ABSTRACT
Despite aggressive treatment regimens based on surgery and radiochemotherapy, the prognosis of patients with grade IV glioblastoma multiforme (GBM) remains extremely poor, calling for alternative options such as immunotherapy. Immunological mechanisms including the Natural Killer Group 2 member D (NKG2D) receptor-ligand system play an important role in tumor immune surveillance and targeting the NKG2D system might be beneficial. However, before considering any kind of immunotherapy, a precise characterization of the immune system is important, particularly in GBM patients where conventional therapies with impact on the immune system are frequently co-administered. Here we performed an in-depth immunophenotyping of GBM patients and age-matched healthy controls and analyzed NKG2D ligand expression on primary GBM cells ex vivo. We report that GBM patients have a compromised innate immune system irrespective of steroid (dexamethasone) medication. However, dexamethasone drastically reduced the number of immune cells in the blood of GBM patients. Moreover, higher counts of immune cells influenced by dexamethasone like CD45+ lymphocytes and non-Vδ2 γδ T cells were associated with better overall survival. Higher levels of NKG2D ligands on primary GBM tumor cells were observed in patients who received radiochemotherapy, pointing towards increased immunogenic potential of GBM cells following standard radiochemotherapy. This study sheds light on how steroids and radiochemotherapy affect immune cell parameters of GBM patients, a pre-requisite for the development of new therapeutic strategies targeting the immune system in these patients.
Abbreviations
| Dex | = | dexamethasone |
| FCM | = | flow cytometry |
| GBM | = | glioblastoma multiforme |
| HCs | = | healthy controls |
| MICA/B | = | MHC class I related chain A/B |
| NKG2D | = | natural killer group 2 member D receptor |
| pAg | = | phosphoantigen |
| RCT | = | radiochemotherapy |
| SDF-1α | = | stromal cell-derived factor 1α |
| sNKG2DL | = | soluble NKG2D ligand(s) |
| TCR | = | T-cell receptor |
| TIL | = | tumor-infiltrating lymphocyte(s) |
| TMZ | = | temozolomide |
| ULBP | = | UL16-binding protein |
Introduction
Grade IV glioblastoma multiforme (GBM) is the most malignant primary brain tumor in adults. Despite aggressive treatment strategies such as surgery and radiochemotherapy (RCT),Citation1,Citation2 the median overall survival is only between 12–15 months. It was believed that brain tumors are not accessible to the immune system, due to the blood brain barrier, a view which has been recently challenged. Although immunosuppression is a hallmark of GBM, targeting and activating distinct immune cell subsets via immunotherapeutic approaches may be beneficial for GBM patient survival.Citation3 Recent advances in the field of immunotherapy led to successful clinical application of immune checkpoint inhibitors targeting the Programmed cell Death protein 1 (PD-1) and its ligand PD-L1 or the Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4).Citation4-6 Furthermore, adoptive immune cell transfer has been explored in various solid or hematological malignancies.Citation7 These results encouraged multiple clinical trials to address the efficacy of immunotherapy in various tumor entities including GBM.Citation8 γδ T cells, a minor subset (3–5%) of peripheral blood T cells, recognize tumor-derived phosphoantigens (pAg) and kill GBM cells without MHC involvement.Citation9 Interestingly, the endogenous production of pAg can be stimulated by nitrogen-containing bisphosphonates such as zoledronic acid which induces potent γδ T cell activation.Citation10,Citation11 Adoptive cell therapy with in vitro expanded γδ T cells expressing the Vγ9Vδ2 TCR was well tolerated and revealed promising effects in some cancer patientsCitation12 and in GBM model systems.Citation13,Citation14 Other γδ T cell subsets (“non-Vδ2”), which usually express the Vδ1 T-cell receptor (TCR), contribute to the immune surveillance of malignant and virally infected cells, for instance in the case of cytomegalovirus (CMV) infection, which is frequently associated with GBM development.Citation15-17 Apart from conventional αβ T cells and γδ T cells, Natural Killer (NK) cells may also contribute to immune defense against GBM. NK cells recognize and kill GBM cells which overexpress transformation-induced ligands for activating NK receptors. Thus, MHC-class I-related molecules A and B (MICA, MICB) and 6 members of UL16-binding protein family (ULBP1–6) are recognized by Natural Killer Group 2 member D (NKG2D) receptor.Citation18 While present on stressed and malignant tissues, the ligands for NKG2D receptor (NKG2DLs) are generally absent on healthy cells, so that the immune system can distinguish cancer cells from normal tissue. Ligand binding to NKG2D triggers cytotoxic effector activity and hence, the NKG2D system plays an important role in GBM immune surveillance. However, tumor cells including GBM cells release NKG2DLs in soluble form (sNKG2DLs) via different pathways.Citation19,Citation20 Elevated serum levels of sNKG2DLs have been considered as a tumor escape mechanism and are associated with poor prognosis in various tumor entities.Citation21
Standard GBM care includes tumor resection followed by radiotherapy (60 Gy) and adjuvant chemotherapy with temozolomide (TMZ), a DNA methylating agent inducing genotoxic stress and apoptosis of tumor cells.Citation1 Radiochemotherapy has a profound effect on the immune system, mainly affecting CD4 T cell counts in peripheral blood cells but simultaneously enhancing the immunogenicity of GBM cells via induction of genotoxic stress.Citation22 In addition, dexamethasone (Dex) is also frequently used to reduce clinically relevant brain edema typically surrounding the GBM thus ameliorating neurologic symptoms of GBM patients.Citation23 Dex effectively reduces intracranial edema but has multiple adverse effects and strongly influences immune cell countsCitation24 and cytotoxic activity of T cells.Citation25 Therefore, a precise understanding of the immune status before administration of immunotherapeutic regimens is crucially important, especially in the case of GBM where patients routinely receive RCT and Dex. The aim of our study was an in-depth analysis of the immune status in GBM patients with a special focus on the effects of RTC and Dex. Our results clearly demonstrate that the alteration of immune cell parameters in GBM patients is mainly due to the steroid medication. However, we also found that tumor cells of untreated patients expressed low levels of NKG2DLs, whereas higher expression was detected in tumor cells of GBM patients with recurrent disease who had already been treated with RCT. We discuss the translational aspects of our results with regard to their prognostic relevance for GBM patients.
Results
Impact of steroid treatment on peripheral blood immune cells in GBM patients
Blood samples of GBM patients collected before tumor resection (n = 35) and of healthy controls (HCs, n = 22) were analyzed by 11- and 3-color-based flow cytometry (FCM) for distinct lymphoid and myeloid populations (gating strategy shown in Suppl. Fig. 1). To determine absolute cell numbers, samples were analyzed in parallel using the BD Multitest™ 6-color TBNK reagent with BD Truecount™ tubes (Suppl. Fig. 2A). Based on CD3+ T cell numbers, the cell counts of each defined population were quantified. In addition, αβ T cells and Vδ2/non-Vδ2 subsets of γδ T cells were quantified by staining of whole blood in BD TrueCount™ tubes as describedCitation26 (Suppl. Fig. 2B). 22 patients received dexamethasone treatment before surgery (see Suppl. Table 1 for clinical details of patients). To address the effects of steroids on the immune cell distribution, we separated the patients in 2 subgroups, GBM patients who did not receive steroid treatment (GBM, n = 13) and GBM patients treated with Dex (GBM-Dex, n = 22). Immune cell populations of GBM patients were compared with sex- and age-matched HCs (n = 22). For comparison of results between the steroid-untreated patients and HCs, 14 sex- and age-matched HCs were selected (HC-I) out of the total group of 22 HCs. The results obtained from patients in the GBM-Dex group were compared with the data of all 22 HCs included in the study (see for details). Thus, HC-I represents a subgroup of HC. We analyzed the relative distribution (%) of each cell population and also the absolute cell numbers per µL of blood (cells/µL). Since treatment strategies such as Dex or RCT differentially affected distinct immune cell populations, we chose to use the absolute cell count data for statistical analysis. When characterizing the distinct subsets of various cell populations, the relative distribution (%) of those subsets within the population was also analyzed. As expected, total lymphocytes (SSClowCD45+) and most lymphocyte populations were negatively influenced by steroid treatment with the exception of CD19+ B cells (). The absolute numbers of αβ T cells, CD3+CD8+ and CD3+CD4+ T cells were significantly lower in GBM-Dex patients. The distribution of Treg cells, defined as % of CD25highCD127− cells among CD3+CD4+ T cells based on gating strategy shown in Suppl. Fig. 1 A (gate A1.5.1) was not significantly different between GBM and HC groups (not shown). However, the absolute number of Treg cells was significantly lower in GBM-Dex compared with HCs and steroid-untreated GBM patients ().
Table 1. Short description of the study populations.
Figure 1. Immune cell subsets in the peripheral blood of GBM patients. Immune cell populations (cells/uL) were determined by FCM in GBM patients, treated with steroids (GBM-Dex, n = 22) or not (GBM, n = 13), and in age-matched HCs groups, all HC (n = 22) and HC-I (n = 14), respectively. Data between groups GBM/GBM-Dex, GBM/HC-I and GBM-Dex/HC were compared for immune cell subsets as indicated: (A) Lymphocytes (CD45+ cells), B cells (CD19+ cells), αβ T cells, CD3+CD8+ T cells, CD3+CD4+ T cells, and Tregs, defined as CD127−CD25high CD3+CD4+ T cells. (B) Neutrophils (CD66b+ cells), monocytes (CD14+ cells). (C) The data obtained from GBM-Dex patients before surgery (BT, before therapy) was compared with the data from a later time point (after 2–5 months; AT, after therapy). Results shown for CD3+CD8+ cells (left panel), and neutrophils (right panel). The median values in (A) and (B) are compared by 2-tailed Wilcoxon's rank-sum test. Statistical significance is displayed as *** for p < 0.001, ** for p < 0.01 and * for p < 0.05.
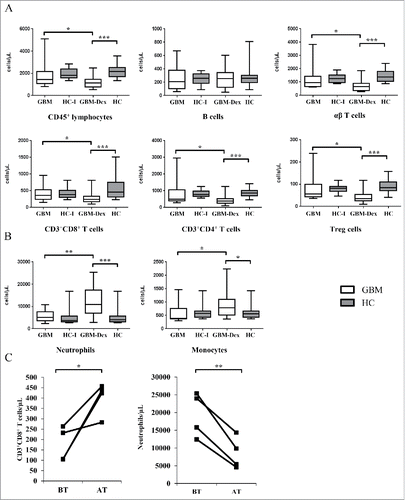
In contrast to the lymphoid compartment, steroid treatment increased the number of cells of myeloid origin. CD66b+ neutrophils (see Suppl. Fig. 1B, gate B2) were drastically increased in the GBM-Dex group (), in line with studies documenting the anti-apoptoticCitation27 and mobilizing effects of Dex on granulocytes.Citation28,Citation29 CD14+ monocytes were also increased in steroid-treated GBM patients ().
To confirm the steroid-mediated effects on immune cell subsets, we analyzed the data of 4 selected patients who had received high doses of steroids before surgery (before treatment, BT). These patients were immunphenotyped again 2–5 months after surgery (after treatment, AT), i.e. at a time point when the patients should have recovered from steroid-induced effects on immune cells. As expected, immune cell populations which were negatively influenced by steroid intake before the treatment were normalized again at later time points, shown for CD3+CD8+ T cells in (left). In contrast, the numbers of neutrophils (, right) and monocytes (not shown, p = 0.08), which were elevated in steroid-treated patients before the treatment, had decreased at the later time point.
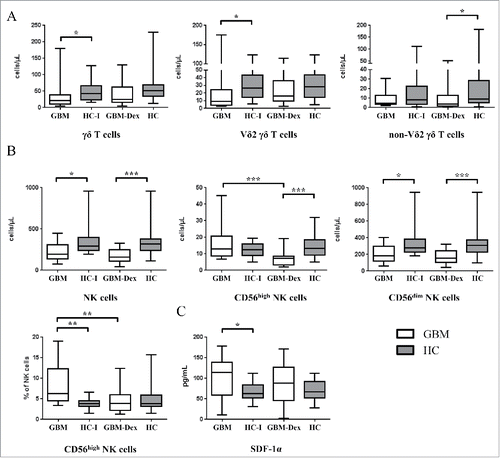
γδ T cells and NK cells, important innate-like anti-tumor effector cells, were both affected in GBM patients irrespective of Dex treatment (). In line with recent reports documenting low levels of circulating Vδ2 γδ T cells in GBM patients compared with HCs,Citation30 we also observed that the reduced γδ T cell numbers in the steroid-untreated GBM group were mainly due to low Vδ2 γδ T cell counts (, left and middle panel). In the GBM-Dex group however, non-Vδ2 γδ T cells (comprising mainly Vδ1) where reduced when compared with HC (, right panel). In line, absolute counts of Vδ1 γδ T cells were significantly lower in the GBM-Dex group compared with HCs (p = 0.025; not shown). NK cells were reduced in both GBM groups compared with HCs (). More specifically, we analyzed CD56high and CD56dim NK cell subsets, which have been identified as cytokine-producing versus cytotoxic effector NK cells, respectively.Citation31,Citation32 As illustrated in (upper row, middle panel), CD56high NK cells were selectively influenced by steroid treatment; hence the proportion of CD56high among total NK cells was higher in the steroid-untreated patient group (, lower row, left panel). We have also analyzed 14 selected cytokines and chemokines in the sera of the patients and HCs (see Materials and Methods). With the exception of stromal cell-derived factor 1α (SDF-1α) and macrophage migration inhibitory factor (MIF), these proteins were detected at very low levels in the sera of GBM patients and HCs. In GBM patients the serum levels of SDF-1α were higher compared with HCs reaching statistical significance in the non-steroid GBM group ().
Figure 2. γδ T cells and NK cells in the peripheral blood of GBM patients. (A) Total γδ T cells (left panel) and γδ T cell subsets (Vδ2, middle panel; non-Vδ2, right panel) and (B) total NK cells (left panel) and NK cell-subsets (CD56high, middle panel; CD56dim, right panel) were analyzed in GBM patients treated with steroids (GBM-Dex, n = 22) or not (GBM, n = 13) and in age matched HCs groups, all HCs (n = 22) and HC-I (n = 14). Median values of numbers were compared between the GBM and GBM-Dex groups, GBM and HC-I groups and GBM-Dex and HC groups. (C) Serum levels of SDF-1α in GBM patients and HCs. Significance was analyzed by 2-tailed Wilcoxon's rank-sum test. Statistical significance is displayed as *** for p < 0.001, ** for p < 0.01 and * for p < 0.05.
To exclude the influence of RCT and the recurrence of disease, we compared the data for all immunological parameters within GBM (n = 13) and GBM-Dex (n = 22) patient groups with respect to primary and recurrent GBM (Suppl. Table 2, primary vs. recurrent for GBM group: 7 vs. 6 patients; for GBM-Dex group: 11 vs. 11 patients). In both groups the CD4/CD8 ratio differed significantly between primary and recurrent disease. Patients with primary GBM in the GBM-Dex group had higher neutrophil counts compared with patients with recurrent disease in this group. Interestingly, in patients who did not receive steroids before surgery, non-Vδ2 γδ T cell numbers were lower in the primary GBM group compared with patients with recurrent disease. Serum levels of IL-18 and MICA mRNA levels in leukocytes were also low in patients with primary disease compared with patients with recurrent GBM (Suppl. Table 2).
Expression of NKG2D and its ligands in GBM patients and HCs
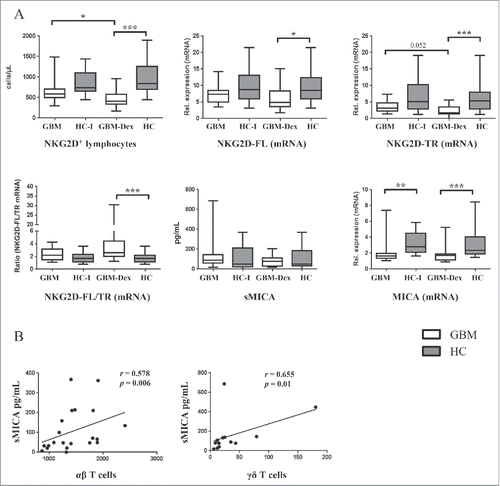
Next, we quantified NKG2D+ cells by FCM and the expression levels of 2 NKG2D isoforms and NKG2DLs MICA/B and ULBP1/2/3/4/5 by qRT-PCR in the blood of study participants. In parallel, serum levels of soluble NKG2DLs were measured by Luminex assay. As expected, NKG2D+ cells were reduced in the steroid-treated GBM-Dex patient group, due to the compromised number of immune cells expressing the NKG2D receptor, such as CD3+CD8+ T cells, NK cells and γδ T cells (, upper left panel). In line with FCM data, qRT-PCR analysis also revealed low levels of mRNA of both isoforms of NKG2D, full length (NKG2D-FL) and truncated variant (NKG2D-TR) in GBM patients compared with HCs (, upper middle and upper right panel, respectively). The ratio of NKG2D-FL/TR was significantly lower in HCs compared GBM patients (, lower left panel). The recently described truncated NKG2D isoform negatively regulates the function of the full length receptor as it attenuates the NKG2D-mediated interferon-γ release and cytotoxic activity.Citation33 The analysis of soluble NKG2DLs in the serum did not reveal significant differences between GBM patients and HCs (shown for sMICA in , lower middle panel). Interestingly, mRNA levels of MICA (, lower right panel) and ULBP3/ULBP5 (not shown) were detected by qRT-PCR at lower levels in the blood of GBM patients compared with HCs. We also correlated serum sMICA levels to other immune parameters. In HCs, but not in GBM patients, there was a positive correlation of sMICA concentration and the number of αβ T cells (, left panel), whereas in GBM patients who were not treated with steroids, the numbers of total γδ T cells, Vδ2 γδ T cells and CD3+CD8+ T cells were positively correlated with serum levels of sMICA (, right panel, shown for γδ T cells). In this patient group the serum level of IL-18 was also positively associated with absolute γδ T cell numbers (not shown).
Figure 3. Analysis of NKG2D/NKG2DLs in GBM patients and HCs. (A) Absolute numbers of NKG2D+ lymphocytes (upper left panel). mRNA levels of full-length (NKG2D-FL) and truncated NKG2D (NKG2D-TR) (upper middle and right panel), and MICA (lower right panel) were measured by qRT-PCR. The ratio between NKG2D-FL and NKG2D-TR (lower left panel) was calculated by dividing the NKG2D-FL value by the NKG2D-TR value and compared between patients groups and HCs. Serum concentrations of sMICA were measured by Luminex assay in the sera of GBM patients and HCs (lower middle panel). (B) Spearman correlation between sMICA and αβ T-cells in HCs (left part). Spearman correlation between sMICA and γδ T cells in steroid untreated GBM patient group (right part). Spearman´s Rho (r) correlation coefficient is depicted in the respective graphs with the corresponding p-value. Statistical significance was analyzed by 2-tailed Wilcoxon's rank-sum test. Statistical significance is displayed as *** for p < 0.001, ** for p < 0.01 and * for p < 0.05.
We used random forests with Boruta algorithm to rank the immunological parameters. Random forests is a powerful statistical test which computes the list of relevant variables with the greatest impact on the “outcome," in this case the variables that better explain the difference between 2 compared groups.Citation34,Citation35 Boruta algorithm on the other hand defines whether the importance of any given variable is significant by indicating confirmed, tentative or unimportant variables.Citation36 The 5 variables ranked in the descending order of importance for the group-wise comparison were identified as follows: for GBM vs. HC-I: CD56high NK cells (% of NK cells), MICA mRNA, NKG2D− lymphocytes/µL, neutrophils (% of leukocytes), CD56dim NK cells (% of NK cells); for GBM-Dex vs. HCs: lymphocytes (% of leukocytes), neutrophils (% of leukocytes), αβ T cells/µL, CD8+ NK cells/µL, CD45+ cells/µL; and for GBM vs. GBM-Dex: lymphocytes (% of leukocytes), CD56high NK cells/µL, neutrophils (% of leukocytes), Tregs/µL, neutrophils/µL (Suppl. Table 3). The confirmed and the tentative variables are listed in Suppl. Table 3 in the descending order of importance.
Short-term cultured primary GBM tumor cells express and release NKG2DLs
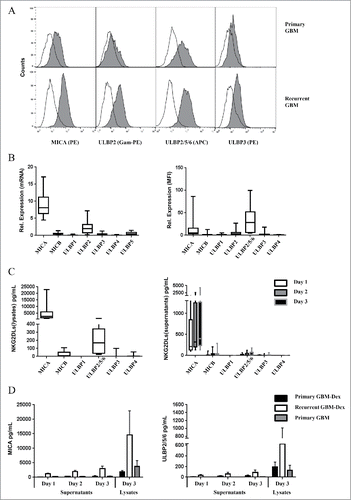
We established short-term cultures of GBM tumor cells to study NKG2DLs expression. Established short-term GBM cell cultures were seeded at a density of 3 × 105 cells per well in 6-well plates and cultured for 3 d. We collected cell culture supernatants to determine levels of soluble NKG2DLs. On day 3, cell surface expression of NKG2DLs was analyzed by FCM. In parallel, to check the overall NKG2DL content in cells, we lysed the cells on day 3 of culture. Most short-term GBM cell lines expressed low levels of NKG2DLs on the cell surface as illustrated for selected NKG2DLs in a representative example in the upper panel of . Of note, the expression level of NKG2DLs was highest on tumor cells from patients with recurrent gliosarcoma, a very aggressive subtype of GBM (, lower panel). MICA and ULBP2 were the highest expressed cell surface molecules of all analyzed NKG2DLs (, right). This observation was in line with qRT-PCR (, left) and Luminex data where in addition to MICA and ULBP2, MICB was also detected at low levels in cell lysates (, left) and culture supernatants (, right). To evaluate the effects of RCT and recurrence of the disease, the samples were divided into 3 groups, i.e., patients with primary (n = 7) and recurrent (n = 3) GBM with pre-surgical treatment with Dex, and patients with primary GBM not treated with Dex (n = 2). Patients with primary GBM had not received any RCT yet, whereas patients in the recurrence group had already received TMZ and radiotherapy. Interestingly, short-term GBM cell cultures established from recurrent tumors expressed and released MICA (, left) and ULBP2 (, right) as well as MICB (not shown) at higher levels compared with cell cultures established from primary tumors, irrespective of whether patients were treated with Dex or not. Due to the small number of cases where we could successfully establish primary GBM tumor cells, we did not present statistical analysis (non-parametric Wilcoxon's rank-sum test) in and . However when comparing soluble MICA at day 1 in the 2 groups with primary and recurrent GBM-Dex (7 vs. 3 patients), the results reached statistical significance (p = 0.0167).
Figure 4. NKG2DLs are expressed and released from short-term GBM tumor cell cultures. Short term GBM primary tumor cells were analyzed for NKG2DLs expression by FCM and qRT-PCR. (A) Representative FCM analysis of NKG2DLs surface expression after staining with mAb MICA-PE, ULBP2/5/6-APC, ULBP3-PE, or ULBP2 followed by goat-anti-mouse (Gam) Ig-PE as indicated (gray histograms). Open histograms represent appropriate isotype controls. (B) Summary of data obtained from 12 short-term GBM primary cell cultures. mRNA levels (left panel) and FCM results (MFI normalized to isotype controls; right panel) of all analyzed NKG2DLs are depicted. (C) NKG2DLs levels in cell lysates (left panel) and cell culture supernatants (right panel) of GBM short-term cell cultures were analyzed with Luminex assay. Culture media of GBM short-term cell cultures were collected at 3 time points (day 1,2,3) and cells were lysed at the end of the experiment (on day 3). The median values of the amounts of sNKG2DLs (pg/mL) of 9 different GBM short-term cell cultures are depicted. (D) Soluble and cellular MICA (left panel) and ULBP2/5/6 (right panel) were analyzed separately in short-term GBM cell cultures from patients with primary GBM (Primary GBM, n = 2), patients with primary GBM treated with steroids (Primary GBM-Dex, n = 7), and patients with recurrent GBM (Recurrent GBM-Dex, n = 3).
Prognostic value of immune parameters for overall survival of GBM patients
240 d median Overall Survival (OS) (95% confidence interval 207–273) was estimated as the time elapsed between inclusion of the GBM patients into the study and death (total n = 26: GBM-Dex 17 (primary/recurrent 7/10); GBM 9 (primary/recurrent 3/6) or the date of the last follow up (censored, n = 6, all primary GBM; GBM-Dex 3; GBM 3). All parameters were dichotomized at the median and analyzed with Kaplan-Meier approach using the log rank test to compare 2 groups. Survival analysis showed that high CD45+ (median survival 188 vs 254 days, , upper left panel) and non-Vδ2 γδ T cell numbers (median survival 196 vs 259 days, , middle left panel) were associated with better survival, whereas Vδ2 γδ T cell counts did not correlate with better survival (, middle right panel). Interestingly, the proportion of CD3+ T cells (defined as % CD3+ of CD45+ cells) was also positively correlated with survival (median survival 188 vs 259 days; , upper right panel), whereas the distribution of CD8+ NK cells (% of NK cells) was negatively associated with overall survival (median survival 260 vs 231 days; , lower left panel). Of note, high cellular content of ULBP2/5/6 in short-term primary tumor cells was associated with poor survival (median survival 370 vs 86 days; ). In an univariate cox regression analysis, CD45+ cells, non-Vδ2 γδ T cells and the percentage of CD3+ cells were indicators of better prognosis with hazard ratios (HR) of 0.398, 0.402 and 0.398, respectively, whereas CD8+ NK cells (% of NK cells, HR 2.32) and the amount of ULBP2/5/6 in lysates of short-term primary GBM cells (HR 9.574) correlated with poorer survival (, upper part). To identify one or a combination of parameters which would correlate best with survival, we used a multiple cox regression model (forward, stepwise), where the CD8+ NK cells in combination with non-Vδ2 γδ T cells were the strongest indicator of prognosis (HR ratios 0.230 and 4.272, respectively; , lower part). When we introduced Dex-medication, TMZ-medication, or the recurrence of the disease as the covariates in the model, the results did not change (not shown).
Figure 5. Kaplan-Meier survival analysis of GBM patients. (A) CD45+ cell and the percentages of CD3+ cells (% of CD45+ cells) (upper panel), non-Vδ2 γδ and Vδ2 γδ T cell numbers per µL blood (middle panel) and CD8+ NK cells (% of NK cells) (lower panel), and (B) the cellular content of ULBP2/5/6 (pg/mL) in short-term primary GBM cells was dichotomized (median as a cut off) and used to generate Kaplan-Meier curves in the GBM patient cohort (n = 32, censored 6). The levels of variables above (high) and below (low) median were compared by log-rank test (depicted p-values).
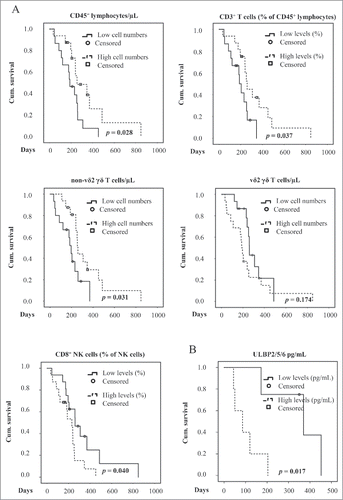
Table 2. Survival analysis using Cox proportional hazard modeling.
Discussion
GBM is a highly malignant tumor which urgently requires innovative (immuno)therapeutic concepts. The identification of distinct TCR repertoire signatures in tumor-infiltrating lymphocytes (TIL) as compared with peripheral blood is in line with an on-going (yet ineffective) local T cell response in GBM.Citation37 In fact, T cells isolated from GBM TIL are functionally compromised and require blocking of inhibitory pathways (such as IL-10) to regain proliferative capacity in vitro.Citation38 GBM tumor cells themselves possess a large arsenal of immunosuppressive mechanisms, spanning from programming tumor-associated macrophages into M2 macrophages and recruiting myeloid-derived suppressor cells (MDSC) to secretion of inhibitory molecules such as TGF-β, IL-10, prostaglandins, and many others.Citation39 Current efforts to increase the anti-tumor immune response in GBM patients include (but are not restricted to) tumor vaccination,Citation40 application of checkpoint inhibitors,Citation41 and the targeting of unconventional lymphocytes including γδ T cells.Citation13,Citation14,Citation42 A precondition for any kind of immunotherapeutic strategy is a precise characterization of the immune system in GBM patients, and particularly the modulation by conventional (yet unsatisfactory) therapies. Along this line, extensive phenotyping has been performed,Citation43 and interesting parameters such as the identification of dysfunctional regulatory T cellsCitation44 or the significance of the neutrophil-to-lymphocyte ratioCitation45 have been described.
In this study, we have performed an in-depth immunophenotyping in a mixed group of GBM patients, with a special focus on the effects of steroids (Dex), RCT and recurrence of the disease. Steroid treatment affected the main immune cell populations including neutrophils, CD4+ T cells, CD8+ T cells, non-Vδ2 γδ T cells and NK cells, and especially the CD56high NK cell subset. Moreover, initially high levels of neutrophils due to steroid treatment were drastically reduced at a later time point when the immune system had recovered from steroid-induced effects, whereas steroid-induced reduction of CD8+ T cell counts were conversely normalized at later time point. Some immune cell populations like Vδ2 γδ T cells and NK cells were present at low levels in GBM patients irrespective of Dex treatment, pointing toward compromised innate killer cells in GBM patients, in line with recent reports by other groups.Citation30,Citation42 Furthermore, random forests test ranked the CD56high NK cell subset (determined as % of NK cells) as the most different variable between GBM and HC-I group, whereas in the steroid treated group the distribution of lymphocytes (determined as % of leukocytes) was the most different variable probably due to reduced lymphocyte numbers and in addition elevated neutrophil counts under steroid treatment. This requires careful interpretation, since almost half of the GBM study participants had a recurrent GBM with medical history of RCT at some point during treatment. TMZ, the standard chemotherapeutic drug in GBM therapy, can negatively influence immune responses and also impacts on immune cell numbers.Citation46 TMZ induces apoptosis in human monocytesCitation47 and also triggers selective CD4+ T cell depletion in melanoma patients, where the effects of TMZ have been studied in the absence of steroid application.Citation48 With respect to NK cells, reported TMZ effects are somewhat controversial. In some studies, TMZ was found to up-regulate multi-drug transporter Abcc3 in murine NK cells and thereby to protect NK cells from toxic effects of chemotherapeutic drugs. In addition, in this study TMZ promoted the chemotactic migration of NK cells toward tumor tissues in murine models of GBM.Citation49 On the other hand, Fadul and coworkers reported decreased counts of peripheral NK cells and CD8+CD56+ cells in GBM patients upon TMZ treatment, possibly due to migration of NK cells into GBM tissue.Citation22 When we compared the results of patients with primary and recurrent disease in both GBM groups in our relatively small cohort, the main difference between groups was the CD4/CD8 T cell ratio, pointing to the negative influence of RCT on the CD4+ T cells (Suppl. Table 2). Redistribution and migration into tumor tissue could also contribute to low counts of immune cells in the peripheral blood. A substantial proportion (up to 10%) of tumor-infiltrating lymphocytes in GBM are CD3+ T cells, mainly consisting of CD4+ T cells, 1% CD8+ T cells and around 2% NK cells, and high CD3+ and CD8+ cell numbers among TIL have been associated with prolonged survival.Citation50
High concentrations of soluble NKG2DL are present in the serum of patients with various tumors (reviewed inCitation18). A widely accepted scenario classifies sNKG2DLs as a tumor immune escape mechanism since high serum levels of sNKG2DLs have been associated with poor prognosis and excess levels of NKG2DLs inhibit NKG2D receptor activity via different mechanisms.Citation18 Surprisingly, however we did not observe significant differences in serum levels of sNKG2DLs between GBM patients and HCs, and mRNA levels of NKG2DLs in the blood were even higher in HCs compared with GBM patients. As serum of healthy individuals contains sNKG2DLs (as shown here and elsewhereCitation51), cells other than tumor cells are obviously capable of releasing soluble NKG2DLs. In the context of GBM, it has been shown that the release of lactate dehydrogenase 5 (LDH5) from GBM cells up-regulates the expression of NKG2DLs on monocytes and contributes to immunosuppression.Citation52 However the precise conditions under which soluble NKG2DLs can be released in a non-malignant setting are not yet well understood. Here we found that sMICA was positively correlated with αβ or γδ T cell numbers in HCs and in GBM patients, respectively, supporting the hypothesis that immune cells might contribute to the release of NKG2DLs. It is thus possible that treatment modalities with a negative impact on immune subsets will “mask” high levels of tumor-derived sNKG2DL by reducing the amount of “background” soluble NKG2DLs in the patient serum.
We observed low expression levels of NKG2DL on short-term GBM cell lines with the exception of cells from patients with previous history of RCT/recurrent GBM. This is in line with previous reports that expression levels of NKG2DLs (MICA, ULBP2, MICB, but not ULBP1) were downregulated on grade IV GBM compared with grade II and grade III tumorsCitation53 hinting to active NKG2D mediated immune surveillance. However, GBM tumor cells of patients with recurrent disease expressed and released high levels of NKG2DLs compared with GBM cells of patients with primary disease. It has been shown by us and others that NKG2DLs are upregulated following TMZ treatment in GBM cells.Citation20,Citation54 We also found that mRNA levels of MICA were elevated in patients with recurrent disease compared with patients with primary GBM (in steroid-untreated group). Interestingly, IL-18 levels were also elevated in this patient group (Suppl. Table 2), in line with reports showing that IL-18 can be produced at increased levels by non-malignant brain resident microglia when stimulated by tumor cells in a GBM mouse modelCitation55 and in response to irradiation.Citation56,Citation57 Of note, recent reports indicate that IL-18 can upregulate the NKG2DL RAE-1 on mouse DCs and macrophages in a MyD88-PI3K-dependent manner.Citation58 Therefore, locally produced IL-18 might contribute to γδ T cell and NK cell anti-tumor immune responses via direct effects but also via upregulation of NKG2D ligands on tumor cells. Altogether, we show that RCT results in upregulated NKG2DLs expression when patient derived tumor cells are analyzed ex-vivo, suggesting that adoptive cell therapies which depend on NKG2DL-NKG2D interaction might be more effective after RCT.
Although the number of patients enrolled in our study is limited, we attempted to identify immune parameters correlating with increased survival. Interestingly, we observed that high levels of non-Vδ2 T cells, but not of Vδ2 γδ T cells, correlated with better survival. Previous studies have documented reduced total γδ T cells numbers and compromised proliferative capacity in GBM patients before surgery and further treatment.Citation42 A preferential reduction of Vδ2 T cells in GBM patients was also reported by Marcu-Malina et al.Citation30 It appears that such losses spare certain γδ T cell subsets, notably Vδ1 T cells. Recent reports indicate that non-Vδ2 (mostly Vδ1) γδ T cells have a high reactivity against certain leukemia and tumor cell targets including GBM.Citation9,Citation17,Citation59-61 Hence, high levels of non-Vδ2 γδ T cells might be an indicator of a better cytotoxic response to GBM correlating with an increased overall survival. On the other hand, it is known that CMV-infection, frequently associated with GBM, can also trigger the expansion of non-Vδ2 T cells.Citation15,Citation62 We did not evaluate the CMV-status of our GBM patients. However, Knight et al (2013) showed that CMV-positive GBM cells are not better killed by non-Vδ2 T cells as compared with CMV-negative GBM cells suggesting that the CMV status in our study participants would not have a direct impact on the tumor cell susceptibility to non-Vδ2 T cells.Citation59 Interestingly, a benefit of high levels of Vδ1 γδ T cell counts on the treatment outcome has also been observed in hematological malignancies.Citation60,Citation61,Citation63 Taken together, it will be important to explore the therapeutic potential of Vδ1 T cells in GBM in more detail.Citation64 Recently, novel protocols for the large-scale in vitro expansion of Vδ1 T cells for adoptive immunotherapy have been developed.Citation65 A possible route of application in GBM patients could be the intracranial infusions of expanded γδ T cells which has been shown to increased overall survival in a mouse model with human GBM xenografts.Citation13 Importantly, preclinical studies indicate that adoptive transfer of allogeneic γδ T cells is likely to be safe. It has been shown that it targets GBM tumor cells but does not harm normal brain tissue even after potentially stress-inducing cytotoxic therapy.Citation66 Obviously, however, more preclinical studies including sophisticated humanized mouse models are required before application of γδ T cell immunotherapy in GBM patients can be considered.Citation67
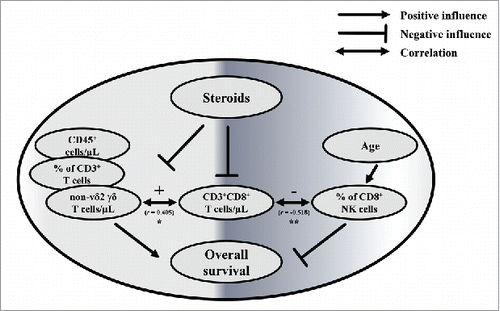
Overall, the strongest independent predictor of better survival in our study was the combination of high non-Vδ2 γδ T cell numbers and low proportions of CD8+ NK cells. Although it is difficult to delineate definitive connections between these variables, it is interesting that CD3+CD8+ T cell numbers seem to correlate with both parameters. Of note, CD8+ NK (determined as % of NK cells) cells negatively correlated with the age of GBM patients (not shown) but not of healthy controls; however age itself was not connected to the survival of GBM patients. Interestingly, the immune cell populations which correlated best with survival are the variables influenced by steroid treatment. Complex treatment regimens such as the combination of steroids with RCT make it more difficult to draw clear conclusions, but still allowed us to reveal the negative influence of Dex for GBM survival. A schematic representation of positive and negative variables based on our results is presented in . Our results support the recent observations of Wong et al. (2015), where in GBM patients with recurrent disease alternative electric fields (TTfields) treatment was compared with standard RCT regimens. The undesirable effects of steroids became more obvious in patient groups who did not receive RCT but TTfields. Patients who received steroids above 4.1 mg/day had a reduced OS (4.8 months) compared with patients who received Dex less than 4.1 mg a day (11 months). In the control RCT group, OS was 6 vs 8.9 months, respectively. Of note, OS was correlated with T cell counts.Citation68 The prognostic value of CD4+ T cell counts for GBM patients has been documented in another study, where CD4+ T cell numbers higher than 200 per μL of blood were associated with better survival.Citation69
Figure 6. Interplay between immunological parameters associated with survival of GBM patients. Interplay between the parameters associated with survival, CD3+CD8+ T cells/µL, steroid medication, recurrence of the disease, RCT and age of GBM patients (n = 26; GBM-Dex/GBM 17/9). Positive and negative influences are indicated with arrows and lines with flat ending, respectively. Negative and positive correlations between parameters are shown as double-headed arrows. For statistical analysis Spearman´s Rank correlation was used. Spearman´s Rho (r) correlation coefficient is depicted with statistical significance displayed as ** for p < 0.01 and * for p < 0.05.
In summary, our study sheds light on how steroids (and RCT) affect immune cell parameters in the blood of GBM patients. This information is particularly important when considering immunotherapeutic approaches for GBM treatment; especially since the exact immune characteristics of many cancers including GBM are not yet well understood. A precise analysis might help to predict the efficacy of such novel therapeutic strategies including adoptive transfer of γδ T cells.Citation70
Materials and methods
Patients
35 GBM patients and 22 age-matched healthy controls (HCs) were enrolled in this study (). Clinical details of GBM patients are listed in Suppl. Table 1. EDTA blood of patients was obtained shortly before surgery (n = 35) and 3.3 months after inclusion in the study (n = 9). In parallel, 16 primary tumor specimens were obtained during surgical resection, and short-term primary tumor cell cultures were established and controlled for purity as described previously.Citation71 The diagnosis was established through histological and molecular evaluation by a pathologist (see Table I and Suppl. Table I). All samples were analyzed by flow cytometry and cryopreserved in DMEM/10% DMSO (#109678, Merck Millipore) until RNA extraction for qRT-PCR. The study was approved by the Ethics committee of the Medical Faculty of University of Kiel (D485/13 and D405/14), and informed consent was obtained from all GBM patients and HCs. Guidelines of the Helsinki Declaration of 1975 were followed. Survival of GBM patients was determined as time elapsed from the date of inclusion in the study to death or last follow-up. In case of 3 patients follow-up was lost.
Flow cytometry
To determine the absolute cell number of leukocyte subpopulations, whole blood was analyzed using the BD Multitest™ 6-color TBNK reagent with BD Truecount™ tubes (#337166, BD Biosciences, Heidelberg, Germany) following the protocol provided by the supplier. In addition, αβ T cells and subsets of γδ T cells were quantified by staining of whole blood in BD TrueCount™ tubes (#340334, BD Biosciences) as described.Citation26 Samples were measured on a FACS Canto flow cytometer using FACS Diva software (BD Biosciences). To study the NKG2D expression on distinct immune cell subsets, whole blood was stained with the following mAb: CD3-BV605 (clone OKT3, #317322, Biolegend,), CD4-BV510 (clone OKT4, #317444, Biolegend), CD25-APC (clone CD25–3G10, #MCD2505, Thermo Fisher Scientific), CD127-PE (clone HIL-7R-M21, #557938, BD Biosciences), CD8-APC/Cy7 (clone SK1, #344714, Biolegend), CD16-Pacific Blue (clone 3G8, #302032, Biolegend), CD56-PE-eFluor 610 (clone CMSSB, #61–0567–42, eBioscience), pan γδ TCR-PE-Cy7 (clone 11F2, #655410, BD Biosciences), Vδ2 TCR-FITC (clone IMMU 389, #IM1464, Beckman Coulter), CD19-AF700 (clone HIB19, #302226, Biolegend), and NKG2D-PerCP-eFluor® 710 (clone 1D11, #46–5878–42, eBioscience). After red blood cell lysis with 1.5 mL BD Lysing-solution (#349202, BD Biosciences), cells were spun down and washed with FACS washing buffer (WB) (PBS containing 1% BSA and 0.1% sodium azide). After resuspending in 1% BSA and 0.1% sodium azide, samples were measured on a BD LSR Fortessa (BD Biosciences) using FACS Diva software. In addition, the numbers of peripheral blood monocytes and granulocytes were analyzed based on CD14 (CD14-APC, clone MFE2, #555399, BD Biosciences) and CD66b (CD66b-FITC, clone 04, # 11729-MM04-F, Sino Biologicals) expression, respectively and NKG2D expression was determined with anti-NKG2D-PE (clone 1D11, #12–5878–42) or anti-NKG2D-PE (clone 149810, #FAB139P, R&D systems) on a FACS Calibur using FACS CellQuest Pro software (BD Biosciences). Data collected on FACS Calibur and BD LSR Fortessa was analyzed with FlowJo software (FlowJo 10.0.8r1) and the absolute cell numbers were quantified based on the numbers of CD3+ cells obtained from Multitest™ TBNK reagent with BD Truecount™ tubes.
The following mAb were used for cell surface staining of NKG2DL on short-term cultured GBM cells: MICA-PE (clone 159227, #FAB1300P, R&D systems), MICB-PE (clone 236511, #FAB1599P, R&D systems), ULBP1-PE (clone 170818, #FAB1380P, R&D systems), ULBP2 mAb (clone MM0593–7F33, #ab89930, Abcam), ULBP2/5/6-APC (clone 165903, #FAB1298 A, R&D systems), ULBP3-PE (clone 166510, #FAB1517P, R&D systems) and ULBP4 (clone 79/6/13/16, #ab95202, Abcam). Tumor cells were washed with WB and blocked with Fc-receptor blocking reagent (#130–059–901, Miltenyi Biotec GmbH). After an additional washing step, the cells were incubated for 30 min with specific antibodies or matched isotype controls. In case of unconjugated antibodies, cells were further incubated with a polyclonal PE-conjugated goat-anti-mouse Ab (#A10543, Invitrogen). After a washing step, cells were fixed in 1% paraformaldehyde. 5000 cells were analyzed on a FACS Calibur flow cytometer using CellQuestPro Software. Data collected was analyzed with FlowJo software.
Measurement of soluble analytes in serum
To detect serum levels of sNKG2DL (MICA, MICB, ULBP1, ULBP2/5/6, ULBP3 and ULBP4), a polystyrene Luminex® Multiplex screening Assay was designed (#LXAP06, R&D systems). In addition, up to 14 analytes were measured in the serum of study participants using Magnetic Luminex® ProcartaPlex Screening Assays (Macrophage migration inhibitory factor (MIF, #EPX01 A-12127–901), SDF-1α (#EPX01 A-12138–901), Macrophage inflammatory protein 3 a (MIP-3 a, #EPX01 A-12128–901), Macrophage inflammatory protein 1 a (MIP-1 a, #EPX01 A-12029–901), IL-1β (#EPX01 A-10224–901), IL-2 (#EPX01 A-10221–901), IL-8 (#EPX01 A-10204–901), IL-10 (#EPX01 A-10215–901), IL-17 F (#EPX01 A-12160–901), IL-13 (#EPX01 A-10231–901), G-CSF (#EPX01 A-12001–901), GM-CSF (#EPX01 A-10283–901), Monocyte chemoattractant protein 1 (MCP1, #EPX01B-10281–901), IL-18 (#EPX01 A-10267–901) all purchased from eBioscience) according to the manufacturer's instructions. Both Luminex® assays were performed with 1+1 diluted serum samples and measured on a Luminex LX100 system (Luminex, Austin, USA). Data acquired using the Luminex xPonent 2.3/3.1 software represents the median of the fluorescence intensity of the respective analyte. For each standard curve, a curve fit was applied according to the manufacturer's manual. The sample concentrations were interpolated from the resulting regression equation.
Quantitative RT-PCR
Fresh blood from study participants and the tumor cells from GBM patients were cryopreserved until RNA extraction. To this end, 110 µL DMSO was added to 1 mL fresh blood or to a primary tumor cell suspension in 1 mL DMEM medium containing 20% FCS and cryopreserved in liquid nitrogen. Thawed blood samples were resuspended in 40 mL RBC (#420301, Biolegend) lysing buffer (10 min, RT). Lysed whole blood and tumor samples were washed twice with PBS. Pellets were resuspended in 1 mL PeqGold TriFast (#30–2010, Peqlab) and immediately stored at −80 °C for further analysis. RNA extraction, cDNA synthesis and qRT-PCR were performed as described previously.Citation72 All PCR reactions were run in duplicates using 2.5 μl of the cDNA (≈10 ng total RNA). qRT-PCR was performed using a Rotorgene 3000 (Corbett, LTF, Wasserburg, Germany) with SYBR green-based qPCR mix (#95072–05 K, Quanto Biosciences). Primers for NKG2DLs and receptors were designed using the Web-based primer3 software (http://primer3.wi.mit.edu/) and purchased from TIB MOLBIOL (Berlin, Germany), together with primers for housekeeping genes β-actin (ACTB), β-2-microglobulin (B2M) and RNA (18 S) (sequences listed in Suppl. Table 4). The sequence for the forward primer targeting MICB transcript is according to ref.Citation73 Threshold levels for Ct-determination were chosen manually. Data analysis was performed according to the ΔCt method.Citation74 Briefly, the mean Ct value of 3 housekeeping genes was subtracted from the Ct value of the gene of interest (ΔCt) and transformed with equation X = 2−ΔCt. For the simpler presentation of the data all values were multiplied by the factor of 1 × 106.
Statistical analysis
We used Graph Pad Prism 6, SPSS 24 (IBM) and R version 3.2.3 (packages ‘Boruta’ and ‘randomForest’). Two-tailed Wilcoxon's rank-sum test was used to compare immunological parameters without adjusting for multiple testing between GBM patients and HCs. Moreover, the predictive importance of the compared immunological parameters to the corresponding classification were ranked with machine learning tools (random forest, Boruta algorithms).Citation34,Citation35 To quantify the association between variables, Spearman rank correlation test was performed. GBM-associated overall survival was analyzed with Kaplan-Meier approach and cox regression models. All variables were dichotomized based on the respective median value as a cut off and a log rank test was run to determine differences in the survival distribution for the 2 groups. A 2-tailed p-value of 0.05 was considered statistically significant.
Conflict of interest
The authors declare no competing conflict of interest.
Supplementary_materials.zip
Download Zip (956.3 KB)Acknowledgments
The expert technical assistance of Monika Kunz, Jörg Krause, Kyong Yoo-Ott, Hilke Clasen and Kristina Dudda is greatly appreciated. Sequencing of PCR products was performed at the Institute of Clinical and Molecular Biology (University of Kiel). This work was supported by grants of the Else-Kröner-Fresenius Foundation (DK), Medical Faculty of Kiel University (GC, CF), Kreitz Foundation (GC, JB), Family Mehdorn Foundation (GC, CF) and Cluster of Excellence EXC 306 funded by the German Research Council (DK, GC, SFW). This work forms part of the PhD thesis of GC.
References
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi:10.1056/NEJMoa043330. PMID:15758009
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi:10.1056/NEJMra0708126. PMID:18669428
- Razavi SM, Lee KE, Jin BE, Aujla PS, Gholamin S, Li G. Immune Evasion Strategies of Glioblastoma. Front Surg. 2016;3(1):11. doi:10.3389/fsurg.2016.00011. PMID:26973839
- Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(13):1270–71. doi:10.1056/NEJMc1509660. PMID:26398076
- Marrone KA, Brahmer JR. Using Immune Checkpoint Inhibitors in Lung Cancer. Oncology (Williston Park). 2016;30(8):713–21. PMID:27528240
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi:10.1056/NEJMoa1003466. PMID:20525992
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–33. doi:10.1056/NEJMoa1103849. PMID:21830940
- Tivnan A, Heilinger T, Lavelle EC, Prehn JH. Advances in immunotherapy for the treatment of glioblastoma. J Neurooncol. 2017;131(1):1–9. doi:10.1007/s11060-016-2299-2. PMID:27743144
- Nakazawa T, Nakamura M, Park YS, Motoyama Y, Hironaka Y, Nishimura F, Nakagawa I, Yamada S, Matsuda R, Tamura K, et al. Cytotoxic human peripheral blood-derived γδ T cells kill glioblastoma cell lines: implications for cell-based immunotherapy for patients with glioblastoma. J Neurooncol. 2014;116(1):31–39. doi:10.1007/s11060-013-1258-4. PMID:24062140
- Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197(2):163–8.doi:10.1084/jem.20021500. PMID:12538656
- Roelofs AJ, Jauhiainen M, Monkkonen H, Rogers MJ, Monkkonen J, Thompson K. Peripheral blood monocytes are responsible for γδ T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144(2):245–50. doi:10.1111/j.1365-2141.2008.07435.x. PMID:19016713
- Fowler DW, Bodman-Smith MD. Harnessing the power of Vδ2 cells in cancer immunotherapy. Clin Exp Immunol. 2015;180(1):1–10. doi:10.1111/cei.12564. PMID:25469879
- Jarry U, Chauvin C, Joalland N, Leger A, Minault S, Robard M, Bonneville M, Oliver L, Vallette FM, Vie H, et al. Stereotaxic administrations of allogeneic human Vγ9Vδ2 T cells efficiently control the development of human glioblastoma brain tumors. Oncoimmunology. 2016;5(6):e1168554. doi:10.1080/2162402X.2016.1168554. PMID:27471644
- Bryant NL, Gillespie GY, Lopez RD, Markert JM, Cloud GA, Langford CP, Arnouk H, Su Y, Haines HL, Suarez-Cuervo C, et al. Preclinical evaluation of ex vivo expanded/activated γδ T cells for immunotherapy of glioblastoma multiforme. J Neurooncol. 2011;101(2):179–88. doi:10.1007/s11060-010-0245-2. PMID:20532954
- Dechanet J, Merville P, Lim A, Retiere C, Pitard V, Lafarge X, Michelson S, Meric C, Hallet MM, Kourilsky P, et al. Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest. 1999;103(10):1437–49. doi:10.1172/JCI5409. PMID:10330426
- Cobbs CS, Soroceanu L, Denham S, Zhang W, Britt WJ, Pieper R, Kraus MH. Human cytomegalovirus induces cellular tyrosine kinase signaling and promotes glioma cell invasiveness. J Neurooncol. 2007;85(3):271–80. doi:10.1007/s11060-007-9423-2. PMID:17589804
- Scheper W, van Dorp S, Kersting S, Pietersma F, Lindemans C, Hol S, Heijhuurs S, Sebestyen Z, Grunder C, Marcu-Malina V, et al. γδ T cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia. 2013;27(6):1328–38. doi:10.1038/leu.2012.374. PMID:23277330
- Chitadze G, Bhat J, Lettau M, Janssen O, Kabelitz D. Generation of soluble NKG2D ligands: proteolytic cleavage, exosome secretion and functional implications. Scand J Immunol. 2013;78(2):120–9. doi:10.1111/sji.12072. PMID:23679194
- Chitadze G, Lettau M, Bhat J, Wesch D, Steinle A, Furst D, Mytilineos J, Kalthoff H, Janssen O, Oberg HH, et al. Shedding of endogenous MHC class I-related chain molecules A and B from different human tumor entities: heterogeneous involvement of the “a disintegrin and metalloproteases” 10 and 17. Int J Cancer. 2013;133(7):1557–66. doi:10.1002/ijc.28174. PMID:23526433
- Chitadze G, Lettau M, Luecke S, Wang T, Janssen O, Furst D, Mytilineos J, Wesch D, Oberg HH, Held-Feindt J, et al. NKG2D- and T-cell receptor-dependent lysis of malignant glioma cell lines by human γδ T cells: Modulation by temozolomide and A disintegrin and metalloproteases 10 and 17 inhibitors. Oncoimmunology. 2016;5(4):e1093276. doi:10.1080/2162402X.2015.1093276. PMID:27141377
- Zhang J, Basher F, Wu JD. NKG2D Ligands in Tumor Immunity: Two Sides of a Coin. Front Immunol. 2015;6(1):97. doi:10.3389/fimmu.2015.00097. PMID:25788898
- Fadul CE, Fisher JL, Gui J, Hampton TH, Cote AL, Ernstoff MS. Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro Oncol. 2011;13(4):393–400. doi:10.1093/neuonc/noq204. PMID:21339188
- Vecht CJ, Hovestadt A, Verbiest HB, van Vliet JJ, van Putten WL. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: a randomized study of doses of 4, 8, and 16 mg per day. Neurology. 1994;44(4):675–80. doi:10.1212/WNL.44.4.675. PMID:8164824
- Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4(2):233–42. doi:10.1586/ecp.11.1. PMID:21666852
- Fauci AS. Mechanisms of corticosteroid action on lymphocyte subpopulations. II. Differential effects of in vivo hydrocortisone, prednisone and dexamethasone on in vitro expression of lymphocyte function. Clin Exp Immunol. 1976;24(1):54–62. PMID:1084818
- Oberg HH, Grage-Griebenow E, Adam-Klages S, Jerg E, Peipp M, Kellner C, Petrick D, Gonnermann D, Freitag-Wolf S, Rocken C, et al. Monitoring and functional characterization of the lymphocytic compartment in pancreatic ductal adenocarcinoma patients. Pancreatology. 2016;16(6):1069–79. doi:10.1016/j.pan.2016.07.008
- Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol. 1995;154(9):4719–25. PMID:7722324
- Nakagawa M, Terashima T, D'Yachkova Y, Bondy GP, Hogg JC, van Eeden SF. Glucocorticoid-induced granulocytosis: contribution of marrow release and demargination of intravascular granulocytes. Circulation. 1998;98(21):2307–13.doi:10.1161/01.CIR.98.21.2307. PMID:9826319
- Waisman D, Van Eeden SF, Hogg JC, Solimano A, Massing B, Bondy GP. L-selectin expression on polymorphonuclear leukocytes and monocytes in premature infants: reduced expression after dexamethasone treatment for bronchopulmonary dysplasia. J Pediatr. 1998;132(1):53–56. doi:10.1016/S0022-3476(98)70484-6. PMID:9470000
- Marcu-Malina V, Garelick D, Peshes-Yeloz N, Wohl A, Zach L, Nagar M, Amariglio N, Besser MJ, Cohen ZR, Bank I. Peripheral blood-derived, γ9δ2 T cell-enriched cell lines from glioblastoma multiforme patients exert anti-tumoral effects in vitro. J Biol Regul Homeost Agents. 2016;30(1):17–30. PMID:27049073
- Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97(10):3146–51. doi:10.1182/blood.V97.10.3146. PMID:11342442
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–40. doi:10.1016/S1471-4906(01)02060-9. PMID:11698225
- Karimi MA, Aguilar OA, Zou B, Bachmann MH, Carlyle JR, Baldwin CL, Kambayashi T. A truncated human NKG2D splice isoform negatively regulates NKG2D-mediated function. J Immunol. 2014;193(6):2764–71. doi:10.4049/jimmunol.1400920. PMID:25092887
- Kursa MB RW. Feature Selection with the Boruta Package. J Stat Softw. 2010;11(36):1–13. doi:10.18637/jss.v036.i11
- Breiman L. Random Forests. Machine Learning. 2001;45(1):5–32. doi:10.1023/A:1010933404324
- Dauwan M, van der Zande JJ, van Dellen E, Sommer IE, Scheltens P, Lemstra AW, Stam CJ. Random forest to differentiate dementia with Lewy bodies from Alzheimer's disease. Alzheimers Dement (Amst). 2016;4(1):99–106. doi:10.1016/j.dadm.2016.07.003 PMID:27722196
- Sims JS, Grinshpun B, Feng Y, Ung TH, Neira JA, Samanamud JL, Canoll P, Shen Y, Sims PA, Bruce JN. Diversity and divergence of the glioma-infiltrating T-cell receptor repertoire. Proc Natl Acad Sci U S A. 2016;113(25):E3529–37. doi:10.1073/pnas.1601012113. PMID:27261081
- Han S, Ma E, Wang X, Yu C, Dong T, Zhan W, Wei X, Liang G, Feng S. Rescuing defective tumor-infiltrating T-cell proliferation in glioblastoma patients. Oncol Lett. 2016;12(4):2924–9. doi:10.3892/ol.2016.4944. PMID:27703529
- Magana-Maldonado R, Chavez-Cortez EG, Olascoaga-Arellano NK, Lopez-Mejia M, Maldonado-Leal FM, Sotelo J, Pineda B. Immunological Evasion in Glioblastoma. Biomed Res Int. 2016;2016(1):7487313. doi:10.1155/2016/7487313. PMID:27294132
- Dejaegher J, Van Gool S, De Vleeschouwer S. Dendritic cell vaccination for glioblastoma multiforme: review with focus on predictive factors for treatment response. Immunotargets Ther. 2014;3(1):55–66. doi:10.2147/ITT.S40121. PMID:27471700
- Mirzaei R, Sarkar S, Yong VW. T Cell exhaustion in glioblastoma: Intricacies of immune checkpoints. Trends Immunol. 2017;38(2):104–15. doi:10.1016/j.it.2016.11.005. PMID:27964820
- Bryant NL, Suarez-Cuervo C, Gillespie GY, Markert JM, Nabors LB, Meleth S, Lopez RD, Lamb LS, Jr. Characterization and immunotherapeutic potential of γδ T-cells in patients with glioblastoma. Neuro Oncol. 2009;11(4):357–67. doi:10.1215/15228517-2008-111. PMID:19211933
- Lamano JB, Ampie L, Choy W, Kesavabhotla K, DiDomenico JD, Oyon DE, Parsa AT, Bloch O. Immunomonitoring in glioma immunotherapy: current status and future perspectives. J Neurooncol. 2016;127(1):1–13. doi:10.1007/s11060-015-2018-4. PMID:26638171
- Lowther DE, Goods BA, Lucca LE, Lerner BA, Raddassi K, van Dijk D, Hernandez AL, Duan X, Gunel M, Coric V, et al. PD-1 marks dysfunctional regulatory T cells in malignant gliomas. JCI Insight. 2016;1(5):e85935. doi:10.1172/jci.insight.85935. PMID:27182555
- Han S, Liu Y, Li Q, Li Z, Hou H, Wu A. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer. 2015;15(1):617. doi:10.1186/s12885-015-1629-7. PMID:26341881
- Sengupta S, Marrinan J, Frishman C, Sampath P. Impact of temozolomide on immune response during malignant glioma chemotherapy. Clin Dev Immunol. 2012;2012(1):831090. doi:10.1155/2012/831090. PMID:23133490
- Bauer M, Goldstein M, Heylmann D, Kaina B. Human monocytes undergo excessive apoptosis following temozolomide activating the ATM/ATR pathway while dendritic cells and macrophages are resistant. PLoS One. 2012;7(6):e39956. doi:10.1371/journal.pone.0039956. PMID:22768182
- Su YB, Sohn S, Krown SE, Livingston PO, Wolchok JD, Quinn C, Williams L, Foster T, Sepkowitz KA, Chapman PB. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22(4):610–6. doi:10.1200/JCO.2004.07.060. PMID:14726505
- Pessina S, Cantini G, Kapetis D, Cazzato E, Di Ianni N, Finocchiaro G, Pellegatta S. The multidrug-resistance transporter Abcc3 protects NK cells from chemotherapy in a murine model of malignant glioma. Oncoimmunology. 2016;5(5):e1108513. doi:10.1080/2162402X.2015.1108513. PMID:27467914
- Kmiecik J, Poli A, Brons NH, Waha A, Eide GE, Enger PO, Zimmer J, Chekenya M. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol. 2013;264(1-2):71–83. doi:10.1016/j.jneuroim.2013.08.013. PMID:24045166
- Holdenrieder S, Eichhorn P, Beuers U, Samtleben W, Stieber P, Nagel D, Peterfi A, Steinle A, Salih HR. Soluble NKG2D ligands in hepatic autoimmune diseases and in benign diseases involved in marker metabolism. Anticancer Res. 2007;27(4 A):2041–5. PMID:17649819
- Crane CA, Austgen K, Haberthur K, Hofmann C, Moyes KW, Avanesyan L, Fong L, Campbell MJ, Cooper S, Oakes SA, et al. Immune evasion mediated by tumor-derived lactate dehydrogenase induction of NKG2D ligands on myeloid cells in glioblastoma patients. Proc Natl Acad Sci U S A. 2014;111(35):12823–8. doi:10.1073/pnas.1413933111. PMID:25136121
- Eisele G, Wischhusen J, Mittelbronn M, Meyermann R, Waldhauer I, Steinle A, Weller M, Friese MA. TGF-β and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain. 2006;129(9):2416–25. doi:10.1093/brain/awl205. PMID:16891318
- Lamb LS, Jr., Bowersock J, Dasgupta A, Gillespie GY, Su Y, Johnson A, Spencer HT. Engineered drug resistant γδ T cells kill glioblastoma cell lines during a chemotherapy challenge: a strategy for combining chemo- and immunotherapy. PLoS One. 2013;8(1):e51805. doi:10.1371/journal.pone.0051805. PMID:23326319
- Yeh WL, Lu DY, Liou HC, Fu WM. A forward loop between glioma and microglia: glioma-derived extracellular matrix-activated microglia secrete IL-18 to enhance the migration of glioma cells. J Cell Physiol. 2012;227(2):558–68. doi:10.1002/jcp.22746. PMID:21442623
- Zhu C, Huang Z, Gao J, Zhang Y, Wang X, Karlsson N, Li Q, Lannering B, Bjork-Eriksson T, Georg Kuhn H, et al. Irradiation to the immature brain attenuates neurogenesis and exacerbates subsequent hypoxic-ischemic brain injury in the adult. J Neurochem. 2009;111(6):1447–56. doi:10.1111/j.1471-4159.2009.06413.x. PMID:19799713
- Kast RE. The role of interleukin-18 in glioblastoma pathology implies therapeutic potential of two old drugs-disulfiram and ritonavir. Chin J Cancer. 2015;34(4):161–5. doi:10.1186/s40880-015-0010-1. PMID:25963312
- Brandstadter JD, Chen H, Jiang S, Huang X, Yang Y. IL-18-dependent NKG2D ligand upregulation on accessory cells is mediated by the PI3 K/GSK-3 pathway. J Leukoc Biol. 2017;101(6):1317–23. doi:10.1189/jlb.2A0816-342R. PMID:28283665
- Knight A, Arnouk H, Britt W, Gillespie GY, Cloud GA, Harkins L, Su Y, Lowdell MW, Lamb LS. CMV-independent lysis of glioblastoma by ex vivo expanded/activated Vδ1+ γδ T cells. PLoS One. 2013;8(8):e68729. doi:10.1371/journal.pone.0068729. PMID:23950874
- Meeh PF, King M, O'Brien RL, Muga S, Buckhalts P, Neuberg R, Lamb LS, Jr. Characterization of the γδ T cell response to acute leukemia. Cancer Immunol Immunother. 2006;55(9):1072–80. doi:10.1007/s00262-005-0094-6. PMID:16328383
- Poggi A, Venturino C, Catellani S, Clavio M, Miglino M, Gobbi M, Steinle A, Ghia P, Stella S, Caligaris-Cappio F, et al. Vdelta1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated by trans-retinoic acid. Cancer Res. 2004;64(24):9172–9179. doi:10.1158/0008-5472.CAN-04-2417. PMID:15604289
- Knight A, Madrigal AJ, Grace S, Sivakumaran J, Kottaridis P, Mackinnon S, Travers PJ, Lowdell MW. The role of Vδ2-negative γδ T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood. 2010;116(12):2164–72. doi:10.1182/blood-2010-01-255166. PMID:20576814
- Catellani S, Poggi A, Bruzzone A, Dadati P, Ravetti JL, Gobbi M, Zocchi MR. Expansion of Vdelta1 T lymphocytes producing IL-4 in low-grade non-Hodgkin lymphomas expressing UL-16-binding proteins. Blood. 2007;109(5):2078–85. doi:10.1182/blood-2006-06-028985. PMID:16973957
- Siegers GM, Lamb LS, Jr. Cytotoxic and regulatory properties of circulating Vδ1+ γδ T cells: a new player on the cell therapy field? Mol Ther. 2014;22(8):1416–22. doi:10.1038/mt.2014.104. PMID:24895997
- Almeida AR, Correia DV, Fernandes-Platzgummer A, da Silva CL, da Silva MG, Anjos DR, Silva-Santos B. Delta One T Cells for Immunotherapy of Chronic Lymphocytic Leukemia: Clinical-Grade Expansion/Differentiation and Preclinical Proof of Concept. Clin Cancer Res. 2016;22(23):5795–804. doi:10.1158/1078-0432.CCR-16-0597. PMID:27307596
- Pereboeva L, Harkins L, Wong S, Lamb LS. The safety of allogeneic innate lymphocyte therapy for glioma patients with prior cranial irradiation. Cancer Immunol Immunother. 2015;64(5):551–62. doi:10.1007/s00262-015-1662-z. PMID:25676710
- Xiang Z, Liu Y, Zheng J, Liu M, Lv A, Gao Y, Hu H, Lam KT, Chan GC, Yang Y, et al. Targeted activation of human Vγ9Vδ2-T cells controls epstein-barr virus-induced B cell lymphoproliferative disease. Cancer Cell. 2014;26(4):565–76. doi:10.1016/j.ccr.2014.07.026. PMID:25220446
- Wong ET, Lok E, Gautam S, Swanson KD. Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. Br J Cancer. 2015;113(2):232–41. doi:10.1038/bjc.2015.238. PMID:26125449
- Gousias K, Voulgaris S, Vartholomatos G, Voulgari P, Kyritsis AP, Markou M. Prognostic value of the preoperative immunological profile in patients with glioblastoma. Surg Neurol Int. 2014;5:89. doi:10.4103/2152-7806.134104. PMID:25024889
- Chitadze G, Oberg HH, Wesch D, Kabelitz D. The ambiguous role of γδ T lymphocytes in antitumor immunity. Trends Immunol. 2017 accessed July 11, 2017. http://www.cell.com/trends/immunology/abstract/S1471-4906(17)30117-5. PMID:28709825
- Hattermann K, Gebhardt H, Krossa S, Ludwig A, Lucius R, Held-Feindt J, Mentlein R. Transmembrane chemokines act as receptors in a novel mechanism termed inverse signaling. Elife. 2016;5(1):e10820. doi:10.7554/eLife.10820. PMID:26796342
- Kalyan S, Chandrasekaran V, Quabius ES, Lindhorst TK, Kabelitz D. Neutrophil uptake of nitrogen-bisphosphonates leads to the suppression of human peripheral blood γδ T cells. Cell Mol Life Sci. 2014;71(12):2335–46. doi:10.1007/s00018-013-1495-x. PMID:24162933
- Leung WH, Vong QP, Lin W, Janke L, Chen T, Leung W. Modulation of NKG2D ligand expression and metastasis in tumors by spironolactone via RXRγ activation. J Exp Med. 2013;210(12):2675–92. doi:10.1084/jem.20122292. PMID:24190430
- Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 2006;1(3):1559–82. doi:10.1038/nprot.2006.236. PMID:17406449
