ABSTRACT
Immunotherapy clinical trials targeting the programmed-death ligand axis (PD-1/PD-L1) show that most head and neck squamous cell carcinoma (HNSCC) patients are resistant to PD-1/PD-L1 inhibition. We investigated whether local radiation to the tumor can transform the immune landscape and render poorly immunogenic HNSCC tumors sensitive to PD-L1 inhibition. We used the first novel orthotopic model of HNSCC with genetically distinct murine cell lines. Tumors were resistant to PD-L1 checkpoint blockade, harbored minimal PD-L1 expression and tumor infiltrating lymphocytes at baseline, and were resistant to radiotherapy. The combination of radiation and PD-L1 inhibition significantly enhanced tumor control and improved survival. This was mediated in part through upregulation of PD-L1 on tumor cells and increased T-cell infiltration after RT, resulting in a highly inflamed tumor. Depletion of both CD4 and CD8 T-cells completely abrogated the effect of anti PD-L1 with radiation on tumor growth. Our findings provide evidence that radiation to the tumor can induce sensitivity to PD-L1 checkpoint blockade in orthotopic models of HNSCC. These findings have direct relevance to high risk HNSCC patients with poorly immunogenic tumors and who may benefit from combined radiation and checkpoint blockade.
Synopsis
Radiation induced sensitivity to PD-L1 blockade in a novel orthotopic model of HNSCC devoid of PD-L1 expression and resistant to anti-PD-L1 inhibitors. RT increased expression of PD-L1 and MHC-I on cancer cells and intra-tumoral infiltration and activation of T-cells. The combination of RT and PD-L1 blockade significantly inhibited tumor growth and improved survival.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a debilitating and deadly disease. Over 600,000 patients are diagnosed annually with HNSCC worldwide.Citation1 Despite aggressive multi-modality treatment involving chemotherapy and radiotherapy (RT), patients with advanced disease and heavy smoking history have an overall survival rate below 50% after 5 years.Citation1,2 The significant mortality and morbidity associated with HNSCC has fueled the pursuit of alternate therapeutic strategies, including immunotherapy. Specifically, the programmed-death-1/programmed-death ligand-1 (PD-1/PD-L1) axis has been implicated in evasion of immune recognition in several cancers and its blockade has shown significant survival benefits in patients with advanced melanoma, renal cell carcinoma and non-small cell lung cancer.Citation3-5 The role of the PD-1/PD-L1 axis in HNSCC tumors is still being characterized but early data suggest that human papilloma virus (HPV)-driven HNSCCs may be more immunogenic compared with non-HPV-driven HNSCCs given their increased T cell infiltration and associated increase in PD-L1 levels.Citation6 Specifically, a recent study in 127 HNSCC tumors showed PD-L1 expression by immunohistochemistry to be predominantly present in HPV-positive tumors (n = 64, 70% PD-L1 positive) compared with HPV-negative tumors (n = 63, 43% PD-L1 positive).Citation7 In addition, HPV-positive HNSCCs demonstrate increased T-cell infiltration compared with HPV-negative tumors, increasing their likelihood of response to immunotherapy.Citation8 Recent data from the CHECKMATE-141 clinical trial on the efficacy of PD-1 blockade in 361 unselected patients with recurrent/metastatic HNSCC have shown a response rate of 13.3%.Citation9 This low response rate may be attributed to factors in the tumor microenvironment, such as lack of appropriate rejection antigens, deficient immune surveillance, or the presence of immunosuppressive mediators.Citation10 Given that most HNSCC tumors do not respond to PD-1/PD-L1 inhibition, it is necessary to investigate mechanisms by which poorly immunogenic tumors can be rendered susceptible to checkpoint blockade. RT has the potential to transform the tumor microenvironment, promoting inflammation leading to infiltration of T-cells in poorly immunogenic tumors and activating T-cells in tumors with pre-existing T-cell populations.Citation11 In particular, RT has been shown in different tumor models to increase tumor antigen presentation and stimulate T cell secretion of interferon gamma (IFNγ).Citation12-15 To date studies investigating mechanisms of RT-mediated immune modulation have been limited to flank xenograft animal models.Citation14,16,17 In head neck cancer, various components of the tumor microenvironment, including the oral mucosa play a critical role in shaping the immune landscape.Citation18 In this study, we hypothesized that combining RT with PD-L1 blockade in orthotopic HNSCC models can increase T-cell infiltration and enhance tumor cell kill. Our findings reveal a critical role for local tumor irradiation in promoting T-cell inflammation and mediating the response to immunotherapy.
Materials and methods
CIBERSORT and TCGA analysis
The HNSCC data set was downloaded from the cancer genome atlas (TCGA) and gene-expression profiles were sorted according to the average expression of CD8A, CD8B, IFNG, GRZMB, and PRF1. Patients on this spectrum were divided into quartiles, with the highest and lowest corresponding to highly T-cell inflamed and poorly T-cell inflamed, respectively. Patients in each group were assessed for overall survival (OS), disease-free survival (DFS) and expression of the MHC class I genes, HLA-A, B, C, E, F, and G. Significance in survival was assessed using the log-rank (Mantel-Cox) test. For analysis of leucocyte populations, a set of 547 genes previously validated to represent leucocyte gene signatures was filtered and input into the online analytical tool CIBERSORT (Cell type Identification By Estimating Relative Subsets Of known RNA Transcripts). The matrix was constructed according to instructions provided by the developers (https://cibersort.stanford.edu/).Citation19 The CIBERSORT LM22 matrix transforms gene-expression data into relative fractions of haematopoietic cells phenotypes, including CD8 T-cell populations. CIBERSORT implements Monte Carlo sampling to generate an empirical P-value for the deconvolution. Only cases with a p-value < 0.05, which indicated a reliable estimation of immune cell infiltration, were used for further survival analysis.
Cell lines and cell culture
Murine B4B8 and LY2 squamous cell carcinoma cells were obtained from the laboratory of Dr. Nadarajah Vigneswaran (UTHealth, Houston, TX). The LY2 cell line was isolated from lymph node metastases that developed in BALB/c mice after inoculation of PAM 212 squamous cell carcinoma cellsCitation20 whereas B4B8 is a murine SCC cell line derived from BALB/c oral keratinocytes treated with chemical carcinogen 4NQO.Citation21 Both cell lines are wildtype for TP53, KRAS, NRAS and EGFR. Cells were cultured in DMEM-F12 media (SigmaAldrich) at 37°C and 5% CO2.
Animal tumor model
Six-week-old female BALB/c mice were purchased from Charles River. For tumor cell inoculation, B4B8 and LY2 cells were grown to 75% confluence and harvested and resuspended in serum-free DMEM media. Cell suspensions were mixed with equal volumes of Matrigel (10 mg/mL, BD Biosciences, San Jose, CA) and injected sub-mucosally via the intraoral route into the buccal mucosa at a final concentration of 1 × 106/0.1 ml per animal. Forty mice per cell line were inoculated at a single site in the right buccal. Mice were randomized to receive IgG2b control (BioXcell, NH), anti PD-L1 (10mg/kg BioXcell, NH), RT or anti PD-L1+RT. Anti PD-L1 and IgG2b treatment was administered i.p. 3 d before RT and maintained twice per week for 3-weeks. The dose of RT was 10Gy delivered directly to the tumor. Treatment was started when average tumor size reached 50 mm3(5–6 d post inoculation). Tumor size was measured weekly with digital calipers and tumor volumes were estimated using the formula (V = A × B2/2 mm3), where A and B are the longer and shorter diameters of the tumor. Mice were also assessed by proton-density and T2-weighted magnetic resonance imaging (MRI) using a Bruker 4.7 Tesla PharmaScan to visualize and quantify tumor burden. Mice exhibiting signs of morbidity according to the guidelines set by the Institutional Animal Care and Use Committee (IACUC) were killed immediately. Primary tumors, regional lymph nodes, spleens and lungs were harvested upon sacrifice. For histopathological and immunohistochemistry (IHC) studies, tissues were fixed in 10% neutral buffered formalin, embedded in paraffin and cut into serial sections. All protocols for animal tumor models were approved by the IACUC of the University of Colorado Denver.
Irradiation
Irradiation was performed using the RS-2000 irradiator (Rad Source Technologies, GA) at 160 kVp, 10 mA with 0.3 mm Cu filter. For in vitro experiments, cells were plated in 6-well plates and irradiated with 0, 4, 8, or 25 Gy X-rays 24 hours after plating. For animal experiments, mice were shielded using a custom Pb 1cm shield and laid on the side. The right buccal was exposed and 10 Gy was delivered at a dose rate of 1.05 Gy/min. The irradiation field contained involved regional cervical lymph nodes as they are in close proximity to the tumor. Low-level neck nodes, mediastinal lymph nodes and contralateral neck nodes were excluded from the field.
Flow cytometry
For flow cytometric analysis of tumor tissue, tumors were digested into single-cell suspension as previously reported.Citation22 Briefly, tumors were finely cut and placed in HBSS solution containing 200U of Collagenase III (Worthington) for 60 minutes with gentle shaking every 15 minutes. After the incubation period, tumor pieces were passed through a 100um nylon mesh. The resulting cell suspension was centrifuged and re-suspended in red blood cell lysis buffer for 2 minutes. HBSS was added to inactivate RBC lysis buffer, cell suspensions were centrifuged, re-suspended and counted using an automated cell counter. Draining lymph nodes and spleens were also collected and processed into single-cell suspensions through mechanical separation. Trypan blue was used to determine cell viability. For flow cytometric analysis 1 × 106 live cells were plated in 24-well plates and cultured for 5 hours in the presence of monensin to prevent release of cytokines and PMA to stimulate cytokine production. After the incubation period, cells were plated in 96-plate wells and blocked with anti-CD16/32 antibody. For analysis of immune cells, the following conjugated antibodies were used: APC-eFluor780-CD8 (Clone 53–6.7, eBioscience), eFluor450-CD4 (Clone RM4–5, eBioscience) AlexaFluor700-CD45 (Clone 30-F11, eBioscience), DyLight350-CD3 (Clone 145–2C11, Novus), FITC-CD44 (Clone IM7, eBioscience), PE-PD-1 (Clone RMP1–30, eBioscience), PECyanine7-IFNγ (Clone XMG1.2, eBioscience). For analysis of surface markers on tumor cells, 1 × 106 cells were plated directly into 96-well plates. Cell surface staining on tumor cells was performed using conjugated antibodies: PE-H2Kd (Clone SF1–1.1.1, eBioscience), BV605-CD80 (Clone 16–10A1, BD Horizon), PerCP-eFluor710-PD-L1 (Clone MIH5, eBioscience) and CD45. For proper compensation of flow cytometry channels, beads and single-stain samples were used. For gating, isotype controls and fluorescence minus-one (FMO) controls were applied. Both mean fluorescence intensity (MFI) and proportion of positively stained cells were analyzed. Stained cells were run on the Yeti Cell Analyzer at the University of Colorado Denver Cancer Flow Cytometry Core. Data was analyzed using Kaluza Analysis software.
T-cell depletion
For depletion of T-cell populations, antibodies (BioXcell, NH) against CD4 (clone GK1.5), CD8 (clone 53–6.7) or both were administered i.p. twice per week starting at 1 week before tumor implantation at a concentration of 3 mg/kg. Control IgG2A and IgG2B antibodies were administered to the control group at the same concentration. Equivalent amounts of depletion antibodies were administered to all groups. T-cell depletion was confirmed on the day of tumor inoculation through flow cytometric analysis of peripheral blood.
Immunohistochemistry
Harvested tumor tissue was formalin-fixed and processed for paraffin embedding. For IHC, 7um thick sections were deparaffinized with xylene and rehydrated with increasing concentrations of ethanol. Heat-mediated antigen retrieval was performed using citrate buffer. Tissues were blocked with goat-serum for 1 hour and stained with CD3 (ThermoFisher, Rockford, IL) antibody overnight at 4°C.
ELISA assays
Conditioned media was collected from irradiated and non-irradiated LY2 and B4B8 cells. CXCL9 and CXCL10 levels were measured using the Invitrogen ELISA Kit (Invitrogen, Minneapolis, MN, USA) following manufacturer's instructions. The sensitivity of detection is reported at 3 pg/mL. Absorbance was measured at 450nm. The measured concentration in each sample was normalized to the number of cells counted at the time of harvesting.
Quantitative real-time PCR
Total RNA was purified from tumors using RNeasy mini prep kits (Qiagen), and aliquots (5 ug) were reverse transcribed in a volume of 20 uL using Maxima First Strand cDNA Synthesis Kit (Thermo Scientific). Aliquots (2 uL) of a 1:25 dilution of the reverse transcription reactions were submitted to quantitative real-time PCR (RT-PCR) in 10 uL reactions with SYBR Select Master Mix (Thermo Fisher Scientific) with rat GAPDH (Forward primer: 5′ CGTGGAGTCTACTGGCGTCTT 3′, Reverse primer: 5′ CGGAGATGATGACCCTTTTGG 3′), mouse CXCL10 (Forward primer: 5′ TCATTTTCTGCCTCATCCTGCT 3′, Reverse primer: 5′ CCGTCATCGATATGGATGCAGT 3′), mouse CXCL9 (Forward primer: 5′ CGTCGTCGTTCAAGGAAGACTA 3′, Reverse primer: 5′ CCAGGGAAGGCTTTTCAGTACA 3′), and mouse CD274/PD-L1 (Forward primer: 5′ AGCAAGTGATTCAGTTTGTGGC 3′, Reverse primer: 5′ CCTTCAAAAGCTGGTCCTTTGG 3′) using a My iQ real time-PCR detection system (BioRad). GAPDH mRNA levels were measured as a housekeeper gene for normalization of the different mRNA expression values.
Statistical analysis
Two-way analysis of variance (ANOVA) was performed to assess differences in the expression of markers across treatment groups. Student's T-test was used for analysis of flow cytometry data comparing irradiated and non-irradiated conditions. For survival analysis, Kaplan-Meir curves were analyzed based on the Log-rank (Mantel-Cox) test for comparison of all groups. Hazard ratios were generated between pairs of groups. For assessment of tumor growth differences, 2-way ANOVA was performed. Analysis of patient and tumor characteristics in TCGA was performed using 2-sided Fisher's exact test and the Chi-squared test. For the purposes of this study, classifications of alveolar ridge, buccal mucosa, lip, oral tongue and floor of mouth were re-classified to oral cavity. Tonsil was re-classified to oropharynx.
Results
Tumor CD8 T-cell infiltration correlates with patient outcome in HNSCC
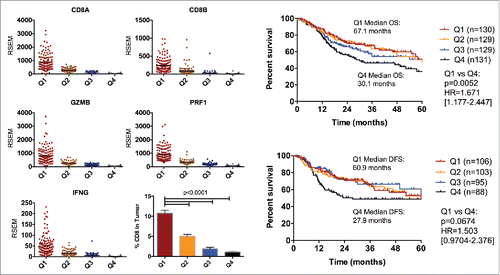
We stratified patients in the HNSCC TCGA database based on a CD8 T-cell genomic signature comprised of the genes: CD8A, CD8B, GRZMB, IFNG, PRF1. The average expression of these genes was determined for each patient. Patients in the upper and lower quartiles were selected for analysis (). The expression of CD8A and CD8B is predominantly specific to CD8 T-cells whereas the expression of GRZMB, IFNG and PRF1 is related to the cytotoxic function of the CD8 cells.Citation23,24 To further validate the significance of the genomic signature, we performed CIBERSORT analysis to determine immune populations within the patient tumors as described previously.Citation19 Tumors from patients in the upper quartile on average had 10 times more CD8+ T-cell infiltrates compared with patients in the lower quartile (10.6% vs 1.0% respectively) (). Bivariate analysis of patient and tumor characteristics showed that patients with poorly T-cell infiltrated tumors tended to be HPV negative (p < 0.0001), more likely to be black or African American (p = 0.0490) and were more likely to have advanced tumor stage (p = 0.0023), higher TP53 mutation rate (p < 0.0001) and EGFR amplification (p = 0.0005) (supplementary Table 1). We further analyzed patients in both groups for overall survival and disease-free survival. Patients with highly T-cell infiltrated tumors performed significantly better in terms of overall survival and disease-free survival (median 67.8 months and 60.9 months, respectively) compared to patients with poorly T-cell infiltrated tumors (median 30.1 months and 27.9 months, respectively) (). Our analysis provides evidence that there is a spectrum of tumor CD8 T cell infiltration in HNSCCs and that poorly T-cell infiltrated tumors correlated with worse outcomes.
Figure 1. Analysis of HNSCC tumors in TCGA based on CD8 T cell genomic signature. (A) Patients were selected based on the lower and upper quartile of mRNA expression of CD8, IFNG, PRF1 and GRZMB. Patients in the lower quartile were classified as poorly CD8 infiltrated and patients in the upper quartile were classified as highly CD8 infiltrated. (B) Analysis of the proportion of CD8 T-cells in tumors from each patient group based on CIBERSORT. (C) Analysis of overall survival and disease-free survival in HNSCC patients based on CD8 T-cell profile. Log-ranks hazard ratios are reported.
RT sensitizes anti PD-L1-resistant HNSCC orthotopic tumors
To model the clinical phenotype of poorly immunogenic tumors and assess the response to immune modulatory therapy, we developed a murine orthotopic model of HNSCC. B4B8 tumor cells were derived from normal oral kertatinocytes that were exposed to the carcinogen 4NQO and which lost CD80 expression when transformed to malignant cells.Citation21 LY2 tumor cells were derived from a nodal metastatic tumor from the squamous cell carcinoma cell line Pam212.Citation25 Both cell lines were confirmed to retain keratin and integrin markers of squamous epithelial origin including Desmoglein3 (DSG3), P-cadherin (CDH3) and Cytokeratin 14/15/16 by RNA sequencing (data not shown). On histological assessment, both tumors appeared poorly differentiated with scant cytoplasm, high mitotic index, and lacked keratin production (supplementary Figure 1). Treatment with anti-PD-L1 antibody or IgG was initiated when average tumor volume reached 50 mm3 (days 5–6 after inoculation). Both LY2 and B4B8 tumor-bearing mice were resistant to PD-L1 checkpoint blockade and demonstrated tumor growth and weight loss similar to mice in the IgG control group (). RT resulted in slightly retarded tumor growth in both models compared with either the IgG controls or anti-PD-L1 group, but this did not reach significance (). The combination of RT and PD-L1 inhibition, on the other hand, significantly enhanced the delay in tumor growth as revealed with caliper measurements and MRI and prevented clinical symptoms including weight loss ( and supplementary Figure 2). In LY2 mice treated with RT+anti PD-L1, average tumor volume on day 20 when all mice were still alive was 128.6 mm3 compared with 469.2 mmCitation3 in the IgG control group, 345.6mm3 in the PD-L1 group and 332.2 mmCitation3 in the RT group. In B4B8 mice, average tumor volume on day 36 in the RT+anti PD-L1 was 48.8mmCitation3 compared with 195.5mmCitation3 in the IgG control group, 172.1mmCitation3 in the PD-L1 group and 141.5mmCitation3 in the RT group (). Two mice in the LY2 group did not respond to RT+anti PD-L1 and 2 mice in the B4B8 group had complete tumor eradication (individual tumor growth curves are shown in supplementary Figure 3). Survival analysis demonstrated a statistically significant advantage for RT+anti PD-L1 therapy over either treatment alone (). In LY2 mice, median survival with RT+anti PD-L1 was 33.0 d compared with 25.50 d with RT, 23.0 d with anti PD-L1 and 22.50 d with IgG. In B4B8 mice, median survival with RT+anti PD-L1 was 93.0 d compared with 51.0 d with RT, 65.0 d with PD-L1 and 52.0 d with IgG. Taken together, these data demonstrate the ability of a single dose of RT to sensitize HNSCC tumors to PD-L1 blockade.
Figure 2. Response of LY2 and B4B8 tumors to treatment with anti PD-L1 monoclonal antibody alone and in combination with RT (single arm RT and IgG were used as control groups). (A) Schematic illustration of treatment schedule. (B) Tumor growth analysis of tumor-bearing mice. Mice received PD-L1 mAb or IgG on day 5 and RT of 10Gy on day 8. PD-L1 or IgG were delivered 2x/week until end of experiment. Statistical analysis was performed on day 20 for LY2 mice and day 36 for B4B8 mice using 2-way analysis of variance (p < 0.0001 in both groups). (C) Average weight of mice in each group (D) Survival analysis of tumor-bearing mice in each group. Hazard ratios were generated based on Log-ranks comparison of the RT+PD-L1 group with each of the other groups. (E) Assessment of tumor growth differences at the last time point when all mice were alive (day 20 for LY2 and day 36 for B4B8). Two-way ANOVA was performed to assess significance. (F) Analysis of tumor-infiltrating lymphocytes 2-weeks after treatment in LY2 mice. Quantification of CD3+ cells was performed by counting the number of positively staining cells per 40x power field. Bars represent SEM from 3–4 independent samples per group. At least 7 fields were quantified per sample. Two-way ANOVA was performed to assess significance.
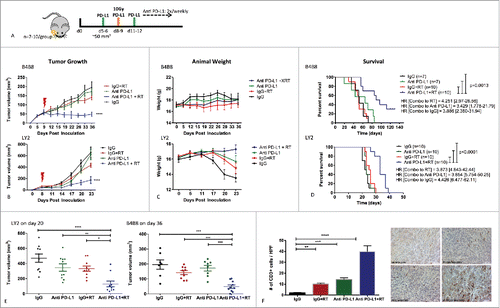
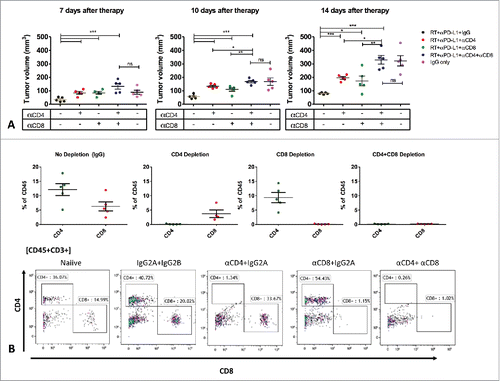
Figure 3. Deciphering the role of T-cell populations in mediating response to RT+anti PD-L1. Mice (n = 5/group) received T-cells depletion antibodies (CD4, CD8 or both) or IgG control 1-week before tumor inoculation. Mice were treated with RT+anti PD-L1 or IgG as described in the methods. Tumor volume was assessed to determine the effect of CD4 and CD8 T-cell depletion on tumor growth.
Efficacy of RT and anti PD-L1 in HNSCC tumors is T-cell dependent
To determine the impact of RT and PD-L1 blockade on the extent of T-cell infiltration, we quantified the number of tumor-infiltrating lymphocytes (TILs) 2 weeks after treatment initiation, using IHC. We observed a significant increase in T-cell infiltration in the combined RT and anti PD-L1 group compared with all other groups (18.9-fold increase relative to IgG, 3.8-fold increase relative to RT and 2.8-fold-increase relative to anti PD-L1) (). RT and anti PD-L1 increased TILs to a lesser extent compared with the IgG control group (4.9-fold and 6.8-fold increase relative to IgG respectively). To confirm the role of specific CD4 and CD8 T-cell populations in the response to RT+anti PD-L1, we inoculated LY2 tumors cells into mice that were depleted for CD4 T cells, CD8 T cells or both. T-cell depletion was confirmed by flow cytometry on blood samples from each mouse (). T-cell depletion efficiency was 98–99%. Our data shows complete abrogation of the efficacy of RT+anti PD-L1 only in mice with both CD4 and CD8 T-cell depletion (, supplementary Figure 4). The depletion of either population alone resulted in partial abrogation of RT+anti PD-L1 efficacy (). These results clearly demonstrate that the efficacy of RT+anti PD-L1 is dependent on both CD4 and CD8 T-cell mediated cytotoxicity.
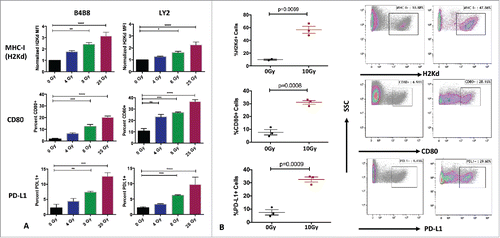
Figure 4. Effects of RT on tumor immunogenicity. (A) Flow cytometric analysis of expression of MHC-I, CD80 and PD-L1 on LY2 and B4B8 HNSCC tumor cells after exposure to increasing doses of RT. Two-way ANOVA was performed to compare statistical significance between the groups .(B) Flow cytometric analysis of the expression of MHC-I, CD80 and PD-L1 on LY2 tumors harvested 72 hours after RT or sham. AquaVi and CD45 staining was performed to gate for live and CD45 negative cells. Bars represent SEM from 3 independent tumor samples. P-values represent significance based on Unpaired T-test analysis.
RT increases tumor cell immunogenicity in vitro and in vivo
To examine the impact of RT on tumor immunogenicity, we irradiated B4B8 and LY2 cells with increasing doses of radiation (0, 4, 8, 25 Gy) in vitro and assessed surface expression of H2Kd (murine analog of HLA proteins). RT induced a dose-dependent increase in the mean fluorescence intensity (MFI) of H2Kd in both cell lines indicating increased antigen presentation on tumor cells (). The expression of H2Kd was significantly higher at doses of 8 Gy and 25 Gy, but not 4Gy in both cell lines. The proportion of tumor cells expressing H2Kd was unchanged. To further understand how RT alters accessory T cell interacting molecules in the cell lines, we assessed surface expression of PD-L1 and the co-activating marker CD80 following RT. At baseline, neither cell line expressed PD-L1 in vitro. However, after exposure to RT, the proportion of cells expression PD-L1 and CD80 increased in a dose dependent manner in both cell lines (). To validate these findings in vivo, we performed flow cytometry on LY2 tumors harvested 72 hours after exposure to 10 Gy and assessed expression of H2Kd, CD80 and PD-L1. Tumor cells were negatively gated based on CD45 expression. RT to the tumor induced a 2.9-fold increase in the proportion of tumor cells expressing H2Kd, 2.6-fold increase in proportion of CD80+ tumor cells and 2.1-fold increase in PD-L1+ tumor cells relative to sham-treated tumors (). We further confirmed the upregulation of PD-L1 by RT-PCR on tumors harvested 72 hours after exposure to 10Gy in comparison to sham-treated tumors (supplementary Figure 5). This data demonstrates that local tumor irradiation can increase tumor immunogenicity in HNSCC models.
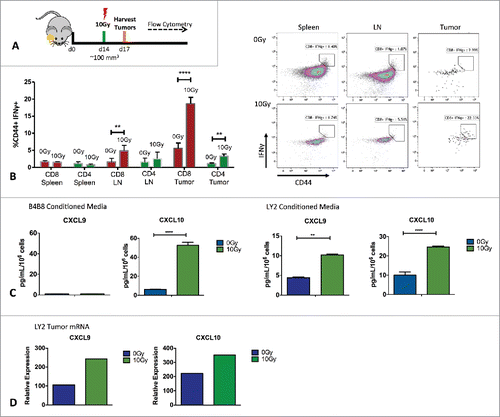
Figure 5. Analysis of T-cell IFNγ activity and in vivo and IFNγ inducible chemokines. (A) Schematic illustration of in vivo experimental setup. (B) Flow cytometric analysis of IFNγ production by activated (CD44+), CD8 and CD4 T-cells from harvested LY2 tumors, spleens and draining lymph nodes. Gating was performed on CD45+, CD3+, live, single cells. Gate assignment was based on FMO and isotype controls. Bars represent SEM from 3 independent experiments with at least 3–4 mice per experimental group. Unpaired T-test was used to assess significance between 0Gy and 10Gy groups. (C) Radiation-induced chemokine production based on ELISA analysis of CXCL9 and CXCL10 in conditioned media from tumor cells irradiated with 10Gy. Conditioned media was harvested 72 hours after irradiation. Unpaired t-test was used for analysis. (C) RT-PCR analysis of CXCL9 and CXCL10 levels in vivo from tumors harvested 72 hours after RT. Experiments were repeated 3 independent times. One representative experiment is shown.
Since MHC class I expression positively correlates with CD8 T-cell infiltration,Citation26 we analyzed RNAseq data from the TCGA patients we selected previously based on CD8 infiltrates. Patients with a highly inflamed T-cell phenotype exhibited significantly higher expression of all MHC-class I genes compared with patients with a poorly inflamed T-cell phenotype (supplementary Figure 6). These data underscore the relevance of MHC class I expression to tumor CD8 T-cell infiltration in HNSCC patients.
PD-L1 upregulation is mediated through T-cell secretion of interferon γ and interferon γ inducible chemokines
Since PD-L1 is an interferon responsive gene known to be induced by T-cell secretion of IFNγ,Citation27 we sought to investigate if RT to the tumor in our HNSCC model results in increased IFNγ production. Using flow cytometry, we analyzed T-cells in LY2 tumors, draining lymph nodes and spleens harvested 72 hours after local RT to the tumor (). Tumors irradiated with 10 Gy had significantly increased IFNγ+ CD8+ T cells compared with non-irradiated tumors (). A similar increase in IFNγ production in CD8+ T cells was also observed in draining lymph nodes of mice treated with 10Gy compared with non-irradiated tumors. The proportion of IFNγ+ CD8+ T cells was unchanged in spleens from both groups. We also observed a significant increase in the proportion of IFNγ+ CD4+ T cells in tumors from irradiated mice (). We further analyzed whether IFNγ inducible chemokines, CXCL9 and CXCL10 are increased with RT. We assessed levels of CXCL9 and CXCL10 in vitro to determine whether these chemokines are produced by LY2 and B4B8 tumor cells directly. Our ELISA analysis of conditioned media from irradiated LY2 and B4B8 tumor cells showed a significant increase in CXCL10 in both cell lines (). In addition, LY2 but not B4B8 cells also upregulated CXCL9 in response to 10Gy RT (). We further assessed the levels of CXCL9 and CXCL10 in vivo at the tumor site by RT-PCR. LY2 tumors irradiated with 10Gy had significantly higher levels of both CXCL9 and CXCL10 compared with non-irradiated tumors (). This data provides evidence that RT to the tumor can increase the production of IFNγ as well as IFNγ inducible chemokines, which can directly impact the upregulation of PD-L1 on tumor cells. Our data provide a possible mechanism by which non-inflamed tumors which are resistant to PD-L1 checkpoint blockade can be transformed to highly inflamed tumors sensitive to anti PD-L1 therapy.
Discussion
Immune-modulating therapy for cancer patients has emerged as a promising strategy for tumor control. However, clinical trials with immune checkpoint inhibitors have thus far only yielded a 15–20% response rate.Citation28 In this study, LY2 and B4B8 murine HNSCC cells are representative of the ∼80% of patients that will not respond to monotherapy with immune checkpoint inhibitors. Indicators of positive response to immune therapy include the presence of tumor-infiltrating lymphocytes, expression of PD-L1 on tumor cells and high mutation burden.Citation3,29,30 Mechanisms that can induce such changes hold great potential for increasing the response to immunotherapy.Citation31 Data generated with checkpoint inhibitors and radiation have thus far been limited to studies in flank xenograft models. Here, we used a novel orthotopic model of HNSCC to study mechanisms of synergy between RT and PD-L1 blockade. An important aspect of our work is the clinical resemblance of the murine model to HPV-negative, smoking driven HNSCC, which have poor survival outcomes, harbor minimal TILs and minimal PD-L1 expression and thus less likely to respond to single-modality immune therapies.Citation7,8,32-36
The application of RT as a strategy to modulate tumor immune response represents an important paradigm shift in the field of radiation oncology.Citation37,38 The standard of care for HNSCC patients includes conventional RT comprised of 66–74Gy delivered in daily 2Gy fractions.Citation39 However, when combining RT with immunomodulatory therapy, the biologic determinant of the response to RT can be different than when RT is used as a sole modality. Several studies have shown that conventionally fractionated fractionated radiation results in a significant and extended loss of lymphocytes while treatment with hypofractionated radiation therapy avoided the loss of lymphocytes associated with conventional fractionation.Citation40-46 In melanoma cells, Lugade et al. compared a single dose of 15Gy to 5 fractions of 3Gy and found that single fraction RT resulted in increased antigen presentation compared with the fractionated group.Citation47 Similarly, Lee et al. showed an increase in T cell infiltration with a single dose of 20Gy that was significantly higher compared with tumors irradiated with 20Gy delivered in 4 fractions.Citation14 In both cases RT was delivered to flank tumors. Our choice of RT dose for HNSCC tumors (10Gy) was guided by our in vitro studies showing a dose-dependent increase in tumor antigen presentation as well as increased secretion of CXCL9 and CXCL10. In addition, the orthotopic nature of our model, limits the amount of dose that can be delivered to the buccal region while minimizing toxicity to normal tissue. This is of particular relevance in HNSCC patients where normal tissue toxicity plays a major role in patient outcomes and patient morbidity.Citation48 Our results demonstrate that a single RT dose of 10Gy can sensitize HNSCC tumors to anti PD-L1 therapy.
Tumor immunogenicity has been reported as an important indicator of response to PD-1/PD-L1 blockade.Citation49 Increasingly, patients receiving therapy targeted against the PD-1/PD-L1 axis are selected based on levels of PD-L1 expression.Citation50 In both of our genetically distinct HNSCC mouse models, we observed minimal expression of PD-L1 at baseline by flow cytometry. This observation correlated well with the lack of clinical response to PD-L1 blockade in our HNSCC models. However, 72-hours after local irradiation, we observed a robust increase in the proportion of tumor cells expressing PD-L1. This is consistent with a previous study in murine bladder cancer demonstrating increased PD-L1 72 hours after RT which was gradually eliminated by day 7.Citation51 The transient nature of PD-L1 expression during stages of tumor progression and exposure to treatments such as RT suggests that it may not be a practical indicator of patient response. In our model, we observed a significant and durable increase in TILs when RT is combined with PD-L1 inhibition up to 2-weeks after treatment. These data suggest that assessment of TILs maybe a better indicator of response to PD-1/PD-L1 inhibition due to the transient nature of PD-L1 expression.
The synergistic lethality of combining anti PD-1/PD-L1 therapy with RT has been demonstrated in pre-clinical models of lung cancer,Citation16 triple negative breast cancer,Citation52,53 gliomaCitation54 and melanoma.Citation47 However, the precise mechanism by which RT synergizes with immunotherapy remains elusive. A recent study demonstrated that RT increases diversity of the T cell receptor (TCR) repertoire in TILs from melanoma and mammary flank tumors.Citation12 However, the cause and consequence of TCR changes remains to be defined. Previous studies have primarily demonstrated a role for CD8 T-cells in mediating tumor cytotoxicity.Citation14,55-57 In a murine model of lymphoma, Dovedi et al, investigated the effect of RT (single dose of 10Gy) when combined with a CD40 antagonist on tumor growth and survival. The study showed remarkable inhibition of tumor growth and prolonged survival which was completely abrogated with CD8, but not CD4 T-cell depletion.Citation55 Our data in orthotopic HNSCC murine models reveal an important role for RT in promoting secretion of IFNγ by CD4 and CD8 T-cells. The production of IFNγ by lymphocytes in the tumor microenvironment has been shown to induce expression of PD-L1 and MHC class-I on tumor cellsCitation58,59 thus providing a mechanism by which poorly inflamed tumors can be transformed to highly inflamed tumors. In our HNSCC model, CD4 and CD8 T-cells are indispensable for increasing tumor immunogenicity after RT and sensitizing tumors to RT+anti PD-L1 therapy.
In conclusion, our study provides evidence that RT can sensitize HNSCC tumors resistant to PD-L1 immune checkpoint blockade. These findings have direct clinical implications for enhancing the therapeutic efficacy of immunotherapy in HNSCC patients.
Disclosure of potential conflicts of interest
No potential conflicts of interest exist.
Supplementary_materials.zip
Download Zip (1.9 MB)References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74-108. doi:10.3322/canjclin.55.2.74. PMID:15761078
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137-50. doi:10.1200/JCO.2005.05.2308. PMID:16682732
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064-74. doi:10.1158/1078-0432.CCR-13-3271. PMID:24714771
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020-30. doi:10.1200/JCO.2013.53.0105. PMID:24590637
- Li H, Chiappinelli KB, Guzzetta AA, Easwaran H, Yen RW, Vatapalli R, Topper MJ, Luo J, Connolly RM, Azad NS, et al. Immune regulation by low doses of the DNA methyltransferase inhibitor 5-azacitidine in common human epithelial cancers. Oncotarget. 2014;5(3):587-98. doi:10.18632/oncotarget.1782. PMID:24583822
- Swanson MS, Sinha UK. Rationale for combined blockade of PD-1 and CTLA-4 in advanced head and neck squamous cell cancer-review of current data. Oral Oncol. 2015;51(1):12-15. doi:10.1016/j.oraloncology.2014.10.010. PMID:25459157
- Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, Freeman GJ, Ferris RL. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNgamma that induce PD-L1 expression in head and neck cancer. Cancer Res. 2016;76(5):1031-43. doi:10.1158/0008-5472.CAN-15-2001
- Oguejiofor K, Hall J, Slater C, Betts G, Hall G, Slevin N, Dovedi S, Stern PL, West CM. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br J Cancer. 2015;113(6):886-93. doi:10.1038/bjc.2015.277. PMID:26313665
- Ferris R, Blumenschein G, Fayette J, Guigay J, Colevas A, Licitra L, Harrington K, Kasper S, Vokes E, Even C. Further evaluations of nivolumab (nivo) versus investigator's choice (IC) chemotherapy for recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): CheckMate 141. J Clin Oncol. 2016;34(suppl; abstr 6009).
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014-22. doi:10.1038/ni.2703. PMID:24048123
- Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: A beneficial liaison? Nat Rev Clin Oncol. 2017;14(6):365-79. doi:10.1038/nrclinonc.2016.211. PMID:28094262.
- Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373-7. doi:10.1038/nature14292. PMID:25754329
- Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687-95. doi:10.1172/JCI67313. PMID:24382348
- Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood. 2009;114(3):589-95. doi:10.1182/blood-2009-02-206870. PMID:19349616
- Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. doi:10.4161/onci.28518. PMID:25071979
- Wang X, Schoenhals JE, Li A, Valdecanas DR, Ye H, Zang F, Tang C, Tang M, Liu CG, Liu X, et al. Suppression of type I IFN signaling in tumors mediates resistance to anti-PD-1 treatment that can be overcome by radiotherapy. Cancer Res. 2017;77(4):839-50. doi:10.1158/0008-5472.CAN-15-3142. PMID:27821490
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458-68. doi:10.1158/0008-5472.CAN-14-1258. PMID:25274032
- Curry JM, Sprandio J, Cognetti D, Luginbuhl A, Bar-ad V, Pribitkin E, Tuluc M. Tumor microenvironment in head and neck squamous cell carcinoma. Semin Oncol. 2014;41(2):217-34. doi:10.1053/j.seminoncol.2014.03.003. PMID:24787294
- Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453-7. doi:10.1038/nmeth.3337. PMID:25822800
- Chen Z, Smith CW, Kiel D, Van Waes C. Metastatic variants derived following in vivo tumor progression of an in vitro transformed squamous cell carcinoma line acquire a differential growth advantage requiring tumor-host interaction. Clin Exp Metastasis. 1997;15(5):527-37. doi:10.1023/A:1018474910432. PMID:9247255
- Thomas GR, Chen Z, Oechsli MN, Hendler FJ, Van Waes C. Decreased expression of CD80 is a marker for increased tumorigenicity in a new murine model of oral squamous-cell carcinoma. Int J Cancer. 1999;82(3):377-84. doi:10.1002/(SICI)1097-0215(19990730)82:3%3c377::AID-IJC11%3e3.0.CO;2-9. PMID:10399955
- Petit V, Massonnet G, Maciorowski Z, Touhami J, Thuleau A, Nemati F, Laval J, Chateau-Joubert S, Servely JL, Vallerand D, et al. Optimization of tumor xenograft dissociation for the profiling of cell surface markers and nutrient transporters. Lab Invest. 2013;93(5):611-21. doi:10.1038/labinvest.2013.44. PMID:23459372
- Clark WR, Walsh CM, Glass AA, Huang MT, Ahmed R, Matloubian M. Cell-mediated cytotoxicity in perforin-less mice. Int Rev Immunol. 1995;13(1):1-14. doi:10.3109/08830189509061734. PMID:7494105
- Tishon A, Lewicki H, Rall G, Von Herrath M, Oldstone MB. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology. 1995;212(1):244-50. doi:10.1006/viro.1995.1477. PMID:7676639
- Dong G, Loukinova E, Chen Z, Gangi L, Chanturita TI, Liu ET, Van Waes C. Molecular profiling of transformed and metastatic murine squamous carcinoma cells by differential display and cDNA microarray reveals altered expression of multiple genes related to growth, apoptosis, angiogenesis, and the NF-kappaB signal pathway. Cancer Res. 2001;61(12):4797-808. doi:10.1158/0008-5472.CAN-04-4621. PMID:11406555
- Al-Batran SE, Rafiyan MR, Atmaca A, Neumann A, Karbach J, Bender A, Weidmann E, Altmannsberger HM, Knuth A, Jager E. Intratumoral T-cell infiltrates and MHC class I expression in patients with stage IV melanoma. Cancer Res. 2005;65(9):3937-41. doi:10.1158/0008-5472.CAN-04-4621. PMID:15867394.
- Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, Oh S, Shin JG, Yao S, Chen L, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett. 2006;580(3):755-62. doi:10.1016/j.febslet.2005.12.093. PMID:16413538
- Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974-82. doi:10.1200/JCO.2014.59.4358. PMID:25605845
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189-99. doi:10.1056/NEJMoa1406498. PMID:25409260
- Ribas A, Hu-Lieskovan S. What does PD-L1 positive or negative mean? J Exp Med. 2016;213(13):2835-40. doi:10.1084/jem.20161462. PMID:27903604
- Gajewski TF. The next hurdle in cancer immunotherapy: Overcoming the non-T-cell-inflamed tumor microenvironment. Semin Oncol. 2015;42(4):663-71. doi:10.1053/j.seminoncol.2015.05.011. PMID:26320069
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563-7. doi:10.1038/nature14011. PMID:25428504
- Oguejiofor K, Galletta-Williams H, Dovedi SJ, Roberts DL, Stern PL, West CM. Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV- oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget. 2017;8(9):14416-27. doi:10.18632/oncotarget.14796. PMID:28122336.
- Balermpas P, Rodel F, Rodel C, Krause M, Linge A, Lohaus F, Baumann M, Tinhofer I, Budach V, Gkika E, et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG). Int J Cancer. 2016;138(1):171-81. doi:10.1002/ijc.29683. PMID:26178914
- Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73(6):1733-41. doi:10.1158/0008-5472.CAN-12-2384
- Mandal R, Senbabaoglu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, Lee KW, Ganly I, Hakimi AA, Chan TA, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1(17):e89829. doi:10.1172/jci.insight.89829. PMID:27777979
- Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR. The tipping point for combination therapy: Cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin Oncol. 2012;39(3):323-39. doi:10.1053/j.seminoncol.2012.02.006. PMID:22595055
- Derer A, Frey B, Fietkau R, Gaipl US. Immune-modulating properties of ionizing radiation: Rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol Immunother. 2016;65:779-86. doi:10.1007/s00262-015-1771-8. PMID:26590829.
- Pfister DG, Spencer S, Brizel DM, Burtness B, Busse PM, Caudell JJ, Cmelak AJ, Colevas AD, Dunphy F, Eisele DW, et al. Head and neck cancers, version 2. 2014. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2014;12(10):1454-87. doi:10.6004/jnccn.2014.0142. PMID:25313184
- Crocenzi T, Cottam B, Newell P, Wolf RF, Hansen PD, Hammill C, Solhjem MC, To YY, Greathouse A, Tormoen G, et al. A hypofractionated radiation regimen avoids the lymphopenia associated with neoadjuvant chemoradiation therapy of borderline resectable and locally advanced pancreatic adenocarcinoma. J Immunother Cancer. 2016;4:45. doi:10.1186/s40425-016-0149-6. PMID:27532020
- Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, Campian J, Laheru DA, Zheng L, Wolfgang CL, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2015;38(3):259-65. doi:10.1097/COC.0b013e3182940ff9. PMID:23648440
- Stewart CC, Perez CA. Effect of irradiation on immune responses. Radiology. 1976;118(1):201-10. doi:10.1148/118.1.201. PMID:1105662
- Savage AM, Pritchard JA, Deeley TJ, Davies BH. Immunological state of patients with carcinoma of the bronchus before and after radiotherapy. Thorax. 1980;35(7):500-5. doi:10.1136/thx.35.7.500. PMID:7434311
- Hoppe RT, Fuks ZY, Strober S, Kaplan HS. The long term effects of radiation of T and B lymphocytes in the peripheral blood after regional irradiation. Cancer. 1977;40(5):2071-8. doi:10.1002/1097-0142(197711)40:5%3c2071::AID-CNCR2820400513%3e3.0.CO;2-V. PMID:144554
- Grossman SA, Ellsworth S, Campian J, Wild AT, Herman JM, Laheru D, Brock M, Balmanoukian A, Ye X. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw. 2015;13(10):1225-31. doi:10.3109/07357907.2012.762780. PMID:26483062
- Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: Modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31(2):140-4. doi:10.3109/07357907.2012.762780. PMID:23362951
- Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516-23. doi:10.4049/jimmunol.174.12.7516. PMID:15944250
- Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, Forastiere A, Ang KK. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol. 2008;26(21):3582-9. doi:10.1200/JCO.2007.14.8841. PMID:18559875
- Lechner MG, Karimi SS, Barry-Holson K, Angell TE, Murphy KA, Church CH, Ohlfest JR, Hu P, Epstein AL. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J Immunother. 2013;36(9):477-89. doi:10.1097/01.cji.0000436722.46675.4a. PMID:24145359
- Ma W, Gilligan BM, Yuan J, Li T. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol. 2016;9(1):47. doi:10.1186/s13045-016-0277-y. PMID:27234522
- Wu CT, Chen WC, Chang YH, Lin WY, Chen MF. The role of PD-L1 in the radiation response and clinical outcome for bladder cancer. Sci Rep. 2016;6:19740. doi:10.1038/srep19740. PMID:26804478
- Verbrugge I, Hagekyriakou J, Sharp LL, Galli M, West A, McLaughlin NM, Duret H, Yagita H, Johnstone RW, Smyth MJ, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72(13):3163-74. doi:10.1158/0008-5472.CAN-12-0210. PMID:22570253
- Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2 Pt 1):728-34. doi:10.1016/j.ijrobp.2012.12.025. PMID:15701862
- Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343-9. doi:10.1016/j.ijrobp.2012.12.025. PMID:23462419
- Dovedi SJ, Lipowska-Bhalla G, Beers SA, Cheadle EJ, Mu L, Glennie MJ, Illidge TM, Honeychurch J. Antitumor efficacy of radiation plus immunotherapy depends upon dendritic cell activation of effector CD8+ T cells. Cancer Immunol Res. 2016;4(7):621-30. doi:10.1158/2326-6066.CIR-15-0253. PMID:27241845.
- Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208(10):2005-16. doi:10.1084/jem.20101159. PMID:21930765
- Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, Schwendener R, Pruschy M, Knuth A, van den Broek M. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189(2):558-66. doi:10.4049/jimmunol.1200563. PMID:22685313
- Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112(9):1501-9. doi:10.1038/bjc.2015.101. PMID:25867264
- Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180(5):3132-9. doi:10.4049/jimmunol.180.5.3132. PMID:18292536
