ABSTRACT
Despite the high interest and concern due to an increasing incidence and death rate, patients who develop muscle invasive bladder cancer (MIBC) have few options available. However, the past decade has produced many candidate bladder tumor-specific markers but further development of these markers is still needed for creating effective targeted medications to solve this urgent need. Interleukin-5 receptor α-subunit (IL-5Rα) has recently been reported to be involved in MIBC progression. Thus, we aimed to validate IL-5Rα as a target for antibody-conjugates to better manage patients with MIBC. Patients were recruited and their tumors were processed for IL-5Rα immunohistochemical analysis. NOD/SCID mice were also heterotopically implanted with the human MIBC HT-1376 and HT-B9 cell lines and established xenografts immunohistochemically evaluated for IL-5Rα and compared against patient tumors. Using the mAb A14, an antibody-drug conjugate (ADC) and a radiolabeled immunoconjugate (RIC) were developed by conjugating to vinblastine and to the positron emitter copper-64 (64Cu), respectively. As a proof-of-concept for ADC and RIC efficacy, in vitro cytotoxicity and in vivo positron emission tomography (PET) imaging in tumor-bearing mice were performed, respectively. In addition, as rapid internalization and accumulation are important components for effective antibody-conjugates, we evaluated these aspects in response to IL-5 and 64Cu-A14 treatments. Our findings suggest that although IL-5Rα protein expression is preferentially increased in MIBC, it is rapid IL-5Rα-mediated internalization allowing vinblastine-A14 to have cytotoxic activity and 64Cu-A14 to detect MIBC tumors in vivo. This is the first report to elucidate the potential of IL-5Rα as an attractive MIBC target for antibody-conjugate applications.
Introduction
Bladder cancer is one of the most prevalent cancers impacting adults worldwide.Citation1 Although early stages of the disease can be successfully treated, there is no effective therapy for stages where cancer has progressed into the surrounding muscle layer (clinically classified as muscle invasive bladder cancer (MIBC)). Recent attempts to improve treatment efficacy of MIBC with novel combinations of chemotherapeutics have mostly benefitted in improving patient tolerability and not therapeutic efficacy.Citation2,3 Thus, MIBC has continued with a high rate of recurrence and poor overall prognosis for the past two decades.Citation2,3
In addition, novel imaging approaches are needed to more accurately stage pre-treatment bladder cancer. Patients often have local and distant disease at the time of initial diagnosis of the primary tumor.Citation4 This is significant as patients with organ confined bladder cancer undergo different treatment regimens compared with patients with metastatic disease. However, the decision for the optimal treatment strategy is mainly based on the results from imaging.Citation5 Unfortunately, current imaging methods, including, computed tomography, magnetic resonance imaging, and positron emission tomography (PET) using metabolic tracers, have difficulty in accurately identifying patients with metastatic disease.Citation5
The improved understanding of molecular contributions to bladder cancer progression is helping to identify new markers and develop targeted treatments. For example, the association between pro-inflammatory mediators expressed by bladder tumor cells or immune cells and their role in the progression of bladder cancer has been well pointed out.Citation6 Many studies have discovered intricate roles for cytokines that promote bladder cancer progression and metastasis.Citation7-9 To this end, the role of interleukin-5 (IL-5) and its ligand-specific receptor (IL-5Rα) in bladder cancer progression had not been extensively ascertained. Recently, Lee and coworkers discovered IL-5, and IL-5Rα mRNA expression were elevated in resected tumor tissues from patients with MIBC tumors relative to non-invasive bladder tumors and healthy urothelium.Citation10 Moreover, IL-5 treatment of invasive bladder cancer cell lines increased IL-5Rα mRNA expression and was dose dependent. IL-5 treatment amplified components associated with cancer invasion, such as enhanced cellular migration, expression of matrix metalloproteinases (MMPs), and the arrest of cellular proliferation.Citation11 However, not much else is known about IL-5Rα and its role as a marker or target for MIBC.
We aimed to determine the potential of IL-5Rα as a target for antibody conjugates by investigating key target properties namely expression and internalization. Immunohistochemistry (IHC) was used to determine the total protein content of IL-5Rα in bladder tumor specimens in relation to pathological staging. In addition, NOD/SCID mice were implanted with human MIBC cell lines and tumors immunohistochemically evaluated for IL-5Rα and compared against patient tumors. A radiolabeled immunoconjugate (RIC) and an antibody-drug conjugate (ADC) were developed and evaluated for tumor imaging by positron emission tomography (PET) and for cytotoxic activity, respectively. In addition, IL-5Rα-mediated internalization and intracellular accumulation in response to 64Cu-A14 treatments were evaluated to further dissect the potential of IL-5Rα as a targetable antigen for antibody conjugates.
We report our findings on the interplay between IL-5Rα expression, internalization, and accumulation dynamics and their contributions to the attractiveness of IL-5Rα as an MIBC target for antibody-conjugate applications.
Results
IL-5Rα increased expression is preferential to invasive bladder tumor specimens
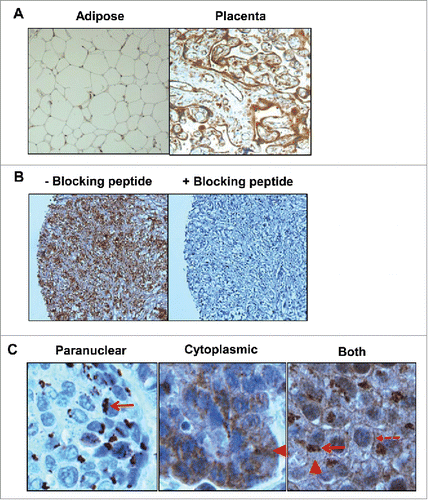
The optimized dilution of the polyclonal antibody LS-C165211 was 1/750, which demonstrated negative staining in IL-5Rα-negative adipose tissues and high-intensity staining in naturally high-expressing placental tissue (). IL-5Rα staining with 1/750 dilution of LS-C165211was completely blocked in the presence of 100-fold excess blocking peptide (). IHC staining revealed the distribution of IL-5Rα protein among different tumor specimens and was divided as predominantly paranuclear, cytoplasmic, or paranuclear and cytoplasmic with membrane surface staining ().
Figure 1. Determination of specificity for IL-5Rα staining by immunohistochemistry (IHC). (A) IL-5Rα-negative and -positive adipose and placental tissue, respectively, were stained in the presence of the IL-5Rα-specific polyclonal antibody. (B) IL-5Rα-positive tissue was stained with the IL-5Rα-specific polyclonal antibody in the presence of 100-fold excess blocking peptide. (C) IL-5Rα staining of bladder cancer specimens is predominantly paranuclear, cytoplasmic, and plasma membrane, respectively (arrow), (arrowhead), and (dashed arrow).
Tumors were classified according to the World Health Organization classification for pathologic tumor-node-metastasis (pTNM) staging, which describes the extent of invasion. As indicated in , invasive bladder tumors (p≥T1) contained significantly more specimens with high levels of IL-5Rα total cellular staining relative to non-invasive (pTa) tumors and normal urothelium (p = 8.6382E−7 and 8.0611E−7, respectively). The majority of Ta and normal urothelial specimens had comparable IL-5Rα staining intensities, which was low or negative. As muscle invasion is a poor prognostic indicator, patients were evaluated at the earliest muscle invasive stage (pT2). pT2 specimens also had significantly increased IL-5Rα staining relative to pTa and normal urothelium (p = 8.91E−9 and 1.1151E−8, respectively). Combining all cases with extensive bladder cancer invasion (>pT2), IL-5Rα stained high in a significantly greater number of specimens relative to pTa and normal urothelium (p = 0.000026 and 0.000661, respectively). IL-5Rα staining in carcinoma in situ (CIS) specimens was significantly elevated relative to normal urothelium (p = 0.023478) but not pTa specimens. IL-5Rα expression was negative or low in the majority of tissues with benign bladder disease or inflammation. There was no significant association between IL-5Rα expression and IL-5Rα intracellular staining location or percentage of positive IL-5Rα tumor cells.
Table 1. IL-5Rα expression in bladder cancer.
IL-5Rα increased expression is proportional to advanced stages in individual tumor specimens and in patientswith matched recurrences
Of the tumors that contained different areas with unique TNM stages or corresponding urothelial tissue without cancer, 50% of tumors contained specimens that increased IL-5Rα staining intensity in accordance with increased pTNM staging. For example, one tumor whose bladder contained tissues with invasive pT1, pT2, and pT3 stages and matched healthy urothelial tissue expressed high IL-5Rα levels in the invasive samples but low levels in the normal urothelium (). The pT1 specimen contained homogeneous high IL-5Rα expression in the urothelium with IL-5Rα-positive tumor cell islets within the lamina propria. The pT2 specimen showed clear dissemination of tumor cells into the muscle layer with high IL-5Rα expression. High-expressing IL-5Rα-positive tumor cells were also present in the perivesical adipose tissue in the pT3 specimen. In a second example, IL-5Rα stained negative in normal urothelium. In contrast, high-expressing IL-5Rα-positive tumor cells were present as large sheets invading the muscle layer in the pT2 specimen (). Of the remaining tumors, 39% contained samples with unchanged IL-5Rα staining despite different TNM stages and 11% of tumors contained samples where IL-5Rα staining was inversely proportional with increasing TNM stage.
Figure 2. IL-5Rα expression in normal and bladder cancer primary specimens at various pathology stages by IHC. Representative examples from individual patients of the increase in IL-5Rα protein expression levels in healthy versus cancer specimens. (A) One patient whose bladder contained normal urothelium and tumors with TNM stages pT1, pT2, and pT3. In the pT1 specimen, IL-5Rα-positive nests are observed (red arrow) within the lamina propria. In the pT2 and pT3 specimens, there is dissemination of IL-5Rα-positive tumor cells in the lamina propria and fat, respectively. (B) Another patient whose bladder contained normal urothelium versus pT2 tumor tissue. (C) Patient with tumor specimens obtained at initial visit and follow-up visit for tumor recurrence. Images are at ×40 magnification except for image of pT3 tumor in A, which is at ×400 magnification.
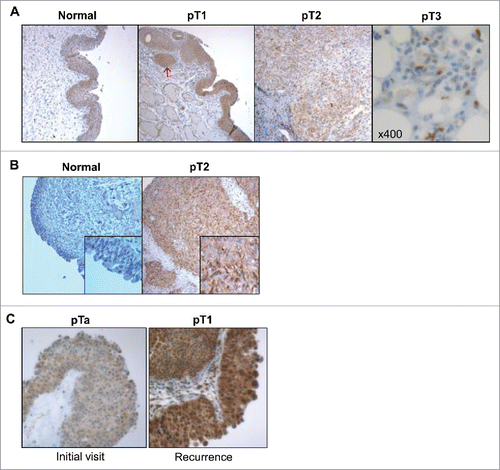
In the patients who underwent second tumor resections during our recruitment period 100% of recurring tumors stained positive for IL-5Rα. Recurrent tumors from 37% of patients had proportional increases in IL-5Rα expression with increasing pathological severity. One example shows IL-5Rα stained low in the non-invasive tumor but increased to high expression on the recurrent invasive tumor (). The TNM and IL-5Rα expression on initial and recurrent visit is summarized in .
Table 2. Bladder tumor recurrence and IL-5Rα expression.
Development of a pathologically similar model of human IL-5Rα-positive MIBC
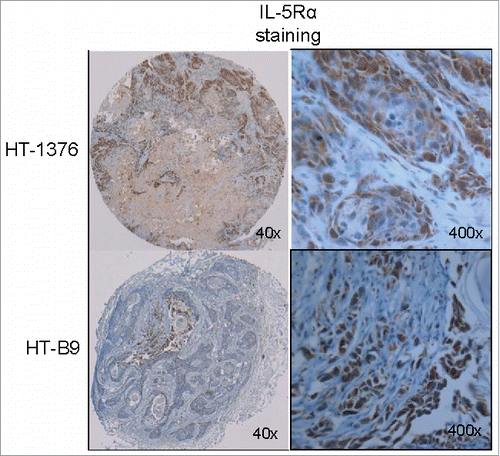
Pathology examination of HT-1376 and HT-B9 xenografts revealed important differences in IL-5Rα staining intensity, percentage of positive tumor cells, and intracellular localization (). HT-1376 xenografts displayed inhomogeneous IL-5Rα staining. Although, IL-5Rα-positive HT-1376 cells comprised >66% of total cells in the xenograft, there was a distinct mixture of intensity for IL-5Rα. Tumor cell clusters stained for IL-5Rα with either strong (dark brown) or weak (light brown) intensity. In contrast, only ∼11% of IL-5Rα-positive HT-B9 cells were contained in developed tumors. Interestingly, IL-5Rα expression was more intense and homogeneous in HT-B9 cells. IL-5Rα localization in HT-1376 appeared predominantly in the cytoplasm with reduced membrane staining. In contrast, IL-5Rα localization in HT-B9 cells was predominantly paranuclear. This heterogeneous IL-5Rα cellular localization pattern was similar to that observed in the human tumor specimens ().
Cell surface expression of IL-5Rα
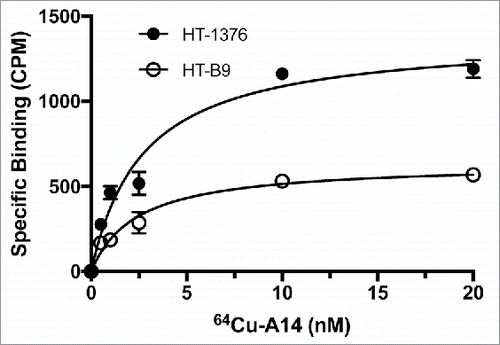
The labeling efficiency and radiopurity of the RIC 64Cu-A14 was >95%. In addition, <10% of the 64Cu dissociated when placed in media for 72 h. A14 cell binding as a function of increasing concentrations of 64Cu-A14 revealed specific binding approached saturation at concentrations of 3–5 nM (). The Kd for 64Cu-A14 on HT-1376 and HT-B9 cells was 2.7 ± 0.6 nM and 1.2 ± 0.3 nM, respectively. The calculated number of IL-5Rα molecules per cell was 616 and 157 receptors for HT-1376 and HT-B9, respectively. Flow cytometry demonstrated both HT-1376 and HT-B9 cells expressed IL-5Rα on the cell surface (Fig. S1). Although, IHC staining revealed elevated IL-5Rα levels in primary MIBC tumors, these radioligand and flow cytometry binding studies reveal modest cell surface expression on HT-1376 and HT-B9 cells.
64Cu-A14 shows IL-5Rα-specific accumulation in MIBC HT-1376 and HT-B9 xenografts as determined by PET imaging and region-of-interest (ROI) analysis
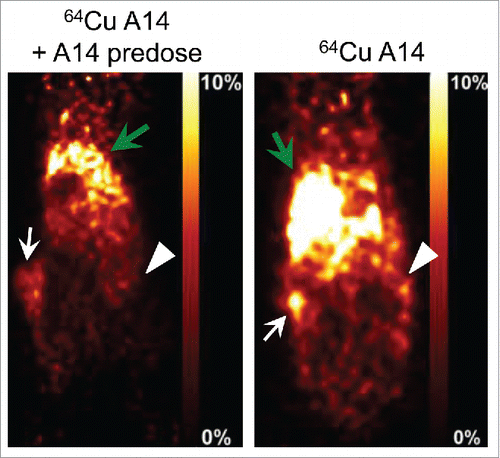
To determine whether IL-5Rα-positive MIBC tumors could be specifically targeted in vivo, PET imaging was used to evaluate tumor accumulation. Tracer doses (7 MBq) of 64Cu-A14 were intravenously injected into NOD/SCID mice bearing bilateral heterotopic HT-1376 and HT-B9 tumors. 64Cu-A14 accumulated in both HT-1376 and HT-B9 tumors sufficiently for them to be visualized on PET images at 48 h post-injection (). The visible uptake in HT-1376 tumors appeared as an intense foci surrounded by diffuse and less intense signal caused by circulating 64Cu-A14 typical for RICs 48 h after injection. In comparison, 64Cu-A14 uptake in HT-B9 tumors was relatively difficult to visualize due to less accumulation and, hence, reduced PET tumor signal. ROI image analysis was then converted into quantifiable tumor or tissue accumulation data expressed as the percent of injected-dose/gram of tissue (%ID/g). 64Cu-A14 accumulated in HT-1376 and HT-B9 tumors with levels of 8.0 ± 1.3%ID/g and 5.0 ± 2.3%ID/g, respectively. The calculated radioactivity uptake in the blood, muscle, and liver were 13.6 ± 1.2%ID/g, 2.2 ± 0.5%ID/g, and 16.9 ± 2.1%ID/g, respectively. For HT-1376 tumors, this resulted in tumor-to-blood, -muscle, and -liver contrast scores of 0.6, 3.6, and 0.5, respectively. In comparison, for HT-B9 xenografts, the tumor-to-blood, -muscle, and -liver contrast scores were 0.4, 2.3, and 0.3, respectively. In mice injected with 25 mg/kg of excess unlabeled A14, ROI analysis and PET imaging revealed a significant reduction in uptake in both HT-1376 (p <0.001) and HT-B9 (p <0.05) tumors (). The %ID/g in the HT-1376 and HT-B9 tumors in mice pre-dosed with blocking A14 was reduced to 4.1 ± 1.2%ID/g and 2.2 ± 0.6%ID/g, respectively.
Figure 5. In vivo PET imaging of HT-1376 and HT-B9 tumors by 64Cu-A14. 48 h post-injection representative PET images of tumor-bearing NOD/SCID mice intravenously injected with 64Cu-A14 with and without A14 pre-dosing to block IL-5Rα sites. White arrows indicate HT-1376 tumors, white arrowheads indicate HT-B9 tumors, and green arrows indicate the liver, which has the highest uptake due to normal antibody metabolism. The reductions in the tumor uptakes in A14-pre-dosed mice are statistically significant (p < 0.05).
IL-5Rα is rapidly internalized and re-expressed following exposure to IL-5
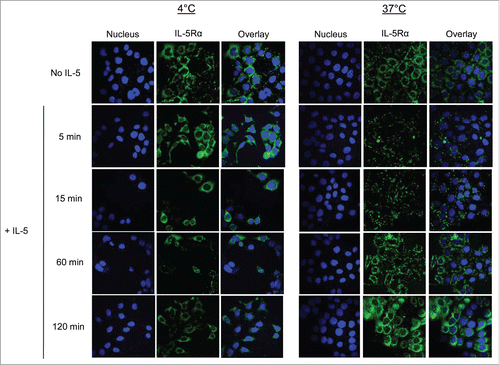
As PET imaging demonstrated that IL-5Rα-positive MIBC tumors could be imaged despite the majority of the protein localized inside the cell, we evaluated IL-5Rα internalization as rapid receptor-mediated internalization is a vital component for an effective antibody-conjugate. In addition, IL-5Rα internalization in an MIBC cancer setting has yet to be addressed. We used confocal microscopy to initially explore IL-5Rα internalization and re-expression dynamics upon exposure to its natural ligand IL-5. The immunofluorescence signals indicating the presence of IL-5Rα were predominantly localized to the cell surface and within the cytoplasm in the absence of IL-5 treatment at both 4°C and 37°C (). Within the cytoplasm, immunofluorescence appeared as small perinuclear foci that were homogeneously distributed throughout the intracellular space. This supported our IHC findings that IL-5Rα was present in higher numbers in the cytoplasm compared with the membrane in HT-1376 cells (). After only 5 min of exposure to IL-5 at 37° C, there was >50% decrease in fluorescence signal with only few intracellular foci able to be visualized suggesting rapid IL-5Rα internalization and degradation. Fluorescence signal intensity remained low for minutes 15 and 30. However, analysis at minute 60 revealed nearly 100% re-expression of IL-5Rα. The IL-5Rα levels at minute 120 were comparable to cells without IL-5 treatment.
64Cu-A14 is internalized resulting in effective intracellular accumulation
The IL-5-induced IL-5Rα internalization efficiency indicated this was most likely an important mechanism contributing to the ability of 64Cu-A14 to image IL-5Rα-positive MIBC tumors. Thus, we treated HT-1376 and HT-B9 cells with 64Cu-A14 and 64Cu-IgG and evaluated radioactivity internalization. Internalized radioactivity in HT-1376 and HT-B9 cells at 37°C increased 2.7- and 4.7-fold (p <0.0001), respectively, relative to 4°C after 1 h treatment ( and ). There was no change in the levels of internalized 64Cu at 4°C and 37°C with 64Cu-IgG.
Figure 7. Internalization and intracellular radioactivity accumulation in MIBC cells after treatment with 64Cu-A14. The relative radioactivity intracellular accumulation increase in HT-1376 (A and C) and HT-B9 (B and D) cells after treatment with 64Cu-A14 and 64Cu-IgG at 1 h at 37°C compared with 4°C and when treated under increasing incubation times. p-values of < 0.05, <0.001, and <0.0001 are indicated by *, **, ***, respectively. For clarity, p-values are focused on the favorable 64Cu-A14-specific increase of intracellular radioactivity accumulation over time and the retention of intracellular versus extracellular radioactivity.
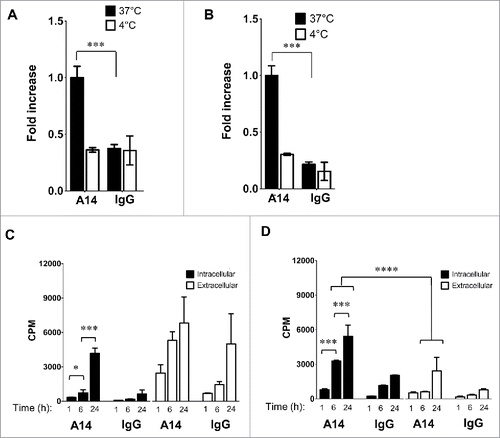
Accumulation and subsequent retention of delivered payloads are key properties for attractive receptor/antibody-conjugate systems. Therefore, cells were treated with 64Cu-A14 and 64Cu-IgG at increasing time points and intracellular and extracellular radioactivity levels measured. HT-1376 intracellular radioactivity significantly increased from 1 h to 6 h (p <0.05) and from 6 h to 24 h (p <0.001; ). In addition, 64Cu-A14 significantly (p <0.001) increased intracellular radioactivity accumulation at 6 h and 24 h by factors of 4.0 and 6.5, respectively, relative to 64Cu-IgG. However, 64Cu-A14 treatments revealed significant (p <0.01) increased extracellular radioactivity levels relative to intracellular levels at all-time points. Interestingly, HT-1376 cells treated with 64Cu-IgG resulted in unexpected increases in extracellular radioactivity relative to intracellular radioactivity levels. In particular, at 24 h extracellular radioactivity was increased by a factor of 7.8.
HT-B9 cells demonstrated specific radioactivity accumulation and improved retention when treated with 64Cu-A14 (). HT-B9 intracellular radioactivity significantly increased from 1 h to 6 h (p < 0.001) and from 6 h to 24 h (; p < 0.001). 64Cu-A14 significantly (p < 0.001) increased intracellular radioactivity at 6 h and 24 h by factors of 2.8 and 2.7, respectively, relative to 64Cu-IgG. Importantly, intracellular radioactivity levels remained significantly (p < 0.0001) elevated relative to extracellular radioactivity levels. Unlike HT-1376 cells, HT-B9 extracellular radioactivity levels remained low.
Vinblastine-A14 possesses cytotoxic activity against HT-1376 cells
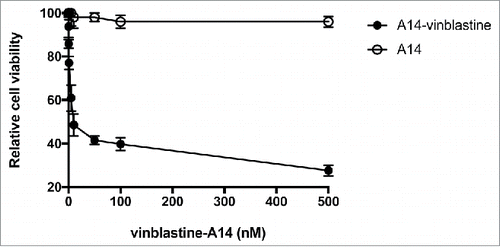
To demonstrate IL-5Rα could be targeted for therapy we conjugated A14 to vinblastine, a vinca alkaloid used in MIBC chemotherapy regimens.Citation12 Vinblastine-A14 was designed with a thioester-based noncleavable linker. This linker used a 5-atom chain to have a length of 6 nm providing it with standard linker lengths incorporated in currently approved ADCs.Citation13 The drug-to-antibody molar ratio was 3.2. Vinblastine-A14 showed increased potency relative to unmodified A14 with a relative IC50 of 4.2 nmol/L (). In contrast, there was no cytotoxicity observed with unmodified A14.
Discussion
We have shown IL-5Rα is a potentially attractive target for antibody-conjugates against MIBC due to its increased preferential expression and favorable internalization dynamics. Immunohistochemical analysis revealed IL-5Rα protein was preferentially expressed at increased levels in primary MIBC tumors. IL-5Rα had increased expression on tumor cells present in the urothelium and invading into and past the adjacent muscle layer. In contrast, IL-5Rα expression was low or negative on normal urothelial and non-invasive bladder tumor cells. IHC further demonstrated IL-5Rα expression was proportional to advanced stages in different tumor specimens from specific individuals. In addition, increased IL-5Rα expression was present on recurrent tumors with advanced stage. An animal model of human IL-5Rα-positive MIBC was developed with the HT-1376 and HT-B9 cell lines. These xenografts were highly similar in IL-5Rα-positive tumor cell patterns observed in the human tumors evaluated by IHC. Thus, we developed 64Cu-A14 and vinblastine-A14, for PET imaging and therapy applications, respectively. However, binding studies with 64Cu-A14 revealed that IL-5Rα is modestly expressed on the cell-surface of both HT-1376 and HT-B9 cells. Nevertheless, PET imaging revealed that 64Cu-A14 could specifically image these MIBC tumors. Therefore, we investigated whether IL-5Rα-mediated internalization and accumulation of 64Cu-A14 were potential important contributors involved in favorable PET imaging and cytotoxicity. In response to IL-5 exposure, confocal microscopy revealed that internalization and re-expression was rapid in HT-1376 cells. 64Cu-A14 uptake data in the HT-1376 and HT-B9 cell lines showed that 64Cu was effectively accumulated. Thus, our work suggests that internalization of IL-5Rα leading to sufficient intracellular accumulation of the delivered payload by the A14 conjugates is a vital component for the effective cytotoxicity by vinblastine-A14 and tumor visualization by 64Cu-A14.
A better understanding of the role of IL-5Rα in bladder cancer progression would increase opportunities for the development of effective targeted agents. IL-5 and IL-5Rα are well characterized for their orchestration of inflammatory processes in response to allergies and in eosinophilia related diseases.Citation14 Until recently, IL-5 and IL-5Rα had limited roles in cancer.Citation15,16 Along with identifying increased IL-5Rα gene expression in MIBC tissues, Lee and colleagues also demonstrated IL-5-stimulated MIBC cells increased their cellular migration through the activation of ERK1/2 and the cell cycle inhibitor P21WAF1.Citation11 RNA interference of IL-5Rα resulted in the inhibition of P21WAF1 and ERK1/2 activation, the reduced expression of MMPs, and the stoppage of cell migration. Increased MMP expression and their activity in accordance with increased tumor stage and invasion has long been associated with bladder cancerCitation17 as well as the activation of ERK1/2.Citation18 A separate group demonstrated that IL-5 protein was elevated in the blood of patients with invasive bladder cancer relative to patients with low grade tumors and patients with no cancer.Citation19 Thus, IL-5 and IL-5Rα appear to have a significant and novel role in central mechanisms involved in bladder cancer invasion.
Although the immunohistochemical findings in this study break ground that IL-5Rα protein expression is significantly associated with MIBC in patients, our study opens the way for additional investigations. For example, IHC revealed IL-5Rα was localized in the paranucleus, cytoplasm or on the plasma membrane (). IHC and confocal microscopy evaluation of HT-1376 and HT-B9 cells also revealed heterogeneous cellular IL-5Rα localization ( and ). The paranuclear staining of cancer markers have previously been correlated with tumor progression and shortened patient survival.Citation20 It is currently unknown what is the significance for IL-5Rα paranuclear staining as it was not associated with the expression of IL-5Rα. In addition, in primary invasive bladder tumors, the tumor cells were nearly all positive for IL-5Rα (). In contrast, IL-5Rα-positive tumor cells were a subpopulation in specimens that progressed past the muscle layer (). It is possible that increased IL-5Rα-positive tumor cells are required during the initial invasion stages and IL-5Rα-positive tumor cell numbers are reduced once cells progress past the surrounding muscle layers. Although this study contained 134 specimens only 11 were from tumors that invaded past the perivesical tissue and did not include any metastatic tumors. Increasing the numbers of metastatic tumors should provide definitive information on the status of IL-5Rα-positive tumor cells and the association with different stages of advanced bladder cancer including whether IL-5Rα cellular localization is associated.
It is also unclear whether IL-5-mediated bladder tumor cell migration is mostly governed by IL-5 expressed via MIBC cells or IL-5-responsive inflammatory cells. Although, Lee and coworkers demonstrated IL-5-enhanced MIBC cell invasion, this phenomena only occurred with the external addition of IL-5.Citation11 Since inflammation is well documented as a cause for bladder cancer,Citation21 concurrent contributions from bladder cancer cells and the inflammatory circuitry is a most likely scenario. Staining for IL-5 and inflammatory immune cells in patient tumors could illuminate this mystery. In addition, the expression of the β-chain of the IL-5R was not examined in this study. If present, this would further support that the IL-5R is functional and involved in invasive bladder cancer pathogenesis.
To determine if IL-5Rα-positive MIBC tumors could be targeted in vivo, mice were implanted with bilateral HT-1376 and HT-B9 xenografts on their flanks and characterized by IHC. Staining revealed these xenografts were heterogeneous in percentage of IL-5Rα-positive tumor cells, IL-5Rα expression, and intracellular localization (). Thus, these xenografts provided a good tumor model of patient heterogeneity. However, HT-1376 and HT-B9 cells expressed modest cell surface levels of IL-5Rα () compared with the other well-known tumor receptor antigens.Citation22 Nevertheless, injecting tracer doses of 64Cu-A14, both tumors were visualized with good contrast (). PET images showed 64Cu-A14 targeting was IL-5Rα specific as pre-dosing with excess unlabeled A14 significantly blocked uptake in both xenografts (). Because 64Cu has a short half-life (t1/2 = 12.7 h), imaging could only be performed up to 48 h post-injection. This resulted in high background due to the slow blood clearance of A14 (days-weeks).
Delivery of molecular payloads by antibodies for imaging and treatment applications is limited by antigen expression and efficient internalization of the cell-surface bound antigen-antibody-conjugate complex.Citation23 Thus, we tested whether IL-5Rα internalization and subsequent intracellular accumulation are important factors for 64Cu-A14. When cells were treated with IL-5, the expression of IL-5Rα was reduced after only 5 min (). IL-5Rα levels returned to non-IL-5 treatment baseline levels by 120 min. Interleukin receptors are known for rapid internalization, including the IL-5 receptor.Citation24-26 IL-5Rα on HT-1376 and HT-B9 cells effectively internalized bound 64Cu-A14 in a receptor-specific manner ( and ). When radioactivity accumulation and retention over time were evaluated there were differences between HT-1376 and HT-B9. 64Cu-A14-treated HT-1376 and HT-B9 cells increased radioactivity accumulation at each successive time point tested. However, only HT-B9 cells retained the intracellular radioactivity as HT-1376 cells had extracellular radioactivity levels higher than intracellular levels ( and ). This is most likely due to the increased recycling of 64Cu-A14 back outside the cell or exportation of free 64Cu that occurs in HT-1376 cells that does not occur in HT-B9 cells. In addition, there was very little intracellular accumulation in HT-1376 cells treated with 64Cu-IgG. This suggests that HT-1376 cells may also express Fc receptors and this potentially contributes to the increased extracellular levels with 64Cu-A14.
Recently, Pan and coworkers developed a fluorescently labeled CD47 antibody as a molecular imaging agent to increase the detection of tumor cells during trans-urethral resection of bladder tumors.Citation27 The CD47 mAb was conjugated to either FITC or the quantum dot, Qdot625 and was tested with confocal endomicroscopy or blue light cystoscopy for detecting tumors in excised bladders. Both imaging agents demonstrated good specificity for bladder tumors. RICs such as 64Cu-A14 possess unique advantages for imaging bladder cancer over the CD47 fluorescent mAb. First, PET is whole body compared with confocal microscopy that its small field of view (240 μm) is impractical for clinical applications. Second, quantum dots are highly toxic while 64Cu-A14 is administered in tracer doses that are typically non-toxic. Third, although CD47 is overexpressed on bladder tumor cells relative to normal urothelial cells, it does not differentiate between invasive and non-invasive bladder cancer. If matched with a longer lived positron-emitting radioisotope such as zirconium-89 (t1/2 = 3.3 d), we anticipate that tumor visualization could improve with A14 as a PET imaging agent so tumors with reduced IL-5Rα expression and/or IL-5Rα-positive tumor cells could be more effectively detected.
ADCs are an innovative therapeutic approach that has recently gained FDA approval for the treatment of advanced Her2-positive breast cancer and CD30-positive Hodgkin's and anaplastic large cell lymphomas.Citation13 The principal advantage of ADCs over traditional chemotherapy is the localization of chemotherapeutics to the tumor and, hence, minimizing systemic toxicity. For bladder cancer two ADCs are advancing into human phase trials.Citation28,29 These ADCs use the microtubule inhibitor monomethyl auristatin E (MMAE). Vinblastine is also a microtubule inhibitor and a component of a traditional chemotherapeutic regimen administered to invasive bladder cancer patients.Citation12 Thus, as a proof-of-concept, we explored the feasibility of developing A14 into an ADC. The IC50 of 4.2 nmol/L cytotoxicity achieved () was in range with the IC50 values with approved ADCs. Thus, delivering chemotherapeutics via the mAb A14 to IL-5Rα-positive tumor cells is a potential option for treating MIBC. Future studies are planned in our MIBC mouse models where an assessment of therapeutic efficacy and toxicity can be evaluated between vinblastine-A14 and free vinblastine.
Precision medicine using antibody-conjugates is becoming an increasing reality to improve tumor staging and provide increased survival benefit for patients with several forms of cancer. The data provided in this report reveal IL-5Rα is a novel MIBC target and that expression, internalization dynamics, and tumor cell density all contribute to successful PET imaging and treatment using developed RICs and ADCs that can potentially improve tumor detection and treatment, respectively. We anticipate that our findings could contribute to the development of new IL-5Rα targeted strategies for therapy or tumor detection and elucidation of important links to MIBC for improving the management of these patients.
Patients and methods
Clinical study design
Patient enrollment and tissue collection
One hundred and thirty four patients were recruited prospectively based on the inclusion criteria that a transurethral resection of bladder tumor (TURBT; n = 91), a biopsy (n = 9), a radical cystoprostatectomy (n = 32), or an anterior extenteration (n = 2) were surgically indicated for their condition. Patient consent and collection of specimens were performed under a protocol reviewed and approved by the Center Hospitalier Universitaire de Sherbrooke (CHUS) Ethical Review Committee. Following surgery, bladder specimens were processed using routine IHC procedures (i.e., formalin-fixation and paraffin-embedding) for pathological evaluation. Additional slides from embedded tissues were generated and used for IL-5Rα IHC (as detailed below). A total of 251 non-duplicate specimens were generated (1 sample for 17 patients, ≥2 samples for 117).
IHC of patient specimens
Tissue microarrays (TMAs) were prepared for immunohistochemical staining by fixation of the specimens in 10% formalin followed by paraffin embedding. TMAs consisted of 3 μm cut 2-mm diameter punches. Tumors of sufficient size had ≥2 locations selected for punching (typically one representing the brush-border urothelium and one representing the stroma). Deparaffinization and rehydration were performed according to the Dako automated staining system (Autostainer Link 48; Dako, Ontario, Canada). Antigen retrieval was performed with Target Retrieval Solution, pH 9 (Dako). IL-5Rα-specific and isotype control rabbit polyclonal antibodies (clones LS-C165211 and PA5–23090, respectively) and specific antibody blocking peptide (LS-E7786) were obtained from Cedarlane (Ontario, Canada). LS-C165211 was specific against the extracellular epitope amino acids 49–76 of IL-5Rα.
For IL-5Rα-specific staining, we evaluated various dilutions of LS-C165211 with and without the addition of 100-fold excess of blocking peptides and processed in the Autostainer Link 48. All cases were reviewed by pathologists N.E. and L.V. IL-5Rα-positive staining intensity standards were first identified using paraffin sections from human adipose and placental tissue known for their negative and positive expression of IL-5Rα, respectively. Positive cases were defined by the presence of total cellular staining, as seen in positive controls. IL-5Rα staining intensity on patient specimens was scored as negative, low, or high. The association between IL-5Rα expression and TNM stage, percentage of IL-5Rα-positive tumor cells, and the intracellular distribution of IL-5Rα were analyzed using the Pearson χ2 and Fisher's exact tests (SSPS Statistics Software ver. 17.0.2 (SSPS Inc., Chicago, IL).
MAb A14 and cell lines
mAb A14 (obtained from Dr LopezCitation30,31) was purified from hybridoma supernatant by protein A column chromatography (ThermoFisher, Ontario, Canada). MIBC HT-1376 and HT-B9 cells (ATCC, Manassas, VA) were incubated at 37°C (5% CO2) in RPMI medium supplemented with 1% sodium pyruvate, 1% Amphotericin B, 1% Penicillin/Streptomycin, and 10% FBS (all reagents supplied by Wisent, Québec, Canada).
IL-5Rα internalization and expression dynamics following exposure to IL-5
Cells grown on glass coverslips were stimulated with IL-5 (BD Biosciences, Ontario, Canada) at 50 ng/mL for the indicated time at 4°C and 37°C. Cells were then washed, fixed, and permeabilized as described previously.Citation32 Cells were then probed with 1/500 rabbit anti-IL-5Rα followed by washing in PBS and incubation with 1/500 secondary anti-murine AlexaFluor 647-labeled Fc polyclonal antibody (Life Technologies, Ontario, Canada). Nuclei were stained with Hoescht (1 μg/mL) for 5 min. Cells were mounted onto glass slides with SlowFade mounting media (Life Technologies). Images were acquired and fluorescence analyzed as described previously.Citation32
Preparation of 64Cu-A14
A 50-fold molar excess of 2,2′-(7-(2-((2,5-dioxopyrrolidin-1-yl)oxy)-2-oxoethyl)-1,4,7-triazonane-1,4-diyl)diacetic acid (NOTA-NHS; CheMatech, Dijon, France) was reacted with A14 in 0.1 M sodium bicarbonate at pH 8.6 for 1 h at room temperature. NOTA-A14 was buffer exchanged using an Ultra-0.5 mL (50 kDa) Centrifugal Filter (EMD Millipore, Ontario, Canada) in 0.1 M ammonium acetate pH 5.5. NOTA-A14 (250 µg) was then reacted with 100 MBq of 64CuCl2 for 1 h at 37°C.Citation33 64Cu-A14 was purified and buffer exchanged in PBS using centrifugal column filtration as described previously.Citation34 Thin-layer chromatography (0.1 M, pH 5.5 sodium citrate) was performed to determine 64Cu-A14 purity.
In vitro binding of HT-1376 and HT-B9 cells by 64Cu-A14
64Cu intracellular accumulation
Cells were treated with 100 nM of 64Cu-A14 in serum-free media at 4°C and 37°C for the indicated times followed by washing and treatment with tryspin (0.25%) for 15 min at 37°C to lift cells. Trypsin also served for the removal of surface IL-5Rα and bound 64Cu-A14 as previously performed.Citation32 Radioactive supernatant (termed extracellular in this study) was removed and saved. Cells were then washed and centrifuged. The wash supernatant was added to the previously collected extracellular fractions. Cells and extracellular contents were placed in a tube and radioactivity measured by gamma counting. 64Cu intracellular accumulation at 37°C is represented as the fold-increase relative to 4°C. Intracellular and extracellular fractions over time are displayed in counts per min (cpm) and are decay-corrected. Experiments were realized three times in triplicate.
Saturation binding
64Cu-A14 at concentrations ranging from 0.5 to 20 nM in duplicate were added to 1 × 106 cells per mL and mixed. To estimate nonspecific binding, duplicate samples of 64Cu-A14 at concentrations ranging from 0.5 to 20 nM were mixed with cells in the presence of 100-fold molar excess of unlabeled A14. After 1 h incubation on ice, the cells were washed and total bound antibody samples and total nonspecific bound antibody samples were counted. Specific binding (total bound antibody – nonspecific bound antibody) was graphed against reactive free 64Cu-A14 (nM) used in the assay. The dissociation constant (Kd) and the number of receptors per cell (Bmax) were determined by nonlinear regression using GraphPad Prism version 7.
64Cu-A14 in vivo IL-5Rα-positive MIBC tumor targeting
Animal model
Mice were handled in accordance with approval from our institution's Ethics Committee for Animal Experiments. Four-week-old female NOD/SCID mice (Charles River, Quebec, Canada) were anesthetized using 2% isoflurane at an oxygen flow of 1.5 L/min. Five million HT-1376 and HT-B9 cells were subcutaneously injected on different flanks. Studies commenced when tumors were 65–100 mm3 (∼10 d post-injection). One mouse was killed by overinhalation of isoflurane followed by placement in a CO2 chamber. HT-1376 and HT-B9 xenografts were collected and processed for IHC IL-5Rα staining and analyzed as described previously.
PET imaging and ROI analysis
Mice (n = 5) were intravenously injected with 64Cu-A14 (∼25 µg; ∼7 MBq; radiochemical purity ≥98%). At 48 h post-injection, mice were placed under anesthesia with isoflurane (1% at 2 L/min oxygen flow). Mice were then placed in prone position on the bed of a PET/CT Triumph™ scanner (Trifoil, CA, USA). PET scans were acquired for 45 min. The images were reconstructed using 20 iterations of a 3D Maximum Likelihood Expectation Maximization (MLEM) algorithm implementing a physical description of the detectors in the system matrix.Citation35 ROI drawing and quantification of the uptake in the tumors and visually assessable organs (liver, muscle, and heart) were performed using the AMIDE software. ROI signals were determined by taking the average of at least three ROIs per tumor/tissue. A cylindrical phantom (24.8 mL) containing 5 MBq of 64Cu at day 0 was used to obtain a calibration factor to convert the radioactive counts per second into %ID/g.
64Cu-A14 in vivo specificity for IL-5Rα
A second study of PET imaging explored tumor uptake by 64Cu-A14 of the IL-5Rα in a group of HT-1376 and HT-B9 tumor-bearing NOD/SCID mice (n = 5) when 25 mg/kg of unlabeled A14 was pre-injected 12 h before 64Cu-A14 injection. The injection dose 64Cu-A14 was also ∼25 µg; ∼7 MBq. PET imaging and analysis was performed as described previously.
A14-vinblastine construction and treatment of HT-1376 cells
Vinblastine-sulfate was purchased from LC Laboratories (Woburn, MA). Vinblastine-16-hydrazide-17-OH was obtained by reaction with hydrazine monohydrate in methanol as described previously.Citation36 The corresponding hydrazide (0.075 g, 0.1 mmol, 1 eq.) was treated for 10 min with 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU) (0.046 g, 0.12 mmol, 1.2 eq.), diisopropylethylamine (DIPEA)(52ul, 0.3 mmol, 3 eq.) and 3-tritylsulfanylpropionic acid (0.041 g, 0.12 mmol, 1.2 eq.) to obtain vinblastine-C10-STrt. The reaction was diluted with water, extracted several times with dichloromethane, and the combined organic phases were washed with water and NaCl solution, dried over anhydrous Na2S04, and the solvent evaporated in vacuo. The crude trityle-protected compound was treated with a mixture of TFA/DCM/TIPS/H2O (50/45/2.5/2.5) for 30 min. After evaporation of the volatiles, acetonitrile was added and vinblastine-C2-SH was directly purified by reverse-phase preparative HPLC-MS using the following gradient: 18 to 33% (acetonitrile:water) in 15 min (Column XSelect, 19 × 100 mm). Purity (UPLC-MS): <95%, ESI-MS (m / z): 858.1 [M + H]+. For conjugating A14 to vinblastine-C2-SH, maleimide groups were introduced into A14 by reaction of 10 mg/mL A14 in PBS, pH 7.6, with 10-fold molar excess of sulfosuccinimidyl-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (sulfo-SMCC; VWR, Quebec, Canada) at room temperature for 1 h. Maleimide-derivatized A14 was purified and prepared as described previouslyCitation32 and then reacted with 50-fold molar excess of vinblastine-C2-SH overnight at 4°C. A14-vinblastine was purified by Sephadex-G50 chromatography in phosphate-buffered saline, pH 7.4 HT-1376 cells (1 × 105 per well) were treated with increasing concentrations of A14-vinblastine for 72 h. XTT colorimetric assay was used to determine cell survival and calculate the IC50 value (ThermoFisher).
Statistical analysis
Students t-test or two-way ANOVA with Holm–Sidak post-analysis for multiple comparisons were used in the analysis of in vitro and in vivo studies (GraphPad Prism, La Jolla, CA). p-values below 0.05 (*), <0.01 (**), <0.001 (**), and <0.0001 (***) were deemed as significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1331195_supplementary_data.zip
Download Zip (503.7 KB)Acknowledgments
Thank you to Catherine Allard and CIMS technical staff for conducting the biostatistics calculations and for radiochemistry-PET imaging support, respectively.
Funding
This work was supported by funds from the Cancer Research Society, the Banting Research Foundation, the Axis Cancer of the CRCHUS, and the CIMS.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics 2012. CA Cancer J Clin 2015; 65:87-108; PMID:25651787; https://doi.org/10.3322/caac.21262
- Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet 2009; 374:239-49; PMID:19520422; https://doi.org/10.1016/S0140-6736(09)60491-8
- Park JC, Citrin DE, Agarwal PK, Apolo AB. Multimodal management of muscle-invasive bladder cancer. Curr Probl Cancer 2014; 38:80-108; PMID:25087173; https://doi.org/10.1016/j.currproblcancer.2014.06.001
- Herr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol 2002; 167:1295-8; PMID:11832716; https://doi.org/10.1097/00005392-200203000-00019 10.1016/S0022-5347(05)65284-6
- Maurer T, Horn T, Heck M, Gschwend JE, Eiber M, Beer AJ. Current staging procedures in urinary bladder cancer. Diagnostics (Basel) 2013; 3:315-24; PMID:26824925; https://doi.org/10.3390/diagnostics3030315
- Nesi G, Nobili S, Cai T, Caini S, Santi R. Chronic inflammation in urothelial bladder cancer. Virchows Arch 2015; 467:623-33; PMID:26263854; https://doi.org/10.1007/s00428-015-1820-x
- Chen C, Qi XJ, Cao YW, Wang YH, Yang XC, Shao SX, Niu HT. Bladder tumor heterogeneity: The impact on clinical treatment. Urol Int 2015; 95:1-8; PMID:25823547; https://doi.org/10.1159/000370165
- Inoue K, Slaton JW, Kim SJ, Perrotte P, Eve BY, Bar-Eli M, Radinsky R, Dinney CP. Interleukin 8 expression regulates tumorigenicity and metastasis in human bladder cancer. Cancer Res 2000; 60:2290-9; PMID:10786697; https://doi.org/10.1097/00005392-199904010-00483
- Joshi BH, Leland P, Lababidi S, Varrichio F, Puri RK. Interleukin-4 receptor alpha overexpression in human bladder cancer correlates with the pathological grade and stage of the disease. Cancer Med 2014; 3:1615-28; PMID:25208941; https://doi.org/10.1002/cam4.330
- Lee SJ, Lee EJ, Kim SK, Jeong P, Cho YH, Yun SJ, Kim S, Kim GY, Choi YH, Cha EJ et al. Identification of pro-inflammatory cytokines associated with muscle invasive bladder cancer; the roles of IL-5, IL-20, and IL-28A. PLoS One 2012; 7:e40267; PMID:22962576; https://doi.org/10.1371/journal.pone.0040267
- Lee EJ, Lee SJ, Kim S, Cho SC, Choi YH, Kim WJ, Moon SK. Interleukin-5 enhances the migration and invasion of bladder cancer cells via ERK1/2-mediated MMP-9/NF-kappaB/AP-1 pathway: Involvement of the p21WAF1 expression. Cell Signal 2013; 25:2025-38; PMID:23770289; https://doi.org/10.1016/j.cellsig.2013.06.004
- Yin M, Joshi M, Meijer RP, Glantz M, Holder S, Harvey HA, Kaag M, Fransen van de Putte EE, Horenblas S, Drabick JJ. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: A systematic review and two-step meta-analysis. Oncologist 2016; 21:708-15; PMID:27053504; https://doi.org/10.1634/theoncologist.2015-0440
- Alley SC, Okeley NM, Senter PD. Antibody-drug conjugates: Targeted drug delivery for cancer. Curr Opin Chem Biol 2010; 14:529-37; PMID:20643572; https://doi.org/10.1016/j.cbpa.2010.06.170
- Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol 2008; 20:288-94; PMID:18511250; https://doi.org/10.1016/j.coi.2008.04.001
- Huang M, Wang J, Lee P, Sharma S, Mao JT, Meissner H, Uyemura K, Modlin R, Wollman J, Dubinett SM. Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res 1995; 55:3847-53; PMID:7641203; https://doi.org/10.1016/0169-5002(96)85851-x
- Zaynagetdinov R, Sherrill TP, Gleaves LA, McLoed AG, Saxon JA, Habermann AC, Connelly L, Dulek D, Peebles RS Jr, Fingleton B et al. Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res 2015; 75:1624-34; PMID:25691457; https://doi.org/10.1158/0008-5472.CAN-14-2379
- Davies B, Waxman J, Wasan H, Abel P, Williams G, Krausz T, Neal D, Thomas D, Hanby A, Balkwill F. Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res 1993; 53:5365-9; PMID:8221672; http://cancerres.aacrjournals.org/content/53/22/5365.long
- Monami G, Gonzalez EM, Hellman M, Gomella LG, Baffa R, Iozzo RV, Morrione A. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res 2006; 66:7103-10; PMID:16849556; https://doi.org/10.1158/0008-5472.CAN-06-0633
- Satyam A, Singh P, Badjatia N, Seth A, Sharma A. A disproportion of TH1/TH2 cytokines with predominance of TH2, in urothelial carcinoma of bladder. Urol Oncol 2011; 29:58-65; PMID:19837616; https://doi.org/10.1016/j.urolonc.2009.06.002
- Rajendiran S, Kpetemey M, Maji S, Gibbs LD, Dasgupta S, Mantsch R, Hare RJ, Vishwanatha JK. MIEN1 promotes oral cancer progression and implicates poor overall survival. Cancer Biol Ther 2015; 16:876-85; PMID:25996585; https://doi.org/10.1080/15384047.2015.1040962
- Michaud DS. Chronic inflammation and bladder cancer. Urol Oncol 2007; 25:260-8; PMID:17483025; https://doi.org/10.1016/j.urolonc.2006.10.002
- Yan M, Schwaederle M, Arguello D, Millis SZ, Gatalica Z, Kurzrock R. HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev 2015; 34:157-64; PMID:25712293; https://doi.org/10.1007/s10555-015-9552-6
- Chari RV, Miller ML, Widdison WC. Antibody-drug conjugates: An emerging concept in cancer therapy. Angew Chem Int Ed Engl 2014; 53:3796-827; PMID:24677743; https://doi.org/10.1002/anie.201307628
- Mita S, Takaki S, Tominaga A, Takatsu K. Comparative analysis of the kinetics of binding and internalization of IL-5 in murine IL-5 receptors of high and low affinity. J Immunol 1993; 151:6924-32; PMID:8258700; https://doi.org/10.1093/intimm/2.2.181
- Subtil A, Hemar A, Dautry-Varsat A. Rapid endocytosis of interleukin 2 receptors when clathrin-coated pit endocytosis is inhibited. J Cell Sci 1994; 107(Pt 12):3461-8; PMID:7706397; https://doi.org/10.1016/0248-4900(96)81369-4
- Von Hoegen I, Falk W, Kojouharoff G, Krammer PH. Internalization of interleukin 1 (IL 1) correlates with IL 1-induced IL 2 receptor expression and IL 2 secretion of EL4 thymoma cells. Eur J Immunol 1989; 19:329-34; PMID:2522880; https://doi.org/10.1002/eji.1830190217
- Pan Y, Volkmer JP, Mach KE, Rouse RV, Liu JJ, Sahoo D, Chang TC, Metzner TJ, Kang L, van de Rijn M et al. Endoscopic molecular imaging of human bladder cancer using a CD47 antibody. Sci Transl Med 2014; 6:260ra148; PMID:25355698; https://doi.org/10.1126/scitranslmed.3009457
- Challita-Eid PM, Satpayev D, Yang P, An Z, Morrison K, Shostak Y, Raitano A, Nadell R, Liu W, Lortie DR et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res 2016; 76:3003-13; PMID:27013195; https://doi.org/10.1158/0008-5472.CAN-15-1313
- Morrison K, Challita-Eid PM, Raitano A, An Z, Yang P, Abad JD, Liu W, Lortie DR, Snyder JT, Capo L et al. Development of ASG-15ME, a Novel antibody-drug conjugate targeting SLITRK6, a new urothelial cancer biomarker. Mol Cancer Ther 2016; 15:1301-10; PMID:26944921; https://doi.org/10.1158/1535-7163.MCT-15-0570
- Sun Q, Woodcock JM, Rapoport A, Stomski FC, Korpelainen EI, Bagley CJ, Goodall GJ, Smith WB, Gamble JR, Vadas MA et al. Monoclonal antibody 7G3 recognizes the N-terminal domain of the human interleukin-3 (IL-3) receptor alpha-chain and functions as a specific IL-3 receptor antagonist. Blood 1996; 87:83-92; PMID:8547680; www.bloodjournal.org/content/87/1/83.long?sso-checked=true
- Yamada T, Sun Q, Zeibecoglou K, Bungre J, North J, Kay AB, Lopez AF, Robinson DS. IL-3, IL-5, granulocyte-macrophage colony-stimulating factor receptor alpha-subunit, and common beta-subunit expression by peripheral leukocytes and blood dendritic cells. J Allergy Clin Immunol 1998; 101:677-82; PMID:9600506; https://doi.org/10.1016/S0091-6749(98)70177-0
- Beaudoin S, Rondeau A, Martel O, Bonin MA, van Lier JE, Leyton JV. ChAcNLS, a novel modification to antibody-conjugates permitting target cell-specific endosomal escape, localization to the nucleus, and enhanced total intracellular accumulation. Mol Pharm 2016; 13:1915-26; PMID:27112376; https://doi.org/10.1021/acs.molpharmaceut.6b00075
- Guérin B, Ait-Mohand A, Tremblay MC, Dumulon-Perreault V, Fournier P, Bénard F. Total solid-phase synthesis of NOTA-functionalized peptides for PET or SPECT imaging. Org Lett 2010; 12:280-3; PMID:20014826; https://doi.org/10.1021/ol902601x
- Zeglis BM, Mohindra P, Weissmann GI, Divilov V, Hilderbrand SA, Weissleder R, Lewis JS. Modular strategy for the construction of radiometalated antibodies for positron emission tomography based on inverse electron demand Diels-Alder click chemistry. Bioconjug chem 2011; 22:2048-59; PMID:21877749; https://doi.org/10.1021/bc200288d
- Selivanov V, Picard Y, Cadorette J, Rodrigue S, Lecomte R. Detector response models for statistical iterative image reconstruction in high resolution PET. IEEE Trans Nucl Sci 2000; 47:1168-75; https://doi.org/10.1109/NSSMIC.1998.774409
- Bánóczi Z, Gorka-Kereskényi Á, Reményi J, Orbán E, Hazai L, Tökési N, Oláh J, Ovádi J, Béni Z, Háda V et al. Synthesis and in vitro effect of vinblastine derivative-oligoarginine conjugates. Bioconjug Chem 2010; 21:1948-55; PMID:20973492; https://doi.org/10.1021/bc100028z