ABSTRACT
Dendritic cell (DC)-based vaccines against cancer have been extensively developed over the past two decades. Typically DC-based cancer immunotherapy entails loading patient-derived DCs with an appropriate source of tumor-associated antigens (TAAs) and efficient DC stimulation through a so-called “maturation cocktail” (typically a combination of pro-inflammatory cytokines and Toll-like receptor agonists), followed by DC reintroduction into patients. DC vaccines have been documented to (re)activate tumor-specific T cells in both preclinical and clinical settings. There is considerable clinical interest in combining DC-based anticancer vaccines with T cell-targeting immunotherapies. This reflects the established capacity of DC-based vaccines to generate a pool of TAA-specific effector T cells and facilitate their infiltration into the tumor bed. In this Trial Watch, we survey the latest trends in the preclinical and clinical development of DC-based anticancer therapeutics. We also highlight how the emergence of immune checkpoint blockers and adoptive T-cell transfer-based approaches has modified the clinical niche for DC-based vaccines within the wide cancer immunotherapy landscape.
Abbreviations
| ACT | = | adoptive T-cell transfer |
| APC | = | antigen-presenting cell |
| CTL | = | cytotoxic T lymphocyte |
| DAMP | = | damage-associated molecular pattern |
| DC | = | dendritic cell |
| ICB | = | immune checkpoint blocker |
| iDC | = | immature DC |
| IFN | = | interferon |
| IL | = | interleukin |
| MAMP | = | microbe-associated molecular pattern |
| mDC | = | mature DC |
| MRC1 | = | mannose receptor C type 1 |
| MUC1 | = | mucin 1 |
| pDC | = | plasmacytoid DC |
| TAA | = | tumor-associated antigen |
| TIL | = | tumor-infiltrating lymphocyte |
| TLR | = | Toll-like receptor |
| Treg | = | regulatory T cell |
| WT1 | = | Wilms tumor 1 |
Introduction
Dendritic cells (DCs) are one of the major antigen-presenting cells (APCs) of the innate immune system. Due to their proficiency at antigen presentation, including the capacity to cross-present antigens (i.e., presenting extracellular antigens on MHC class I molecules), DCs engage a decisive spot at the interface between innate and adaptive immunity. DCs were first discovered in 1973 by Ralph Steinman, who was later awarded a Nobel Prize for this achievement.Citation1-4 DCs are named as such due to their peculiar, tree-like morphology (“Dendron” is the Greek word for “tree”).Citation5-7 Since their discovery nearly three decades ago, a considerable number of studies have focused on the unique phenotypic and functional characteristics of DCs.Citation8
DCs are a relatively ubiquitous population of myeloid cells (which differentiate from common myeloid bone marrow progenitors) exhibiting heterogeneity at various levels, including morphology, ontogeny and immunological features.Citation9 This heterogeneity is the basis for the classification of DCs into various subsets. DC subsets tend to specialize in specific immunological functions: (1) processing and presenting antigens (e.g., murine CD8α+ DCs and their human counterparts, CD141+ DCs, are particularly efficient at antigen cross-presentation),Citation10-17 (2) specifically interfacing with selected cells from the adaptive immune system (e.g., CD14+ dermal DCs or epidermal Langerhans cells preferentially facilitate humoral or CD8+ T-cell responses, respectively)Citation18-20 and (3) mediating specific interferon (IFN) responses (e.g., plasmacytoid DCs (pDCs) tend to react to microbial stimuli by secreting high amounts of type I IFNs).Citation21-24
In absence of pathophysiological insults, circulating or tissue-resident DCs tend to exist in a perpetual immature state. Immature DCs (iDCs) are highly effective elicitors of immunological tolerance, typically due to their capacity to facilitate the suppression or clonal deletion of auto-reactive T cells as well as the clonal expansion of immunosuppressive CD4+CD25+FOXP3+ regulatory T cells (Tregs).Citation25-28 This immature state is connoted by (1) continuous engulfment of extracellular material coupled to the secretion of limited amounts of cytokines or chemokines, (2) retention of MHC class II molecules within late endosomes, (3) expression of some assorted chemokine receptors and (4) negligible expression of co-stimulatory ligands like CD86, CD40, CD83, CD70, CD80 and tumor necrosis factor (ligand) superfamily, member 4 (TNFSF4, also known as OX40L) on the cell surface.Citation29,30
However, several microbial (e.g., microbe-associated molecular patterns (MAMPs))Citation31 or endogenous (e.g., damage-associated molecular patterns (DAMPs))Citation32 cues can drive iDCs into maturation.Citation33-37 Mature DCs (mDCs) exhibit little-to-null phagocytic activity and efficiently home toward a nearby lymph node for antigen presentation to T cells rather than ensuring continuous phagocytic clearance (the latter function is mainly executed by local macrophages or neutrophils).Citation7,29,38 Generally, mDCs are characterized by increased expression of antigen-bound MHC class II molecules and co-stimulatory molecules (e.g., CD80, CD40, CD83, CD70, CD86 and OX40L) on the cell surface, upregulation of chemokine (C–C motif) receptor 7 (CCR7) and abundant secretion of immunostimulatory cytokines and inflammatory chemokines.Citation7,27,29,39-41 These molecules are used by mDCs to co-stimulate T cells during antigen presentation.Citation42-45
Owing to their significant contribution to antigen processing and presentation as well as to immunomodulation of T-cell functions, DCs have a strong impact on oncogenesis, tumor progression and response to anticancer therapy.Citation46-50 This crucial role of DCs has been documented in a plethora of preclinical tumor models (e.g., through vaccination, antibody-dependent depletion or genetic ablation) as well as in several clinical studies (e.g., by correlating intra-tumoral DC levels with disease outcome in various cohorts of cancer patients).Citation46-49,51-54 An established tumor usually strives to limit antigen availability to DCs and to enforce immunosuppression to disrupt the generation of antigen-specific T-cell responses.Citation8,55-59 This is one of the reasons underlying the emergence of DC-based vaccines for cancer immunotherapy. Indeed, DCs can be obtained from the monocytes of a cancer patient, loaded with an appropriate source of tumor-associated antigens (TAAs) ex vivo, matured in a proper manner ex vivo, and infused back into the patient. At least theoretically, this provides cancer patients with a pool of autologous DCs capable of priming tumor-targeting cytotoxic T lymphocytes (CTLs).Citation46,47,60-62
In the past few decades, significant experimental and clinical resources have been devoted to the development of anticancer DC-based vaccines.Citation46,47,60,63 Various types of DC-based vaccines have been generated so far, which can be classified depending on the protocol for loading DCs with TAAs or for the biochemical manipulation of DCs.Citation64,65 These include (but are not limited to) (1) unstimulated DCs or DCs exposed to specific maturation cocktails (consisting of various immunostimulatory cytokines and toll-like receptor (TLR) agonists);Citation66-70 (2) DCs exposed ex vivo to tumor-cell lysates or other preparations enriched in one or more TAAs;Citation71-121 (3) direct delivery of TAAs to DCs in vivo;Citation122-134 (4) in situ anticancer vaccination by intra-tumoral administration of immunomodulatory molecules to activate local DCsCitation135 and (5) exosomes derived from DCs.Citation136-141 However, the DC-based vaccine most commonly used so far involves the loading of DCs with a source of TAAs followed by their stimulation with defined maturation cocktails.Citation142,143 Ex vivo, DC loading with TAAs is typically accomplished by (1) co-culturing iDCs with either autologous or allogeneic tumor-cell lysatesCitation71-80,144-146 or recombinant TAAs;Citation81-88,147 (2) transfecting DCs with vectors or RNAs coding for TAA(s), or even bulk RNA derived from tumor cells;Citation41,89-113,148-152 and (3) creating fusions between DCs and incapacitated malignant cells (also known as “dendritomes”).Citation114-121 Alternatively, TAAs can be specifically conveyed to DCs in vivo by (1) fusing them to monoclonal antibodies, polypeptides or carbohydrates that specifically target DC-specific receptors (e.g., mannose receptor, C type 1 (MRC1), CD209 (also known as DC-SIGN), and lymphocyte antigen 75 (LY75, also known as DEC-205)),Citation122-128,130,131,133,153 or DC-associated glycolipids (e.g., glycosphingolipid globotriaosylceramide (Gb3));Citation154,155 (2) encapsulating them in immunoliposomes that target DCs,Citation156-158 or (3) encoding them in vectors targeting DCs.Citation159-162 In the latter case, to overcome the tolerogenic activity of iDCs,Citation123,124 strategies aiming to target DCs in vivo also need to simultaneously integrate DC-activating stimuli such as TLR agonists and/or pro-inflammatory cytokines.Citation163 The concept of targeting TAAs to DCs in vivo has recently been harnessed for the development of CDX1401 (Celldex Therapeutics, USA), a DC-based vaccine consisting of a fully human anti-DEC205 monoclonal antibody (3G9) fused to the human TAA cancer/testis antigen 1B (CTAG1B; also known as NY-ESO-1).Citation164 In an early phase clinical trial, cancer patients receiving CDX1401 in combination with TLR3 and TLR7/8 agonists experienced efficient generation of NY-ESO-1-directed T-cell and humoral responses.Citation164
Another strategy of DC-based vaccination that has begun to receive attention involves specific naturally occurring DC subsets, which can be isolated via high-performance antibody-coated magnetic beads.Citation165,166 Accumulating clinical evidence demonstrates that DC-based vaccines consisting of pDC or CD1c+ DCs loaded with TAA-derived peptides achieve promising efficacy in melanoma patients.Citation165,166 In fact, CD1c+ DC-based vaccines induced long-term progression-free survival (1–3 y) in ∼28% of treated melanoma patients.Citation166 Last but not least, while most of DC-based vaccines are administered to cancer patients in a curative setting, DC-based vaccines have recently been used also as neoadjuvant treatment.Citation167 Breast cancer patients exhibiting overexpression of Erb-B2 receptor tyrosine kinase 2 (ERBB2; also known as HER2) are often susceptible to disease recurrence. In this setting, DC-based vaccine pulsed with HER2-derived MHC class I and II-binding peptides is being applied before surgical resection of the tumor.Citation167 While clinical trials based on this strategy are ongoing (e.g., NCT02063724), preliminary evidence suggests that this vaccine efficiently induces HER2-targeting immunity and decreases the burden of residual HER2high tumor cells in breast cancer patients.Citation167
In general, the efficacy of DC-based vaccination is influenced by various factors including the amount of functional activation that can be achieved, the nature and source of TAAs, the immunological fitness of the host, the type of DC receptors engaged and the specific DC-subset that was targeted.Citation168-172 Moreover, the presence of some cytokines like interleukin 12 (IL-12) can also be decisive for patients administered with DC-based vaccines to achieve clinical responses. Thus, the combinatorial administration of IL-12 or CD40 and TLR ligands (which stimulate IL-12 secretion) is generally encouraged.Citation173,174 It is important to mention that only one cellular therapy involving DCs (but not restricted to them) is presently licensed for use in humans, namely, sipuleucel-T (branded as Provenge®). Since 2010, sipuleucel-T has been approved for the treatment of asymptomatic or minimally-symptomatic metastatic castration-resistant prostate cancer.Citation175-178 The company manufacturing Provenge®, however, filed for bankruptcy a few years later, and has now been acquired by a multinational specialty pharmaceutical company, casting doubts on the actual cost-effectiveness of their lead product.
In this Trial Watch, we recapitulate the latest developments in the field of DC-based cancer immunotherapy, discussing the results of major preclinical and clinical studies published recently, and ongoing clinical trials relative to the publication of our previous Trial Watch on this topic. Moreover, we analyze the broad impact of cancer immunotherapies including immune checkpoint blockers (ICBs),Citation179,180 and adoptive T-cell transfer (ACT)Citation181,182 on the field of DC-based vaccines.Citation183,184 Indeed, ongoing clinical trials are identifying a niche for DC-based vaccines in a cancer immunotherapy landscape dominated by ICBs and ACT.
Recent preclinical developments
A number of interesting preclinical studies dealing with DC-based anticancer immunotherapy have been published in the last 2.5 y (September 2014 to February 2017, i.e., the approximate period since the publication of the latest Trial Watch on this topic). While summarizing all of these studies here is not possible, we found the following ones to be of particular interest and, in some cases, largely representing the general trends in this field (not mentioned in any particular order): (1) Mitchell et al. documented that pre-conditioning the intended site for DC-based vaccination with the tetanus/diphtheria toxoid (acting as a “recall antigen”) significantly improves the lymph node-homing of DCs thereby inducing (CCL3-dependent) anti-glioblastoma immunity following DC-based vaccination in mice and patients;Citation185 (2) Carreno et al. reported that a DC-based vaccine boosts (naturally-occurring) neoantigen-specific anti-melanoma immunity (comprising an increase in diversity and clonality of neoantigen-specific T cells) associated with de novo emergence of MHC class I-restricted neoantigens;Citation186 (3) Garg et al. found that next-generation DC-based vaccines relying on immunogenic cell death (ICD) elicited by hypericin-dependent photodynamic therapy (Hyp-PDT) in glioma cells induce potent anti-glioblastoma immunity in an orthotopic murine model (both in prophylactic and curative settings, alone or in combination with chemotherapy), accompanied by a shift from a Treg-dominated intracranial immune contexture to a Th1/Th17/CTLs-dominated one;Citation187 (4) Ohshio et al. observed that targeting immunosuppressive cancer-associated fibroblasts with an anti-fibrotic agent, tranilast, in combination with DC-based vaccination drastically improves (CTL- and NK-cell-driven) antitumor immunity against subcutaneously transplanted lymphomas, lung carcinomas and melanomas;Citation188 (5) Xiang et al. documented that ovalbumin-loaded upconversion nanoparticles (i.e., nanoparticles exhibiting photon upconversion, a process wherein two or more photons of low energy are absorbed and converted into a single emitted photon of higher energy) can be used for antigen loading and maturation of DCs, which can also be tracked in vivo (via luminescence imaging), so that these DCs eventually drive potent ovalbumin-specific immunity;Citation189 (6) Carmi et al. discovered that DC-based vaccines relying on tumor cells coated with naturally occurring tumor-binding IgG antibodies or DC-based vaccines administered in combination with allogeneic IgG antibodies (two strategies aimed at facilitating allogeneic tumor rejection) elicit strong antitumor immunity in mouse models of (autologous or autochthonous) melanoma as well as breast, pancreatic and lung carcinoma;Citation190 (7) Vandenberk et al. reported that DC-based vaccines relying on irradiated, freeze/thawed (F/T) glioma cells induce significant anti-glioblastoma immunity, driven (at least in part) by CTL responses elicited by oxidation-associated molecular patterns (OAMPs);Citation191 (8) Lu et al. observed that a DC-based vaccine generated with a cancer stem cell (CSC)-lysate administered in combination with localized radiotherapy induce potent antitumor immunity driven (at least in part) by B cell-dependent humoral responses and a significant shift in the “chemokinome” of the tumor;Citation192 (9) Jung et al. discovered that using Mycobacterium tuberculosis heat shock protein X (HspX) as an adjuvant for DC-based vaccines facilitates Th1/CTL-driven antitumor immunity;Citation193 (10) Willemen et al. reported that electroporating DCs with an IFN-α-coding mRNA significantly enhances their ability to drive TAA-specific CTLs and antitumor NK-cell responses;Citation194 and (11) Martin et al. discovered that ansamitocin P3, a microtubule depolymerizing agent, enhances DC activation thereby making DC-based vaccines potent inducers of antigen-specific T cells, especially in combination with ICBs targeting programmed cell death 1 (PDCD1, also known as PD-1) and cytotoxic T lymphocyte-associated protein 4 (CTLA4).Citation195
Completed clinical trials
In the last 2.5 y (September 2014 to February 2017), 43 peer-reviewed publications have documented the outcome of various clinical trials evaluating DC-based vaccines as therapeutic anticancer interventions. These publications were acquired from PubMed (http://www.ncbi.nlm.nih.gov/pubmed), by using the following search string: “(cancer OR tumor OR tumor) AND ((DC OR dendritic OR “dendritic cells” OR “dendritic cell”) AND (vaccine OR vaccination OR infusion OR injection OR immunotherapy)).” The initial list was manually curated to ensure relevance for this Trial Watch.
More than half of these studies () involved autologous DCs exposed to autologous tumor-derived RNA,Citation196 tumor-cell lysates,Citation197-203 autologous tumor stem cell lysates,Citation204 self-renewing and proliferating autologous tumor cells,Citation205 allogeneic cancer cell line lysates,Citation206-208 TAAs or TAA-derived peptidesCitation209-218 or a combination thereof.Citation219 Most clinical studies based on the latter approach preferred melanoma-associated differentiation antigens including premelanosome protein (PMEL; also known as gp100), antigens belonging to the melanoma antigen gene (MAGE) family, tyrosinase (TYR) and Melan-A (MLANA; also known as MART1) (). In addition, a few trials tested autologous DCs manipulated by electroporation or (viral) transfection with TAA-coding mRNA ().Citation185,220-222 Finally, a few studies preferred to use “unpulsed” autologous (matured ex vivo) DCs, administered in combination with cytokine-induced killer cells (CIKs), chemotherapy and/or radiotherapy.Citation223-226 Other approaches included the use of autologous dendritomas,Citation227 allogeneic peripheral blood mononuclear cells matured ex vivo into DCs,Citation228,229 or autologous unpulsed DCs combined with ACT.Citation230
Figure 1. Overview of cancer types and tumor-associated antigens (TAAs) in published and ongoing clinical trials testing DC-based vaccination in cancer patients. A survey of the PubMed database (for published clinical trials; left) or Clinicaltrials.gov database (for ongoing clinical trials; right) concerning the use of DC-based vaccines in cancer patients is presented relative to (1) cancer type; (2) source of antigens; and (3) specific TAAs (when relevant). Only entities applicable to ≥ 2 clinical trials (published studies) or ≥ 3 clinical trials (ongoing studies) are shown. DC, dendritic cell; GBM, glioblastoma; HGG, high-grade glioma; RCC, renal cell carcinoma; TAA, tumor-associated antigens.
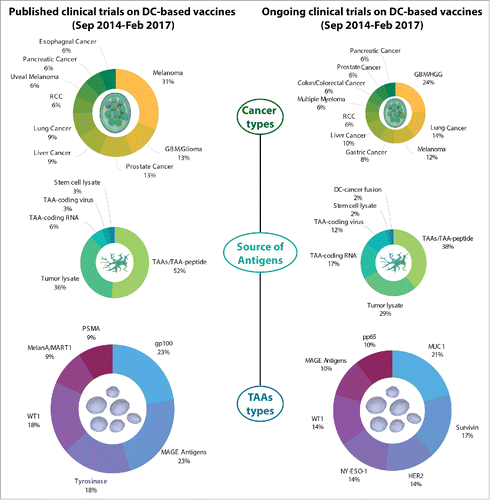
Regarding the distribution across different cancer types (), patients harboring melanoma were most commonly enrolled in these trials,Citation186,196,198,205,213,221,223,227,231-234 followed by patients with prostate cancer,Citation206,208,212,215 glioma or glioblastoma (GBM),Citation185,197,202,219 hepatocellular carcinoma,Citation204,211,220 non-small cell lung carcinoma (NSCLC),Citation207,214,230 renal cell carcinoma (RCC),Citation201,203 esophageal carcinoma,Citation216,226 and pancreatic ductal adenocarcinoma,Citation209,217 among others. Of note, only two trials included a wide range of advanced solid tumors refractory to previous treatments.Citation218,219
Most of these publications documented DC-based vaccines to elicit immune responses (generally in combination with standard-of-care therapies) achieving disease stabilization in most cases and partial or complete responses at a comparatively lower frequency. In several instances, DC-based vaccines have been administered in combination with conventional immunostimulatory agents including cytokines (e.g., IL-2, colony stimulating factor 2 (CSF2; also known as GM-CSF), IFN-α2B, IFNγ or IFNβ),Citation199,205,221,224,227 the tetanus toxoid,Citation185 or TLR agonists.Citation211 Interestingly, a few clinical studies also tested DC-based vaccines in combination with ACT and ICBs.Citation198,230,235 The combination of a DC-based vaccine with ipilimumab (an anti-CTLA4 ICB currently approved for the treatment of melanoma patients) achieved a promising overall response rate of 38% (eight patients with complete responses and seven with partial responses) and a 6-mo disease control rate of 51%.Citation235 Similarly, among eight Stage IV melanoma patients treated with DC-based vaccination followed by tumor-infiltrating lymphocyte (TIL)-based ACT, one patient achieved complete remission and two others achieved stable disease (although in this case the study size prevented from drawing robust conclusions).Citation198 Interestingly, a Phase III trial demonstrated that unpulsed DCs in combination with adoptively transferred autologous activated CTLs derived from patient lymph nodes (delivered in combination with platinum-based chemotherapy) significantly improved long-term survival in NSCLC patients when compared with chemotherapy alone.Citation230
Many of these studies also brought into focus various predictive biomarkers of clinical responses to DC-based vaccination. For instance, an increased overlap in the TCR repertoire of TILs and blood-borne T cells correlated with improved responses to DC-based vaccination and overall survival in glioma patients.Citation202 Conversely, a T-cell polarization skewed toward an immunosuppressive Th2 phenotype was found to reduce the therapeutic efficacy of DC-CIK co-administration in NSCLC patients.Citation207 Finally, increased plasma levels of pro-inflammatory cytokines like IL-6 and IL-8 negatively correlated with overall survival following DC-based vaccination in pancreatic ductal adenocarcinoma patients.Citation236
Collectively, these studies highlight that DC-based vaccines are well-tolerated by cancer patients, with mild flu-like symptoms and local irritation at the injection site being the most common side effects, and can elicit antitumor immune response of therapeutic value.
Ongoing clinical trials
When this Trial Watch was being redacted (February 2017), official sources listed no less than 72 clinical trials initiated after 1st of September, 2014 to be evaluating anticancer DC-based vaccines in the ClinicalTrials.gov database (http://www.clinicaltrials.gov/). These were retrieved by using the following search string: “(cancer OR tumor OR tumor) AND ((DC OR dendritic OR “dendritic cells” OR “dendritic cell”) AND (vaccine OR vaccination OR infusion OR injection)).” The initial list was manually curated to ensure relevance for this Trial Watch. The specific details of the different short-listed clinical trials are elaborated in the .
Table 1. Clinical trials currently testing the therapeutic efficacy of dendritic cell-based anticancer immunotherapy.
The majority of these studies involved autologous DCs exposed to autologous tumor-derived RNA, tumor-cell lysates and TAAs or TAA-derived peptides ( and ). The rest consisted of either autologous DCs loaded with autologous tumor stem cell lysates, autologous DCs (virally) transfected with TAAs-coding mRNA, or autologous dendritomas, among others (). Most clinical studies focusing on TAAs or TAA-derived peptides selected following antigens: mucin 1 (MUC1), baculoviral IAP repeating containing 5 (BIRC5; also known as survivin), HER2, NY-ESO-1 and Wilms Tumor 1 (WT1) ( and ). Also, several ongoing clinical trials relied on loading a complex mixture of TAAs or TAA-derived peptides onto the autologous DCs for vaccine production (). Regarding trial distribution across cancer types, patients harboring glioma or GBM were the most commonly enrolled, followed by patients with lung cancer, melanoma, liver cancer and gastric cancer, among others ( and ). Of note, there is no ongoing Phase III clinical trial administering DC-based vaccines.
Most ongoing clinical trials are testing the administration of DC-based vaccines in combination with standard-of-care chemo- or radiotherapy (including known ICD inducers) ().Citation33,237,238 However, several chemotherapies that are unable to promote ICD are also being combined with DC-based vaccination, wherein cisplatin is the preferred option ().Citation35 Furthermore, combinatorial regimens included targeted therapies such as the broad spectrum receptor tyrosine kinase inhibitor sunitinib, the CD25-targeting antibody basiliximab, the CD20-targeted antibody tituximab and HER2-targeted antibodies like trastuzumab and pertuzumab. Interestingly, a few studies are testing DC-based vaccines in combination with anti-inflammatory drugs (e.g., celecoxib). It will be interesting to see whether such drugs increase or decrease the efficacy of DC-based vaccination.
Finally, multiple ongoing clinical trials are combining DC-based vaccines with immunostimulatory cytokines (IL-2, GM-CSF, IFN-α2B), the tetanus diphtheria toxoid, or TLR agonists (e.g., rintatolimod, hiltonol). Notably, there has been a palpable surge in the number of clinical trials combining DC-based vaccines with the tetanus diphtheria toxoid in GBM patients. Finally, several ongoing clinical trials are testing DC-based vaccines in combination with CIK cells, ICBs and ACT ().
Status update on clinical trials
The following clinical trials, enlisted in the previous edition of Trial Watch dealing with this topic,Citation239 have changed status since: NCT02042053 and NCT02107378 have been “Terminated;” NCT02115126 and NCT02033616, have been “Withdrawn;” NCT02063724, NCT02049489, NCT01974661, NCT02107937, NCT02107950, NCT02107404, NCT02137746, NCT01956630, NCT02129075 and, NCT01981122, were previously “Recruiting” but are now listed as “Active, Not recruiting;” NCT01883297, NCT02070406, NCT02151448, NCT01983748 and, NCT02170389, were previously “Not yet recruiting,” but are now listed as “Recruiting;” NCT01926639 and NCT02159950, have since been “Completed” (source http://www.clinicaltrials.gov). NCT02115126 and NCT02033616 have been withdrawn due to unknown reasons. NCT02107378 has been terminated due to a not-better specified decision by the trial sponsors, and NCT02042053 has been terminated because the principal investigator of the trial left the designated institute.
Concluding remarks
A plethora of strategies have been developed in the past decades to exploit the anticancer activity of DCs – culminating in the paradigm of DC-based cancer immunotherapy. The studies presented in this survey show that – despite the emergence of ICBs and ACT – the field of DC-based vaccination is still vibrant (both preclinically and clinically). However, ICBs and ACT have definitely influenced the clinical application of DC-based vaccines. For instance, many clinical studies that have been published in the period between September 2014 and February 2017 were initiated before or just around the time ICBs gained clinical foothold. In these studies, DC-based vaccines were mostly administered to melanoma patients and hence were based on melanoma-associated differentiation antigensCitation240 (). However, the major survival advantage provided to melanoma patients by ICBs has introduced a change in the patient populations enrolled in clinical studies currently testing DC-based vaccines ( and ). In fact, these ongoing clinical trials are often enrolling patients with glioma or glioblastoma ( and ) – a cancer type that appears to be particularly resistant to ICBs but responsive to DC-based vaccines.Citation184,187,241 Interestingly, recombinant TAAs, TAA-derived peptides and tumor-cell lysates remain the preferred source of antigens for loading DCs for DC-based vaccines across both published and ongoing clinical studies ( and ). These trends illustrate that the field of DC-based vaccines is readily adapting to the shift in cancer immunotherapy.
As it stands, there is an urgent need for clinical studies demonstrating that DC-based vaccines can induce durable objective responses and improve long-term survival in cancer patients. While the field of DC-based vaccination gained momentum with the approval of sipuleucel-T for the immunotherapy of prostate cancer patients,Citation242 the overall development of DC-based vaccines has been facing multiple obstacles. For instance, DC-based vaccines have failed to achieve more than 15% objective response rates in cancer patients.Citation48 Moreover, most of the clinical efficacy data in this setting rely on short-term criteria (derived from Phase I/II clinical trials) rather than on long-term survival, also reflecting a paucity in Phase III clinical trials testing anticancer DC-based vaccines.Citation48 Besides efficacy issues, the development of DC-based vaccination for clinical use has also been hampered by the relatively high financial and human resources associated with production in good manufacturing practice (GMP) facilities.Citation47 Immunologically speaking, the relatively low avidity of TAA-specific T cells and the robust immunosuppression imposed on TILs have constituted major obstacles to the efficacy of DC-based vaccines even when vaccines could successfully generate tumor-specific, peripheral CTLs (a typical mechanistic biomarker of the immunostimulatory potential of DC-based vaccines in patients).Citation47,243-245 At least theoretically, this latter problem could be overcome by combining DC-based vaccines with ICBs or ACT – an endeavor currently being pursued by many investigators. That said, highly reliable predictive biomarkers of the clinical efficacy of DC-based vaccines are still missing. In conclusion, there is an urgent need to reduce the costs associated with the manufacturing of DC-based vaccines and to increase the homogeneity of the process (which would enable for multi-center Phase III clinical trials). Moreover, biomarker-guided clinical trials involving highly efficacious DC-based vaccines combined with ICBs, ACT or other (immuno)therapies will have to be designed to elucidate the actual clinical potential of DC-based vaccination.
Disclosure of potential conflicts of interest
LG provides remunerated consulting to OmniSEQ (Buffalo, NY).
Funding
ADG, MVP and MS are recipients of FWO Postdoctoral or Doctoral Fellowships from FWO-Vlaanderen, Belgium. PA is supported by grants from FWO (G060713N, G076617N), KU Leuven (C16/15/073) and Belgian State (IAP7/32). GK is supported by Ligue contre le Cancer (équipe labelisée); Agence National de la Recherche (ANR); Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; AXA Chair for Longevity Research; Institut National du Cancer (INCa); Fondation Bettencourt-Schueller; Fondation de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LabEx Immuno-Oncology; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI). LG is supported by WCMC (intramural funds) and Sotio a.c. (Prague, Czech Republic)
References
- Lanzavecchia A, Sallusto F, Ralph M. Steinman 1943–2011. Cell 2011; 147:1216-7; PMID:22263224; https://doi.org/10.1016/j.cell.2011.11.040
- Nussenzweig MC, Mellman I. Ralph Steinman (1943–2011). Nature 2011; 478:460; PMID:22031432; https://doi.org/10.1038/478460a
- Mellman I, Nussenzweig M. Retrospective. Ralph M. Steinman (1943–2011). Science 2011; 334:466; PMID:22034425; https://doi.org/10.1126/science.1215136
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 1973; 137:1142-62; PMID:4573839; https://doi.org/10.1084/jem.137.5.1142
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392:245-52; PMID:9521319; https://doi.org/10.1038/32588
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007; 449:419-26; PMID:17898760; https://doi.org/10.1038/nature06175
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 2013; 31:563-604; PMID:23516985; https://doi.org/10.1146/annurev-immunol-020711-074950
- Dudek AM, Martin S, Garg AD, Agostinis P. Immature, semi-mature, and fully mature dendritic cells: Toward a DC-cancer cells interface that augments anticancer immunity. Front Immunol 2013; 4:438; PMID:24376443; https://doi.org/10.3389/fimmu.2013.00438
- Morello S, Pinto A, Blandizzi C, Antonioli L. Myeloid cells in the tumor microenvironment: Role of adenosine. OncoImmunology 2016; 5:e1108515; PMID:27141365; https://doi.org/10.1080/2162402X.2015.1108515
- Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med 2010; 207:1273-81; PMID:20479115; https://doi.org/10.1084/jem.20100348
- Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, Vu Manh TP, Baranek T, Storset AK, Marvel J et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med 2010; 207:1283-92; PMID:20479118; https://doi.org/10.1084/jem.20100223
- Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med 2010; 207:1247-60; PMID:20479116; https://doi.org/10.1084/jem.20092140
- Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, Keller AM, Joffre O, Zelenay S, Nye E et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med 2010; 207:1261-71; PMID:20479117; https://doi.org/10.1084/jem.20092618
- Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol 2012; 12:557-69; PMID:22790179; https://doi.org/10.1038/nri3254
- Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev 2010; 234:18-31; PMID:20193009; https://doi.org/10.1111/j.0105-2896.2009.00870.x
- Laoui D, Keirsse J, Morias Y, Van Overmeire E, Geeraerts X, Elkrim Y, Kiss M, Bolli E, Lahmar Q, Sichien D et al. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat Commun 2016; 7:13720; PMID:28008905; https://doi.org/10.1038/ncomms13720
- Di Blasio S, Wortel IMN, van Bladel DAG, de Vries LE, Duiveman-de Boer T, Worah K, de Haas N, Buschow SI, de Vries IJ, Figdor CG et al. Human CD1c+ DCs are critical cellular mediators of immune responses induced by immunogenic cell death. OncoImmunology 2016; 5:e1192739; PMID:27622063; https://doi.org/10.1080/2162402X.2016.1192739
- Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity 2008; 29:497-510; PMID:18789730; https://doi.org/10.1016/j.immuni.2008.07.013
- Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di Pucchio T, Connolly J, Fay JW, Pascual V et al. Dendritic cell subsets in health and disease. Immunol Rev 2007; 219:118-42; PMID:17850486; https://doi.org/10.1111/j.1600-065X.2007.00551.x
- Van Acker HH, Anguille S, Van Tendeloo VF, Lion E. Empowering gamma delta T cells with antitumor immunity by dendritic cell-based immunotherapy. OncoImmunology 2015; 4:e1021538; PMID:26405575; https://doi.org/10.1080/2162402X.2015.1021538
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science 1999; 284:1835-7; PMID:10364556; https://doi.org/10.1126/science.284.5421.1835
- Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol 2000; 1:305-10; PMID:11017101; https://doi.org/10.1038/79747
- Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, O'Garra A, Liu YJ. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med 2002; 195:953-8; PMID:11927638; https://doi.org/10.1084/jem.20020045
- van Beek JJP, Gorris MAJ, Sköld AE, Hatipoglu I, Van Acker HH, Smits EL, de Vries IJ, Bakdash G. Human blood myeloid and plasmacytoid dendritic cells cross activate each other and synergize in inducing NK cell cytotoxicity. OncoImmunology 2016; 5:e1227902; PMID:27853652; https://doi.org/10.1080/2162402X.2016.1227902
- Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, Mucida D, Merad M, Steinman RM. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest 2013; 123:844-54; PMID:23298832; https://doi.org/10.1172/JCI65260
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol 2003; 21:685-711; PMID:12615891; https://doi.org/10.1146/annurev.immunol.21.120601.141040
- Garg AD, Romano E, Rufo N, Agostinis P. Immunogenic versus tolerogenic phagocytosis during anticancer therapy: Mechanisms and clinical translation. Cell Death Differ 2016; 23:938-51; PMID:26891691; https://doi.org/10.1038/cdd.2016.5
- Maddur MS, Bayry J. B cells drive Th2 responses by instructing human dendritic cell maturation. OncoImmunology 2015; 4:e1005508; PMID:26155405; https://doi.org/10.1080/2162402X.2015.1005508
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12:265-77; PMID:22437871; https://doi.org/10.1038/nrc3258
- Fellermeier S, Beha N, Meyer J-E, Ring S, Bader S, Kontermann RE, Müller D. Advancing targeted co-stimulation with antibody-fusion proteins by introducing TNF superfamily members in a single-chain format. OncoImmunology 2016; 5:e1238540; PMID:27999756; https://doi.org/10.1080/2162402X.2016.1238540
- Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remédios C et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med 2014; 20:1301-9; PMID:25344738; https://doi.org/10.1038/nm.3708
- Garg AD, Dudek AM, Agostinis P. Cancer immunogenicity, danger signals, and DAMPs: What, when, and how? Biofactors 2013; 39:355-67; PMID:23900966; https://doi.org/10.1002/biof.1125
- Garg AD, Galluzzi L, Apetoh L, Baert T, Birge RB, Bravo-San Pedro JM, Breckpot K, Brough D, Chaurio R, Cirone M et al. Molecular and translational classifications of DAMPs in immunogenic cell death. Front Immunol 2015; 6:588; PMID:26635802; https://doi.org/10.3389/fimmu.2015.00588
- Fucikova J, Moserova I, Urbanova L, Bezu L, Kepp O, Cremer I, Salek C, Strnad P, Kroemer G, Galluzzi L et al. Prognostic and predictive value of DAMPs and DAMP-associated processes in cancer. Front Immunol 2015; 6:402; PMID:26300886; https://doi.org/10.3389/fimmu.2015.00402
- Bezu L, Gomes-de-Silva LC, Dewitte H, Breckpot K, Fucikova J, Spisek R, Galluzzi L, Kepp O, Kroemer G. Combinatorial strategies for the induction of immunogenic cell death. Front Immunol 2015; 6:187; PMID:25964783; https://doi.org/10.3389/fimmu.2015.00275 10.3389/fimmu.2015.00187
- Land WG, Agostinis P, Gasser S, Garg AD, Linkermann A. DAMP - induced allograft and tumor rejection: The circle is closing. Am J Transplant 2016; 16:3322-37; PMID:27529775; https://doi.org/10.1111/ajt.14012
- Land WG, Agostinis P, Gasser S, Garg AD, Linkermann A. Transplantation and damage-associated molecular patterns (DAMPs). Am J Transplant 2016; 16:3338-61; PMID:27421829; https://doi.org/10.1111/ajt.13963
- Garg AD, Vandenberk L, Fang S, Fasche T, Van Eygen S, Maes J, Van Woensel M, Koks C, Vanthillo N, Graf N et al. Pathogen response-like recruitment and activation of neutrophils by sterile immunogenic dying cells drives neutrophil-mediated residual cell killing. Cell Death Differ 2017; 24:832-843; PMID:28234357; https://doi.org/10.1038/cdd.2017.15
- Baert T, Garg AD, Vindevogel E, A VANH, Verbist G, Agostinis P, Vergote I, Coosemans AN. In vitro generation of murine dendritic cells for cancer immunotherapy: An optimized protocol. Anticancer Res 2016; 36:5793-801; PMID:27793901; https://doi.org/10.21873/anticanres.11163
- Stachura J, Wachowska M, Kilarski WW, Güç E, Golab J, Muchowicz A. The dual role of tumor lymphatic vessels in dissemination of metastases and immune response development. OncoImmunology 2016; 5:e1182278; PMID:27622039; https://doi.org/10.1080/2162402X.2016.1182278
- Bol KF, Figdor CG, Aarntzen EHJG, Welzen MEB, van Rossum MM, Blokx WAM, van de Rakt MW, Scharenborg NM, de Boer AJ, Pots JM et al. Intranodal vaccination with mRNA-optimized dendritic cells in metastatic melanoma patients. OncoImmunology 2015; 4:e1019197; PMID:26405571; https://doi.org/10.1080/2162402X.2015.1019197
- Steinman RM. Decisions about dendritic cells: Past, present, and future. Annu Rev Immunol 2012; 30:1-22; PMID:22136168; https://doi.org/10.1146/annurev-immunol-100311-102839
- Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med 1999; 189:587-92; PMID:9927520; https://doi.org/10.1084/jem.189.3.587
- Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci U S A 1999; 96:1036-41; PMID:9927689; https://doi.org/10.1073/pnas.96.3.1036
- Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG et al. Differential antigen processing by dendritic cell subsets in vivo. Science 2007; 315:107-11; PMID:17204652; https://doi.org/10.1126/science.1136080
- Sabado RL, Balan S, Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res 2016; 27:74-95; PMID:28025976; https://doi.org/10.1038/cr.2016.157
- Bol KF, Schreibelt G, Gerritsen WR, de Vries IJ, Figdor CG. Dendritic cell-based immunotherapy: State of the art and beyond. Clin Cancer Res 2016; 22:1897-906; PMID:27084743; https://doi.org/10.1158/1078-0432.CCR-15-1399
- Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol 2014; 15:e257-67; PMID:24872109; https://doi.org/10.1016/S1470-2045(13)70585-0
- Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013; 39:38-48; PMID:23890062; https://doi.org/10.1016/j.immuni.2013.07.004
- Hanoteau A, Moser M. Chemotherapy and immunotherapy: A close interplay to fight cancer? OncoImmunology 2016; 5:e1190061; PMID:27622046; https://doi.org/10.1080/2162402X.2016.1190061
- Hargadon KM. The extent to which melanoma alters tissue-resident dendritic cell function correlates with tumorigenicity. OncoImmunology 2016; 5:e1069462; PMID:26942090; https://doi.org/10.1080/2162402X.2015.1069462
- Toma M, Wehner R, Kloß A, Hübner L, Fodelianaki G, Erdmann K, Füssel S, Zastrow S, Meinhardt M, Seliger B et al. Accumulation of tolerogenic human 6-sulfo LacNAc dendritic cells in renal cell carcinoma is associated with poor prognosis. OncoImmunology 2015; 4:e1008342; PMID:26155414; https://doi.org/10.1080/2162402X.2015.1008342
- Stoll G, Iribarren K, Michels J, Leary A, Zitvogel L, Cremer I, Kroemer G. Calreticulin expression: Interaction with the immune infiltrate and impact on survival in patients with ovarian and non-small cell lung cancer. OncoImmunology 2016; 5:e1177692; PMID:27622029; https://doi.org/10.1080/2162402X.2016.1177692
- Stoll G, Bindea G, Mlecnik B, Galon J, Zitvogel L, Kroemer G. Meta-analysis of organ-specific differences in the structure of the immune infiltrate in major malignancies. Oncotarget 2015; 6:11894-909; PMID:26059437; https://doi.org/10.18632/oncotarget.4180
- Flies DB, Higuchi T, Harris JC, Jha V, Gimotty PA, Adams SF. Immune checkpoint blockade reveals the stimulatory capacity of tumor-associated CD103+ dendritic cells in late-stage ovarian cancer. OncoImmunology 2016; 5:e1185583; PMID:27622059; https://doi.org/10.1080/2162402X.2016.1185583
- Cornwall SMJ, Wikstrom M, Musk AW, Alvarez J, Nowak AK, Nelson DJ. Human mesothelioma induces defects in dendritic cell numbers and antigen-processing function which predict survival outcomes. OncoImmunology 2016; 5:e1082028; PMID:27057464; https://doi.org/10.1080/2162402X.2015.1082028
- Suryawanshi A, Manicassamy S. Tumors induce immune tolerance through activation of β-catenin/TCF4 signaling in dendritic cells: A novel therapeutic target for cancer immunotherapy. OncoImmunology 2015; 4:e1052932; PMID:26587326; https://doi.org/10.1080/2162402X.2015.1052932
- Garg AD, Elsen S, Krysko DV, Vandenabeele P, de Witte P, Agostinis P. Resistance to anticancer vaccination effect is controlled by a cancer cell-autonomous phenotype that disrupts immunogenic phagocytic removal. Oncotarget 2015; 6:26841-60; PMID:26314964; https://doi.org/10.18632/oncotarget.4754
- Ye J, Peng G. Controlling T cell senescence in the tumor microenvironment for tumor immunotherapy. OncoImmunology 2015; 4:e994398; PMID:25949919; https://doi.org/10.4161/2162402X.2014.994398
- Romero P, Banchereau J, Bhardwaj N, Cockett M, Disis ML, Dranoff G, Gilboa E, Hammond SA, Hershberg R, Korman AJ et al. The human vaccines project: A roadmap for cancer vaccine development. Sci Transl Med 2016; 8:334ps9; PMID:27075624; https://doi.org/10.1126/scitranslmed.aaf0685
- Wimmers F, Aarntzen EHJG, Duiveman-deBoer T, Figdor CG, Jacobs JFM, Tel J, de Vries IJ. Long-lasting multifunctional CD8+ T cell responses in end-stage melanoma patients can be induced by dendritic cell vaccination. OncoImmunology 2016; 5:e1067745; PMID:26942087; https://doi.org/10.1080/2162402X.2015.1067745
- Fromm PD, Papadimitrious MS, Hsu JL, Van Kooten Losio N, Verma ND, Lo TH, Silveira PA, Bryant CE, Turtle CJ, Prue RL et al. CMRF-56+ blood dendritic cells loaded with mRNA induce effective antigen-specific cytotoxic T-lymphocyte responses. OncoImmunology 2016; 5:e1168555; PMID:27471645; https://doi.org/10.1080/2162402X.2016.1168555
- Boudewijns S, Bol KF, Schreibelt G, Westdorp H, Textor JC, van Rossum MM, Scharenborg NM, de Boer AJ, van de Rakt MW, Pots JM et al. Adjuvant dendritic cell vaccination induces tumor-specific immune responses in the majority of stage III melanoma patients. OncoImmunology 2016; 5:e1191732; PMID:27622047; https://doi.org/10.1080/2162402X.2016.1191732
- Gilboa E. DC-based cancer vaccines. J Clin Invest 2007; 117:1195-203; PMID:17476349; https://doi.org/10.1172/JCI31205
- Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: From ex vivo loading to in vivo targeting. Nat Rev Immunol 2007; 7:790-802; PMID:17853902; https://doi.org/10.1038/nri2173
- Satoh Y, Esche C, Gambotto A, Shurin GV, Yurkovetsky ZR, Robbins PD, Watkins SC, Todo S, Herberman RB, Lotze MT et al. Local administration of IL-12-transfected dendritic cells induces antitumor immune responses to colon adenocarcinoma in the liver in mice. J Exp Ther Oncol 2002; 2:337-49; PMID:12440225; https://doi.org/10.1046/j.1359-4117.2002.01050.x
- Nishioka Y, Hirao M, Robbins PD, Lotze MT, Tahara H. Induction of systemic and therapeutic antitumor immunity using intratumoral injection of dendritic cells genetically modified to express interleukin 12. Cancer Res 1999; 59:4035-41; PMID:10463604.
- Yang SC, Hillinger S, Riedl K, Zhang L, Zhu L, Huang M, Atianzar K, Kuo BY, Gardner B, Batra RK et al. Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity. Clin Cancer Res 2004; 10:2891-901; PMID:15102698; https://doi.org/10.1158/1078-0432.CCR-03-0380
- Hu J, Yuan X, Belladonna ML, Ong JM, Wachsmann-Hogiu S, Farkas DL et al. Induction of potent antitumor immunity by intratumoral injection of interleukin 23-transduced dendritic cells. Cancer Res 2006; 66:8887-96; PMID:16951206; https://doi.org/10.1158/0008-5472.CAN-05-3448
- Endo H, Saito T, Kenjo A, Hoshino M, Terashima M, Sato T, Anazawa T, Kimura T, Tsuchiya T, Irisawa A et al. Phase I trial of preoperative intratumoral injection of immature dendritic cells and OK-432 for resectable pancreatic cancer patients. J Hepatobiliary Pancreat Sci 2012; 19:465-75; PMID:21983893; https://doi.org/10.1007/s00534-011-0457-7
- Nair SK, Snyder D, Rouse BT, Gilboa E. Regression of tumors in mice vaccinated with professional antigen-presenting cells pulsed with tumor extracts. Int J Cancer 1997; 70:706-15; PMID:9096653; https://doi.org/10.1002/(SICI)1097-0215(19970317)70:6%3c706::AID-IJC13%3e3.0.CO;2-7
- DeMatos P, Abdel-Wahab Z, Vervaert C, Hester D, Seigler H. Pulsing of dendritic cells with cell lysates from either B16 melanoma or MCA-106 fibrosarcoma yields equally effective vaccines against B16 tumors in mice. J Surg Oncol 1998; 68:79-91; PMID:9624036; https://doi.org/10.1002/(SICI)1096-9098(199806)68:2%3c79::AID-JSO3%3e3.0.CO;2-H
- DeMatos P, Abdel-Wahab Z, Vervaert C, Seigler HF. Vaccination with dendritic cells inhibits the growth of hepatic metastases in B6 mice. Cell Immunol 1998; 185:65-74; PMID:9636684; https://doi.org/10.1006/cimm.1998.1277
- Fields RC, Shimizu K, Mule JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci U S A 1998; 95:9482-7; PMID:9689106; https://doi.org/10.1073/pnas.95.16.9482
- Chen Z, Moyana T, Saxena A, Warrington R, Jia Z, Xiang J. Efficient antitumor immunity derived from maturation of dendritic cells that had phagocytosed apoptotic/necrotic tumor cells. Int J Cancer 2001; 93:539-48; PMID:11477558; https://doi.org/10.1002/ijc.1365
- Paczesny S, Beranger S, Salzmann JL, Klatzmann D, Colombo BM. Protection of mice against leukemia after vaccination with bone marrow-derived dendritic cells loaded with apoptotic leukemia cells. Cancer Res 2001; 61:2386-9; PMID:11289101
- Kokhaei P, Choudhury A, Mahdian R, Lundin J, Moshfegh A, Osterborg A, Mellstedt H. Apoptotic tumor cells are superior to tumor cell lysate, and tumor cell RNA in induction of autologous T cell response in B-CLL. Leukemia 2004; 18:1810-5; PMID:15385926; https://doi.org/10.1038/sj.leu.2403517
- Kokhaei P, Rezvany MR, Virving L, Choudhury A, Rabbani H, Osterborg A, Mellstedt H. Dendritic cells loaded with apoptotic tumour cells induce a stronger T-cell response than dendritic cell-tumour hybrids in B-CLL. Leukemia 2003; 17:894-9; PMID:12750703; https://doi.org/10.1038/sj.leu.2402913
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 1998; 392:86-9; PMID:9510252; https://doi.org/10.1038/32183
- Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med 1998; 188:1359-68; PMID:9763615; https://doi.org/10.1084/jem.188.7.1359
- Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med 1995; 1:1297-302; PMID:7489412; https://doi.org/10.1038/nm1295-1297
- Ossevoort MA, Feltkamp MC, van Veen KJ, Melief CJ, Kast WM. Dendritic cells as carriers for a cytotoxic T-lymphocyte epitope-based peptide vaccine in protection against a human papillomavirus type 16-induced tumor. J Immunother Emphasis Tumor Immunol 1995; 18:86-94; PMID:8574470; https://doi.org/10.1097/00002371-199508000-00002
- Mayordomo JI, Loftus DJ, Sakamoto H, De Cesare CM, Appasamy PM, Lotze MT, Storkus WJ, Appella E, DeLeo AB. Therapy of murine tumors with p53 wild-type and mutant sequence peptide-based vaccines. J Exp Med 1996; 183:1357-65; PMID:8666894; https://doi.org/10.1084/jem.183.4.1357
- Paglia P, Chiodoni C, Rodolfo M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med 1996; 183:317-22; PMID:8551239; https://doi.org/10.1084/jem.183.1.317
- Mackey MF, Gunn JR, Maliszewsky C, Kikutani H, Noelle RJ, Barth RJ, Jr. Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J Immunol 1998; 161:2094-8; PMID:9725199
- Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: Dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med 1996; 183:87-97; PMID:8551248; https://doi.org/10.1084/jem.183.1.87
- Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Garcia-Prats MD, DeLeo AB, Lotze MT. Bone marrow-derived dendritic cells serve as potent adjuvants for peptide-based antitumor vaccines. Stem Cells 1997; 15:94-103; PMID:9090785; https://doi.org/10.1002/stem.150094
- Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: Cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med 1999; 5:405-11; PMID:10202929; https://doi.org/10.1038/7403
- Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med 1996; 184:465-72; PMID:8760800; https://doi.org/10.1084/jem.184.2.465
- Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med 1997; 186:1177-82; PMID:9314567; https://doi.org/10.1084/jem.186.7.1177
- Boczkowski D, Nair SK, Nam JH, Lyerly HK, Gilboa E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res 2000; 60:1028-34; PMID:10706120
- Irvine AS, Trinder PK, Laughton DL, Ketteringham H, McDermott RH, Reid SC, Haines AM, Amir A, Husain R, Doshi R et al. Efficient nonviral transfection of dendritic cells and their use for in vivo immunization. Nat Biotechnol 2000; 18:1273-8; PMID:11101806; https://doi.org/10.1038/82383
- Manickan E, Kanangat S, Rouse RJ, Yu Z, Rouse BT. Enhancement of immune response to naked DNA vaccine by immunization with transfected dendritic cells. J Leukoc Biol 1997; 61:125-32; PMID:9021916
- Song W, Kong HL, Carpenter H, Torii H, Granstein R, Rafii S, Moore MA, Crystal RG. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J Exp Med 1997; 186:1247-56; PMID:9334364; https://doi.org/10.1084/jem.186.8.1247
- Tuting T, DeLeo AB, Lotze MT, Storkus WJ. Genetically modified bone marrow-derived dendritic cells expressing tumor-associated viral or “self” antigens induce antitumor immunity in vivo. Eur J Immunol 1997; 27:2702-7; PMID:9368629; https://doi.org/10.1002/eji.1830271033
- Wan Y, Bramson J, Carter R, Graham F, Gauldie J. Dendritic cells transduced with an adenoviral vector encoding a model tumor-associated antigen for tumor vaccination. Hum Gene Ther 1997; 8:1355-63; PMID:9295130; https://doi.org/10.1089/hum.1997.8.11-1355
- McArthur JG, Mulligan RC. Induction of protective anti-tumor immunity by gene-modified dendritic cells. J Immunother 1998; 21:41-7; PMID:9456435; https://doi.org/10.1097/00002371-199801000-00005
- Ishida T, Chada S, Stipanov M, Nadaf S, Ciernik FI, Gabrilovich DI, Carbone DP. Dendritic cells transduced with wild-type p53 gene elicit potent anti-tumour immune responses. Clin Exp Immunol 1999; 117:244-51; PMID:10444254; https://doi.org/10.1046/j.1365-2249.1999.00913.x
- Tuting T, Steitz J, Bruck J, Gambotto A, Steinbrink K, DeLeo AB, Robbins P, Knop J, Enk AH. Dendritic cell-based genetic immunization in mice with a recombinant adenovirus encoding murine TRP2 induces effective anti-melanoma immunity. J Gene Med 1999; 1:400-6; PMID:10753065; https://doi.org/10.1002/(SICI)1521-2254(199911/12)1:6%3c400::AID-JGM68%3e3.3.CO;2-4 10.1002/(SICI)1521-2254(199911/12)1:6%3c400::AID-JGM68%3e3.0.CO;2-D
- Wan Y, Emtage P, Zhu Q, Foley R, Pilon A, Roberts B, Gauldie J. Enhanced immune response to the melanoma antigen gp100 using recombinant adenovirus-transduced dendritic cells. Cell Immunol 1999; 198:131-8; PMID:10648127; https://doi.org/10.1006/cimm.1999.1585
- Klein C, Bueler H, Mulligan RC. Comparative analysis of genetically modified dendritic cells and tumor cells as therapeutic cancer vaccines. J Exp Med 2000; 191:1699-708; PMID:10811863; https://doi.org/10.1084/jem.191.10.1699
- Ribas A, Butterfield LH, Hu B, Dissette VB, Chen AY, Koh A, Amarnani SN, Glaspy JA, McBride WH, Economou JS. Generation of T-cell immunity to a murine melanoma using MART-1-engineered dendritic cells. J Immunother 2000; 23:59-66; PMID:10687138; https://doi.org/10.1097/00002371-200001000-00008
- Okada N, Saito T, Masunaga Y, Tsukada Y, Nakagawa S, Mizuguchi H, Mori K, Okada Y, Fujita T, Hayakawa T et al. Efficient antigen gene transduction using Arg-Gly-Asp fiber-mutant adenovirus vectors can potentiate antitumor vaccine efficacy and maturation of murine dendritic cells. Cancer Res 2001; 61:7913-9; PMID:11691812
- Koido S, Kashiwaba M, Chen D, Gendler S, Kufe D, Gong J. Induction of antitumor immunity by vaccination of dendritic cells transfected with MUC1 RNA. J Immunol 2000; 165:5713-9; PMID:11067929; https://doi.org/10.4049/jimmunol.165.10.5713
- Nair SK, Heiser A, Boczkowski D, Majumdar A, Naoe M, Lebkowski JS, Vieweg J, Gilboa E. Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nat Med 2000; 6:1011-7; PMID:10973321; https://doi.org/10.1038/79519
- Yamanaka R, Zullo SA, Tanaka R, Blaese M, Xanthopoulos KG. Enhancement of antitumor immune response in glioma models in mice by genetically modified dendritic cells pulsed with Semliki forest virus-mediated complementary DNA. J Neurosurg 2001; 94:474-81; PMID:11235953; https://doi.org/10.3171/jns.2001.94.3.0474
- Insug O, Ku G, Ertl HC, Blaszczyk-Thurin M. A dendritic cell vaccine induces protective immunity to intracranial growth of glioma. Anticancer Res 2002; 22:613-21; PMID:12014629
- Van Meirvenne S, Straetman L, Heirman C, Dullaers M, De Greef C, Van Tendeloo V, Thielemans K. Efficient genetic modification of murine dendritic cells by electroporation with mRNA. Cancer Gene Ther 2002; 9:787-97; PMID:12189529; https://doi.org/10.1038/sj.cgt.7700499
- Minami T, Nakanishi Y, Izumi M, Harada T, Hara N. Enhancement of antigen-presenting capacity and antitumor immunity of dendritic cells pulsed with autologous tumor-derived RNA in mice. J Immunother 2003; 26:420-31; PMID:12973031; https://doi.org/10.1097/00002371-200309000-00005
- Schmidt T, Ziske C, Marten A, Endres S, Tiemann K, Schmitz V, Gorschlüter M, Schneider C, Sauerbruch T, Schmidt-Wolf IG. Intratumoral immunization with tumor RNA-pulsed dendritic cells confers antitumor immunity in a C57BL/6 pancreatic murine tumor model. Cancer Res 2003; 63:8962-7; PMID:14695214
- Jung CW, Kwon JH, Seol JG, Park WH, Hyun JM, Kim ES, Kim ST, Lee SJ, Kim BK, Lee YY. Induction of cytotoxic T lymphocytes by dendritic cells pulsed with murine leukemic cell RNA. Am J Hematol 2004; 75:121-7; PMID:14978690; https://doi.org/10.1002/ajh.10471
- Liu BY, Chen XH, Gu QL, Li JF, Yin HR, Zhu ZG, Lin YZ. Antitumor effects of vaccine consisting of dendritic cells pulsed with tumor RNA from gastric cancer. World J Gastroenterol 2004; 10:630-3; PMID:14991927; https://doi.org/10.3748/wjg.v10.i5.630
- Zeis M, Siegel S, Wagner A, Schmitz M, Marget M, Kuhl-Burmeister R, Adamzik I, Kabelitz D, Dreger P, Schmitz N et al. Generation of cytotoxic responses in mice and human individuals against hematological malignancies using survivin-RNA-transfected dendritic cells. J Immunol 2003; 170:5391-7; PMID:12759413; https://doi.org/10.4049/jimmunol.170.11.5391
- Celluzzi CM, Falo LD, Jr. Physical interaction between dendritic cells and tumor cells results in an immunogen that induces protective and therapeutic tumor rejection. J Immunol 1998; 160:3081-5; PMID:9531260
- Wang J, Saffold S, Cao X, Krauss J, Chen W. Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. J Immunol 1998; 161:5516-24; PMID:9820528
- Tanaka H, Shimizu K, Hayashi T, Shu S. Therapeutic immune response induced by electrofusion of dendritic and tumor cells. Cell Immunol 2002; 220:1-12; PMID:12718934; https://doi.org/10.1016/S0008-8749(03)00009-1
- Orentas RJ, Schauer D, Bin Q, Johnson BD. Electrofusion of a weakly immunogenic neuroblastoma with dendritic cells produces a tumor vaccine. Cell Immunol 2001; 213:4-13; PMID:11747351; https://doi.org/10.1006/cimm.2001.1864
- Kjaergaard J, Shimizu K, Shu S. Electrofusion of syngeneic dendritic cells and tumor generates potent therapeutic vaccine. Cell Immunol 2003; 225:65-74; PMID:14698141; https://doi.org/10.1016/j.cellimm.2003.09.005
- Sukhorukov VL, Reuss R, Endter JM, Fehrmann S, Katsen-Globa A, Gessner P, Steinbach A, Müller KJ, Karpas A, Zimmermann U et al. A biophysical approach to the optimisation of dendritic-tumour cell electrofusion. Biochem Biophys Res Commun 2006; 346:829-39; PMID:16780801; https://doi.org/10.1016/j.bbrc.2006.05.193
- Cathelin D, Nicolas A, Bouchot A, Fraszczak J, Labbe J, Bonnotte B. Dendritic cell-tumor cell hybrids and immunotherapy: What's next? Cytotherapy 2011; 13:774-85; PMID:21299362; https://doi.org/10.3109/14653249.2011.553593
- Errington F, Jones J, Merrick A, Bateman A, Harrington K, Gough M, O'Donnell D, Selby P, Vile R, Melcher A. Fusogenic membrane glycoprotein-mediated tumour cell fusion activates human dendritic cells for enhanced IL-12 production and T-cell priming. Gene Ther 2006; 13:138-49; PMID:16136162; https://doi.org/10.1038/sj.gt.3302609
- Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med 2004; 199:815-24; PMID:15024047; https://doi.org/10.1084/jem.20032220
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med 2002; 196:1627-38; PMID:12486105; https://doi.org/10.1084/jem.20021598
- Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med 2001; 194:769-79; PMID:11560993; https://doi.org/10.1084/jem.194.6.769
- Adotevi O, Vingert B, Freyburger L, Shrikant P, Lone YC, Quintin-Colonna F, Haicheur N, Amessou M, Herbelin A, Langlade-Demoyen P et al. B subunit of Shiga toxin-based vaccines synergize with alpha-galactosylceramide to break tolerance against self antigen and elicit antiviral immunity. J Immunol 2007; 179:3371-9; PMID:17709554; https://doi.org/10.4049/jimmunol.179.5.3371
- Berraondo P, Nouze C, Preville X, Ladant D, Leclerc C. Eradication of large tumors in mice by a tritherapy targeting the innate, adaptive, and regulatory components of the immune system. Cancer Res 2007; 67:8847-55; PMID:17875726; https://doi.org/10.1158/0008-5472.CAN-07-0321
- Tacken PJ, de Vries IJ, Gijzen K, Joosten B, Wu D, Rother RP, Faas SJ, Punt CJ, Torensma R, Adema GJ et al. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood 2005; 106:1278-85; PMID:15878980; https://doi.org/10.1182/blood-2005-01-0318
- Klechevsky E, Flamar AL, Cao Y, Blanck JP, Liu M, O'Bar A, Agouna-Deciat O, Klucar P, Thompson-Snipes L, Zurawski S et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood 2010; 116:1685-97; PMID:20530286; https://doi.org/10.1182/blood-2010-01-264960
- Cruz LJ, Tacken PJ, Pots JM, Torensma R, Buschow SI, Figdor CG. Comparison of antibodies and carbohydrates to target vaccines to human dendritic cells via DC-SIGN. Biomaterials 2012; 33:4229-39; PMID:22410170; https://doi.org/10.1016/j.biomaterials.2012.02.036
- Schreibelt G, Klinkenberg LJ, Cruz LJ, Tacken PJ, Tel J, Kreutz M, Adema GJ, Brown GD, Figdor CG, de Vries IJ. The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells. Blood 2012; 119:2284-92; PMID:22234694; https://doi.org/10.1182/blood-2011-08-373944
- Tacken PJ, Ginter W, Berod L, Cruz LJ, Joosten B, Sparwasser T, Figdor CG, Cambi A. Targeting DC-SIGN via its neck region leads to prolonged antigen residence in early endosomes, delayed lysosomal degradation, and cross-presentation. Blood 2011; 118:4111-9; PMID:21860028; https://doi.org/10.1182/blood-2011-04-346957
- Tacken PJ, Ter Huurne M, Torensma R, Figdor CG. Antibodies and carbohydrate ligands binding to DC-SIGN differentially modulate receptor trafficking. Eur J Immunol 2012; 42:1989-98; PMID:22653683; https://doi.org/10.1002/eji.201142258
- Tacken PJ, Zeelenberg IS, Cruz LJ, van Hout-Kuijer MA, van de Glind G, Fokkink RG, Lambeck AJ, Figdor CG. Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood 2011; 118:6836-44; PMID:21967977; https://doi.org/10.1182/blood-2011-07-367615
- Van der Jeught K, Van Lint S, Thielemans K, Breckpot K. Intratumoral delivery of mRNA: Overcoming obstacles for effective immunotherapy. OncoImmunology 2015; 4:e1005504; PMID:26155403; https://doi.org/10.1080/2162402X.2015.1005504
- Hammerich L, Bhardwaj N, Kohrt HE, Brody JD. In situ vaccination for the treatment of cancer. Immunotherapy 2016; 8:315-30; PMID:26860335; https://doi.org/10.2217/imt.15.120
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat Med 1998; 4:594-600; PMID:9585234; https://doi.org/10.1038/nm0598-594
- Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol 1999; 147:599-610; PMID:10545503; https://doi.org/10.1083/jcb.147.3.599
- Viaud S, Thery C, Ploix S, Tursz T, Lapierre V, Lantz O, Zitvogel L, Chaput N. Dendritic cell-derived exosomes for cancer immunotherapy: What's next? Cancer Res 2010; 70:1281-5; PMID:20145139; https://doi.org/10.1158/0008-5472.CAN-09-3276
- Consortium E-T, Van Deun J, Mestdagh P, Agostinis P, Akay O, Anand S, Anckaert J, Martinez ZA, Baetens T, Beghein E et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Met 2017; 14:228-32; PMID:28245209; https://doi.org/10.1038/nmeth.4185
- Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F, Laplanche A et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. OncoImmunology 2016; 5:e1071008; PMID:27141373; https://doi.org/10.1080/2162402X.2015.1071008
- Liu Y, Gu Y, Cao X. The exosomes in tumor immunity. OncoImmunology 2015; 4:e1027472; PMID:26405598; https://doi.org/10.1080/2162402X.2015.1027472
- Iribarren K, Bloy N, Buqué A, Cremer I, Eggermont A, Fridman WH, Fucikova J, Galon J, Špíšek R, Zitvogel L et al. Trial watch: Immunostimulation with toll-like receptor agonists in cancer therapy. OncoImmunology 2016; 5:e1088631; PMID:27141345; https://doi.org/10.1080/2162402X.2015.1088631
- Vacchelli E, Aranda F, Bloy N, Buqué A, Cremer I, Eggermont A, Fridman WH, Fucikova J, Galon J, Spisek R et al. Trial Watch—Immunostimulation with cytokines in cancer therapy. OncoImmunology 2016; 5:e1115942; PMID:27057468; https://doi.org/10.1080/2162402X.2015.1115942
- Fucikova J, Rozkova D, Ulcova H, Budinsky V, Sochorova K, Pokorna K, Bartůňková J, Špíšek R. Poly I: C-activated dendritic cells that were generated in CellGro for use in cancer immunotherapy trials. J Transl Med 2011; 9:223; PMID:22208910; https://doi.org/10.1186/1479-5876-9-223
- Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spísek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res 2011; 71:4821-33; PMID:21602432; https://doi.org/10.1158/0008-5472.CAN-11-0950
- Bercovici N, Haicheur N, Massicard S, Vernel-Pauillac F, Adotevi O, Landais D, Gorin I, Robert C, Prince HM, Grob JJ et al. Analysis and characterization of antitumor T-cell response after administration of dendritic cells loaded with allogeneic tumor lysate to metastatic melanoma patients. J Immunother 2008; 31:101-12; PMID:18157017; https://doi.org/10.1097/CJI.0b013e318159f5ba
- Pol J, Bloy N, Buqué A, Eggermont A, Cremer I, Sautès-Fridman C, Galon J, Tartour E, Zitvogel L, Kroemer G et al. Trial Watch: Peptide-based anticancer vaccines. OncoImmunology 2015; 4:e974411; PMID:26137405; https://doi.org/10.4161/2162402X.2014.974411
- Li M, You S, Ge W, Ma S, Ma N, Zhao C. Induction of T-cell immunity against leukemia by dendritic cells pulsed with total RNA isolated from leukemia cells. Chin Med J (Engl) 2003; 116:1655-61; PMID:14642130
- Lee J, Boczkowski D, Nair S. Programming human dendritic cells with mRNA. Methods Mol Biol 2013; 969:111-25; PMID:23296931; https://doi.org/10.1007/978-1-62703-260-5_8
- Garg NK, Dwivedi P, Prabha P, Tyagi RK. RNA pulsed dendritic cells: An approach for cancer immunotherapy. Vaccine 2013; 31:1141-56; PMID:23306369; https://doi.org/10.1016/j.vaccine.2012.12.027
- Kyte JA, Aamdal S, Dueland S, Sæbøe-Larsen S, Inderberg EM, Madsbu UE, Skovlund E, Gaudernack G, Kvalheim G. Immune response and long-term clinical outcome in advanced melanoma patients vaccinated with tumor-mRNA-transfected dendritic cells. OncoImmunology 2016; 5:e1232237; PMID:27999747; https://doi.org/10.1080/2162402X.2016.1232237
- Borch TH, Engell-Noerregaard L, Zeeberg Iversen T, Ellebaek E, Met Ö, Hansen M, Andersen MH, Thor Straten P, Svane IM. mRNA-transfected dendritic cell vaccine in combination with metronomic cyclophosphamide as treatment for patients with advanced malignant melanoma. OncoImmunology 2016; 5:e1207842; PMID:27757300; https://doi.org/10.1080/2162402X.2016.1207842
- Apostolopoulos V, Thalhammer T, Tzakos AG, Stojanovska L. Targeting antigens to dendritic cell receptors for vaccine development. J Drug Deliv 2013; 2013:869718; PMID:24228179; https://doi.org/10.1155/2013/869718
- Vingert B, Adotevi O, Patin D, Jung S, Shrikant P, Freyburger L, Eppolito C, Sapoznikov A, Amessou M, Quintin-Colonna F et al. The Shiga toxin B-subunit targets antigen in vivo to dendritic cells and elicits anti-tumor immunity. Eur J Immunol 2006; 36:1124-35; PMID:16568496; https://doi.org/10.1002/eji.200535443
- Haicheur N, Bismuth E, Bosset S, Adotevi O, Warnier G, Lacabanne V, Regnault A, Desaymard C, Amigorena S, Ricciardi-Castagnoli P et al. The B subunit of Shiga toxin fused to a tumor antigen elicits CTL and targets dendritic cells to allow MHC class I-restricted presentation of peptides derived from exogenous antigens. J Immunol 2000; 165:3301-8; PMID:10975847; https://doi.org/10.4049/jimmunol.165.6.3301
- Copland MJ, Baird MA, Rades T, McKenzie JL, Becker B, Reck F, Tyler PC, Davies NM. Liposomal delivery of antigen to human dendritic cells. Vaccine 2003; 21:883-90; PMID:12547598; https://doi.org/10.1016/S0264-410X(02)00536-4
- van Broekhoven CL, Parish CR, Demangel C, Britton WJ, Altin JG. Targeting dendritic cells with antigen-containing liposomes: A highly effective procedure for induction of antitumor immunity and for tumor immunotherapy. Cancer Res 2004; 64:4357-65; PMID:15205352; https://doi.org/10.1158/0008-5472.CAN-04-0138
- Badiee A, Davies N, McDonald K, Radford K, Michiue H, Hart D, Kato M. Enhanced delivery of immunoliposomes to human dendritic cells by targeting the multilectin receptor DEC-205. Vaccine 2007; 25:4757-66; PMID:17512099; https://doi.org/10.1016/j.vaccine.2007.04.029
- Yang L, Yang H, Rideout K, Cho T, Joo KI, Ziegler L, Elliot A, Walls A, Yu D, Baltimore D et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat Biotechnol 2008; 26:326-34; PMID:18297056; https://doi.org/10.1038/nbt1390
- Hangalapura BN, Oosterhoff D, de Groot J, Boon L, Tuting T, van den Eertwegh AJ, Gerritsen WR, van Beusechem VW, Pereboev A, Curiel DT et al. Potent antitumor immunity generated by a CD40-targeted adenoviral vaccine. Cancer Res 2011; 71:5827-37; PMID:21747119; https://doi.org/10.1158/0008-5472.CAN-11-0804
- Thacker EE, Nakayama M, Smith BF, Bird RC, Muminova Z, Strong TV, Timares L, Korokhov N, O'Neill AM, de Gruijl TD et al. A genetically engineered adenovirus vector targeted to CD40 mediates transduction of canine dendritic cells and promotes antigen-specific immune responses in vivo. Vaccine 2009; 27:7116-24; PMID:19786146; https://doi.org/10.1016/j.vaccine.2009.09.055
- Korokhov N, de Gruijl TD, Aldrich WA, Triozzi PL, Banerjee PT, Gillies SD, Curiel TJ, Douglas JT, Scheper RJ, Curiel DT. High efficiency transduction of dendritic cells by adenoviral vectors targeted to DC-SIGN. Cancer Biol Ther 2005; 4:289-94; PMID:15753654; https://doi.org/10.4161/cbt.4.3.1499
- Vindevogel E, Baert T, A VANH, Verbist G, Vande Velde G, Garg AD, Agostinis P, Vergote I, Coosemans AN. The use of toll-like receptor 4 agonist to reshape the immune signature in ovarian cancer. Anticancer Res 2016; 36:5781-92; PMID:27793900; https://doi.org/10.21873/anticanres.11162
- Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, Chuang E, Sanborn RE, Lutzky J, Powderly J et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med 2014; 6:232ra51; PMID:24739759; https://doi.org/10.1126/scitranslmed.3008068
- Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, Boerman OC, Croockewit S, Oyen WJ, van Rossum M et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res 2013; 73:1063-75; PMID:23345163; https://doi.org/10.1158/0008-5472.CAN-12-2583
- Schreibelt G, Bol KF, Westdorp H, Wimmers F, Aarntzen EH, Duiveman-de Boer T, van de Rakt MW, Scharenborg NM, de Boer AJ, Pots JM et al. Effective clinical responses in metastatic melanoma patients after vaccination with primary myeloid dendritic cells. Clin Cancer Res 2016; 22:2155-66; PMID:26712687; https://doi.org/10.1158/1078-0432.CCR-15-2205
- Czerniecki BJ, Koski GK, Koldovsky U, Xu S, Cohen PA, Mick R, Nisenbaum H, Pasha T, Xu M, Fox KR et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res 2007; 67:1842-52; PMID:17293384; https://doi.org/10.1158/0008-5472.CAN-06-4038
- Duluc D, Joo H, Ni L, Yin W, Upchurch K, Li D, Xue Y, Klucar P, Zurawski S, Zurawski G et al. Induction and activation of human Th17 by targeting antigens to dendritic cells via dectin-1. J Immunol 2014; 192:5776-88; PMID:24835401; https://doi.org/10.4049/jimmunol.1301661
- Li D, Romain G, Flamar AL, Duluc D, Dullaers M, Li XH, Zurawski S, Bosquet N, Palucka AK, Le Grand R et al. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J Exp Med 2012; 209:109-21; PMID:22213806; https://doi.org/10.1084/jem.20110399
- Yu CI, Becker C, Wang Y, Marches F, Helft J, Leboeuf M, Anguiano E, Pourpe S, Goller K, Pascual V et al. Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF-beta. Immunity 2013; 38:818-30; PMID:23562160; https://doi.org/10.1016/j.immuni.2013.03.004
- Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G et al. Mucosal imprinting of vaccine-induced CD8(+) T cells is crucial to inhibit the growth of mucosal tumors. Sci Transl Med 2013; 5:172ra20; PMID:23408053; https://doi.org/10.1126/scitranslmed.3004888
- Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and anticancer immunosurveillance. Cell 2016; 165:276-87; PMID:27058662; https://doi.org/10.1016/j.cell.2016.03.001
- Carreno BM, Becker-Hapak M, Huang A, Chan M, Alyasiry A, Lie W-R, Aft RL, Cornelius LA, Trinkaus KM, Linette GP. IL-12p70–producing patient DC vaccine elicits Tc1-polarized immunity. J Clin Invest 2013; 123:3383-94; PMID:23867552; https://doi.org/10.1172/JCI68395
- Lasek W, Zagożdżon R, Jakobisiak M. Interleukin 12: Still a promising candidate for tumor immunotherapy? Cancer Immunol Immunother 2014; 63:419-35; PMID:24514955; https://doi.org/10.1007/s00262-014-1523-1
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411-22; PMID:20818862; https://doi.org/10.1056/NEJMoa1001294
- Higano CS, Small EJ, Schellhammer P, Yasothan U, Gubernick S, Kirkpatrick P, Kantoff PW. Sipuleucel-T. Nat Rev Drug Discov 2010; 9:513-4; PMID:20592741; https://doi.org/10.1038/nrd3220
- Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin Cancer Res 2011; 17:3520-6; PMID:21471425; https://doi.org/10.1158/1078-0432.CCR-10-3126
- Brunet LR, Hagemann T, Gaya A, Mudan S, Marabelle A. Have lessons from past failures brought us closer to the success of immunotherapy in metastatic pancreatic cancer? OncoImmunology 2016; 5:e1112942; PMID:27141395; https://doi.org/10.1080/2162402X.2015.1112942
- Felix J, Lambert J, Roelens M, Maubec E, Guermouche H, Pages C, Sidina I, Cordeiro DJ, Maki G, Chasset F et al. Ipilimumab reshapes T cell memory subsets in melanoma patients with clinical response. OncoImmunology 2016; 5:1136045; PMID:27622012; https://doi.org/10.1080/2162402X.2015.1136045
- Vacchelli E, Pol J, Bloy N, Eggermont A, Cremer I, Fridman WH, Galon J, Marabelle A, Kohrt H, Zitvogel L et al. Trial watch: Tumor-targeting monoclonal antibodies for oncological indications. OncoImmunology 2015; 4:e985940; PMID:25949870; https://doi.org/10.4161/2162402X.2014.985940
- Kayser S, Boβ C, Feucht J, Witte K-E, Scheu A, Bülow H-J, Joachim S, Stevanović S, Schumm M, Rittig SM et al. Rapid generation of NY-ESO-1-specific CD4+ THELPER1 cells for adoptive T-cell therapy. OncoImmunology 2015; 4:e1002723; PMID:26155389; https://doi.org/10.1080/2162402X.2014.1002723
- Aranda F, Buqué A, Bloy N, Castoldi F, Eggermont A, Cremer I, Fridman WH, Fucikova J, Galon J, Spisek R et al. Trial Watch: Adoptive cell transfer for oncological indications. OncoImmunology 2015; 4:e1046673; PMID:26451319; https://doi.org/10.1080/2162402X.2015.1046673
- Galluzzi L, Vacchelli E, Bravo-San Pedro JM, Buque A, Senovilla L, Baracco EE, Bloy N, Castoldi F, Abastado JP, Agostinis P et al. Classification of current anticancer immunotherapies. Oncotarget 2014; 5:12472-508; PMID:25537519; https://doi.org/10.18632/oncotarget.2998
- Garg AD, Vandenberk L, Van Woensel M, Belmans J, Schaaf M, Boon L, De Vleeschouwer S, Agostinis P. Preclinical efficacy of immune-checkpoint monotherapy does not recapitulate corresponding biomarkers-based clinical predictions in glioblastoma. Oncoimmunology 2017; Mar 3;6(4):e1295903; PMID:28507806; https://doi.org/10.1080/2162402X.2017.1295903.
- Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, Congdon KL, Reap EA, Archer GE, Desjardins A et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 2015; 519:366-9; PMID:25762141; https://doi.org/10.1038/nature14320
- Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH, Mardis ER et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015; 348:803-8; PMID:25837513; https://doi.org/10.1126/science.aaa3828
- Garg AD, Vandenberk L, Koks C, Verschuere T, Boon L, Van Gool SW, Agostinis P. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci Transl Med 2016; 8:328ra27; PMID:26936504; https://doi.org/10.1126/scitranslmed.aae0105
- Ohshio Y, Teramoto K, Hanaoka J, Tezuka N, Itoh Y, Asai T, Daigo Y, Ogasawara K. Cancer-associated fibroblast-targeted strategy enhances antitumor immune responses in dendritic cell-based vaccine. Cancer Sci 2015; 106:134-42; PMID:25483888; https://doi.org/10.1111/cas.12584
- Xiang J, Xu L, Gong H, Zhu W, Wang C, Xu J, Feng L, Cheng L, Peng R, Liu Z. Antigen-loaded upconversion nanoparticles for dendritic cell stimulation, tracking, and vaccination in dendritic cell-based immunotherapy. ACS Nano 2015; 9:6401-11; PMID:26028363; https://doi.org/10.1021/acsnano.5b02014
- Carmi Y, Spitzer MH, Linde IL, Burt BM, Prestwood TR, Perlman N, Davidson MG, Kenkel JA, Segal E, Pusapati GV et al. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature 2015; 521:99-104; PMID:25924063; https://doi.org/10.1038/nature14424
- Vandenberk L, Garg AD, Verschuere T, Koks C, Belmans J, Beullens M, Agostinis P, De Vleeschouwer S, Van Gool SW. Irradiation of necrotic cancer cells, employed for pulsing dendritic cells (DCs), potentiates DC vaccine-induced antitumor immunity against high-grade glioma. Oncoimmunology 2016; 5:e1083669; PMID:27057467; https://doi.org/10.1080/2162402X.2015.1083669
- Lu L, Tao H, Chang AE, Hu Y, Shu G, Chen Q, Egenti M, Owen J, Moyer JS, Prince ME et al. Cancer stem cell vaccine inhibits metastases of primary tumors and induces humoral immune responses against cancer stem cells. Oncoimmunology 2015; 4:e990767; PMID:25949905; https://doi.org/10.4161/2162402X.2014.990767
- Jung ID, Shin SJ, Lee MG, Kang TH, Han HD, Lee SJ, Kim WS, Kim HM, Park WS, Kim HW et al. Enhancement of tumor-specific T cell-mediated immunity in dendritic cell-based vaccines by Mycobacterium tuberculosis heat shock protein X. J Immunol 2014; 193:1233-45; PMID:24990079; https://doi.org/10.4049/jimmunol.1400656
- Willemen Y, Van den Bergh JM, Lion E, Anguille S, Roelandts VA, Van Acker HH, Heynderickx SD, Stein BM, Peeters M, Figdor CG et al. Engineering monocyte-derived dendritic cells to secrete interferon-alpha enhances their ability to promote adaptive and innate anti-tumor immune effector functions. Cancer Immunol Immunother 2015; 64:831-42; PMID:25863943; https://doi.org/10.1007/s00262-015-1688-2
- Martin K, Muller P, Schreiner J, Prince SS, Lardinois D, Heinzelmann-Schwarz VA, Thommen DS, Zippelius A. The microtubule-depolymerizing agent ansamitocin P3 programs dendritic cells toward enhanced anti-tumor immunity. Cancer Immunol Immunother 2014; 63:925-38; PMID:24906866; https://doi.org/10.1007/s00262-014-1565-4
- Schuler-Thurner B, Bartz-Schmidt KU, Bornfeld N, Cursiefen C, Fuisting B, Grisanti S, Heindl LM, Holbach L, Keserü M, Knorr H et al. Immunotherapy of uveal melanoma: Vaccination against cancer. Multicenter adjuvant phase 3 vaccination study using dendritic cells laden with tumor RNA for large newly diagnosed uveal melanoma. Ophthalmologe 2015; 112:1017-21; PMID:26602097; https://doi.org/10.1007/s00347-015-0162-z
- Hunn MK, Bauer E, Wood CE, Gasser O, Dzhelali M, Ancelet LR, Mester B, Sharples KJ, Findlay MP, Hamilton DA et al. Dendritic cell vaccination combined with temozolomide retreatment: Results of a phase I trial in patients with recurrent glioblastoma multiforme. J Neurooncol 2015; 121:319-29; PMID:25366363; https://doi.org/10.1007/s11060-014-1635-7
- Poschke I, Lovgren T, Adamson L, Nystrom M, Andersson E, Hansson J, Tell R, Masucci GV, Kiessling R. A phase I clinical trial combining dendritic cell vaccination with adoptive T cell transfer in patients with stage IV melanoma. Cancer Immunol Immunother 2014; 63:1061-71; PMID:24993563; https://doi.org/10.1007/s00262-014-1575-2
- Baek S, Kim YM, Kim SB, Kim CS, Kwon SW, Kim Y, Kim H, Lee H. Therapeutic DC vaccination with IL-2 as a consolidation therapy for ovarian cancer patients: A phase I/II trial. Cell Mol Immunol 2015; 12:87-95; PMID:24976269; https://doi.org/10.1038/cmi.2014.40
- Ahrens ET, Helfer BM, O'Hanlon CF, Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med 2014; 72:1696-701; PMID:25241945; https://doi.org/10.1002/mrm.25541 10.1002/mrm.25454
- Wang H, Feng F, Zhu M, Wang R, Wang X, Wu Y, Zhuang Z. Therapeutic efficacy of dendritic cells pulsed by autologous tumor cell lysate in combination with CIK cells on advanced renal cell carcinoma. Xi bao yu fen zi mian yi xue za zhi 2015; 31:67-71; PMID:25575061
- Hsu MS, Sedighim S, Wang T, Antonios JP, Everson RG, Tucker AM, Du L, Emerson R, Yusko E, Sanders C et al. TCR sequencing can identify and track glioma-infiltrating T cells after DC vaccination. Cancer Immunol Res 2016; 4:412-8; PMID:26968205; https://doi.org/10.1158/2326-6066.CIR-15-0240
- Zhao X, Zhang Z, Li H, Huang J, Yang S, Xie T, Huang L, Yue D, Xu L, Wang L et al. Cytokine induced killer cell-based immunotherapies in patients with different stages of renal cell carcinoma. Cancer Lett 2015; 362:192-8; PMID:25843292; https://doi.org/10.1016/j.canlet.2015.03.043
- Wang X, Bayer ME, Chen X, Fredrickson C, Cornforth AN, Liang G, Cannon J, He J, Fu Q, Liu J et al. Phase I trial of active specific immunotherapy with autologous dendritic cells pulsed with autologous irradiated tumor stem cells in hepatitis B-positive patients with hepatocellular carcinoma. J Surg Oncol 2015; 111:862-7; PMID:25873455; https://doi.org/10.1002/jso.23897
- Dillman RO, McClay EF, Barth NM, Amatruda TT, Schwartzberg LS, Mahdavi K, de Leon C, Ellis RE, DePriest C. Dendritic versus tumor cell presentation of autologous tumor antigens for active specific immunotherapy in metastatic melanoma: Impact on long-term survival by extent of disease at the time of treatment. Cancer Biother Radiopharm 2015; 30:187-94; PMID:26083950; https://doi.org/10.1089/cbr.2015.1843
- Podrazil M, Horvath R, Becht E, Rozkova D, Bilkova P, Sochorova K, Hromadkova H, Kayserova J, Vavrova K, Lastovicka J et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget 2015; 6:18192-205; PMID:26078335; https://doi.org/10.18632/oncotarget.4145
- Zhao P, Bu X, Wei X, Sun W, Xie X, Li C, Guo Q, Zhu D, Wei X, Gao D. Dendritic cell immunotherapy combined with cytokine-induced killer cells promotes skewing toward Th2 cytokine profile in patients with metastatic non-small cell lung cancer. Int Immunopharmacol 2015; 25:450-6; PMID:25698555; https://doi.org/10.1016/j.intimp.2015.02.010
- Rexer H. [Study and therapy of metastatic castration-resistant prostate cancer: A randomized, double blind, multicenter, parallel-group, phase III study to evaluate efficacy and safety of DCVAC/PCa versus placebo in men with metastatic castration resistant prostate cancer eligible for first line chemotherapy (VIABLE) - AUO study AP 78/13]. Urologe A 2015; 54:555-7; PMID:25758238; https://doi.org/10.1007/s00120-015-3801-8
- Tsukinaga S, Kajihara M, Takakura K, Ito Z, Kanai T, Saito K, Takami S, Kobayashi H, Matsumoto Y, Odahara S et al. Prognostic significance of plasma interleukin-6/-8 in pancreatic cancer patients receiving chemoimmunotherapy. World J Gastroenterol 2015; 21:11168-78; PMID:26494971; https://doi.org/10.3748/wjg.v21.i39.11168
- Qiu Y, Yun MM, Dong X, Xu M, Zhao R, Han X, Zhou E, Yun F, Su W, Liu C et al. Combination of cytokine-induced killer and dendritic cells pulsed with antigenic alpha-1,3 galactosyl epitope-enhanced lymphoma cell membrane for effective B-cell lymphoma immunotherapy. Cytotherapy 2016; 18:91-8; PMID:26549382; https://doi.org/10.1016/j.jcyt.2015.09.012
- Lee JH, Lee Y, Lee M, Heo MK, Song JS, Kim KH, Lee H, Yi NJ, Lee KW, Suh KS et al. A phase I/IIa study of adjuvant immunotherapy with tumour antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Br J Cancer 2015; 113:1666-76; PMID:26657650; https://doi.org/10.1038/bjc.2015.430
- Prue RL, Vari F, Radford KJ, Tong H, Hardy MY, D'Rozario R, Waterhouse NJ, Rossetti T, Coleman R, Tracey C et al. A phase I clinical trial of CD1c (BDCA-1)+ dendritic cells pulsed with HLA-A*0201 peptides for immunotherapy of metastatic hormone refractory prostate cancer. J Immunother 2015; 38:71-6; PMID:25658616; https://doi.org/10.1097/CJI.0000000000000063
- Bol KF, Mensink HW, Aarntzen EH, Schreibelt G, Keunen JE, Coulie PG, de Klein A, Punt CJ, Paridaens D, Figdor CG et al. Long overall survival after dendritic cell vaccination in metastatic uveal melanoma patients. Am J Ophthalmol 2014; 158:939-47; PMID:25038326; https://doi.org/10.1016/j.ajo.2014.07.014
- Zhang L, Xu Y, Shen J, He F, Zhang D, Chen Z, Duan Y, Sun J. Feasibility study of DCs/CIKs combined with thoracic radiotherapy for patients with locally advanced or metastatic non-small-cell lung cancer. Radiat Oncol 2016; 11:60; PMID:27097970; https://doi.org/10.1186/s13014-016-0635-5
- Xi HB, Wang GX, Fu B, Liu WP, Li Y. Survivin and PSMA loaded dendritic cell vaccine for the treatment of prostate cancer. Biol Pharm Bull 2015; 38:827-35; PMID:25787895; https://doi.org/10.1248/bpb.b14-00518
- Narita M, Kanda T, Abe T, Uchiyama T, Iwafuchi M, Zheng Z, Liu A, Kaifu T, Kosugi S, Minagawa M et al. Immune responses in patients with esophageal cancer treated with SART1 peptide-pulsed dendritic cell vaccine. Int J Oncol 2015; 46:1699-709; PMID:25625346; https://doi.org/10.3892/ijo.2015.2846
- Mayanagi S, Kitago M, Sakurai T, Matsuda T, Fujita T, Higuchi H, Taguchi J, Takeuchi H, Itano O, Aiura K et al. Phase I pilot study of Wilms tumor gene 1 peptide-pulsed dendritic cell vaccination combined with gemcitabine in pancreatic cancer. Cancer Sci 2015; 106:397-406; PMID:25614082; https://doi.org/10.1111/cas.12621
- Krishnadas DK, Shusterman S, Bai F, Diller L, Sullivan JE, Cheerva AC, George RE, Lucas KG. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol Immunother 2015; 64:1251-60; PMID:26105625; https://doi.org/10.1007/s00262-015-1731-3
- Sakai K, Shimodaira S, Maejima S, Udagawa N, Sano K, Higuchi Y, Koya T, Ochiai T, Koide M, Uehara S et al. Dendritic cell-based immunotherapy targeting Wilms' tumor 1 in patients with recurrent malignant glioma. J Neurosurg 2015; 123:989-97; PMID:26252465; https://doi.org/10.3171/2015.1.JNS141554
- Maeda Y, Yoshimura K, Matsui H, Shindo Y, Tamesa T, Tokumitsu Y, Hashimoto N, Tokuhisa Y, Sakamoto K, Sakai K et al. Dendritic cells transfected with heat-shock protein 70 messenger RNA for patients with hepatitis C virus-related hepatocellular carcinoma: A phase 1 dose escalation clinical trial. Cancer Immunol Immunother 2015; 64:1047-56; PMID:25982372; https://doi.org/10.1007/s00262-015-1709-1
- Wilgenhof S, Corthals J, Van Nuffel AM, Benteyn D, Heirman C, Bonehill A, Thielemans K, Neyns B. Long-term clinical outcome of melanoma patients treated with messenger RNA-electroporated dendritic cell therapy following complete resection of metastases. Cancer Immunol Immunother 2015; 64:381-8; PMID:25548092; https://doi.org/10.1007/s00262-014-1642-8
- Si Y, Deng Z, Lan G, Du H, Wang Y, Si J, Wei J, Weng J, Qin Y, Huang B et al. The safety and immunological effects of rAd5-EBV-LMP2 vaccine in nasopharyngeal carcinoma patients: A phase I clinical trial and two-year follow-up. Chem Pharm Bull 2016; 64:1118-23; PMID:27477649; https://doi.org/10.1248/cpb.c16-00114
- Rozera C, Cappellini GA, D'Agostino G, Santodonato L, Castiello L, Urbani F, Macchia I, Aricò E, Casorelli I, Sestili P et al. Intratumoral injection of IFN-alpha dendritic cells after dacarbazine activates anti-tumor immunity: Results from a phase I trial in advanced melanoma. J Transl Med 2015; 13:139; PMID:25933939; https://doi.org/10.1186/s12967-015-0473-5
- Kolstad A, Kumari S, Walczak M, Madsbu U, Hagtvedt T, Bogsrud TV, Kvalheim G, Holte H, Aurlien E, Delabie J et al. Sequential intranodal immunotherapy induces antitumor immunity and correlated regression of disseminated follicular lymphoma. Blood 2015; 125:82-9; PMID:25293773; https://doi.org/10.1182/blood-2014-07-592162
- Zhao X, Ji CY, Liu GQ, Ma DX, Ding HF, Xu M, Xing J. Immunomodulatory effect of DC/CIK combined with chemotherapy in multiple myeloma and the clinical efficacy. Int J Clin Exp Pathol 2015; 8:13146-55; PMID:26722513
- Yan L, Wu M, Ba N, Wang LJ, Zhang HQ, Shi GY, Zhang ZS, Wang XJ. Efficacy of dendritic cell-cytokine-induced killer immunotherapy plus intensity-modulated radiation therapy in treating elderly patients with esophageal carcinoma. Genet Mol Res 2015; 14:898-905; PMID:25730028; https://doi.org/10.4238/2015.February.2.13
- Greene JM, Schneble EJ, Jackson DO, Hale DF, Vreeland TJ, Flores M, Martin J, Herbert GS, Hardin MO, Yu X et al. A phase I/IIa clinical trial in stage IV melanoma of an autologous tumor-dendritic cell fusion (dendritoma) vaccine with low dose interleukin-2. Cancer Immunol Immunother 2016; 65:383-92; PMID:26894495; https://doi.org/10.1007/s00262-016-1809-6
- Ho VT, Kim HT, Kao G, Cutler C, Levine J, Rosenblatt J, Joyce R, Antin JH, Soiffer RJ, Ritz J et al. Sequential infusion of donor-derived dendritic cells with donor lymphocyte infusion for relapsed hematologic cancers after allogeneic hematopoietic stem cell transplantation. Am J Hematol 2014; 89:1092-6; PMID:25132538; https://doi.org/10.1002/ajh.23825
- Saito S, Yanagisawa R, Yoshikawa K, Higuchi Y, Koya T, Yoshizawa K, Tanaka M, Sakashita K, Kobayashi T, Kurata T et al. Safety and tolerability of allogeneic dendritic cell vaccination with induction of Wilms tumor 1-specific T cells in a pediatric donor and pediatric patient with relapsed leukemia: A case report and review of the literature. Cytotherapy 2015; 17:330-5; PMID:25484308; https://doi.org/10.1016/j.jcyt.2014.10.003
- Kimura H, Matsui Y, Ishikawa A, Nakajima T, Yoshino M, Sakairi Y. Randomized controlled phase III trial of adjuvant chemo-immunotherapy with activated killer T cells and dendritic cells in patients with resected primary lung cancer. Cancer Immunol Immunother 2015; 64:51-9; PMID:25262164; https://doi.org/10.1007/s00262-014-1613-0
- Wilgenhof S, Corthals J, Heirman C, van Baren N, Lucas S, Kvistborg P, Thielemans K, Neyns B. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol 2016; 34:1330-8; PMID:26926680; https://doi.org/10.1200/JCO.2015.63.4121
- van den Hout MF, Sluijter BJ, Santegoets SJ, van Leeuwen PA, van den Tol MP, van den Eertwegh AJ, Scheper RJ, de Gruijl TD. Local delivery of CpG-B and GM-CSF induces concerted activation of effector and regulatory T cells in the human melanoma sentinel lymph node. Cancer Immunol Immunother 2016; 65:405-15; PMID:26935057; https://doi.org/10.1007/s00262-016-1811-z
- Sluijter BJ, van den Hout MF, Koster BD, van Leeuwen PA, Schneiders FL, van de Ven R, Molenkamp BG, Vosslamber S, Verweij CL, van den Tol MP et al. Arming the melanoma sentinel lymph node through local administration of CpG-B and GM-CSF: Recruitment and activation of BDCA3/CD141(+) dendritic cells and enhanced cross-presentation. Cancer Immunol Res 2015; 3:495-505; PMID:25633713; https://doi.org/10.1158/2326-6066.CIR-14-0165
- Lipson EJ, Sharfman WH, Chen S, McMiller TL, Pritchard TS, Salas JT, Sartorius-Mergenthaler S, Freed I, Ravi S, Wang H et al. Safety and immunologic correlates of Melanoma GVAX, a GM-CSF secreting allogeneic melanoma cell vaccine administered in the adjuvant setting. J Transl Med 2015; 13:214; PMID:26143264; https://doi.org/10.1186/s12967-015-0572-3
- Wilgenhof S, Corthals J, Heirman C, Baren Nv, Lucas S, Kvistborg P, Thielemans K, Neyns B. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol 2016; 34:1330-8; PMID:26926680; https://doi.org/10.1200/JCO.2015.63.4121
- Tsukinaga S, Kajihara M, Takakura K, Ito Z, Kanai T, Saito K, Takami S, Kobayashi H, Matsumoto Y, Odahara S et al. Prognostic significance of plasma interleukin-6/-8 in pancreatic cancer patients receiving chemoimmunotherapy. World J Gastroenterol 2015; 21:11168-78; PMID:26494971; https://doi.org/10.3748/wjg.v21.i39.11168
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31:51-72; PMID:23157435; https://doi.org/10.1146/annurev-immunol-032712-100008
- Garg AD, De Ruysscher D, Agostinis P. Immunological metagene signatures derived from immunogenic cancer cell death associate with improved survival of patients with lung, breast or ovarian malignancies: A large-scale meta-analysis. Oncoimmunology 2016; 5:e1069938; PMID:27057433; https://doi.org/10.1080/2162402X.2015.1069938
- Bloy N, Pol J, Aranda F, Eggermont A, Cremer I, Fridman WH, Fučíková J, Galon J, Tartour E, Spisek R et al. Trial watch: Dendritic cell-based anticancer therapy. Oncoimmunology 2014; 3:e963424; PMID:25941593; https://doi.org/10.4161/21624011.2014.963424
- Bol KF, Aarntzen EHJG, Hout FEMit, Schreibelt G, Creemers JHA, Lesterhuis WJ, Gerritsen WR, Grunhagen DJ, Verhoef C, Punt CJ et al. Favorable overall survival in stage III melanoma patients after adjuvant dendritic cell vaccination. OncoImmunology 2016; 5:e1057673; PMID:26942068; https://doi.org/10.1080/2162402X.2015.1057673
- Hodges TR, Ferguson SD, Caruso HG, Kohanbash G, Zhou S, Cloughesy TF, Berger MS, Poste GH, Khasraw M, Ba S et al. Prioritization schema for immunotherapy clinical trials in glioblastoma. OncoImmunology 2016; 5:e1145332; PMID:27471611; https://doi.org/10.1080/2162402X.2016.1145332
- Gulley JL, Mulders P, Albers P, Banchereau J, Bolla M, Pantel K, Powles T. Perspectives on sipuleucel-T: Its role in the prostate cancer treatment paradigm. OncoImmunology 2016; 5:e1107698; PMID:27141392; https://doi.org/10.1080/2162402X.2015.1107698
- Simon S, Vignard V, Florenceau L, Dreno B, Khammari A, Lang F, Labarriere N. PD-1 expression conditions T cell avidity within an antigen-specific repertoire. OncoImmunology 2016; 5:e1104448; PMID:26942093; https://doi.org/10.1080/2162402X.2015.1104448
- Chevalier MF, Bobisse S, Costa-Nunes C, Cesson V, Jichlinski P, Speiser DE, Harari A, Coukos G, Romero P, Nardelli-Haefliger D et al. High-throughput monitoring of human tumor-specific T-cell responses with large peptide pools. OncoImmunology 2015; 4:e1029702; PMID:26451296; https://doi.org/10.1080/2162402X.2015.1029702
- Poschke I, Faryna M, Bergmann F, Flossdorf M, Lauenstein C, Hermes J, Hinz U, Hank T, Ehrenberg R, Volkmar M et al. Identification of a tumor-reactive T-cell repertoire in the immune infiltrate of patients with resectable pancreatic ductal adenocarcinoma. OncoImmunology 2016; 5:e1240859; PMID:28123878; https://doi.org/10.1080/2162402X.2016.1240859
