ABSTRACT
The establishment and maintenance of anti-tumor immune responses are the objectives of cancer immunotherapy. Despite recent promising advances, the effectiveness of these approaches has been limited by the multiple immunosuppressive mechanisms developed by tumors (checkpoint). The aim of the present study was to demonstrate intratumor heterogeneity at the levels of immune escape strategies and tumor-host relationships. We focused on well-known checkpoints such as PD1/PDL1 and on a new checkpoint involving HLA-G and its receptors ILT2/ILT4. A prospective study was performed on 19 renal-cell carcinoma patients that were included during hospitalization for surgical tumor resection. Different areas of the tumor were collected for each patient and subjected to both immunohistochemical and flow cytometry analysis. Immune cells from peripheral blood were concomitantly analyzed for each patient. Our results show the heterogeneous expression of PD1/PDL1 and HLA-G/ILT in the various areas of the same tumor. Intratumor heterogeneity was found both at tumor cell and infiltrating immune cell levels. From a clinical point of view, this work highlights the functional redundancies of checkpoints and the need to adapt personalized poly-immunotherapy.
Introduction
It is well established that the immune system recognizes tumor cells and initiates a response to eliminate them.Citation1 However, many mechanisms of anti-tumor immunity inhibition, called checkpoints, help tumor cells escape and disseminate.Citation2 These checkpoints result from the interaction between ligand molecules expressed by the tumor cell and their receptors present on immune cells, mainly T cells.Citation3 Among these checkpoints, CTLA4/B7 interactions specifically inhibit the induction phase of the T cell response while PD1/PDL1 interactions have a prominent role in the effector phase of the T cell response.Citation4 Other checkpoints have been described and may also significantly contribute to tumor immune escape, such as CD47/SIRP1a; TIGIT/PvR; LAG3/MHC-II; BTLA/HVEM; CD200/CD200R; B7-H3; B7-H4; VISTA; CD39/CD38/CD73/CD203a/CD157/ADOR; TIM-3/Galectin9Citation5. In the present study, we focus particularly on the checkpoint resulting from the interaction between HLA-G and its receptors, ILT2 and ILT4.Citation6
Indeed, HLA-G expression has been shown in most cancers with a variable frequency, depending on the tumor type (50% of kidney tumors). Up to now, more than 2,000 cancer patients have been tested, showing that (i) HLA-G expression by tumor cells and/or by tumor-infiltrating immune cells, and (ii) high HLA-G concentrations in the plasma, are both correlated with malignancy, inflammation, and poor prognosis.Citation7 The in vivo role of HLA-G as a tumor escape mechanism has been demonstrated in mice.Citation8,Citation9 Indeed, in a tumor-implantation model, control HLA-G-negative tumors were rejected, whereas HLA-G-expressing tumors grew. In these experiments, blocking of HLA-G by a specific neutralizing antibody prevented the growth of HLA-G-expressing tumors, providing the proof of concept for new antitumor therapeutic strategy.
The 2 major receptors for HLA-G are ILT2 (LILRB1/CD85j) and ILT4 (LILRB2/CD85d) Citation10,11. ILT2 is expressed at the surface of monocytes / macrophages, dendritic cells, B cells, and some T and NK cells. ILT4 is expressed by neutrophils and myeloid cells. The interaction between HLA-G and the ILTs receptors inhibits the function of these immune cells and induces immunosuppressive cells such as Tregs and myeloid suppressive cells.Citation7 Beyond this expression by immune cells, ILT4 was recently described on breast and lung tumor cells. This expression is associated with lymph node metastasis and less number of tumor-infiltrating lymphoid cells.Citation12,Citation13 Such site of expression, quite unexpected for ILT4, is of great interest with respect to how it affects the phenotypic and functional characteristics of tumor cells that express it.
To restore an effective anti-tumor response, blocking checkpoints by monoclonal antibodies is the currently favored immune-therapeutic strategy, already implemented in the context of metastatic melanomaCitation14 and lung cancer.Citation15 In kidney cancer, clinical trials were performed to study the therapeutic effect of antibodies targeting PD1 (nivolumab, BMS; pembrolizumab, Merck) or PDL1 (atezolizumab, Roche), and a survival benefit was demonstrated for nivolumab.Citation16,Citation17,Citation18,Citation19 Despite these striking results, these anti-checkpoint monotherapies are inefficient in the majority of patients, and there is currently no predictive test for efficacy. The reasons might be the involvement of several checkpoints regulating distinct inhibitory pathways through non-overlapping mechanisms of action, and their disparate expression in different tumor areas. In this context, concurrent combination therapies with several anti-checkpoint strategies might be more efficacious than either one alone. This was indeed shown to be the case in a 2013 phase III clinical trial with anti-CTLA4 (ipilimumab) in combination with anti-PD1 (nivolumab) which demonstrated tumor regression in ∼50% of treated patients with advanced melanoma.Citation20 As a result, the combination of nivolumab and ipilimumab for metastatic melanoma was recently approved by the Food and Drug Administration.Citation21 It therefore appears fundamental to characterize changes in the expression of these checkpoints, and to identify their redundancy on a single tumor to prevent the ineffectiveness of anti-checkpoint monotherapy and to customize combined immunotherapy. In this regard, the present work proposes to study spatial heterogeneity of the expression of immune checkpoints in surgical specimens and blood samples in prospective cohorts of patients with renal-cell carcinoma.
Results
Nineteen patients who underwent a partial or radical nephrectomy for renal-cell carcinoma in the urology department of Saint-Louis Hospital (Paris, France) were finally included in this observational prospective population-based study (). Normal and tumor tissues were collected for each patient, and different tumor areas (3–4 zones per tumor) were selected using macroscopic criteria. These different tumor areas were then subjected to an analysis combining immunohistochemical and phenotypic approaches. All the 19 patients exhibited intratumor heterogeneity of immune checkpoint expression at the tumor cell and/or the infiltrate immune cell levels (). We here present detailed results for patients #2, #7, #8 and #10 that were selected as representative examples. Clinical characteristics of these 4 patients are summarized in .
Table 1. Patient characteristics (n = 19).
Table 2. Heterogeneous expression of checkpoint molecules on tumor cells and/or tumor-infiltrating T cells.
Table 3. Characteristics of patients #2, #7, #8 and #10.
Intra-tumor heterogeneity of immune checkpoint expression on tumor-infiltrating immune cells
We found that intra-tumor heterogeneity of tumor-infiltrating immune cells could be great, with respect to the nature of the infiltrating cells themselves, as already known, and also with respect to their expression of immune checkpoint molecules. We selected 2 patients of our cohort (#7 and #8) to illustrate this point.
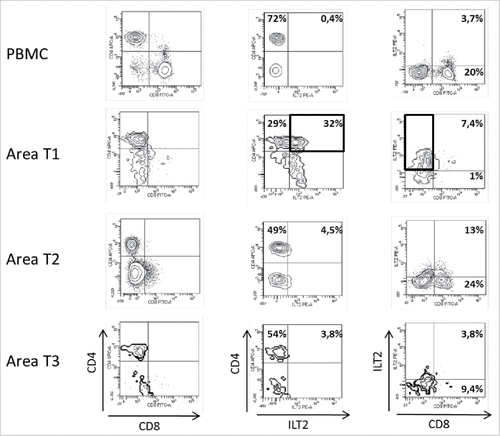
In patient #7 with a clear cell renal-cell carcinoma (ccRCC), immune populations from peripheral blood and 3 tumor areas were compared. The intra-tumor immune infiltrate was almost exclusively constituted of T cells. Other cell populations were barely detectable (not shown). Interestingly, CD3+CD8+ T cells were detected in only one of the tumor areas (). These CD8 T cells which constituted a minority of the infiltrate expressed low levels of CD8 (CD8low). In all tumor samples, CD3+CD4+ T cells made up most of the immune infiltrate. Even within CD3+CD4+ T cells, spatial heterogeneity was evidenced: whereas CD3+CD4+ T cells from PBMC barely expressed the checkpoint receptor ILT2, similarly as to what is seen in healthy blood donors, and whereas CD3+CD4+ T cells from 2 out of 3 intra-tumor areas also showed no ILT2 cell-surface expression, 32% of CD3+ T cells from tumor area T1 expressed ILT2, which is an outstanding feature. ILT2 expression at a tumor site could be indicative of functional inhibition by this molecule, leading to immune escape. Consistently, HLA-G expression was found on the tumor cell counterpart (). It is of note that CD3+ILT2+ T cells negative for CD4 and CD8 are present in the tumor area.
Figure 1. Representative dot plots of the results obtained by flow cytometry analysis for ILT2 cell-surface expression on CD8+ and CD4+ T cells from PBMC and 3 different tumor areas in patient #7 are shown. Tumor-infiltrating cells were obtained after mechanic disruption followed by enzymatic digestion of 3 different areas from the surgically-resected tumor (T1, T2, T3). PBMC and tumor infiltrating cells from each tumor area were stained with conjugated-antibodies directed against CD3, CD4, CD8, and ILT2 and were then analyzed by flow cytometry. Percentages of both ILT2-positive and ILT2-negative populations gated on CD3+ T cells are indicated.
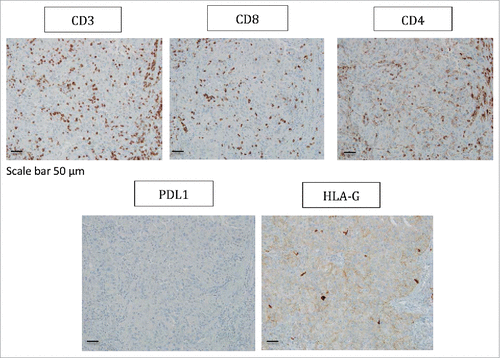
In patient #8 with a type 2 papillary RCC, PBMC, 2 tumor areas (T1, T2), one tumoral thrombus of the renal vein (VT) and one metastatic hilar lymph node (G) were analyzed. Again, all areas of the tumor were infiltrated by immune cells and most of them were made up of CD3+CD4+ T cells ( and ). Regarding CD3+CD8+ T cells, immunohistochemistry shows a significant CD8+ T cell infiltrate (), and flow cytometry shows that these cells are a minority compared with CD4+ T cells, and all expressed low levels of CD8 (). In this patient, as in patient #7, ILT2 expression was site-specific. Indeed, ILT2 expression was detected on <10% of CD8low T cells infiltrating the tumor-invaded lymph node, and on a large proportion of CD3+CD4+ T cells infiltrating the venous thrombus (37%). Here again, HLA-G was found on the corresponding tumor cells, together with ILT4 (, ).
Figure 2. Representative dot plots of the results obtained by flow cytometry analysis for ILT2 cell-surface expression on CD8+ and CD4+ T cells from PBMC and 4 different tumor areas in patient #8 are shown. Tumor-infiltrating cells were obtained after mechanic disruption followed by enzymatic digestion of 4 different areas from the surgically-resected tumor (T1, T2, G, VT). PBMC and tumor infiltrating cells from each tumor area were stained with conjugated-antibodies directed against CD3, CD4, CD8, and ILT2 and were then analyzed by flow cytometry. Percentages of both ILT2-positive and ILT2-negative populations gated on CD3+ T cells are indicated. G, metastatic hilar lymph node; VT, tumoral thrombus of the renal vein
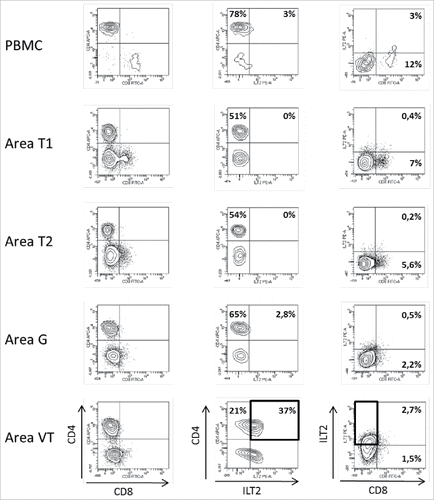
Figure 3. Representative staining obtained by immunohistochemistry analysis for CD3, CD8, CD4, PDL1 and HLA-G expression in tumor biopsy from patient #8 are shown. Formalin-fixed tumor tissue sections from the tumoral thrombus of the renal vein were stained with antibody directed either against CD3, CD8, CD4, PDL1 or HLA-G marker. Brown labeling indicates marker positivity. Scale bar is indicated.
Intratumor heterogeneity of immune checkpoints expression on tumor cells
We found intra-tumor heterogeneity for tumor cell expression of checkpoint molecules. We selected 2 patients of our cohort (#2 and #10) to illustrate this point.
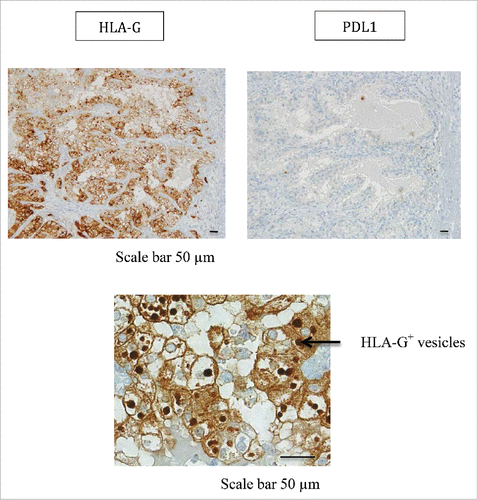
In patient #2 with a ccRCC, from whom 4 tumor areas (T1-T4) and adjacent normal tissue were analyzed, we found high HLA-G expression by the CA9+ ccRCC tumor cells (). However, HLA-G-expression levels differed in each area: 70% in area T1, 37% in area T2, and 58% in areas T3 and T4. This expression was observed by immunohistochemical staining where all tumor areas showed a same diffuse and intense immunostaining ( for area T1). Very interestingly, the expression of HLA-G was found in the cytoplasm of tumor cells but also in intracellular and extracellular vesicles called “hyaline granules” (). These vesicles could serve as HLA-G reservoir which may be conveyed away from the tumor. Notably, a low expression of ILT4 by tumor cells was also observed in 2 out of the 4 zones tested (). In sharp contrast, no PDL1 expression was found on the tumor cells of any of the 4 areas investigated, as exemplified by immunohistochemistry of area T1 in . Thus, in this patient, heterogeneous HLA-G and ILT4 expression was found and associated with no PDL1 expression.
Figure 4. Representative histograms obtained by flow cytometry analysis for CA9 and HLA-G cell-surface expression on tumor cells from 4 different tumor areas in patient #2 are shown. Tumor cells were obtained after mechanic disruption followed by enzymatic digestion of 4 different areas from the surgically-resected tumor (T1, T2, T3, T4). Cells were then cultured for 3 d and then stained with antibody either directed against CD3, CD45, HLA-G or CA9 marker. Tumor cells were considered to be large CD45-negative and CD3-negative cells (data not shown). Percentage of the HLA-G-positive population in CD3− CD45− gated cells is indicated. Blue and red histograms correspond to staining with isotype control and marker, respectively.
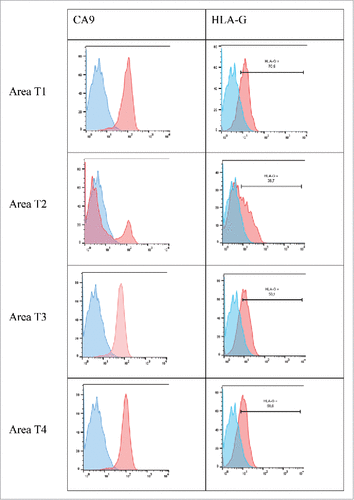
Figure 5. Representative staining obtained by immunohistochemistry analysis for PDL1 and HLA-G expression in tumor area (T1) from patient #2 is shown. Formalin-fixed tumor tissue sections from the tumor area T1 were stained with antibody directed either against PDL1 or HLA-G marker. Brown labeling indicates marker positivity. HLA-G staining is observed in the cytoplasm of tumor cells but also in intracellular and extracellular vesicles. Scale bars are indicated.
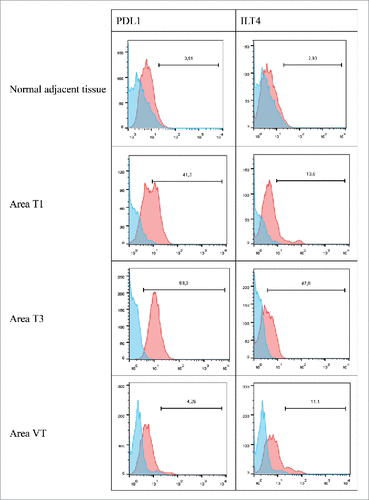
In patient #10 with a ccRCC, from whom 2 tumor areas (T1 and T2), one tumoral thrombus of the renal vein (VT), and adjacent healthy tissue were analyzed, we found no HLA-G expression (not shown), but heterogeneous expressions of PDL1 and ILT4 by tumor cells. Indeed, PDL1 was detected on 41% of CA9+ tumor cells in area T1 and on 98% of CA9+ tumor cells in area T2, but was almost not expressed (4%) on tumor cells of the renal vein thrombus (VT) or in the healthy renal parenchyma distant to the tumor (). In addition, the expression of the inhibitory receptor ILT4, demonstrated here for the first time at the surface of a renal tumor cell, followed the same heterogeneity pattern as PDL1 with a strong expression in area T2 (47%) and a low expression in area T1 (11%), venous tumoral thrombus (13.8%) and no expression on normal parenchyma. The tumor area T3 is particularly interesting because it is the most aggressive tumor area according to its anatomopathological characteristics. In this respect, this area has lost the expression of CA9 (data not shown). This very aggressive area is the only one that highly expressed ILT4 and PDL1 together.
Figure 6. Representative histograms obtained by flow cytometry analysis for PDL1 and ILT4 cell-surface expression on cells from the normal adjacent tissue or on tumor cells from 3 different tumor areas in patient #10 are shown. Cells were obtained after mechanic disruption followed by enzymatic digestion of either the normal adjacent tissue or 3 different areas from the surgically-resected tumor (T1,T3, VT). Cells were then cultured for 3 d and then stained with antibody either directed against CD3, CD45, PDL1 or ILT4 marker. Tumor cells were considered to be large CD45-negative and CD3-negative cells (data not shown). Percentage of the HLA-G-positive population in CD3- CD45- gated cells is indicated. Blue and red histograms correspond to staining with isotype control and marker, respectively. VT, tumoral thrombus of the renal vein.
Discussion
Restoration of anti-tumor immune responses was the goal of cancer immunotherapy during the past 30 years. Despite the promising advances, the success of such therapeutic approaches is counteracted by immunosuppressive mechanisms developed by tumors. Among these mechanisms, the inducible expression of HLA-G appears as an effective strategy to escape the anti-tumor response, and several studies associate its expression with poor prognosis in cancer patients.Citation22 Indeed, HLA-G is capable of inhibiting the activity of all immune cells via its interaction with inhibitory receptors ILT2 and ILT4, thus protecting the tumor cells from antitumor responses.Citation7
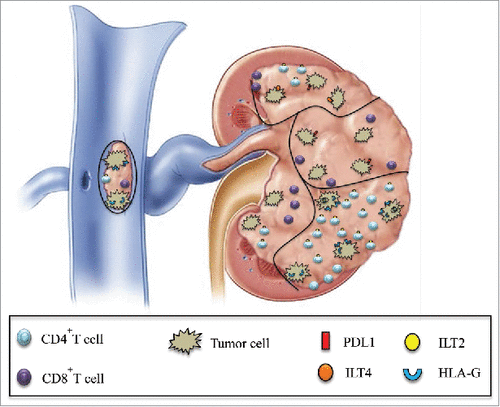
This study aimed at demonstrating intra-tumor heterogeneity in immune escape strategies and host/tumor relationships in kidney cancer, particularly targeting the checkpoint formed by the interaction between HLA-G and its receptors ILT2 and ILT4. From a fundamental point of view, this study highlights the differences in checkpoint molecule expression between separate tumor areas from the same tumor. From a clinical point of view, this work has implications for the choice of immunotherapy strategies adapted to each tumor profile. Our results validated the hypothesis that, within a tumor site, there is much heterogeneity in terms of immune checkpoint expression both in tumor cells and in tumor-infiltrating CD4+ and CD8+ T cells. Interestingly the CD4+ILT2+, CD8+ILT2+ and CD8low T cells found in tumor areas are known in the literature as suppressor cells.Citation23,Citation24,Citation25 One can hypothesize that in such tumor regions where these suppressor cells predominate, the anti-tumor response will be particularly inhibited. Thus, our results show that each tumor area will develop specific escape mechanisms from immunological attack. illustrates this heterogeneity within different tumor areas of kidney cancer at both infiltrating immune cell and tumor cell levels.
Figure 7. Schematic representation of the simultaneous expression of various immune checkpoints in different tumor areas is shown. Intratumor heterogeneity of PD1/PDL1 and HLA-G/ILT expression in various areas of the same tumor, including the tumoral thrombus of the renal vein, can be observed both at tumor cell and infiltrating CD4+ and CD8+ T cell levels.
A remarkable finding of our study is the demonstration of the expression of ILT4 receptor on the surface of tumor cells, which is quite unexpected because this receptor is well-known on cells of the immune system.Citation11 ILT4 belongs to immunoglobulin-like transcript family, is expressed predominantly in myeloid cells: monocytes, macrophages, dendritic cells, and some neutrophils.Citation11 ILT4 binds to classical and nonclassical major histocompatibility complex class I molecules, with a preferential binding to HLA-G.Citation26 ILT4 has been shown to induce inhibitory signaling via immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in its cytoplasmic tail, which is thought to recruit the protein tyrosine phosphatase (SHP)-1.Citation27 Recent studies reported that ILT4 could be also induced in non-small cell lung cancer (NSCLC) and breast cancer cells and was associated with lymph node metastasis and less number of tumor-infiltrating lymphoid cells.Citation12,Citation13 Also, ILT4 could up-regulate vascular endothelial growth factor-C (VEGF-C) expression via extracellular signal regulated kinases (ERK) signaling pathway and up-regulate B7-H3 expression via PI3K/AKT/mTOR signaling in NSCLC cells.Citation28,Citation29 These results point out that both immune regulation-related and non-immune regulation-related processes may be involved in ILT4-mediated progression of NSCLC. Thus, ILT4 may have dual concordant roles in tumor biology, as immune checkpoint molecule and as tumor-sustaining factor.Citation27 More recently, co-expression of ILT4 and HLA-G was described on NSCLC tissues and cells.Citation30 Such co-expression was significantly associated with regional lymph node involvement, advanced stages, and decreased overall survival of patients. These recent results indicate that the ILT4-HLA-G interaction might play an important role in non-small cell lung cancer progression. Whether a similar role is played by ILT4 in renal cancer remains to be defined.
In addition, we here observed in situ HLA-G expression in hyaline granules. Such HLA-G molecules trapped in these granules may represent a new reservoir of inhibitory molecules acting both locally and at distance from the tumor site, since hyaline granules can be released in the tumor microenvironment. HLA-G is a checkpoint molecule and a well-known tumor escape mechanism. Indeed, HLA-G is expressed in many types of primary tumors and metastases, and in malignant effusions. Its clinical relevance in cancer is supported by the following observations: (i) HLA-G expression is associated with malignant transformation and is never observed in healthy surrounding tissues; (ii) HLA-G is found to be expressed in liquid and solid tumors of high histological grades and advanced clinical stages; and (iii) use of HLA-G as a prognostic marker has been proposed since HLA-G expression in biopsies and/or high levels of sHLA-G in plasma of patients have been significantly correlated with poor prognosis in different types of cancer.Citation31
Characterization of intratumor heterogeneity targeting checkpoint such as PD1/PDL1 and HLA-G/ILT2/ILT4 is important for immunotherapy. For 20 years, the use in kidney cancer of interleukin-2 and interferon aimed at stimulating T cell responses, have resulted in dramatic regression of metastases, including lung metastases.Citation6 However, only 10–15% of patients have a beneficial effect of these treatments, that are often poorly supported (fever, flu syndrome, edema, redness and rashes, depression). Then, targeted therapies have been proposed using interferon in combination with anti-VEGF antibody (Bevacizumab). But this treatment is offered to a minority of patients. Since then, targeted immunotherapies have been developed. The purpose of these immunotherapies is to “reactivate” the immune system by blocking various checkpoints to restore immune cell function. The results of early studies are very promising in many cancers, including kidney cancer.Citation19 A Phase 3 study to evaluate the efficacy of anti-PD1 antibody (nivolumab) in kidney cancer in advanced or metastatic clear cell renal carcinoma after failure of one or 2 anti-angiogenic treatments, is under study.Citation32 However, this treatment does not target all tumor areas but only some areas. In view of the poor prognosis of this cancer, it is essential to identify new therapeutic targets to optimize the management of these patients. In this regard, the use of HLA-G protein antagonists or anti-HLA-G or anti-ILT antibodies may be viewed as new immunotherapy strategy to block this checkpoint resulting from in situ interaction between ILT2/ILT4 and HLA-G.
In conclusion, our results clearly demonstrate both intra- and inter- heterogeneity of immune checkpoint expression among and inside patients. The objective of this study was to contribute to the development of a personalized immunotherapy for each patient, able to adapt to the immune evolution of the tumor. In this regard, blocking multiple checkpoints may be necessary to target the entire tumor.
Patients, materials and methods
Patients and samples
Nineteen patients were included during hospitalization for surgical resection of the renal tumor in the urology department of Saint-Louis Hospital (Paris, France) from January 2015 to December 2016 (). They were classified according to the recommendations of the European Association of Urology, i.e. TNM 2010, WHO/ISUP 2016 grading system, WHO classification of tumors of the kidney (WHO, 2016), and Karnofsky index.Citation33 All patients who participated to this study gave their free and informed writing consent. No patient received any neoadjuvant therapy including biologic therapy/immunotherapy. The following samples were collected from each patient: (i) blood for analysis of the peripheral blood mononuclear cell (PBMC) immune profile, (ii) 3 to 4 areas (0.1cm3–0.2cmCitation3)from the surgically-resected primary tumor or local metastasis, and (iii) 1 area from the normal renal parenchyma adjacent to the tumor. All these areas were selected by a pathologist using macroscopic criteria. PBMC were isolated by density-gradient centrifugation following the instructions of Ficoll-Paque Plus (GE Healthcare).
Surgical specimens
Each tumor sample was subjected to mechanical disruption followed by enzymatic digestion to obtain tumor-infiltrating cells on one hand, and tumor cells on the other hand. Briefly, enzymatic digestion was performed using the Tumor Dissociation Kit (Miltenyi Biotech; Bergisch Gladbach, Germany) at 37°C for 30 min (twice). Infiltrating cells were characterized immediately without any culture step by flow cytometry analysis. The expression of different checkpoints on T, NK, NKT, Tγ/δ, MAIT, Tregs, B, Bregs, monocytes, dendritic cells and macrophages were performed by a multi-parameter flow cytometry analysis. Tumor cells were characterized for their expression of checkpoints after a short culture period (3 days), in DMEM medium (Gibco) supplemented with 10% Fetal Bovine Serum (FBS, Sigma) and Penicillin/Streptomycin (P/S, Sigma).
Antibodies and Flow cytometry
PBMC, tumor-infiltrating cells and tumor cells were subjected to phenotype analysis by flow cytometry. For tumor-infiltrating cells all antibodies used were directly conjugated and they were all from Miltenyi, except anti-PD1 (BD PharMingen), and anti-HLA-G MEM-G/9 (Exbio, Praha). The following subpopulations were systematically analyzed: (i) T cells: CD3/CD4/CD8/ILT2, and; (ii) NK cells and NKT cells: CD3/CD56/TCRgd/ILT2; (iii) Tregs, CD3/CD4/CD25; (iv) B cells and Breg cells: CD19/CD24/CD38/ILT2-FITC; (v) monocytes and DC: CD14/ILT2/ILT4. Cell numbers allowing, the following subpopulations were also investigated (i.e., not systematically); (vi) T cells: CD3/CD4/CD8/PD1; (vii) MAIT: CD3/CD161/TCRVa7.2/ILT2; (viii) M2 macrophages: CD14/CD163/CD206/ILT4/HLA-G; and (ix) NK CD56bright and CD56low cells: CD3/CD16/CD56/ILT2.
For tumor cell staining, all antibodies were directly conjugated. Anti-HLA-G MEM-G/9 was from Exbio Praha, anti-hCarbonic Anhydrase IX (CA9) was from R&D Systems, anti-PDL1 was from eBioscience, anti-CD3 and anti-CD45 were from Miltenyi, and anti-ILT2 and anti-ILT4 were from Beckman Coulter. Tumor cells were considered to be large CD45-negative and CD3-negative cells. HLA-G, ILT2, ILT4, PDL1, and CA9 for ccRCC, were tested independently on such gated tumor cells using appropriate isotype antibodies to control background staining.
For flow-cytometry analyses, Fc receptors were blocked by a 30-min incubation with 1μg of pooled purified isotype antibodies in 100 μl PBS1x. All staining steps were performed on ice and isotype-matched control antibodies were systematically used. Samples were acquired on a BD Canto-II flow cytometer and data were analyzed using BD Vista or Beckman Coulter Kaluza software.
Immunohistochemistry
Immunohistochemical studies were performed on 4-µm-thick, formalin-fixed tumor tissue sections from 3 to 5 paraffin blocks for each tumor. Staining was performed on automated slide stainers from Roche (BenchMark ULTRA system, Tucson, AZ) using the OptiView DAB IHC Detection Kit (Roche), Cell Conditioning 1 (CC1) short or standard antigen retrieval, an antibody incubation time of 32 min at 37°C, ultraWash procedure, counterstaining with Hematoxylin II for 4 min and bluing reagent for 8 min. The dilution and sources of antibodies are CD3 (polyclonal rabbit, dilution 1:200, Dako, Glostrup, Denmark), CD8 (C8/144B clone, dilution 1:50, Dako), CD4 (clone SP35, prediluted, Roche), PDL1 (clone E1L3N, dilution 1:100, Cell Signaling Technology, Denver, MA, USA), PD1 (NAT105 clone, dilution 1:50, Abcam, Cambridge, United Kingdom), HLA-G (clone 4H84, dilution 1/200, mAb recognizing all HLA-G isoforms, Santa Cruz Biotechnology, Santa Cruz, CA) and ILT4 (polyclonal goat, dilution 1:50, R&D Systems, Minneapolis, MN). Positive and negative controls gave appropriate results for each procedure. The immunohistochemical study was performed by an uropathologist using a BX51 microscope (Olympus France S.A.S, Rungis).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Romain Luinaud, Marie-Delphine Lanic, Julie Renard, Malika Djouadou and Marina Daouya for their technical help. We thank Drs Clement Dumont and Amaury de Gouvello for patient follow up and analysis.
Funding
This work was supported by the CEA, Universite Paris Diderot and AP-HP.
References
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331(6024):1565-70; PMID:21436444; https://doi.org/10.1126/science.1203486
- Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer–preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol 2010; 37(5):430-9; PMID:21074057; https://doi.org/10.1053/j.seminoncol.2010.09.005
- Kline J, Gajewski TF. Clinical development of mAbs to block the PD1 pathway as an immunotherapy for cancer. Curr Opin Investig Drugs 2010; 11(12):1354-9; PMID:21154117
- Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer 2011; 11(11):805-12; PMID:22020206; https://doi.org/10.1038/nrc3153
- Wolchok JD, Chan TA. Cancer: Antitumour immunity gets a boost. Nature 2014; 515(7528):496-8; PMID:25428495; https://doi.org/10.1038/515496a
- Carosella ED, Ploussard G, LeMaoult J, Desgrandchamps F. A Systematic Review of Immunotherapy in Urologic Cancer: Evolving Roles for Targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur Urol 2015; 68(2):267-9; PMID:25824720; https://doi.org/10.1016/j.eururo.2015.02.032
- Carosella ED, Rouas-Freiss N, Roux DT, Moreau P, LeMaoult J. HLA-G: An Immune Checkpoint Molecule. Adv Immunol 2015; 127:33-144; PMID: 26073983; https://doi.org/10.1016/bs.ai.2015.04.001
- Agaugue S, Carosella ED, Rouas-Freiss N. Role of HLA-G in tumor escape through expansion of myeloid-derived suppressor cells and cytokinic balance in favor of Th2 versus Th1/Th17. Blood 2011; 117(26):7021-31; PMID:21482709; https://doi.org/10.1182/blood-2010-07-294389
- Loumagne L, Baudhuin J, Favier B, Montespan F, Carosella ED, Rouas-Freiss N. In vivo evidence that secretion of HLA-G by immunogenic tumor cells allows their evasion from immunosurveillance. Int J Cancer 2014; 135(9):2107-17; PMID:24623585; https://doi.org/10.1002/ijc.28845
- Colonna M, Navarro F, Bellon T, Llano M, Garcia P, Samaridis J, Angman L, Cella M, López-Botet M.. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med 1997; 186(11):1809-1818; PMID:9382880; https://doi.org/10.1084/jem.186.11.1809
- Colonna M, Samaridis J, Cella M, Angman L, Allen RL, O'Callaghan CA, Dunbar R, Ogg GS, Cerundolo V, Rolink A.. Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J Immunol 1998; 160(7):3096-100; PMID:9531263
- Liu J, Wang L, Gao W, Li L, Cui X, Yang H, Lin W, Dang Q, Zhang N, Sun Y. Inhibitory receptor immunoglobulin-like transcript 4 was highly expressed in primary ductal and lobular breast cancer and significantly correlated with IL-10. Diagnostic pathology 2014; 9:85; PMID:24762057; https://doi.org/10.1186/1746-1596-9-85
- Sun Y, Liu J, Gao P, Wang Y, Liu C. Expression of Ig-like transcript 4 inhibitory receptor in human non-small cell lung cancer. Chest 2008; 134(4):783-8; PMID:18625675; https://doi.org/10.1378/chest.07-1100
- Franklin C, Livingstone E, Roesch A, Schilling B, Schadendorf D. Immunotherapy in melanoma: Recent advances and future directions. Eur J Surg Oncol 2017; 43(3):604-11; PMID:27769635; https://doi.org/10.1016/j.ejso.2016.07.145
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. The New England journal of medicine 2015; 373(17):1627-39; PMID:26412456; https://doi.org/10.1056/NEJMoa1507643
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515(7528):563-7; PMID:25428504; https://doi.org/10.1038/nature14011
- Choueiri TK, Figueroa DJ, Fay AP, Signoretti S, Liu Y, Gagnon R, Deen K, Carpenter C, Benson P, Ho TH, et al. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled trial. Clin Cancer Res 2015; 21(5):1071-7; PMID:25538263; https://doi.org/10.1158/1078-0432.CCR-14-1993
- Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol 2015; 33(13):1430-7; PMID:25452452; https://doi.org/10.1200/JCO.2014.59.0703
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015; 373(19):1803-13; PMID:26406148; https://doi.org/10.1056/NEJMoa1510665
- Hassel JC. Ipilimumab plus nivolumab for advanced melanoma. Lancet Oncol 2016; 17(11):1471-2; PMID:27617662; https://doi.org/10.1016/S1470-2045(16)30409-0
- Kourie HR, Klastersky JA. Side-effects of checkpoint inhibitor-based combination therapy. Curr Opin Oncol 2016; 28(4):306-13; PMID:27136134; https://doi.org/10.1097/CCO.0000000000000295
- Rouas-Freiss N, Moreau P, Ferrone S, Carosella ED. HLA-G proteins in cancer: do they provide tumor cells with an escape mechanism? Cancer research 2005; 65(22):10139-10144; PMID:16287995; https://doi.org/10.1158/0008-5472.CAN-05-0097
- Naji A, Le Rond S, Durrbach A, Krawice-Radanne I, Creput C, Daouya M, Caumartin J, LeMaoult J, Carosella ED, Rouas-Freiss N. CD3+CD4low and CD3+CD8low are induced by HLA-G: novel human peripheral blood suppressor T-cell subsets involved in transplant acceptance. Blood 2007; 110(12):3936-48; PMID:17804694; https://doi.org/10.1182/blood-2007-04-083139
- LeMaoult J, Caumartin J, Daouya M, Favier B, Le Rond S, Gonzalez A, Carosella ED. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood 2007; 109(5):2040-48; PMID:17077329; https://doi.org/10.1182/blood-2006-05-024547
- Djurisic S, Skibsted L, Hviid TV. A Phenotypic Analysis of Regulatory T Cells and Uterine NK Cells from First Trimester Pregnancies and Associations with HLA-G. Am J Reprod Immunol 2015; 74(5):427-44; PMID:26293482; https://doi.org/10.1111/aji.12421
- Shiroishi M, Tsumoto K, Amano K, Shirakihara Y, Colonna M, Braud VM, Allan DS, Makadzange A, Rowland-Jones S, Willcox B, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci U S A 2003; 100(15):8856-61; PMID:12853576; https://doi.org/10.1073/pnas.1431057100
- Kang X, Kim J, Deng M, John S, Chen H, Wu G, Phan H, Zhang CC. Inhibitory leukocyte immunoglobulin-like receptors: Immune checkpoint proteins and tumor sustaining factors. Cell cycle 2016; 15(1):25-40; PMID:26636629; https://doi.org/10.1080/15384101.2015.1121324
- Zhang P, Yu S, Li H, Liu C, Li J, Lin W, Gao A, Wang L, Gao W, Sun Y. ILT4 drives B7-H3 expression via PI3K/AKT/mTOR signalling and ILT4/B7-H3 co-expression correlates with poor prognosis in non-small cell lung cancer. FEBS Lett 2015; 589(17):2248-56; PMID:26149216; https://doi.org/10.1016/j.febslet.2015.06.037
- Zhang P, Guo X, Li J, Yu S, Wang L, Jiang G, Yang D, Wei Z, Zhang N, Liu J, et al. Immunoglobulin-like transcript 4 promotes tumor progression and metastasis and up-regulates VEGF-C expression via ERK signaling pathway in non-small cell lung cancer. Oncotarget 2015; 6(15):13550-63; PMID:25948790; https://doi.org/10.18632/oncotarget.3624
- Zhang Y, Zhao J, Qiu L, Zhang P, Li J, Yang D, Wei X, Han Y, Nie S, Sun Y. Co-expression of ILT4/HLA-G in human non-small cell lung cancer correlates with poor prognosis and ILT4-HLA-G interaction activates ERK signaling. Tumour Biol 2016; 37(8):11187-98; PMID:26939901; https://doi.org/10.1007/s13277-016-5002-5
- Morandi F, Rizzo R, Fainardi E, Rouas-Freiss N, Pistoia V. Recent Advances in Our Understanding of HLA-G Biology: Lessons from a Wide Spectrum of Human Diseases. J Immunol Res 2016; 2016:4326495; PMID:27652273; https://doi.org/10.1155/2016/4326495
- Hammers H. Immunotherapy in kidney cancer: the past, present, and future. Curr Opin Urol 2016; 26(6):543-7; PMID:27533501; https://doi.org/10.1097/MOU.0000000000000338
- Escudier B, Kataja V, Group EGW. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO 2010; 21 Suppl 5:v137-139; PMID:20555064; https://doi.org/10.1093/annonc/mdq206
