis and cancer progression is less well defined.Citation59 CEACAM1-L has been implicated in B cell function, although the literature is contradictory with older and newer studies, suggesting conflicting roles for CEACAM1 in B cells.
The first work on CEACAM1 and B cells reported on the creation of an FCγRIIB-CEACAM1-L chimeric fusion protein that was capable of acting as an inhibitory receptor in B cells, suggesting that CEACAM1-L's cytoplasmic ITIMs could function in B cells.Citation60 On the other hand, it has been shown that cross-linking CEACAM1 on mouse B cells with either antibodies or CEACAM1 transfectants, but only in the presence of B cell receptor (BCR) cross-linking, promotes B cell activation but not immunoglobulin isotype switching.Citation61 This is presumably through CEACAM1 promotion of B cell survival, and suggests that homophilic ligands may be the source of CEACAM1-mediated effects on B cells, but this requires further study.
Consistent with the latter observations, it has recently been demonstrated using Ceacam1−/− mice that CEACAM1 is a critical regulator of B cell survival, influencing B-cell number and protective antiviral antibody responses.Citation62 This phenomenon is mediated via activation of spleen tyrosine kinase (SYK), extracellular signal related kinases (ERK) and nuclear factor kappa-light-chain-enhancer in activated B cells (NFκB).Citation62 While CEACAM1 had little effect on the proliferation of newly formed B cells, the number of mature B cells is reduced significantly in Ceacam1−/− mouse lymph nodes and forced expression of CEACAM1 in T cells leads to increased IgA production in mucosal tissues.Citation12,62 CEACAM1 also promotes efficient production and secretion of anti-viral antibodies consistent with other findings that elevated T lymphocyte CEACAM1 expression causes augmented IgA mucosal production.Citation62 Thus CEACAM1 on B cells may be important for B cell survival during an active immune response. How this relates to CEACAM1-L inhibitory functions in these cells remains to be elucidated.
CEACAM1 expression on human B cells is less well defined. However, CEACAM1 is indeed functional in human B cell lines. CEACAM1 is highly expressed in human activated CD19-positive Daudi B cells stimulated with IL-2 for 3 d,Citation63 where it functions as a negative co-receptor for the BCR. Anti-IgM stimulation of the BCR results in CEACAM1 phosphorylation and SHP-1 recruitment to lipid raft domains with reduced phosphorylation of activated PI3K and increased activation-induced cell death (). How this reconciles with the results in Ceacam1−/− mice, wherein CEACAM1 deficiency is associated with decreased B cell numbers, is unclear.
Due to the ambiguous role of B cells in cancer, either promoting or inhibiting tumor responses,Citation59 and the contradictory role of CEACAM1 in these cells, it is unclear what effects CEACAM1 blockade would have on B cells and on the antitumor response. In light of the mouse-based studies, one can hypothesize scenarios whereby B cells could become apoptotic with decreased antibody production. This may predict an increased risk of infection, consistent with the susceptibility to pathogens such as Listeria monocytogenes observed in Ceacam1−/− mice.Citation12,64 Thus, clinical trials will require close monitoring of humoral immune functions.
Monocytes/macrophages
Macrophages are typically the most abundant immune cells present in the tumor microenvironment where they can have both protumor and antitumor functions.Citation65 Similar to the Th1/Th2 paradigm of T-cell polarization in cancer, macrophages are thought to exert their effect on tumors in a fashion largely dependent upon the polarity of the macrophage population between a continuum of M1 and M2 phenotypes.Citation66 The current evidence suggests that the more classical M1-type macrophages are capable of tumor cell killing while the M2 macrophages, inclined to function in tissue repair, allow tumor progression by suppressing inflammatory responses and promoting angiogenesis. CEACAM1's function in macrophages and in their monocyte precursors has been investigated with certain critical questions remaining on monocyte development and early stage cancer. CEACAM1's role in advanced cancers through its effects on macrophages is ambiguous. Cancer patients with advanced disease eligible for treatment with a CEACAM1 inhibitor in a clinical trial would be expected to have tumors infiltrated with predominantly M2 macrophages. CEACAM1 homophilic binding protects monocytes from apoptosis via a pathway involving PI3K- and AKT-dependent activation of Bcl2, preventing the activation of caspase 367 (). Therefore, inhibition of CEACAM1 could prevent development of circulating monocytes into tumor-associated macrophages, thereby preventing M2 infiltration or persistence in advanced tumors. A CEACAM1-targeted therapy selectively inhibiting M2 macrophage function or survival would theoretically be beneficial to late stage cancer patients.
CEACAM1 expression also defines a new monocyte subtype (Ceacam1+Msr1+Ly6C−F4/80−Mac1+) critical for fibrosis development.Citation68 This suggests that CEACAM1 inhibition may be beneficial to patients whose tumors are characterized by high degrees of fibrosis by halting the establishment of a microenvironmental niche capable of driving tumor progression locally or at distant sites.Citation69 Furthermore, these findings may spur future studies better defining CEACAM1's function in several devastating fibrotic diseases such as idiopathic pulmonary fibrosis.Citation70 Patients with such diseases may also one day benefit from treatment with a CEACAM1 inhibitory antibody.
CEACAM1 expressed by monocytes promotes angiogenesis in a Leishmaniasis model of inflammation.Citation71 While these experiments cannot be directly applied to the setting of advanced cancer, it may suggest that a CEACAM1 inhibitor plays a role in preventing angiogenesis in VEGF-independent tumors. However, CEACAM1 prevents M1 macrophage-mediated angiogenesis, suggesting that CEACAM1 blockade might promote angiogenesis in early breast cancer development.Citation72 This would be further enhanced by cancer cell downregulation of CEACAM1, which occurs during early stages of some types of cancers such as those associated with the colon, in contrast to late stage cancer cells that commonly upregulate CEACAM1 during tumor progression and invasion.Citation72
Granulocytes and neutrophils
While CEACAM1 is expected to act as an inhibitory molecule in granulocytes similarly to other immune cell types, its role is complicated by findings implicating it as an inhibitor of the apoptotic process. In cancer, neutrophils act both as a line of defense against the tumor and can simultaneously promote tumor progression by engaging factors that promote invasion, angiogenesis and metastasis.Citation73 While there is little CEACAM1 on the surface of resting human neutrophils, their activation causes rapid transport of CEACAM1, CEACAM6 and CEACAM8 from intracellular granules to the cell surface.Citation74-76 CEACAM3 is also expressed by human neutrophils where it plays a role in bacterial phagocytosis.Citation77 Sarantis and Gray-Owen have shown that human neutrophil CEACAM1 and CEACAM6 contribute to bacterial internalization without significant neutrophil activation.Citation77 While CEACAM3 is also expressed by neutrophils, this ITAM-containing activating receptor appears to be constitutively expressed at the neutrophil surface, which facilitates its role as a decoy receptor to capture CEACAM-targeting pathogens.Citation78 CEACAM3 engagement causes potent neutrophil degranulation, oxidative burst and inflammatory cytokine expression.Citation78 After neutrophil activation, these four CEACAMs are highly abundant on the neutrophil surface. While not previously appreciated, soluble CEACAM8 is released by degranulated neutrophils and can promote CEACAM1 inhibitory signaling on other cells, such as the SHP-1-dependent suppression of TLR2-dependent inflammatory responses in epithelial cells.Citation79 These results suggest that CEACAM1-specific inhibitors must avoid cross-reactivity with this closely related receptor. In addition, CEACAM1 activation has been reported to delay Fas ligand-induced apoptosis of human neutrophils using SHP1, ERK1/2 and caspase 3-mediated pathways,Citation80 which suggests that CEACAM1 inhibition on neutrophils may promote neutrophil apoptosis. Together, these results suggest that CEACAM1-targeting inhibitors must avoid cross-reactivity with other closely related receptors to avoid unforeseen effects on neutrophils.
Mice do not express CEACAM3, CEACAM6 or CEACAM8, so their contribution to granulocytic (or other) functions cannot be considered in wild type mouse lines. CEACAM1's function in granulopoiesis has been studied in CEACAM1-deficient mice. Interestingly, in contrast to the human studies discussed above, these animals develop systemic neutrophilia in association with slightly faster apoptosis but heightened neutrophil progenitor proliferation, together with an overall slower turnover rate of mature neutrophils.Citation64 Mechanistically, the SHP-1-dependent inhibition of G-CSFR and STAT3 normally provided by CEACAM1 was significantly decreased, accounting for increased responsiveness to neutrophil growth factors.Citation64 Since L. monocytogenes infection depends upon neutrophils for clearance, Ceacam1−/− mice exhibited higher neutrophil counts and increased levels of cytokines, resulting in both increased neutrophil production and Listeria clearance. However, counter-intuitively, this was associated with severe tissue damage, liver necrosis and immunopathology. Furthermore, CEACAM1-deficient mice died more quickly than wild-type mice, implicating neutrophil-induced immunopathology in the absence of CEACAM1 expression. This phenotype was reversed when CEACAM1 expression was re-established in chimeric mice exhibiting CEACAM1-deficient bone marrow. Although the studies with mouse and human neutrophils must be reconciled, these results suggest that neutrophil production and turnover must be closely monitored in clinical trials targeting human CEACAM1.
CEACAM1 also negatively regulates myeloid-dependent tumor angiogenesis by inhibiting the G-CSF and Bv8 pathways in melanoma and colorectal tumor models. Ceacam1−/− mice developing CRC liver metastasis also demonstrated less recruitment of CD11b+Gr1+ myeloid-derived suppressor cells (MDSC) from chimeric bone marrow transplants, and thus showed a significant decrease of liver metastatic development.Citation81 In addition, WT tumor-bearing mice treated with a CEACAM1-blocking antibody exhibited significantly reduced tumor growth and angiogenesis with reduced levels of CD11b+Gr1+ MDSCs in spleen and blood in a CRC model.Citation82 Interestingly, neutrophil infiltration density was correlated with higher CEACAM1 expression in tongue squamous carcinoma than in peritumoral tissue, correlating with lymph node metastasis, recurrence and survival,Citation83 consistent with other types of cancers demonstrating poor prognosis upon neutrophil infiltration.Citation73
These findings suggest a mechanism whereby pharmacological inhibition of CEACAM1 might result in decreased MDSC infiltration into tumors and, potentially, neutrophilia depending on the balance in humans between production and removal. However, it is unclear whether such a neutrophilia would be systemic and functioning in an antitumor role, or in a tumor-infiltrative role that would promote tumor immune evasion. In the context of conventional chemotherapy, where neutropenia is a common complication leading to devastating infections,Citation84 combination with a CEACAM1 inhibitor that may restore neutrophil counts might be beneficial. However, further complicating this issue, recent findings demonstrating that neutrophils suppress NK-mediated tumor cell clearance must be considered given CEACAM1's prominent role in this immune cell compartment.Citation85 It is clear that more studies are required to investigate neutrophil effects by CEACAM1 in different cancer contexts that may respond differently.
Taken together, decades of studies have implicated CEACAM1 in cancer immunology. While more fundamental research is required to further understand its function in B cells, neutrophils and macrophages, CEACAM1 presents as a valuable target for cancer immunotherapy in late stage cancer due to its well-defined inhibitory role in T and NK cells.
CEACAM1 in metabolism and tissue homeostasis
There is extensive knowledge implicating CEACAM1's role in metabolism and tissue homeostasis. This is pertinent in the context of obesity, insulin resistance and cardiovascular function.Citation86 While the direct link between CEACAM1's metabolic and cancer promoting functions have yet to be established in the literature, the metabolic impact of CEACAM1 inhibition in cancer patients can be inferred by its known involvement in metabolic signaling.
Insulin resistance and obesity
CEACAM1 is phosphorylated by the insulin receptor upon insulin stimulation.Citation87 This causes the increase of insulin receptor complex rate of internalization by endocytosis in clathrin-coated vesicles.Citation88-90 By binding to Shc upon phosphorylation, CEACAM1 sequesters Shc, downregulating the mitogenic action of insulin.Citation91 It is thus possible that in the context of CEACAM1 inhibition, Shc stabilization can lead to cancer promoting effects, a phenomenon that should be closely monitored in preclinical and clinical studies.Citation92
These initial findings suggested that CEACAM1 plays an important role in insulin internalization and that inhibition of CEACAM1 would result in hyperinsulinemia which was demonstrated with the creation of the LSACC mouse expressing a Ser phosphorylation-defective CEACAM1 (S503A) exclusively in the liver.Citation93 CEACAM1 Ser503 phosphorylation is required for Tyr488 phosphorylation by the insulin receptor and prevention of Tyr488 phosphorylation leads to mice developing hyperinsulinemia secondary to impaired insulin clearance, impaired glucose tolerance and random hyperglycemia. In addition, these mice display visceral adiposity with increased amounts of plasma free fatty acids and hepatic triglycerides.Citation93
Similar to the LSACC mice, the CEACAM1 knockout mice display diet-induced insulin resistance and are also prone to hepatic steatosis and non-alcoholic steatohepatitis (NASH) through elevated hepatic triglycerides and total serum cholesterol.Citation94,95 This effect is believed to be downstream of elevated fatty acid synthase (FAS) activity and leptin resistance.Citation96 Lee has documented that patients exhibiting high-grade fatty liver and obesity have lower CEACAM1 hepatic levels.Citation97 Monitoring for increased visceral fat, insulin resistance and hyperinsulinemia would then be crucial in patients being treated with a CEACAM1 inhibitor, and we suggest that diabetic patients be excluded from early trials with a CEACAM1 inhibitor.
Interestingly, upon insulin stimulation and subsequent to internalization, CEACAM1 also interacts with and reduces the activity of hepatic FAS, an enzyme that catabolizes fatty acid synthesis. However, the acute effect of insulin on hepatic FAS does not exist in the context of chronic hyperinsulinemia, likely due to the reduced ability of insulin to activate its receptor to induce CEACAM1 phosphorylation, and therefore inhibition of FAS function. Najjar et al. propose that this mechanism of FAS activity downregulation acts to reduce hepatic lipogenesis incurred by insulin pulses during refeeding. This phenotype may be relevant in the setting of CEACAM1 inhibition in cancer, as FAS is a known oncogene that is also being actively explored as a target to treat several cancers.Citation98 In the context of a monoclonal antibody binding to CEACAM1 on the surface of hepatocytes,Citation97 internalization of CEACAM1 may be induced, resulting in increased interaction, and therefore FAS inhibition. On the other hand, as CEACAM1 is expressed at the hepatocyte canalicular membrane, it should be inaccessible to the inhibitory antibody and thus should not perturb metabolic patient status.Citation97
Further validating CEACAM1's role in insulin clearance, removal of SHP-1, a tyrosine phosphatase dephosphorylating CEACAM1, displayed markedly increased insulin clearance in both cultured hepatocytes or mice deficient for a functional SHP-1.Citation99 Because SHP-1's role in cancer is unclear, with studies suggesting it can act as both a tumor suppressor and an oncogene,Citation100 pharmacological inhibition of CEACAM1 may have unpredicted effects due to SHP-1 being free to interact with other proteins.
In addition to a causal relationship between obesity and human CEACAM1,Citation97 C57BL6/J mice fed a high fat diet reduced hepatic CEACAM1 expression by >50% at 21 d and developed hyperinsulinemia, insulin resistance and elevated hepatic triglycerides.Citation101 These symptoms did not develop in mice expressing a liver-inducible Ceacam1 gene.Citation101,102 Furthermore, a murine study suggested that CEACAM1 reduction can be passed on to offspring subjected to prenatal stress in the form of maternal obesity.Citation103 These studies, in combination with findings in the LSACC and CEACAM1 knockout mice demonstrate that it is possible that CEACAM1 loss results in obesity, and vice versa. Other than diet and age studies performed in mouse Ceacam1 mutants, other contributing factors such as hormonal disturbances, human leukocyte antigen type and genetic polymorphisms have not yet been investigated in the context of CEACAM1 inhibition.Citation104 Short term inhibition of CEACAM1 as a cancer therapeutic may, however, prove beneficial in maintaining weight of cancer patients having cachexia.Citation105
In summary, CEACAM1 is a crucial molecule in insulin signaling and metabolism. The insulin signaling CEACAM1 function may prove beneficial in the context of monoclonal antibody inhibition due to its potential for preventing or treating cancer cachexia and inhibiting the function of FAS, a known oncogene. However, the metabolic aspect of CEACAM1's function must be closely monitored in the context of inhibition due to the potential for development of metabolic disorders that can affect the liver, an organ bearing a heavy burden in patients treated with a host of cytotoxic agents. We suggest that diabetic patients and those with liver disease should be carefully monitored and/or considered for exclusion from early trials of CEACAM1 inhibitors in cancer because of the distinct possibility of worsening these conditions due to CEACAM1's function in insulin clearance and preventing NASH.
Vascular homeostasis
CEACAM1 has been extensively studied in the context of vascular homeostasis, endothelial cell permeability, vasculogenesis, tumor angiogenesis and cardiovascular disease.Citation106 CEACAM1 is highly expressed in de novo blood vessels, but not established ones.Citation107-110 CEACAM1 also plays an important role in vascular endothelial lumen formation, given its function as a mediator of VEGF-induced vasculogenesis and angiogenesis, particularly in the context of cancer developmentCitation108-114 In addition, Ceacam1−/− mice develop endothelial dysfunction with these mice demonstrating impaired endothelial integrity with fat deposition and aortic plaque-like lesions in the aortaCitation115,116 due to insulin's regulation of altered nitric oxide production in aortic endothelial cells with CEACAM1 abolishment.
Ceacam1−/− mice also have increased blood pressure with increased activation of the renin–angiotensin system.Citation117,118 This effect is downstream of PI3K-AKT signaling pathways and depends on CREB family and NF-κB transcription factors.
CEACAM1's function in the early development of new vessels applies to angiogenesis in the context of a tumor.Citation119 CEACAM1 inhibitors may act as anti-angiogenic compounds, similar to the class of VEGF inhibitors approved for cancer therapy.Citation119 Since CEACAM1 inhibition will be used in the context of metastatic patients, this negates the risk posed by the possibility of increased vessel leakiness promoting metastasis.Citation114
CEACAM1 is expressed at higher levels in human patient serum in response to myocardial infarction.Citation120 Under these conditions, Ceacam1−/− mice exhibited lower mortality, improved cardiac function and lower myocardial expression of pro-apoptotic genes compared with wild-type littermates.Citation120 CEACAM1 is a novel serum biomarker for pericarditis.Citation121 These findings linking CEACAM1 to hypoxic conditions may stem from the induction of HIF-1α in hypoxia, which in turn induces expression of CEACAM1 through VEGF.Citation108
The link between CEACAM1 loss and cardiovascular function will require close monitoring in clinical trials inhibiting CEACAM1. Furthermore, a therapeutic antibody targeting CEACAM1 must be carefully designed to promote an antitumor angiogenic response rather than one that promotes metastasis.
Considering the major role played by CEACAM1 in cardiometabolic syndromes described above as well as its pertinent role as a co-inhibitory receptor in both lymphoid and myeloid compartments, CEACAM1 loss or its inhibition results in a pro-inflammatory condition due to the expression of various chemokines/cytokines such as interferon γ, IL1β, IL6, TNFα, leptin and TGFβ (reviewed in Ref. Citation[86]). Thus, high-fat feeding of Ceacam1 mutants stimulates a NASH-like condition as seen in human patients, progressive fibrosisCitation86 and renal dysfunctions.Citation118 CEACAM1 is also known to play a role in murine colitis.Citation5,30 These conditions should be appropriately monitored in future clinical trials.
CEACAM1 expression in cancer cells
While a CEACAM1 inhibitor would play an important role in modulating the tumor microenvironment and immune infiltrate, such an inhibitor could also work by attacking cancer cells directly; this is a unique feature of this class of therapies. CEACAM1 is highly expressed in several different cancers and is correlated with tumor progression, metastasis and overall survival.Citation122,123 A monoclonal antibody therapeutic targeting CEACAM1 can have direct antitumor effects, such as inhibiting tumor cell growth or activating immune cell function. If a CEACAM1-specific monoclonal antibody were to effectively inhibit tumor cells directly, while also activating the immune system as explained in the previous section, such an antibody would be a powerful therapeutic in the context of local and metastatic disease. In this section, we outline evidence gathered from cancer-specific CEACAM1 studies in several different cancer types that represent the most likely to benefit from treatment with a CEACAM1 inhibitor ( and ).
Figure 3. CEACAM1's function in cancer cells. CEACAM1 has been studied extensively in several cancer types. Green font represents findings from patient data, while black font represents experimental data. Non-small cell lung cancer (NSCLC): CEACAM1 is a specific biomarker, and its expression is correlated with increased microvessel density, metastasis and shortened overall survival. Pancreatic: CEACAM1 is a sensitive but not specific biomarker for pancreatic adenocarcinoma. shRNA knockdown of CEACAM1 in human PaCa5061 pancreatic adenocarcinoma cells implanted in mice resulted in longer overall survival relative to mice that received wild-type PaCa5061 cells. Colorectal: CEACAM1 is downregulated in colorectal adenomas but its expression is increased in advanced stages of colorectal cancer and correlated with increased invasiveness and metastasis. Bladder: Overexpression of CEACAM1 in bladder cancer cell lines results in slowed proliferation. shRNA knockdown of CEACAM1 in bladder cancer cell lines leads to increased angiogenesis of tumors implanted in mice via VEGF signaling. Melanoma: CEACAM1 expression is a diagnostic and prognostic biomarker for melanoma. High serum CEACAM1 in melanoma patients correlates with decreased survival and reduced responses to autologous vaccine and immunotherapy.
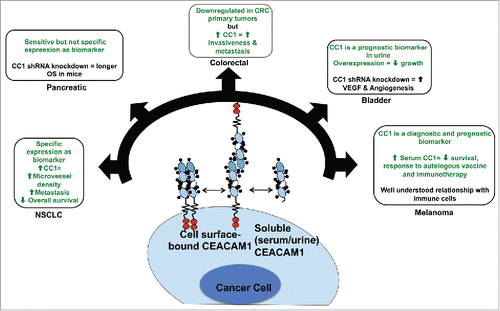
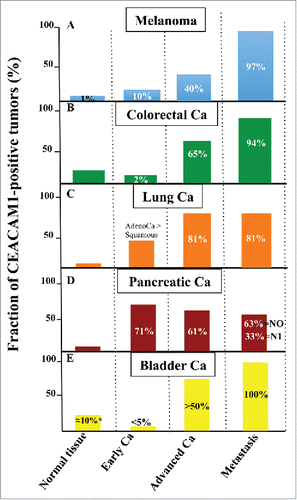
Figure 4. Expression of CEACAM1 in various types of cancers. Relative CEACAM1 expression is indicated in normal tissue, early carcinomas, advanced carcinomas and metastases for melanoma (A), colorectal (B), lung (C), pancreatic (D) and bladder (E) cancers. The numbers indicated in various columns represent fractions of patient samples positive for CEACAM1 expression in percentages. Information for this data has been gathered from (A), ref.124 and 127 (B), Citationref. 11,142 and Citation143 (C), ref.151,152 and 153 (D), ref.159, (E) ref.16 and 161
Melanoma
CEACAM1's role in melanoma is well defined. A significant number of studies have unanimously demonstrated the prognostic value of CEACAM1 expression in melanoma diagnosis and progression and metastasis, solidifying the claim that CEACAM1 can be applied as an improved prognostic biomarker over the commonly used Breslow depth.Citation122,124-127 This function has been confined to the overwhelming expression of CEACAM1-L rather than CEACAM1-S on melanoma cellsCitation56,128 (). Importantly, serum levels of soluble CEACAM1 is a prognostic and predictive biomarker in melanoma, with higher serum CEACAM1 levels correlating with decreased survival, decreased efficacy of autologous vaccination and failure to respond to immunotherapy.Citation14,129,130 Elevated CEACAM1 expression was observed in whole blood samples of melanoma patients treated with ipilimumab who exhibited gastrointestinal immune-related adverse events, thereby defining it as a potential biomarker to detect such pathologies.Citation131
The relationship of CEACAM1 with cells of the immune compartment has been studied in depth in melanoma; a high percentage of circulating NK and CD8+ T lymphocytes are CEACAM1+ in melanoma patients.Citation14 CEACAM1 homophilic interactions between melanoma cells and tumor-infiltrating lymphocytes (TILs) are thought to dampen in vivo TIL functions and thus limit efficacy of TIL adoptive cell transfer therapy in melanoma patients.Citation132 Continuous interferon-γ (IFNγ) production which promotes CEACAM1 expressionCitation133 could lead to CEACAM1 upregulation in surviving melanoma cells and in turn to elevated resistance to TIL-mediated killing.Citation134 A CEACAM1-directed therapy would be important in eliminating this potentially deleterious feedback loop. The CEACAM1-4L isoform downregulates cell surface levels of NKG2D ligands MICA and ULBP2 in a mouse melanoma cell line,Citation56 in line with the established function of CEACAM1 in NK and T cells, as discussed above. Due to the overwhelming evidence outlining CEACAM1's expression on metastatic melanoma cells,Citation122,124 its relationship with the immune system, and the astounding success of immunotherapies in metastatic melanoma, it is desirable to include a monoclonal antibody targeting CEACAM1 in trials with metastatic melanoma patients. CEACAM1's reported value in predicting prognosis raises interesting questions about the role of such an inhibitor when used in combination with already approved immunotherapies.Citation130
Colorectal cancer
CRC was among the first to be explored relative to CEACAM1, mainly because of CEA's discovery and use as a prognostic biomarker in this cancer.Citation135 Early studies demonstrated that CEACAM1 is downregulated early in CRC development as observed in adenomas, suggesting its role as a tumor suppressor.Citation136-139 Later studies demonstrated that an absence of CEACAM1 may promote early CRC development by removing CEACAM1-mediated growth inhibitory signaling since, for example, genetic reconstitution of CEACAM1 expression was observed to inhibit cancer cell proliferation.Citation140 On the other hand, CEACAM1-L expression is increased in advanced stages of CRC and is associated with invasiveness and metastasis when present in humans.Citation11,141,142,143 However, other studies have not demonstrated significant correlation between CEACAM1 expression and invasiveness.Citation144,145
The experimental data relating to CEACAM1 upregulation in CRC is equally debatable. Liver metastasis is decreased when CRC cells are engrafted into Ceacam1−/− mice on one hand,Citation81 while overexpression of CEACAM1 in CRC cells has been observed to decrease their propensity to metastasize to the liver in other xenograft models.Citation81,145 This might depend on the inherent genetic mutations of the murine cell lines used as xenografts or on intrinsic modulation of the transformative properties of CEA and CEACAM6 on the human cell lines used in these studies, which could inhibit cell differentiation or promote anoikis when engaged at the cell surface by CEACAM1 on the human CRC cells.Citation146-148 On the other hand, in a model of azoxymethane-dextran sodium sulfate-induced colitis, CEACAM1-deficient mice exhibited protection from colorectal neoplasia, consistent with the notion that CEACAM1 expression both promotes tumor-autonomous aggression and inhibits the immune system when expressed on the tumor and immune cells, respectively.Citation5 Consistent with the latter, CEACAM1 expression on the TILs in mouse and humans marks the most highly exhausted T cells.Citation5,44 In fact, both CEACAM1 and TIM-3 are highly expressed on circulating CD8+ T lymphocytes and TILs of CRC patients compared with normal tissue, with CRC tumors exhibiting a significant decrease in IFNγ production among the double-positive (CEACAM1+TIM-3+) T cells.Citation44 Thus, use of a CEACAM1-specific monoclonal antibody should be considered in clinical trials in patients with advanced CRC. This would have the potential to cause both the reversal of the inhibition imposed by CEACAM1 expression on immune cells and the potential for strong antitumor specific effects, which would be enabling for a therapeutic agent.
Non-small cell lung cancer
Serum and immunohistochemistry measurements of CEACAM1 have revealed it as a valuable prognostic biomarker in non-small cell lung cancer (NSCLC).Citation149 High CEACAM1-expressing NSCLCs exhibit high microvessel density, distant metastases, shorter median overall survival and progression-free survival.Citation123,150-152 While CEACAM1-L is the predominant form expressed in normal lung tissue, CEACAM1-S is expressed at higher levels in non-small cell and small cell lung adenocarcinoma.Citation153 Despite there being little functional data on CEACAM1's role in lung cancer cells, it is likely that the high level of specific CEACAM1 expression in these tumors will allow a monoclonal antibody to bind to lung cancer cells and have direct antitumor effects. Interestingly, CEACAM1 present in the urine of NSCLC patients represents an excellent biomarker when considered along with 3 or 4 other signature proteins.Citation154 In addition, cytokine-induced killer cells, defined as CD3+CD56+ T cells, as well as CD8+ T lymphocytes derived from NSCLC patients exhibit a sharp elevation of both CEACAM1 and TIM-3, which therefore might represent hallmarks of dysfunctional killer cells.Citation155 The current success of other similarly acting immunotherapies in the context of NSCLC may also predict CEACAM1's success in this disease type.Citation156
Pancreatic cancer
Multiple studies have identified CEACAM1 as a sensitive biomarker present in both the serum and tumor specimens of pancreatic cancer patients compared with healthy controls.Citation15,157-159 In addition, CEACAM1 has been strongly correlated with distant metastasis of pancreatic adenocarcinoma.Citation159 Knockdown of CEACAM1 in human PaCa5061 pancreatic cancer cells resulted in prolonged overall survival in mice subjected to subcutaneous injection of tumor cells, suggesting CEACAM1 increases the aggressiveness of the xenograft.Citation159 Although this was not examined attentively, it remains possible that this occurs through signal responses whereby CEACAM1 silencing leads to significant changes in chemokine/cytokine secretion from tumor cells thus influencing immune cell infiltration, as seen with metastatic CRC.Citation145 Although no information is yet available relative to CEACAM1 expression in immune cells infiltrating pancreatic tumors, immune activation in general and high TIM-3 expression on circulating CD4+ T lymphocytes in particular correlates with improved overall survival of pancreatic cancer patients.Citation160 It will be interesting to characterize CEACAM1 expression on the T cells in this case since it may be consistent with the proposed activating function of TIM-3 in the absence of CEACAM1 expression.Citation5 Taken together, these studies show promise for CEACAM1 as a valuable pancreatic cancer biomarker that could serve as a target of immunotherapy in this disease.
Bladder cancer
CEACAM1 has been proposed as a urinary marker for bladder cancer.Citation16 However, CEACAM1 may be a tumor suppressor in bladder cancer cells based upon analyses of bladder cancer cell lines. In invasive bladder cancers (pT2-T4), endothelial cells of immature blood vessels become CEACAM1+ whereas epithelial cells remain negative but correlate with high VEGF-C and -D.Citation161 Overexpression of CEACAM1 in several bladder cancer cell lines results in repressed growth on one hand, while silencing results in increased VEGF expression and enhanced blood vessel formation.Citation16,161,162 CEACAM1 is highly expressed on endothelial cells of angiogenic blood vessels, suggesting it may be promoting their growth once they are established, which would imply that CEACAM1 inhibition may be anti-angiogenic in this type of cancer.Citation161 TIM-3 is highly expressed in cancer cells, TILs and endothelial cells of bladder uro-epithelial carcinoma specimens, significantly correlating with advanced grade and tumor stage.Citation163 High TIM-3 expression also represents an independent predictor of shortened disease-free and overall survival.Citation163 Despite the anti-proliferative effects that CEACAM1 expression may have on bladder cancer cell lines, bladder cancer patients remain as good candidates to be treated with a CEACAM1-blocking drug because of the success of immunotherapies in early trials of metastatic bladder cancer,Citation164 and because the evidence for increased TIM-3 in the associated immune cells suggests the possibility that these may represent CEACAM1 co-expressing cells that are highly exhausted.Citation5
Conclusion
Decades of studies have characterized the role of CEACAM1 in the context of the immune system and cancer.Citation7 CEACAM1 represents a novel therapeutic target that can be exploited alongside existing immunotherapeutics to treat cancer, particularly due to its well-established inhibitory function in T and NK cells. In addition, CEACAM1 is also a tumor-associated molecule in several cancer types, potentially providing a second angle from which a CEACAM1-specific monoclonal antibody can target cancer cells. Future work will reveal the most appropriate indications for therapeutic agents targeting CEACAM1.
Disclosure of potential conflicts of interest
In accordance with Taylor & Francis policy and our ethical obligation as researchers, RSB, SDG and NB are consultants to Syntalogic which is developing immuno-oncology agents. We have disclosed those interests fully to Taylor & Francis, and we have in place an approved plan for managing any potential conflicts arising from that involvement.
Acknowledgments
The authors acknowledge the constructive feedback of Drs Phil Gold and Peter Siegel as well as the members of the Siegel and Beauchemin laboratory.
Funding
This work was supported by the Canadian Institutes of Health Research under Grant numbers 258962 to NB and MOP-15499 to SDG and National Institutes of Health Grant # DK51362 and AI073748 to RSB.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; https://doi.org/10.1016/j.cell.2011.02.013
- Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol 2010; 10:345-52; PMID:20414207; https://doi.org/10.1038/nri2747
- Ortenberg R, Sapir Y, Raz L, Hershkovitz L, Ben Arav A, Sapoznik S, Barshack I, Avivi C, Berkun Y, Besser MJ et al. Novel immunotherapy for malignant melanoma with a monoclonal antibody that blocks CEACAM1 homophilic interactions. Mol Cancer Ther 2012; 11:1300-10; PMID:22466331; https://doi.org/10.1158/1535-7163.MCT-11-0526
- Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, Hammarstrom S, Holmes KV, Karlsson A, Kuroki M et al. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res 1999; 252:243-9; PMID:11501563; https://doi.org/10.1006/excr.1999.4610
- Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 2015; 517:386-90; PMID:25363763; https://doi.org/10.1038/nature13848
- Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol 2006; 6:433-46; PMID:16724098; https://doi.org/10.1038/nri1864
- Beauchemin N, Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev 2013; 32:643-71; PMID:23903773; https://doi.org/10.1007/s10555-013-9444-6
- Beauchemin N, Kunath T, Robitaille J, Chow B, Turbide C, Daniels E, Veillette A. Association of biliary glycoprotein with protein tyrosine phosphatase SHP-1 in malignant colon epithelial cells. Oncogene 1997; 14:783-90; PMID:9047385; https://doi.org/10.1038/sj.onc.1200888
- Huber M, Izzi L, Grondin P, Houde C, Kunath T, Veillette A, Beauchemin N. The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J Biol Chem 1999; 274:335-44; PMID:9867848; https://doi.org/10.1074/jbc.274.1.335
- Kammerer R, Zimmermann W. Coevolution of activating and inhibitory receptors within mammalian carcinoembryonic antigen families. BMC Biol 2010; 8:12; PMID:20132533; https://doi.org/10.1186/1741-7007-8-12
- Ieda J, Yokoyama S, Tamura K, Takifuji K, Hotta T, Matsuda K, Oku Y, Nasu T, Kiriyama S, Yamamoto N et al. Re-expression of CEACAM1 long cytoplasmic domain isoform is associated with invasion and migration of colorectal cancer. Int J Cancer 2011; 129:1351-61; PMID:21413011; https://doi.org/10.1002/ijc.26072
- Chen L, Chen Z, Baker K, Halvorsen EM, da Cunha AP, Flak MB, Gerber G, Huang YH, Hosomi S, Arthur JC et al. The short isoform of the CEACAM1 receptor in intestinal T cells regulates mucosal immunity and homeostasis via Tfh cell induction. Immunity 2012; 37:930-46; PMID:23123061; https://doi.org/10.1016/j.immuni.2012.07.016
- Markel G, Achdout H, Katz G, Ling KL, Salio M, Gruda R, Gazit R, Mizrahi S, Hanna J, Gonen-Gross T et al. Biological function of the soluble CEACAM1 protein and implications in TAP2-deficient patients. Euro J Immunol 2004; 34:2138-48; PMID:15259011; https://doi.org/10.1002/eji.200425021
- Markel G, Ortenberg R, Seidman R, Sapoznik S, Koren-Morag N, Besser MJ, Bar J, Shapira R, Kubi A, Nardini G et al. Systemic dysregulation of CEACAM1 in melanoma patients. Cancer immunol Immunother 2010; 59:215-30; PMID:19633846; https://doi.org/10.1007/s00262-009-0740-5
- Simeone DM, Ji B, Banerjee M, Arumugam T, Li D, Anderson MA, Bamberger AM, Greenson J, Brand RE, Ramachandran V et al. CEACAM1, a novel serum biomarker for pancreatic cancer. Pancreas 2007; 34:436-43; PMID:17446843; https://doi.org/10.1097/MPA.0b013e3180333ae3
- Tilki D, Singer BB, Shariat SF, Behrend A, Fernando M, Irmak S, Buchner A, Hooper AT, Stief CG, Reich O et al. CEACAM1: a novel urinary marker for bladder cancer detection. Euro Urol 2010; 57:648-54; PMID:19487071; https://doi.org/10.1016/j.eururo.2009.05.040
- Wojtowicz ME, Dunn BK, Umar A. Immunologic approaches to cancer prevention-current status, challenges, and future perspectives. Semin Oncol 2016; 43:161-72; PMID:26970135; https://doi.org/10.1053/j.seminoncol.2015.11.001
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015; 14:561-84; PMID:26228759; https://doi.org/10.1038/nrd4591
- Beck A, Goetsch L, Dumontet C, Corvaia N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov 2017; 16(5):315-37; PMID:28303026; https://doi.org/10.1038/nrd.2016.268
- Moller MJ, Kammerer R, Grunert F, von Kleist S. Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell sub-population. Int J Cancer 1996; 65:740-5; PMID:8631584; https://doi.org/10.1002/(SICI)1097-0215(19960315)65:6%3c740::AID-IJC5%3e3.0.CO;2-Z
- Kammerer R, Hahn S, Singer BB, Luo JS, von Kleist S. Biliary glycoprotein (CD66a), a cell adhesion molecule of the immunoglobulin superfamily, on human lymphocytes: structure, expression and involvement in T cell activation. Eur J Immunol 1998; 28:3664-74; PMID:9842909; https://doi.org/10.1002/(SICI)1521-4141(199811)28:11%3c3664::AID-IMMU3664%3e3.0.CO;2-D
- Nakajima A, Iijima H, Neurath MF, Nagaishi T, Nieuwenhuis EE, Raychowdhury R, Glickman J, Blau DM, Russell S, Holmes KV et al. Activation-induced expression of carcinoembryonic antigen-cell adhesion molecule 1 regulates mouse T lymphocyte function. J Immunol (Baltimore, Md.: 1950) 2002; 168:1028-35; PMID:11801635; https://doi.org/10.4049/jimmunol.168.3.1028
- Morales VM, Christ A, Watt SM, Kim HS, Johnson KW, Utku N, Texieira AM, Mizoguchi A, Mizoguchi E, Russell GJ et al. Regulation of human intestinal intraepithelial lymphocyte cytolytic function by biliary glycoprotein (CD66a). J Immunol (Baltimore, Md.: 1950) 1999; 163:1363-70; PMID:10415036
- Donda A, Mori L, Shamshiev A, Carena I, Mottet C, Heim MH, Beglinger C, Grunert F, Rochlitz C, Terracciano L et al. Locally inducible CD66a (CEACAM1) as an amplifier of the human intestinal T cell response. Euro J Immunol 2000; 30:2593-603; PMID:11009093; https://doi.org/10.1002/1521-4141(200009)30:9%3c2593::AID-IMMU2593%3e3.0.CO;2-0
- Chen D, Iijima H, Nagaishi T, Nakajima A, Russell S, Raychowdhury R, Morales V, Rudd CE, Utku N, Blumberg RS. Carcinoembryonic antigen-related cellular adhesion molecule 1 isoforms alternatively inhibit and costimulate human T cell function. J Immunol (Baltimore, Md.: 1950) 2004; 172:3535-43; PMID:15004154; https://doi.org/10.4049/jimmunol.172.6.3535
- Singer BB, Scheffrahn I, Heymann R, Sigmundsson K, Kammerer R, Obrink B. Carcinoembryonic antigen-related cell adhesion molecule 1 expression and signaling in human, mouse, and rat leukocytes: evidence for replacement of the short cytoplasmic domain isoform by glycosylphosphatidylinositol-linked proteins in human leukocytes. J Immunol (Baltimore, Md.: 1950) 2002; 168:5139-46; PMID:11994468; https://doi.org/10.4049/jimmunol.168.10.5139
- Staub E, Rosenthal A, Hinzmann B. Systematic identification of immunoreceptor tyrosine-based inhibitory motifs in the human proteome. Cell Signal 2004; 16:435-56; PMID:14709333; https://doi.org/10.1016/j.cellsig.2003.08.013
- Chen Z, Chen L, Qiao SW, Nagaishi T, Blumberg RS. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits proximal TCR signaling by targeting ZAP-70. J Immunol (Baltimore, Md.: 1950) 2008; 180:6085-93; PMID:18424730; https://doi.org/10.4049/jimmunol.180.9.6085
- Lee HS, Ostrowski MA, Gray-Owen SD. CEACAM1 dynamics during Neisseria gonorrhoeae suppression of CD4+ T lymphocyte activation. J Immunol (Baltimore, Md.: 1950) 2008; 180:6827-35; PMID:18453603; https://doi.org/10.4049/jimmunol.180.10.6827
- Iijima H, Neurath MF, Nagaishi T, Glickman JN, Nieuwenhuis EE, Nakajima A, Chen D, Fuss IJ, Utku N, Lewicki DN et al. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J Exp Med 2004; 199:471-82; PMID:14970176; https://doi.org/10.1084/jem.20030437
- Dery KJ, Gaur S, Gencheva M, Yen Y, Shively JE, Gaur RK. Mechanistic control of carcinoembryonic antigen-related cell adhesion molecule-1 (CEACAM1) splice isoforms by the heterogeneous nuclear ribonuclear proteins hnRNP L, hnRNP A1, and hnRNP M. J Biol Chem 2011; 286:16039-51; PMID:21398516; https://doi.org/10.1074/jbc.M110.204057
- Nagaishi T, Pao L, Lin SH, Iijima H, Kaser A, Qiao SW, Chen Z, Glickman J, Najjar SM, Nakajima A et al. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity 2006; 25:769-81; PMID:17081782; https://doi.org/10.1016/j.immuni.2006.08.026
- Jin Y, Lin Y, Lin L, Sun Y, Zheng C. Carcinoembryonic antigen related cellular adhesion molecule 1 alleviates dextran sulfate sodium-induced ulcerative colitis in mice. Life Sci 2016; 149:120-8; PMID:26898127; https://doi.org/10.1016/j.lfs.2016.02.065
- Fujita M, Otsuka T, Mizuno M, Tomi C, Yamamura T, Miyake S. Carcinoembryonic antigen-related cell adhesion molecule 1 modulates experimental autoimmune encephalomyelitis via an iNKT cell-dependent mechanism. Am J Pathol 2009; 175:1116-23; PMID:19700760; https://doi.org/10.2353/ajpath.2009.090265
- Watanabe N, Kaminuma O, Kitamura N, Hiroi T. Induced treg cells augment the Th17-mediated intestinal inflammatory response in a CTLA4-dependent manner. PloS One 2016; 11:e0150244; PMID:26950218; https://doi.org/10.1371/journal.pone.0150244
- Song MY, Hong CP, Park SJ, Kim JH, Yang BG, Park Y, Kim SW, Kim KS, Lee JY, Lee SW et al. Protective effects of Fc-fused PD-L1 on two different animal models of colitis. Gut 2015; 64:260-71; PMID:24902766; https://doi.org/10.1136/gutjnl-2014-307311
- Costello CM, Mah N, Hasler R, Rosenstiel P, Waetzig GH, Hahn A, Lu T, Gurbuz Y, Nikolaus S, Albrecht M et al. Dissection of the inflammatory bowel disease transcriptome using genome-wide cDNA microarrays. PLoS Med 2005; 2:e199; PMID:16107186; https://doi.org/10.1371/journal.pmed.0020199
- Dooley TP, Curto EV, Reddy SP, Davis RL, Lambert GW, Wilborn TW, Elson CO. Regulation of gene expression in inflammatory bowel disease and correlation with IBD drugs: screening by DNA microarrays. Inflamm Bowel Dis 2004; 10:1-14; PMID:15058520; https://doi.org/10.1097/00054725-200401000-00001
- Li Y, Shively JE. CEACAM1 regulates Fas-mediated apoptosis in Jurkat T-cells via its interaction with beta-catenin. Exp Cell Res 2013; 319:1061-72; PMID:23499736; https://doi.org/10.1016/j.yexcr.2013.02.020
- Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016; 44:989-1004; PMID:27192565; https://doi.org/10.1016/j.immuni.2016.05.001
- Sada-Ovalle I, Chavez-Galan L, Torre-Bouscoulet L, Nava-Gamino L, Barrera L, Jayaraman P, Torres-Rojas M, Salazar-Lezama MA, Behar SM. The Tim3-galectin 9 pathway induces antibacterial activity in human macrophages infected with Mycobacterium tuberculosis. J Immunol (Baltimore, Md.: 1950) 2012; 189:5896-902; PMID:23180819; https://doi.org/10.4049/jimmunol.1200990
- Cho JL, Roche MI, Sandall B, Brass AL, Seed B, Xavier RJ, Medoff BD. Enhanced Tim3 activity improves survival after influenza infection. J Immunol (Baltimore, Md.: 1950) 2012; 189:2879-89; PMID:22875804; https://doi.org/10.4049/jimmunol.1102483
- Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T et al. Corrigendum: CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 2016; 536:359; PMID:26982724; https://doi.org/10.1038/nature17421
- Zhang Y, Cai P, Li L, Shi L, Chang P, Liang T, Yang Q, Liu Y, Wang L, Hu L. Co-expression of TIM-3 and CEACAM1 promotes T cell exhaustion in colorectal cancer patients. Int Immunopharmacol 2016; 43:210-8; PMID:28038383; https://doi.org/10.1016/j.intimp.2016.12.024
- Marquez-Rodas I, Cerezuela P, Soria A, Berrocal A, Riso A, Gonzalez-Cao M, Martin-Algarra S. Immune checkpoint inhibitors: therapeutic advances in melanoma. Ann Transl Med 2015; 3:267; PMID:26605313; https://doi.org/10.3978/j.issn.2305-5839.2015.10.27
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science (New York, N.Y.) 2011; 331:44-9; PMID:21212348; https://doi.org/10.1126/science.1198687
- Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 2015; 16:7-19; PMID:26694935; https://doi.org/10.1038/nrc.2015.5
- Markel G, Lieberman N, Katz G, Arnon TI, Lotem M, Drize O, Blumberg RS, Bar-Haim E, Mader R, Eisenbach L et al. CD66a interactions between human melanoma and NK cells: a novel class I MHC-independent inhibitory mechanism of cytotoxicity. J Immunol (Baltimore, Md.: 1950) 2002; 168:2803-10; PMID:11884449; https://doi.org/10.4049/jimmunol.168.6.2803
- Dupuis ML, Fiori V, Soriani A, Ricci B, Dominici S, Moricoli D, Ascione A, Santoni A, Magnani M, Cianfriglia M. The human antibody fragment DIATHIS1 specific for CEACAM1 enhances natural killer cell cytotoxicity against melanoma cell lines in vitro. J Immunother (Hagerstown, Md.: 1997) 2015; 38:357-70; PMID:26448580; https://doi.org/10.1097/CJI.0000000000000100
- Markel G, Mussaffi H, Ling KL, Salio M, Gadola S, Steuer G, Blau H, Achdout H, de Miguel M, Gonen-Gross T et al. The mechanisms controlling NK cell autoreactivity in TAP2-deficient patients. Blood 2004; 103:1770-8; PMID:14604968; https://doi.org/10.1182/blood-2003-06-2114
- Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012; 119:3734-43; PMID:22383801; https://doi.org/10.1182/blood-2011-11-392951
- Stern N, Markel G, Arnon TI, Gruda R, Wong H, Gray-Owen SD, Mandelboim O. Carcinoembryonic antigen (CEA) inhibits NK killing via interaction with CEA-related cell adhesion molecule 1. J Immunol (Baltimore, Md.: 1950) 2005; 174:6692-701; PMID:15905509; https://doi.org/10.4049/jimmunol.174.11.6692
- Markel G, Gruda R, Achdout H, Katz G, Nechama M, Blumberg RS, Kammerer R, Zimmermann W, Mandelboim O. The critical role of residues 43R and 44Q of carcinoembryonic antigen cell adhesion molecules-1 in the protection from killing by human NK cells. J Immunol (Baltimore, Md.: 1950) 2004; 173:3732-9; PMID:15356119; https://doi.org/10.4049/jimmunol.173.6.3732
- Stern-Ginossar N, Nedvetzki S, Markel G, Gazit R, Betser-Cohen G, Achdout H, Aker M, Blumberg RS, Davis DM, Appelmelk B et al. Intercellular transfer of carcinoembryonic antigen from tumor cells to NK cells. J Immunol (Baltimore, Md.: 1950) 2007; 179:4424-34; PMID:17878338; https://doi.org/10.4049/jimmunol.179.7.4424
- Chen Z, Chen L, Baker K, Olszak T, Zeissig S, Huang YH, Kuo TT, Mandelboim O, Beauchemin N, Lanier LL et al. CEACAM1 dampens antitumor immunity by down-regulating NKG2D ligand expression on tumor cells. J Exp Med 2011; 208:2633-40; PMID:22143889; https://doi.org/10.1084/jem.20102575
- Ullrich N, Heinemann A, Nilewski E, Scheffrahn I, Klode J, Scherag A, Schadendorf D, Singer BB, Helfrich I. CEACAM1-3S drives melanoma cells into NK cell-mediated cytolysis and enhances patient survival. Cancer Res 2015; 75:1897-907; PMID:25744717; https://doi.org/10.1158/0008-5472.CAN-14-1752
- Hosomi S, Chen Z, Baker K, Chen L, Huang YH, Olszak T, Zeissig S, Wang JH, Mandelboim O, Beauchemin N et al. CEACAM1 on activated NK cells inhibits NKG2D-mediated cytolytic function and signaling. Eur J Immunol 2013; 43:2473-83; PMID:23696226; https://doi.org/10.1002/eji.201242676
- Blake SJ, Stannard K, Liu J, Allen S, Yong MC, Mittal D, Aguilera AR, Miles JJ, Lutzky VP, de Andrade LF et al. Suppression of metastases using a new lymphocyte checkpoint target for cancer immunotherapy. Cancer Discov 2016; 6:446-59; PMID:26787820; https://doi.org/10.1158/2159-8290.CD-15-0944
- Zitvogel L, Kroemer G. Cancer: antibodies regulate antitumour immunity. Nature 2015; 521:35-37; PMID:25924066; https://doi.org/10.1038/nature14388
- Chen T, Zimmermann W, Parker J, Chen I, Maeda A, Bolland S. Biliary glycoprotein (BGPa, CD66a, CEACAM1) mediates inhibitory signals. J Leukoc Biol 2001; 70:335-40; PMID:11493628
- Greicius G, Severinson E, Beauchemin N, Obrink B, Singer BB. CEACAM1 is a potent regulator of B cell receptor complex-induced activation. J Leukoc Biol 2003; 74:126-34; PMID:12832451; https://doi.org/10.1189/jlb.1202594
- Khairnar V, Duhan V, Maney SK, Honke N, Shaabani N, Pandyra AA, Seifert M, Pozdeev V, Xu HC, Sharma P et al. CEACAM1 induces B-cell survival and is essential for protective antiviral antibody production. Nat Commun 2015; 6:6217; PMID:25692415; https://doi.org/10.1038/ncomms7217
- Lobo EO, Zhang Z, Shively JE. Pivotal advance: CEACAM1 is a negative coreceptor for the B cell receptor and promotes CD19-mediated adhesion of B cells in a PI3K-dependent manner. J Leukoc Biol 2009; 86:205-18; PMID:19454653; https://doi.org/10.1189/jlb.0109037
- Pan H, Shively JE. Carcinoembryonic antigen-related cell adhesion molecule-1 regulates granulopoiesis by inhibition of granulocyte colony-stimulating factor receptor. Immunity 2010; 33:620-31; PMID:21029969; https://doi.org/10.1016/j.immuni.2010.10.009
- Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin cancer Biol 2008; 18:349-55; PMID:18467122; https://doi.org/10.1016/j.semcancer.2008.03.004
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014; 41:49-61; PMID:25035953; https://doi.org/10.1016/j.immuni.2014.06.010
- Yu Q, Chow EM, Wong H, Gu J, Mandelboim O, Gray-Owen SD, Ostrowski MA. CEACAM1 (CD66a) promotes human monocyte survival via a phosphatidylinositol 3-kinase- and AKT-dependent pathway. J Biol Chem 2006; 281:39179-93; PMID:17071610; https://doi.org/10.1074/jbc.M608864200
- Satoh T, Nakagawa K, Sugihara F, Kuwahara R, Ashihara M, Yamane F, Minowa Y, Fukushima K, Ebina I, Yoshioka Y et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature 2017; 541:96-101; PMID:28002407; https://doi.org/10.1038/nature20611
- Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem 2007; 101:830-9; PMID:17211838; https://doi.org/10.1002/jcb.21186
- Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell 2016; 166:21-45; PMID:27368099; https://doi.org/10.1016/j.cell.2016.06.028
- Horst AK, Bickert T, Brewig N, Ludewig P, van Rooijen N, Schumacher U, Beauchemin N, Ito WD, Fleischer B, Wagener C et al. CEACAM1+ myeloid cells control angiogenesis in inflammation. Blood 2009; 113:6726-36; PMID:19273835; https://doi.org/10.1182/blood-2008-10-184556
- Samineni S, Zhang Z, Shively JE. Carcinoembryonic antigen-related cell adhesion molecule 1 negatively regulates granulocyte colony-stimulating factor production by breast tumor-associated macrophages that mediate tumor angiogenesis. Int J Cancer 2013; 133:394-407; PMID:23319418; https://doi.org/10.1002/ijc.28036
- Liang W, Ferrara N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res 2016; 4:83-91; PMID:26839309; https://doi.org/10.1158/2326-6066.CIR-15-0313
- Ducker TP, Skubitz KM. Subcellular localization of CD66, CD67, and NCA in human neutrophils. J Leukoc Biol 1992; 52:11-6; PMID:1640165
- Jantscheff P, Nagel G, Thompson J, Kleist SV, Embleton MJ, Price MR, Grunert F. A CD66a-specific, activation-dependent epitope detected by recombinant human single chain fragments (scFvs) on CHO transfectants and activated granulocytes. J Leukoc Biol 1996; 59:891-901; PMID:8691075
- Gray-Owen SD, Dehio C, Haude A, Grunert F, Meyer TF. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J 1997; 16:3435-45; PMID:9218786; https://doi.org/10.1093/emboj/16.12.3435
- Sintsova A, Sarantis H, Islam EA, Sun CX, Amin M, Chan CH, Stanners CP, Glogauer M, Gray-Owen SD. Global analysis of neutrophil responses to Neisseria gonorrhoeae reveals a self-propagating inflammatory program. PLoS Pathog 2014; 10:e1004341; PMID:25188454; https://doi.org/10.1371/journal.ppat.1004341
- Sarantis H, Gray-Owen SD. Defining the roles of human carcinoembryonic antigen-related cellular adhesion molecules during neutrophil responses to Neisseria gonorrhoeae. Infect Immun 2012; 80:345-58; PMID:22064717; https://doi.org/10.1128/IAI.05702-11
- Singer BB, Opp L, Heinrich A, Schreiber F, Binding-Liermann R, Berrocal-Almanza LC, Heyl KA, Muller MM, Weimann A, Zweigner J et al. Soluble CEACAM8 interacts with CEACAM1 inhibiting TLR2-triggered immune responses. PloS One 2014; 9:e94106; PMID:24743304; https://doi.org/10.1371/journal.pone.0094106
- Singer BB, Klaile E, Scheffrahn I, Muller MM, Kammerer R, Reutter W, Obrink B, Lucka L. CEACAM1 (CD66a) mediates delay of spontaneous and Fas ligand-induced apoptosis in granulocytes. Eur J Immunol 2005; 35:1949-59; PMID:15909305; https://doi.org/10.1002/eji.200425691
- Arabzadeh A, Chan C, Nouvion AL, Breton V, Benlolo S, DeMarte L, Turbide C, Brodt P, Ferri L, Beauchemin N. Host-related carcinoembryonic antigen cell adhesion molecule 1 promotes metastasis of colorectal cancer. Oncogene 2013; 32:849-60; PMID:22469976; https://doi.org/10.1038/onc.2012.112
- Lu R, Kujawski M, Pan H, Shively JE. Tumor angiogenesis mediated by myeloid cells is negatively regulated by CEACAM1. Cancer Res 2012; 72:2239-50; PMID:22406619; https://doi.org/10.1158/0008-5472.CAN-11-3016
- Wang N, Feng Y, Wang Q, Liu S, Xiang L, Sun M, Zhang X, Liu G, Qu X, Wei F. Neutrophils infiltration in the tongue squamous cell carcinoma and its correlation with CEACAM1 expression on tumor cells. PloS One 2014; 9:e89991; PMID:24587171; https://doi.org/10.1371/journal.pone.0089991
- Lustberg MB. Management of neutropenia in cancer patients. Clin Adv Hematol Oncol 2012; 10:825-6; PMID:23271355
- Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, Chen MB, Krall JA, DeCock J, Zervantonakis IK et al. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov 2016; 6(6):630-49; PMID:27072748; https://doi.org/10.1158/2159-8290.CD-15-1157
- Najjar SM, Russo L. CEACAM1 loss links inflammation to insulin resistance in obesity and non-alcoholic steatohepatitis (NASH). Semin Immunopathol 2014; 36:55-71; PMID:24258517; https://doi.org/10.1007/s00281-013-0407-3
- Najjar SM, Philippe N, Suzuki Y, Ignacio GA, Formisano P, Accili D, Taylor SI. Insulin-stimulated phosphorylation of recombinant pp120/HA4, an endogenous substrate of the insulin receptor tyrosine kinase. Biochemistry 1995; 34:9341-9; PMID:7626603; https://doi.org/10.1021/bi00029a009
- Li Calzi S, Choice CV, Najjar SM. Differential effect of pp120 on insulin endocytosis by two variant insulin receptor isoforms. Am J Physiol 1997; 273:E801-8; PMID:9357811
- Formisano P, Najjar SM, Gross CN, Philippe N, Oriente F, Kern-Buell CL, Accili D, Gorden P. Receptor-mediated internalization of insulin. Potential role of pp120/HA4, a substrate of the insulin receptor kinase. J Biol Chem 1995; 270:24073-7; PMID:7592607; https://doi.org/10.1074/jbc.270.41.24073
- Najjar SM, Choice CV, Soni P, Whitman CM, Poy MN. Effect of pp120 on receptor-mediated insulin endocytosis is regulated by the juxtamembrane domain of the insulin receptor. J Biol Chem 1998; 273:12923-8; PMID:9582324; https://doi.org/10.1074/jbc.273.21.12923
- Poy MN, Ruch RJ, Fernstrom MA, Okabayashi Y, Najjar SM. Shc and CEACAM1 interact to regulate the mitogenic action of insulin. J Biol Chem 2002; 277:1076-84; PMID:11694516; https://doi.org/10.1074/jbc.M108415200
- Ursini-Siegel J, Muller WJ. The ShcA adaptor protein is a critical regulator of breast cancer progression. Cell Cycle (Georgetown, Tex.) 2008; 7:1936-43; PMID:18604176; https://doi.org/10.4161/cc.7.13.6205
- Poy MN, Yang Y, Rezaei K, Fernstrom MA, Lee AD, Kido Y, Erickson SK, Najjar SM. CEACAM1 regulates insulin clearance in liver. Nat Genet 2002; 30:270-6; PMID:11850617; https://doi.org/10.1038/ng840
- Xu E, Dubois MJ, Leung N, Charbonneau A, Turbide C, Avramoglu RK, DeMarte L, Elchebly M, Streichert T, Levy E et al. Targeted disruption of carcinoembryonic antigen-related cell adhesion molecule 1 promotes diet-induced hepatic steatosis and insulin resistance. Endocrinology 2009; 150:3503-12; PMID:19406938; https://doi.org/10.1210/en.2008-1439
- Ghosh S, Kaw M, Patel PR, Ledford KJ, Bowman TA, McInerney MF, Erickson SK, Bourey RE, Najjar SM. Mice with null mutation of Ceacam I develop nonalcoholic steatohepatitis. Hepat Med 2010; 2010:69-78; PMID:21949477; https://doi.org/10.2147/HMER.S8902
- Heinrich G, Russo L, Castaneda TR, Pfeiffer V, Ghadieh HE, Ghanem SS, Wu J, Faulkner LD, Ergun S, McInerney MF et al. Leptin resistance contributes to obesity in mice with null mutation of carcinoembryonic antigen cell adhesion molecule 1. J Biol Chem 2016; 291(21):11124-32; PMID:27002145; https://doi.org/10.1074/jbc.M116.716431
- Lee W. The CEACAM1 expression is decreased in the liver of severely obese patients with or without diabetes. Diagn Pathol 2011; 6:40; PMID:21569294; https://doi.org/10.1186/1746-1596-6-40
- Baron A, Migita T, Tang D, Loda M. Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem 2004; 91:47-53; PMID:14689581; https://doi.org/10.1002/jcb.10708
- Dubois MJ, Bergeron S, Kim HJ, Dombrowski L, Perreault M, Fournes B, Faure R, Olivier M, Beauchemin N, Shulman GI et al. The SHP-1 protein tyrosine phosphatase negatively modulates glucose homeostasis. Nat Med 2006; 12:549-56; PMID:16617349; https://doi.org/10.1038/nm1397
- Wu C, Sun M, Liu L, Zhou GW. The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene 2003; 306:1-12; PMID:12657462; https://doi.org/10.1016/S0378-1119(03)00400-1
- Al-Share QY, DeAngelis AM, Lester SG, Bowman TA, Ramakrishnan SK, Abdallah SL, Russo L, Patel PR, Kaw MK, Raphael CK et al. Forced hepatic overexpression of CEACAM1 curtails diet-induced insulin resistance. Diabetes 2015; 64:2780-90; PMID:25972571; https://doi.org/10.2337/db14-1772
- Lester SG, Russo L, Ghanem SS, Khuder SS, DeAngelis AM, Esakov EL, Bowman TA, Heinrich G, Al-Share QY, McInerney MF et al. Hepatic CEACAM1 over-expression protects against diet-induced fibrosis and inflammation in white adipose tissue. Front Endocrinol 2015; 6:116; PMID:26284027; https://doi.org/10.3389/fendo.2015.00116
- Balasubramanian P, Varde PA, Abdallah SL, Najjar SM, MohanKumar PS, MohanKumar SM. Differential effects of prenatal stress on metabolic programming in diet-induced obese and dietary-resistant rats. Am J Physiol Endocrinol Metab 2015; 309:E582-8; PMID:26219866; https://doi.org/10.1152/ajpendo.00167.2015
- DeAngelis AM, Heinrich G, Dai T, Bowman TA, Patel PR, Lee SJ, Hong EG, Jung DY, Assmann A, Kulkarni RN et al. Carcinoembryonic antigen-related cell adhesion molecule 1: a link between insulin and lipid metabolism. Diabetes 2008; 57:2296-303; PMID:18544705; https://doi.org/10.2337/db08-0379
- Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014; 14:754-62; PMID:25291291; https://doi.org/10.1038/nrc3829
- Rueckschloss U, Kuerten S, Ergun S. The role of CEA-related cell adhesion molecule-1 (CEACAM1) in vascular homeostasis. Histochem Cell Biol 2016; 146:657-71; PMID:27695943; https://doi.org/10.1007/s00418-016-1505-9
- Sawa H, Kamada K, Sato H, Sendo S, Kondo A, Saito I, Edlund M, Obrink B. C-CAM expression in the developing rat central nervous system. Brain Res Dev Brain Res 1994; 78:35-43; PMID:8004772; https://doi.org/10.1016/0165-3806(94)90006-X
- rgun S, Kilik N, Ziegeler G, Hansen A, Nollau P, Gotze J, Wurmbach JH, Horst A, Weil J, Fernando M et al. CEA-related cell adhesion molecule 1: a potent angiogenic factor and a major effector of vascular endothelial growth factor. Mol Cell 2000; 5:311-20; PMID:10882072; https://doi.org/10.1016/S1097-2765(00)80426-8
- Kilic N, Oliveira-Ferrer L, Wurmbach JH, Loges S, Chalajour F, Neshat-Vahid S, Weil J, Fernando M, Ergun S. Pro-angiogenic signaling by the endothelial presence of CEACAM1. J Biol Chem 2005; 280:2361-69; PMID:15536067; https://doi.org/10.1074/jbc.M409407200
- Tilki D, Irmak S, Oliveira-Ferrer L, Hauschild J, Miethe K, Atakaya H, Hammerer P, Friedrich MG, Schuch G, Galalae R et al. CEA-related cell adhesion molecule-1 is involved in angiogenic switch in prostate cancer. Oncogene 2006; 25:4965-74; PMID:16568082; https://doi.org/10.1038/sj.onc.1209514
- Gu A, Tsark W, Holmes KV, Shively JE. Role of Ceacam1 in VEGF induced vasculogenesis of murine embryonic stem cell-derived embryoid bodies in 3D culture. Exp Cell Res 2009; 315:1668-82; PMID:19285068; https://doi.org/10.1016/j.yexcr.2009.02.026
- Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergun S. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development (Cambridge, England) 2006; 133:1543-51; PMID:16524930; https://doi.org/10.1242/dev.02315
- Horst AK, Ito WD, Dabelstein J, Schumacher U, Sander H, Turbide C, Brummer J, Meinertz T, Beauchemin N, Wagener C. Carcinoembryonic antigen-related cell adhesion molecule 1 modulates vascular remodeling in vitro and in vivo. J Clin Invest 2006; 116:1596-605; PMID:16680193; https://doi.org/10.1172/JCI24340
- Gerstel D, Wegwitz F, Jannasch K, Ludewig P, Scheike K, Alves F, Beauchemin N, Deppert W, Wagener C, Horst AK. CEACAM1 creates a pro-angiogenic tumor microenvironment that supports tumor vessel maturation. Oncogene 2011; 30:4275-88; PMID:21532628; https://doi.org/10.1038/onc.2011.146
- Najjar SM, Ledford KJ, Abdallah SL, Paus A, Russo L, Kaw MK, Ramakrishnan SK, Muturi HT, Raphael CK, Lester SG et al. Ceacam1 deletion causes vascular alterations in large vessels. Am J Physiol Endocrinol Metab 2013; 305:E519-29; PMID:23800882; https://doi.org/10.1152/ajpendo.00266.2013
- Nouvion AL, Oubaha M, Leblanc S, Davis EC, Jastrow H, Kammerer R, Breton V, Turbide C, Ergun S, Gratton JP et al. CEACAM1: a key regulator of vascular permeability. J Cell Sci 2010; 123:4221-30; PMID:21081647; https://doi.org/10.1242/jcs.073635
- Huang J, Ledford KJ, Pitkin WB, Russo L, Najjar SM, Siragy HM. Targeted deletion of murine CEACAM 1 activates PI3K-Akt signaling and contributes to the expression of (Pro)renin receptor via CREB family and NF-kappaB transcription factors. Hypertension 2013; 62:317-23; PMID:23734002; https://doi.org/10.1161/HYPERTENSIONAHA.113.01324
- Li C, Culver SA, Quadri S, Ledford KL, Al-Share QY, Ghadieh HE, Najjar SM, Siragy HM. High-fat diet amplifies renal renin angiotensin system expression, blood pressure elevation, and renal dysfunction caused by Ceacam1 null deletion. Am J Physiol Endocrinol Metab 2015; 309:E802-10; PMID:26374765; https://doi.org/10.1152/ajpendo.00158.2015
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 2007; 6:273-86; PMID:17396134; https://doi.org/10.1038/nrd2115
- Wang Y, Chen Y, Yan Y, Li X, Chen G, He N, Shen S, Chen G, Zhang C, Liao W et al. Loss of CEACAM1, a tumor-associated factor, attenuates post-infarction cardiac remodeling by inhibiting apoptosis. Sci Rep 2016; 6:21972; PMID:26911181; https://doi.org/10.1038/srep21972
- Markel G, Imazio M, Koren-Morag N, Galore-Haskel G, Schachter J, Besser M, Cumetti D, Maestroni S, Altman A, Shoenfeld Y et al. CEACAM1 and MICA as novel serum biomarkers in patients with acute and recurrent pericarditis. Oncotarget 2016; 7(14):17885-95; PMID:26909604; https://doi.org/10.18632/oncotarget.7530
- Thies A, Moll I, Berger J, Wagener C, Brummer J, Schulze HJ, Brunner G, Schumacher U. CEACAM1 expression in cutaneous malignant melanoma predicts the development of metastatic disease. J Clin Oncol 2002; 20:2530-6; PMID:12011132; https://doi.org/10.1200/JCO.2002.05.033
- Thom I, Schult-Kronefeld O, Burkholder I, Schuch G, Andritzky B, Kastendieck H, Edler L, Wagener C, Bokemeyer C, Schumacher U et al. Expression of CEACAM-1 in pulmonary adenocarcinomas and their metastases. Anticancer Res 2009; 29:249-54; PMID:19331157
- Thies A, Berlin A, Brunner G, Schulze HJ, Moll I, Pfuller U, Wagener C, Schachner M, Altevogt P, Schumacher U. Glycoconjugate profiling of primary melanoma and its sentinel node and distant metastases: implications for diagnosis and pathophysiology of metastases. Cancer Lett 2007; 248:68-80; PMID:16822608; https://doi.org/10.1016/j.canlet.2006.05.020
- Kluger HM, Hoyt K, Bacchiocchi A, Mayer T, Kirsch J, Kluger Y, Sznol M, Ariyan S, Molinaro A, Halaban R. Plasma markers for identifying patients with metastatic melanoma. Clin Cancer Res 2011; 17:2417-25; PMID:21487066; https://doi.org/10.1158/1078-0432.CCR-10-2402
- Khatib N, Pe'er J, Ortenberg R, Schachter J, Frenkel S, Markel G, Amer R. Carcinoembryonic antigen cell adhesion molecule-1 (CEACAM1) in posterior uveal melanoma: correlation with clinical and histological survival markers. Invest Ophthalmol Vis Sci 2011; 52:9368-72; PMID:22039239; https://doi.org/10.1167/iovs.10-6006
- Gambichler T, Grothe S, Rotterdam S, Altmeyer P, Kreuter A. Protein expression of carcinoembryonic antigen cell adhesion molecules in benign and malignant melanocytic skin lesions. Am J Clin Pathol 2009; 131:782-7; PMID:19461083; https://doi.org/10.1309/AJCP24KXJVBZXENS
- Ortenberg R, Galore-Haskel G, Greenberg I, Zamlin B, Sapoznik S, Greenberg E, Barshack I, Avivi C, Feiler Y, Zan-Bar I et al. CEACAM1 promotes melanoma cell growth through Sox-2. Neoplasia (New York, N.Y.) 2014; 16:451-60; PMID:24931667; https://doi.org/10.1016/j.neo.2014.05.003
- Sivan S, Suzan F, Rona O, Tamar H, Vivian B, Tamar P, Jacob S, Gal M, Michal L. Serum CEACAM1 correlates with disease progression and survival in malignant melanoma patients. Clin Dev Immunol 2012; 2012:290536; PMID:22291846; https://doi.org/10.1155/2012/290536
- Ortenberg R, Sapoznik S, Zippel D, Shapira-Frommer R, Itzhaki O, Kubi A, Zikich D, Besser MJ, Schachter J, Markel G. Serum CEACAM1 elevation correlates with melanoma progression and failure to respond to adoptive cell transfer immunotherapy. J Immunol Res 2015; 2015:902137; PMID:26688824; https://doi.org/10.1155/2015/902137
- Shahabi V, Berman D, Chasalow SD, Wang L, Tsuchihashi Z, Hu B, Panting L, Jure-Kunkel M, Ji RR. Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events. J Transl Med 2013; 11:75; PMID:23521917; https://doi.org/10.1186/1479-5876-11-75
- Markel G, Seidman R, Stern N, Cohen-Sinai T, Izhaki O, Katz G, Besser M, Treves AJ, Blumberg RS, Loewenthal R et al. Inhibition of human tumor-infiltrating lymphocyte effector functions by the homophilic carcinoembryonic cell adhesion molecule 1 interactions. J Immunol (Baltimore, Md.: 1950) 2006; 177:6062-71; PMID:17056532; https://doi.org/10.4049/jimmunol.177.9.6062
- Takahashi H, Okai Y, Paxton RJ, Hefta LJ, Shively JE. Differential regulation of carcinoembryonic antigen and biliary glycoprotein by gamma-interferon. Cancer Res 1993; 53:1612-9; PMID:8453631
- Markel G, Seidman R, Cohen Y, Besser MJ, Sinai TC, Treves AJ, Orenstein A, Berger R, Schachter J. Dynamic expression of protective CEACAM1 on melanoma cells during specific immune attack. Immunology 2009; 126:186-200; PMID:18557789; https://doi.org/10.1111/j.1365-2567.2008.02888.x
- Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med 1965; 122:467-81; PMID:4953873; https://doi.org/10.1084/jem.122.3.467
- Neumaier M, Paululat S, Chan A, Matthaes P, Wagener C. Biliary glycoprotein, a potential human cell adhesion molecule, is down-regulated in colorectal carcinomas. Proc Natl Acad Sci U S A 1993; 90:10744-8; PMID:7504281; https://doi.org/10.1073/pnas.90.22.10744
- Kunath T, Ordonez-Garcia C, Turbide C, Beauchemin N. Inhibition of colonic tumor cell growth by biliary glycoprotein. Oncogene 1995; 11:2375-82; PMID:8570189
- Nollau P, Scheller H, Kona-Horstmann M, Rohde S, Hagenmuller F, Wagener C, Neumaier M. Expression of CD66a (human C-CAM) and other members of the carcinoembryonic antigen gene family of adhesion molecules in human colorectal adenomas. Cancer Res 1997; 57:2354-57; PMID:9192807
- Turbide C, Kunath T, Daniels E, Beauchemin N. Optimal ratios of biliary glycoprotein isoforms required for inhibition of colonic tumor cell growth. Cancer Res 1997; 57:2781-8; PMID:9205090
- Izzi L, Turbide C, Houde C, Kunath T, Beauchemin N. cis-Determinants in the cytoplasmic domain of CEACAM1 responsible for its tumor inhibitory function. Oncogene 1999; 18:5563-72; PMID:10523833; https://doi.org/10.1038/sj.onc.1202935
- Yeatman TJ, Mao W, Karl RC. Biliary glycoprotein is overexpressed in human colon cancer cells with high metastatic potential. J Gastrointest Sur 1997; 1:292-8; PMID:9834361; https://doi.org/10.1016/S1091-255X(97)80123-0
- Kang WY, Chen WT, Wu MT, Chai CY. The expression of CD66a and possible roles in colorectal adenoma and adenocarcinoma. Int J Colorectal Dis 2007; 22:869-74; PMID:17143599; https://doi.org/10.1007/s00384-006-0247-x
- Song JH, Cao Z, Yoon JH, Nam SW, Kim SY, Lee JY, Park WS. Genetic alterations and expression pattern of CEACAM1 in colorectal adenomas and cancers. Pathol Oncol Res 2011; 17:67-74; PMID:20524097; https://doi.org/10.1007/s12253-010-9282-6
- Ou G, Baranov V, Lundmark E, Hammarstrom S, Hammarstrom ML. Contribution of intestinal epithelial cells to innate immunity of the human gut––studies on polarized monolayers of colon carcinoma cells. Scand J Immunol 2009; 69:150-61; PMID:19170965; https://doi.org/10.1111/j.1365-3083.2008.02208.x
- Arabzadeh A, Dupaul-Chicoine J, Breton V, Haftchenary S, Yumeen S, Turbide C, Saleh M, McGregor K, Greenwood CM, Akavia UD et al. Carcinoembryonic Antigen Cell Adhesion Molecule 1 long isoform modulates malignancy of poorly differentiated colon cancer cells. Gut 2016; 65:821-9; PMID:25666195; https://doi.org/10.1136/gutjnl-2014-308781
- Ilantzis C, DeMarte L, Screaton RA, Stanners CP. Deregulated expression of the human tumor marker CEA and CEA family member CEACAM6 disrupts tissue architecture and blocks colonocyte differentiation. Neoplasia (New York, N.Y.) 2002; 4:151-63; PMID:11896570; https://doi.org/10.1038/sj.neo.7900201
- Ordonez C, Screaton RA, Ilantzis C, Stanners CP. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res 2000; 60:3419-24; PMID:10910050
- Camacho-Leal P, Stanners CP. The human carcinoembryonic antigen (CEA) GPI anchor mediates anoikis inhibition by inactivation of the intrinsic death pathway. Oncogene 2008; 27:1545-53; PMID:17891182; https://doi.org/10.1038/sj.onc.1210789
- Zhou MQ, Du Y, Liu YW, Wang YZ, He YQ, Yang CX, Wang WJ, Gao F. Clinical and experimental studies regarding the expression and diagnostic value of carcinoembryonic antigen-related cell adhesion molecule 1 in non-small-cell lung cancer. BMC Cancer 2013; 13:359; PMID:23885995; https://doi.org/10.1186/1471-2407-13-359
- Dango S, Sienel W, Schreiber M, Stremmel C, Kirschbaum A, Pantel K, Passlick B. Elevated expression of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM-1) is associated with increased angiogenic potential in non-small-cell lung cancer. Lung Cancer (Amsterdam, Netherlands) 2008; 60:426-33; PMID:18215438; https://doi.org/10.1016/j.lungcan.2007.11.015
- Laack E, Nikbakht H, Peters A, Kugler C, Jasiewicz Y, Edler L, Brummer J, Schumacher U, Hossfeld DK. Expression of CEACAM1 in adenocarcinoma of the lung: a factor of independent prognostic significance. J Clin Oncol 2002; 20:4279-84; PMID:12409325; https://doi.org/10.1200/JCO.2002.08.067
- Sienel W, Dango S, Woelfle U, Morresi-Hauf A, Wagener C, Brummer J, Mutschler W, Passlick B, Pantel K. Elevated expression of carcinoembryonic antigen-related cell adhesion molecule 1 promotes progression of non-small cell lung cancer. Clin Cancer Res 2003; 9:2260-6; PMID:12796394
- Wang L, Lin SH, Wu WG, Kemp BL, Walsh GL, Hong WK, Mao L. C-CAM1, a candidate tumor suppressor gene, is abnormally expressed in primary lung cancers. Clin Cancer Res 2000; 6:2988-93; PMID:10955775
- Nolen BM, Lomakin A, Marrangoni A, Velikokhatnaya L, Prosser D, Lokshin AE. Urinary protein biomarkers in the early detection of lung cancer. Cancer Prev Res (Phila) 2015; 8:111-9; PMID:25416410; https://doi.org/10.1158/1940-6207.CAPR-14-0210
- Zhang L, Wang J, Wei F, Wang K, Sun Q, Yang F, Jin H, Zheng Y, Zhao H, Wang L et al. Profiling the dynamic expression of checkpoint molecules on cytokine-induced killer cells from non-small-cell lung cancer patients. Oncotarget 2016; 7:43604-15; PMID:27283895; https://doi.org/10.18632/oncotarget.9871
- Santabarbara G, Maione P, Rossi A, Palazzolo G, Gridelli C. The role of pembrolizumab in the treatment of advanced non-small cell lung cancer. Ann Transl Med 2016; 4:215; PMID:27386489; https://doi.org/10.21037/atm.2016.05.64
- Gong DY, Fu HX, Peng Y, You YQ, Li ZP. Diagnostic value of serum CEACAM1 in patients with pancreatic cancer. Nan Fang Yi Ke Da Xue Xue Bao J Southern Med University 2011; 31:164-6; PMID:21269981
- Giulietti M, Occhipinti G, Principato G, Piva F. Weighted gene co-expression network analysis reveals key genes involved in pancreatic ductal adenocarcinoma development. Cell Oncol (Dordrecht) 2016; 39:379-88; PMID:27240826; https://doi.org/10.1007/s13402-016-0283-7
- Gebauer F, Wicklein D, Horst J, Sundermann P, Maar H, Streichert T, Tachezy M, Izbicki JR, Bockhorn M, Schumacher U. Carcinoembryonic antigen-related cell adhesion molecules (CEACAM) 1, 5 and 6 as biomarkers in pancreatic cancer. PloS One 2014; 9:e113023; PMID:25409014; https://doi.org/10.1371/journal.pone.0113023
- `Farren MR, Mace TA, Geyer S, Mikhail S, Wu C, Ciombor K, Tahiri S, Ahn D, Noonan AM, Villalona-Calero M et al. Systemic immune activity predicts overall survival in treatment-naïve patients with metastatic pancreatic cancer. Clin Cancer Res 2016; 22:2565-74; PMID:26719427; https://doi.org/10.1158/1078-0432.CCR-15-1732
- Oliveira-Ferrer L, Tilki D, Ziegeler G, Hauschild J, Loges S, Irmak S, Kilic E, Huland H, Friedrich M, Ergun S. Dual role of carcinoembryonic antigen-related cell adhesion molecule 1 in angiogenesis and invasion of human urinary bladder cancer. Cancer Res 2004; 64:8932-8; PMID:15604255; https://doi.org/10.1158/0008-5472.CAN-04-0505
- Kleinerman DI, Dinney CP, Zhang WW, Lin SH, Van NT, Hsieh JT. Suppression of human bladder cancer growth by increased expression of C-CAM1 gene in an orthotopic model. Cancer Res 1996; 56:3431-5; PMID:8758907
- Yang M, Yu Q, Liu J, Fu W, Cao Y, Yu L, Shao S, Wang X, Niu H, Wang Y. T-cell immunoglobulin mucin-3 expression in bladder urothelial carcinoma: clinicopathologic correlations and association with survival. J Surg Oncol 2015; 112:430-5; PMID:26265374; https://doi.org/10.1002/jso.24012
- Zibelman M, Plimack ER. Systemic therapy for bladder cancer finally comes into a new age. Future Oncol (London, England) 2016; 12(19):2227-42; PMID:27402371; https://doi.org/10.2217/fon-2016-0135
