ABSTRACT
Immunotherapy has shown great promise in the fight against cancer, as evidenced by the clinical efficacy of chimeric antigen receptor T cells in hematologic malignancies and checkpoint blockade in certain solid tumors. However, a considerable number of patients fail to respond to these therapies. Induction of myeloid-derived suppressor cells (MDSCs) by growing tumors has been shown to be one important factor limiting the efficacy of cancer immunotherapy. Recently, several chemotherapeutic agents used in conventional cancer chemotherapy have been found to reduce MDSC numbers in tumor tissues as well as in the peripheral lymphoid organs, and combining these agents with immunotherapy improved survival of tumor-bearing hosts. In this review, we will highlight the effects of chemotherapeutic agents on MDSC accumulation, and examine the various factors likely to influence these effects.
| Abbreviations | ||
| DD | = | dose-dense |
| Five-FU | = | fluorouracil |
| M-MDSCs | = | monocytic myeloid-derived suppressor cells |
| MDSC | = | myeloid-derived suppressor cells |
| MTD | = | maximum-tolerated dose |
| NKG2D | = | natural killer group 2D |
| PMN-MDSC | = | polymorphonuclear myeloid-derived suppressor cells |
Introduction
Myeloid-derived suppressor cells (MDSCs) are a phenotypically heterogeneous cell population accumulating in tumor sites and peripheral lymphoid organs in tumor-bearing hosts.Citation1,2 In mice, these cells are characterized by the co-expression of the myeloid-cell linage differentiation antigens CD11b and Gr1. MDSCs can be further subdivided into two major subsets, polymorphonuclear (PMN-MDSC, initially termed gMDSCCitation3) of the phenotype CD11b+Ly6G+Ly6Clow, and monocytic (M-MDSCs) with the expression profile CD11b+Ly6G−Ly6Chi.Citation4 In human peripheral blood mononuclear cells, MDSCs can be divided into three subtypes: the equivalent to PMN-MDSC are defined as CD11b+CD14−CD15+ or CD11b+CD14−CD66b+, M-MDSC as CD11b+CD14+HLA-DR−/loCD15−, and early-stage MDSCs of the phenotype Lin− (including CD3, CD14, CD15, CD19, CD56), HLA-DR−CD33+.Citation4 MDSCs mediate immunosuppression through multiple mechanisms: (i) inhibition of proliferation and activation of CD4+ and CD8+ T lymphocytes through arginase-1 or nitrogen oxide synthase 2 as well as indoleamine 2,3-dioxygenase;Citation5,6 (ii) induction of oxidative stress via production of reactive oxygen species and reactive nitrogen species that cause loss of T cell receptor ζ-chain expression and desensitization of the T cell receptor;Citation7 (iii) altering macrophage phenotype toward to a type 2 response and subsequent activity by increasing MDSC production of IL-10;Citation8 (iv) inhibition of the cytotoxicity, natural killer group 2D (NKG2D) expression, and interferon-gamma production of natural killer cells through a cell-contact-dependent mechanism that involves membrane-bound transforming growth factor β;Citation9 (v) induction of immunosuppressive T regulatory cellsCitation10 and (vi) upregulation of programmed death-ligand 1 on MDSC via hypoxia-inducible factor-1α.Citation11 MDSCs are also involved in non-immune suppressive processes that may aid tumor development such as promoting blood vessel formation,Citation12 inducing epithelial–mesenchymal transition,Citation13 and enhancing stemness of cancer cells.Citation14 Data from clinical studies provide strong evidence that the frequency and number of MDSCs in the peripheral blood correlate with cancer stage, metastatic tumor burden, and response to systemic therapy,Citation15-17 rendering these cells a promising predictive biomarker and/or therapeutic target for improving the efficacy and prognosis of patients with cancers.Citation18,19
With the recognition that MDSCs inhibit antitumor immune responses, it is conceivable that efficacy of cancer immunotherapy can be improved by eliminating MDSC accumulation in tumor-bearing hosts. In addition to their intended cytotoxic effects on tumor cells, chemotherapeutic agents have been found to affect the recruitment of MDSCs to tumor sites, peripheral blood, and lymphoid organs.Citation1,20 Most studies to date have shown that combining these agents with immunotherapy decreases MDSC numbers, resulting in enhanced efficacy and a synergistic benefit on survival in tumor-bearing hosts.Citation21-24,25 For example, it was reported that the combination of different chemotherapeutic regimens in association with a specific immunotherapy (adoptive T cell transfer) induced a significant control of progression in MCA203 fibrosarcoma-bearing mice.Citation26 Clinically, MDSC elimination by chemotherapy correlates with the induction of a tumor vaccine-induced immune response in renal cell carcinoma patientsCitation27 and a better immune response after vaccination was detected after MDSC elimination in ovarian and cervical cancer patients.Citation28,29
However, how effectively chemotherapeutic agents deplete MDSCs might impact the efficacy of the concurrent immunotherapy. Efficient screening of suitable chemotherapeutic agents will be a key factor in the design of combinatorial regimens together with immunotherapy. Here, we review the most recent reports on the effects of the most commonly used chemotherapeutic agents in clinic such as gemcitabine, fluorouracil (5-FU), doxorubicin, and paclitaxel on MDSC accumulation, and analyze the possible factors responsible for these effects. In addition, we review the latest findings on the correlation between MDSC number and efficacy of concurrent chemotherapy and immunotherapy.
Various results of chemotherapeutic agents on MDSC accumulation
Gemcitabine (a deoxycytidine analog that inhibits ribonucleotide reductase) and fluorouracil (5-FU, an antimetabolite agent that targets thymidylate synthase) are two generally recognized chemotherapeutic agents that deplete MDSCs in both animal models as well as in patients.Citation30-33 In one study, one of several cytotoxic agents, including cyclophosphamide, paclitaxel, raltitrexed, gemcitabine, doxorubicin, 5-FU, or oxaliplatin were injected into lymphoma tumor-bearing mice.Citation31 The authors found only 5-FU (and to a lesser extent gemcitabine) significantly decreased the number of MDSCs in both the spleen and tumor bed. The elimination of MDSCs by 5-FU increased interferon-γ production by tumor-specific CD8+ T cells and promoted T cell-dependent antitumor responses.Citation31 Further analysis showed that 5-FU was able to contract M-MDSC without (or less) affecting PMN-MDSC accumulation.Citation26,31 Moreover, data reported that 5-FU administration was able to contract M-MDSC in the spleen, although this effect was not detected in the lymph nodes.Citation25 However, findings from several other studies showed conflicting effects of these two agents. In a preclinical model of thymoma, 5-FU administration alone failed to reduce numbers of either M-MDSCs or PMN-MDSCs in the draining lymph node, or PMN-MDSCs in the spleen, although the combination of 5-FU and an adenoviral tumor vaccine had shown a synergistic benefit on survival of tumor-bearing mice.Citation25 In a B16F10 lung metastatic mouse model, application of 5-FU resulted in only minimal effects on the frequency of MDSCs recruited into the tumor microenvironment, while the therapy decreased the frequency of MDSCs in the spleen and lung. These findings suggest that 5-FU decreases MDSCs systemically, which was concomitant with the improved survival of the animals.Citation34 In a clinical study, patients with advanced pancreatic cancer were administered gemcitabine together with capecitabine, a prodrug that is enzymatically converted to 5-FU in the body. The study showed that there was no consistent reduction in blood MDSCs post-treatment. However, in patients with baseline MDSCs greater than the median, those whose MDSC levels did fall seemed to experience more immunological benefit, though the small numbers of patients precluded definitive conclusions.Citation35
FOLFIRI is a chemotherapy regimen for treatment of colorectal cancer, and is made up of 5-FU, irinotecan, and leucovorin. Reports from studies using a FOLFIRI regimen are also inconsistent in terms of impact on MDSC counts. In one study, mice were injected with MC38/CEA2 colon adenocarcinoma cells subcutaneously before FOLFIRI treatment. At day 6 after FOLFIRI treatment of tumor-bearing mice, the number of splenic MDSCs was significantly decreased compared with control mice, leading to enhanced tumor-specific responses.Citation36 In another report, following FOLFIRI treatment in patents with colorectal cancer, the percentage of MDSCs in the peripheral blood was not observed to decrease; to the contrary, it continuously increased.Citation37
Similar apparent discrepancies were encountered when treatments involved doxorubicin, an antineoplastic drug commonly used in the treatment of a wide range of cancers, including hematological malignancies, soft tissue sarcomas, and several types of solid tumors. Using a breast cancer mouse model, it was shown that the proportion and absolute number of MDSCs in the spleen and peripheral blood were significantly reduced following doxorubicin treatment.Citation38 In contrast, a related study using a B-cell lymphoma mouse model reported that doxorubicin induced the expansion of M-MDSCs in the spleen.Citation21
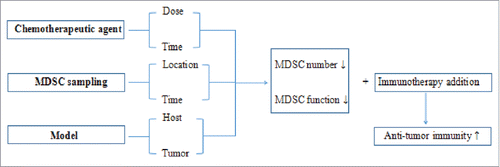
Collectively, the cumulative data indicate that chemotherapeutic agents have diverse effects in regulating MDSC accumulation in different situations. However, it should be noted that these data were derived from independent studies, and although chemotherapeutic agents were identical, as yet unidentified factors, including the chemotherapy dose, time, administration schedule, location of MDSC sampling, as well as the different disease stages in clinic setting, may also have contributed to differences in MDSC levels.
Factors shaping the effect of chemotherapeutic agents on accumulation of MDSCs
Dosage of drugs
Dose-dependent inhibition of MDSC accumulation has been observed in tumor-bearing hosts treated with other anticancer therapies, including molecular target agents. For example, sunitinib is one commonly used tyrosine kinase inhibitor, typically first-line therapy for metastatic renal cell carcinoma. Animal studies have shown that sunitinib treatment dose-dependently depleted MDSCs in the tumor, spleen, and in circulation.Citation39 Combining this MDSCs-decreasing agent with various immune-based cancer therapies, such as an agonistic CD40-antibody,Citation40 an αvirus-based cancer vaccine,Citation41 tumor-infiltrating lymphocytes,Citation42 or a viral vector based cancer vaccine,Citation39 effectively enhances their efficacy. These studies provide evidence that dosage is an important factor affecting the ability of an agent to inhibit MDSC accumulation and subsequent antitumor effect.
Similarly, treatment with paclitaxel, an agent inhibiting microtubule disassembly, also affects MDSC accumulation diversely. In animal studies, at a dose of 1 mg/kg body weight, paclitaxel was shown to cause a significant reduction in frequencies of tumor-infiltrating MDSCs and restore CD8+ T cell effector functions,Citation43 an effect not observed at 36 mg/kg paclitaxel.Citation31 In a clinical study, dose of 175 mg/m2 paclitaxel showed a significant yet modest increase in both the absolute number and percentage of circulating MDSCs.Citation15 Together, this body of evidence suggests that single use of paclitaxel may result in different numbers of circulating MDSC, depending on the specific dosage administered.
Another example of a dose-effect relationship is the application of cyclophosphamide in a melanoma mouse model. At a dose of 50 mg/kg body weight, cyclophosphamide was shown to have negligible influence on MDSC accumulation in either tumor sites or spleens of tumor-bearing mice.Citation30,34 However, at a dosage between 100 and 300 mg/kg, the number of MDSCs was clearly expanding.Citation17 This dose-effect relationship was directly demonstrated in another recent report using the same experimental model, where substantial accumulation of melanoma-infiltrating MDSCs were observed following administration of 2.5 mg cyclophosphamide, but not 1.0 mg per mouse.Citation44
In a recent study using an ovarian cancer model, the effects of different doses of concurrent pacilitaxel and cisplatin on MDSC accumulation were investigated. Tumor-bearing mice were treated with two different regimens of combination chemotherapy: a lower “dose-dense” (DD) regimen and a higher “maximum-tolerated dose” (MTD) regimen. The DD regimen was defined as the combination of 5 mg/kg paclitaxel and 3 mg/kg cisplatin, while the MTD regimen consisted of higher dosages of 12 mg/kg paclitaxel and 7 mg/kg cisplatin. These results clearly showed that DD chemotherapy profoundly reduced the number of MDSCs, more significantly than mice in the MTD group. These data may provide at least a partial explanation as to why the DD regimen produced superior clinical outcomes.Citation45
As described above, diverse effects of dosages of chemotherapeutic agents on MDSC accumulation have been observed. It is important to note that this conclusion is based on the results from separate studies. Different experimental conditions in each study, different ways of measuring MDSCs, etc. could potentially lead to different results. Additional studies directly comparing different doses in the same experimental model are needed for confirmation.
Timing of drug administration
The timing of treatment with chemotherapeutic agents also affects MDSC accumulation. One study using gemcitabine in a mammary carcinoma mouse model supported this point.Citation30 Gemcitabine was administered at two different time points: early gemcitabine was injected on day 5 post tumor inoculation and repeated once weekly; late gemcitabine was given as a single dose on days 20–25. MDSC levels in the spleen were measured at different time points. The authors measured significant increases in both percentage and absolute number of MDSCs in the spleens on day 7 post tumor inoculation compared with naive mice; however, early gemcitabine treatment neither decreased the percentage nor the absolute numbers of MDSCs. On the contrary, in the late gemcitabine group, there were significant decreases in both percentage and absolute numbers in MDSCs, an observation that correlated with greater in vitro proliferative activity by splenic T cells.Citation30
Location of MDSC sampling
From recent studies, it has become clear that depending on the organs selected, different MDSC levels are detected in response to chemotherapeutic agents. One study showed that melanoma-bearing mice treated with 5-FU exhibited no change in the frequency of MDSCs at the tumor site. However, in the spleen and lung, the frequency was significantly decreased.Citation34 The results of a second study also showed that changes in MDSC levels vary by anatomic site. In the spleen, treatment with 5-FU resulted in substantial reductions of M-MDSCs and PMN-MDSCs. In contrast, no decrease in M-MDSCs or PMN-MDSCs was found in the draining lymph nodes.Citation25 Another study using the same mouse melanoma model showed that 5-FU treatment led to statistically significant reduction of the relative and absolute numbers of MDSC peritoneal cells, but did not affect the number of MDSC spleen cells.Citation32 Like 5-FU, paclitaxel was also shown to exert different effects on MDSC accumulation in different organs. As reported in one study, administration of an ultra-low dose of paclitaxel caused a significant reduction in frequency of MDSCs in the tumor, but not in the metastatic lymph nodes, spleen, or bone marrow.Citation43
To evaluate the specific effects of individual chemotherapeutic agents on the accumulation of MDSCs, separate from the additional effects of the tumor, one study used a model using normal mice without tumors.Citation44 This study tested whether changes in MDSC levels could be observed across various organs after cyclophosphamide treatment. When these mice were injected with 2.5 mg cyclophosphamide, an increase in MDSC levels was found in the spleen, peripheral blood, and lymph nodes, but not, however, in the bone marrow, suggesting the effects of chemotherapeutic agents on MDSCs vary by compartment, irrespective of the presence of tumor.
Currently, it is still unknown in which anatomic compartment depletion of MDSCs is the most important to improve antitumor activity of T cells. We selected several studies in which clinically commonly used chemotherapeutic agents in solid tumors were used and their effects on MDSCs were investigated (). It seems that a decrease in MDSCs in the spleen is sufficient to produce an improved therapeutic effect, irrespective of MDSC changes in other anatomic compartments, including the tumor site. However, this assumption needs to be further investigated.
Table 1. The relationship between anatomic compartment depletion of MDSCs and antitumor effect.
Detection time of MDSCs
Several recent studies have investigated the effects of chemotherapeutic agents on the dynamics of MDSC levels using either normal mice or various tumor models, showing that the number of MDSCs measured varies, depending on the time interval between administration of chemotherapeutic agents and detection of the MDSCs. In one study, normal mice were treated with intraperitoneal injections of cyclophosphamide, and MDSCs were detected at different time points post treatment.Citation46 At days 6 and 10, there was a significant increase in MDSC levels in the spleen, both in percentage and absolute numbers. However, by day 20, MDSC numbers had started to fall, still higher than controls at day 20 but lower than day 6–10 levels.Citation46 Similar results were observed in a recent study using both naïve mice and tumor-bearing mice to investigate the effects of cyclophosphamide on MDSC accumulation.Citation21 All mice were treated with a single dose of cyclophosphamide, and spleen cells were harvested at different time points to enumerate the MDSCs. They found that in naïve mice, the numbers of M-MDSCs and PMN-MDSCs were both reduced shortly after cyclophosphamide, reaching the nadir by day 2 and day 4, respectively; and both populations rebounded thereafter, resulting in net increase in cell number by day 10. In tumor-bearing mice, there was an initial reduction in the numbers of M-MDSCs in the spleen (day 2) followed by a rebound thereafter. In the tumor, the rebound of M-MDSCs peaked at day 7, and by day 10 reached a stable level that was elevated compared with the starting point.Citation21
The influence of detection time on MDSC numbers has also been observed for other chemotherapeutic agents, including 5-FU and doxorubicin.Citation34,38 C57BL/6 mice received subcutaneous inoculation of B16F10 syngeneic melanoma cells, with 5-FU administered 3 d post-inoculation. It was shown that neither the frequency of splenic MDSC nor the absolute numbers had changed by day 7 or 14, however, a significant decrease was observed by day 21.Citation34 Another study using doxorubicin in a 4T1 breast cancer mouse model similarly reported results that varied according to time interval, though with an inverse trajectory from the previous study.Citation38 On days 14 and 17, doxorubicin treatment resulted in a significant reduction in the proportion and absolute numbers of MDSCs in the spleen and blood of mice; however, these cells were reconstituted by day 23.Citation38 Besides single chemotherapeutic agents, combinational chemotherapy also showed different results on MDSCs if detected at different time points. For example, a study using FOLFIRI treatment showed a reduction in the numbers of MDSCs on day 6, while significantly higher numbers of MDSCs were detected at day 14.Citation36
One explanation for the various results at different time points is the kinetics of MDSC subtypes in the absence of chemotherapy. It has been reported that there were substantial expansion of PMN-MDSC in blood and spleens over time in different transplantable tumor models and in a spontaneous tumor model. In contrast, the increase in the number of M-MDSC was relatively small.Citation47 Further analysis showed that M-MDSC can generate cells with CD11b+Ly6CloLy6G+phenotype of PMN-MDSC in vitro and in vivo.Citation47 Therefore, the plasticity of M-MDSC to promote PMN-MDSC generation might also contribute to the different kinetics of blood/spleen accumulation of MDSC in chemotherapeutic agent-treated tumor-bearing hosts.
Mouse and tumor models
Another important factor accounting for various effects of a particular chemotherapeutic agent on MDSC accumulation is the mouse model used in studies. Under identical conditions, use of naïve or tumor-bearing mice can result in apparently different findings of chemotherapeutic agent effects on MDSC number, as is observed in two previously reported studies. In one study, naive mice were used and treated with intraperitoneal injections of cyclophosphamide.Citation46 At days 6–10, a significant increase in splenic MDSCs was observed, both in percentage and absolute numbers.Citation46 In contrast, using a mouse model with a tumor, a separate study showed that neither splenic MDSC frequency nor absolute number changed when detected at day 7, although cyclophosphamide was given at the same dosage and the same routes of administration.Citation34
The precise effect of the chemotherapeutic agent on MDSC levels also depends on the tumor type. In a recent report, the authors used two different tumor cell line models, B16F10 melanoma and EL4 T-cell lymphoma, to compare the effects of chemotherapeutic agents on MDSC depletion.Citation48 Mice bearing one of the two tumor types were injected with a single dose of either cisplatin or 5-FU. The authors found that the increase in the percentage of splenic MDSCs for both B16F10 and EL-4 tumor-bearing mice, compared with the MDSCs in spleens of mice lacking tumors, was similar. However, when these two different tumor-bearing mice were compared after treatment with cisplatin or 5-FU, the EL-4-bearing mice exhibited much lower levels of splenic MDSCs.Citation48
In addition, the route of administration of chemotherapeutic agents may affect MDSC levels. Several studies showed that gemcitabine treatment reduces the number of tumor-induced MDSCs in transplantable tumor models.Citation30,49 In most of these models, gemcitabine was administered via the subcutaneous route. Recently, another study used intraperitoneal injection instead, to test its efficiency in reducing MDSC numbers. When the gemcitabine treatment regimen was given to tumor-bearing mice, the absolute number of MDSCs in the peritoneal cavity did not change.Citation32
Conclusion
Based on the above findings that various parameters affect the ability of chemotherapeutic agents to eliminate tumor-induced MDSCs, we propose that most chemotherapeutic agents have the potential ability to inhibit MDSCs, if administered at the proper time and dose in a proper model with a proper administration mode.
MDSCs play a potentially important role in the efficacy of immunotherapy and can be modulated by various chemotherapeutic agents. However, the exact effects of these agents on MDSCs can be difficult to discern based on apparent inconsistencies between published studies. These apparent contradictions can likely be explained by differences in important variables such as drug dose and timing, location and timing of MDSC sampling, and mouse model and tumor type (). These differences in mouse model studies to date preclude definitive conclusions from being drawn about how best to translate these approaches to the clinic. This highlights the need for additional studies linking the impact of these variables with antitumor efficacy to define the relevant biomarkers. Ultimately, human clinical trials with correlative studies examining MDSCs are needed to evaluate the overall effect of chemotherapy regimens on efficacy of immunotherapies and the role MDSC depletion plays in successful treatment.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Dr Torsten Juelich for linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by the national Natural Science Foundation of China under Grant (81000914) and Foundation of He'nan Health Committee under Grant (2011010011).
References
- Draghiciu O, Lubbers J, Nijman HW, Daemen T. Myeloid derived suppressor cells-An overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology 2015; 4(1):e954829; PMID:25949858; https://doi.org/10.4161/21624011.2014.954829
- Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: Expect the unexpected. J Clin Invest 2015; 125(9):3356-64; PMID:26168215; https://doi.org/10.1172/JCI80005
- Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 2008; 181(8):5791-802; PMID:18832739; https://doi.org/10.4049/jimmunol.181.8.5791
- Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7:12150; PMID:27381735; https://doi.org/10.1038/ncomms12150
- Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE, Boulden J, Sutanto-Ward E, Soler AP, Laury-Kleintop LD et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov 2012; 2(8):722-35; PMID:22822050; https://doi.org/10.1158/2159-8290.CD-12-0014
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9(3):162-74; PMID:19197294; https://doi.org/10.1038/nri2506
- Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med 2007; 13(7):828-35; PMID:17603493; https://doi.org/10.1038/nm1609
- Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol 2007; 179(2):977-83; PMID:17617589; https://doi.org/10.4049/jimmunol.179.2.977
- Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol 2009; 182(1):240-9; PMID:19109155; https://doi.org/10.4049/jimmunol.182.1.240
- Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res 2008; 68(13):5439-49; PMID:18593947; https://doi.org/10.1158/0008-5472.CAN-07-6621
- Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014; 211(5):781-90; PMID:24778419; https://doi.org/10.1084/jem.20131916
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 2004; 6(4):409-21; PMID:15488763; https://doi.org/10.1016/j.ccr.2004.08.031
- Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, Kato M, Prevost-Blondel A, Thiery JP, Abastado JP. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol 2011; 9(9):e1001162; PMID:21980263; https://doi.org/10.1371/journal.pbio.1001162
- Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH, Vatan L, Szeliga W, Mao Y, Thomas DG et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity 2013; 39(3):611-21; PMID:24012420; https://doi.org/10.1016/j.immuni.2013.08.025
- Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 2009; 58(1):49-59; PMID:18446337; https://doi.org/10.1007/s00262-008-0523-4
- Huang A, Zhang B, Wang B, Zhang F, Fan KX, Guo YJ. Increased CD14(+)HLA-DR (−/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother 2013; 62(9):1439-51; PMID:23760662; https://doi.org/10.1007/s00262-013-1450-6
- Mizukoshi E, Yamashita T, Arai K, Terashima T, Kitahara M, Nakagawa H, Iida N, Fushimi K, Kaneko S. Myeloid-derived suppressor cells correlate with patient outcomes in hepatic arterial infusion chemotherapy for hepatocellular carcinoma. Cancer Immunol Immunother 2016; 65(6):715-25; PMID:27083166; https://doi.org/10.1007/s00262-016-1837-2
- Wang Z, Zhang Y, Liu Y, Wang L, Zhao L, Yang T, He C, Song Y, Gao Q. Association of myeloid-derived suppressor cells and efficacy of cytokine-induced killer cell immunotherapy in metastatic renal cell carcinoma patients. J Immunother 2014; 37(1):43-50; PMID:24316555; https://doi.org/10.1097/CJI.0000000000000005
- Schneider T, Sevko A, Heussel CP, Umansky L, Beckhove P, Dienemann H, Safi S, Utikal J, Hoffmann H, Umansky V. Serum inflammatory factors and circulating immunosuppressive cells are predictive markers for efficacy of radiofrequency ablation in non-small-cell lung cancer. Clin Exp Immunol 2015; 180(3):467-74; PMID:25644608; https://doi.org/10.1111/cei.12596
- Waldron TJ, Quatromoni JG, Karakasheva TA, Singhal S, Rustgi AK. Myeloid derived suppressor cells: targets for therapy. Oncoimmunology 2013; 2(4):e24117; PMID:23734336; https://doi.org/10.4161/onci.24117
- Ding ZC, Lu X, Yu M, Lemos H, Huang L, Chandler P, Liu K, Walters M, Krasinski A, Mack M et al. Immunosuppressive myeloid cells induced by chemotherapy attenuate antitumor CD4+ T-cell responses through the PD-1-PD-L1 axis. Cancer Res 2014; 74(13):3441-53; PMID:24780756; https://doi.org/10.1158/0008-5472.CAN-13-3596
- Ghansah T, Vohra N, Kinney K, Weber A, Kodumudi K, Springett G, Sarnaik AA, Pilon-Thomas S. Dendritic cell immunotherapy combined with gemcitabine chemotherapy enhances survival in a murine model of pancreatic carcinoma. Cancer Immunol Immunother 2013; 62(6):1083-91; PMID:23604104; https://doi.org/10.1007/s00262-013-1407-9
- Hsu FT, Chen TC, Chuang HY, Chang YF, Hwang JJ. Enhancement of adoptive T cell transfer with single low dose pretreatment of doxorubicin or paclitaxel in mice. Oncotarget 2015; 6(42):44134-50; PMID:26683520; https://doi.org/10.18632/oncotarget.6628
- Wang Z, Liu Y, Li R, Shang Y, Zhang Y, Zhao L, Li W, Yang Y, Zhang X, Yang T et al. Autologous cytokine-induced killer cell transfusion increases overall survival in advanced pancreatic cancer. J Hematol Oncol 2016; 9:6; PMID:26842696; https://doi.org/10.1186/s13045-016-0237-6
- Geary SM, Lemke CD, Lubaroff DM, Salem AK. The combination of a low-dose chemotherapeutic agent, 5-fluorouracil, and an adenoviral tumor vaccine has a synergistic benefit on survival in a tumor model system. PloS One 2013; 8(6):e67904; PMID:23840786; https://doi.org/10.1371/journal.pone.0067904
- Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S, Bronte V. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep 2012; 2(3):628-39; PMID:22959433; https://doi.org/10.1016/j.celrep.2012.08.006
- Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 2012; 18(8):1254-61; PMID:22842478; https://doi.org/10.1038/nm.2883
- Welters MJ, van der Sluis TC, van Meir H, Loof NM, van Ham VJ, van Duikeren S, Santegoets SJ, Arens R, de Kam ML, Cohen AF et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci Transl Med 2016; 8(334):334ra52; PMID:27075626; https://doi.org/10.1126/scitranslmed.aad8307
- Dijkgraaf EM, Santegoets SJ, Reyners AK, Goedemans R, Nijman HW, van Poelgeest MI, van Erkel AR, Smit VT, Daemen TA, van der Hoeven JJ et al. A phase 1/2 study combining gemcitabine, Pegintron and p53 SLP vaccine in patients with platinum-resistant ovarian cancer. Oncotarget 2015; 6(31):32228-43; PMID:26334096; https://doi.org/10.18632/oncotarget.4772
- Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol 2009; 9(7–8):900-9; PMID:19336265; https://doi.org/10.1016/j.intimp.2009.03.015
- Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 2010; 70(8):3052-61; PMID:20388795; https://doi.org/10.1158/0008-5472.CAN-09-3690
- Qu X, Felder MA, Perez Horta Z, Sondel PM, Rakhmilevich AL. Antitumor effects of anti-CD40/CpG immunotherapy combined with gemcitabine or 5-fluorouracil chemotherapy in the B16 melanoma model. Int Immunopharmacol 2013; 17(4):1141-7; PMID:24201083; https://doi.org/10.1016/j.intimp.2013.10.019
- Sawant A, Schafer CC, Jin TH, Zmijewski J, Tse HM, Roth J, Sun Z, Siegal GP, Thannickal VJ, Grant SC et al. Enhancement of antitumor immunity in lung cancer by targeting myeloid-derived suppressor cell pathways. Cancer Res 2013; 73(22):6609-20; PMID:24085788; https://doi.org/10.1158/0008-5472.CAN-13-0987
- Otsubo D, Yamashita K, Fujita M, Nishi M, Kimura Y, Hasegawa H, Suzuki S, Kakeji Y. Early-phase treatment by low-dose 5-Fluorouracil or primary tumor resection inhibits MDSC-mediated lung metastasis formation. Anticancer Res 2015; 35(8):4425-31; PMID:26168482
- Annels NE, Shaw VE, Gabitass RF, Billingham L, Corrie P, Eatock M, Valle J, Smith D, Wadsley J, Cunningham D et al. The effects of gemcitabine and capecitabine combination chemotherapy and of low-dose adjuvant GM-CSF on the levels of myeloid-derived suppressor cells in patients with advanced pancreatic cancer. Cancer Immunol Immunother 2014; 63(2):175-83; PMID:24292263; https://doi.org/10.1007/s00262-013-1502-y
- Kim HS, Park HM, Park JS, Sohn HJ, Kim SG, Kim HJ, Oh ST, Kim TG. Dendritic cell vaccine in addition to FOLFIRI regimen improve antitumor effects through the inhibition of immunosuppressive cells in murine colorectal cancer model. Vaccine 2010; 28(49):7787-96; PMID:20883737; https://doi.org/10.1016/j.vaccine.2010.09.046
- Kanterman J, Sade-Feldman M, Biton M, Ish-Shalom E, Lasry A, Goldshtein A, Hubert A, Baniyash M. Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res 2014; 74(21):6022-35; PMID:25209187; https://doi.org/10.1158/0008-5472.CAN-14-0657
- Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, Katsanis E, Larmonier N. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res 2014; 74(1):104-18; PMID:24197130; https://doi.org/10.1158/0008-5472.CAN-13-1545
- Draghiciu O, Nijman HW, Hoogeboom BN, Meijerhof T, Daemen T. Sunitinib depletes myeloid-derived suppressor cells and synergizes with a cancer vaccine to enhance antigen-specific immune responses and tumor eradication. Oncoimmunology 2015; 4(3):e989764; PMID:25949902; https://doi.org/10.4161/2162402X.2014.989764
- van Hooren L, Georganaki M, Huang H, Mangsbo SM, Dimberg A. Sunitinib enhances the antitumor responses of agonistic CD40-antibody by reducing MDSCs and synergistically improving endothelial activation and T-cell recruitment. Oncotarget 2016; 7(31):50277-89; PMID:27385210; https://doi.org/10.18632/oncotarget.10364
- Draghiciu O, Boerma A, Hoogeboom BN, Nijman HW, Daemen T. A rationally designed combined treatment with an alphavirus-based cancer vaccine, sunitinib and low-dose tumor irradiation completely blocks tumor development. Oncoimmunology 2015; 4(10):e1029699; PMID:26451295; https://doi.org/10.1080/2162402X.2015.1029699
- Guislain A, Gadiot J, Kaiser A, Jordanova ES, Broeks A, Sanders J, van Boven H, de Gruijl TD, Haanen JB, Bex A et al. Sunitinib pretreatment improves tumor-infiltrating lymphocyte expansion by reduction in intratumoral content of myeloid-derived suppressor cells in human renal cell carcinoma. Cancer Immunol Immunother 2015; 64(10):1241-50; PMID:26105626; https://doi.org/10.1007/s00262-015-1735-z
- Sevko A, Michels T, Vrohlings M, Umansky L, Beckhove P, Kato M, Shurin GV, Shurin MR, Umansky V. Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J Immunol 2013; 190(5):2464-71; PMID:23359505; https://doi.org/10.4049/jimmunol.1202781
- Sevko A, Sade-Feldman M, Kanterman J, Michels T, Falk CS, Umansky L, Ramacher M, Kato M, Schadendorf D, Baniyash M et al. Cyclophosphamide promotes chronic inflammation-dependent immunosuppression and prevents antitumor response in melanoma. J Invest Dermatol 2013; 133(6):1610-9; PMID:23223128; https://doi.org/10.1038/jid.2012.444
- Chang CL, Hsu YT, Wu CC, Lai YZ, Wang C, Yang YC, Wu TC, Hung CF. Dose-dense chemotherapy improves mechanisms of antitumor immune response. Cancer Res 2013; 73(1):119-27; PMID:23108141; https://doi.org/10.1158/0008-5472.CAN-12-2225
- Angulo I, de las Heras FG, Garcia-Bustos JF, Gargallo D, Munoz-Fernandez MA, Fresno M. Nitric oxide-producing CD11b(+)Ly-6G(Gr-1)(+)CD31(ER-MP12)(+) cells in the spleen of cyclophosphamide-treated mice: Implications for T-cell responses in immunosuppressed mice. Blood 2000; 95(1):212-20; PMID:10607705
- Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, Villagra A, Antonia S, McCaffrey JC, Fishman M et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol 2013; 14(3):211-20; PMID:23354483; https://doi.org/10.1038/ni.2526
- Gobbo J, Marcion G, Cordonnier M, Dias AM, Pernet N, Hammann A, Richaud S, Mjahed H, Isambert N, Clausse V et al. Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide aptamer. J Natl Cancer Inst 2016; 108(3); PMID:26598503; https://doi.org/10.1093/jnci/djv330
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clinical Cancer Res 2005; 11(18):6713-21; PMID:16166452; https://doi.org/10.1158/1078-0432.CCR-05-0883