ABSTRACT
Inhibitory properties of PD-1 receptor engagement on activated T cells are well established in physiologic and pathological contexts. In cancer, the use of checkpoint blockade, such as anti-PD-1 antibodies, becomes progressively a reference treatment of a growing number of tumors. Nonetheless, it is also established that PD-1 expression on antigen-specific T cells reflects the functional avidity and anti-tumor reactivity of these T cells. We will discuss this dual significance of PD-1 expression on tumor-specific T cells, due to a complex regulation and the opportunity to exploit this expression to define, monitor and exploit tumor-reactive T cells for immunotherapy purposes.
Introduction
PD-1 inhibitory receptor (CD279) has been discovered in 1992, by the group of Pr Honjo.Citation1 Initially identified for its ability to induce apoptosis, its role in the maintenance of peripheral immune tolerance has been further documented using PD-1−/− murine models.Citation2 PD-1 is expressed at the cell surface of activated T cells, NK cells, B cells, macrophages and several subsets of DCs.Citation3,4 PD-1 expression on naïve T cells is induced upon TCR activation.Citation5 This transient expression decreases in absence of TCR signaling but is maintained upon chronic activation with a persisting epitope target such as in chronic viral infections and in cancer.Citation6 PD-1 ligation to its ligands PD-L1 and PD-L2 impairs TCR signaling and CD28 co-stimulation.Citation7,8 Constitutive PD-1 expression by tumor-specific T cells was initially described as associated with the expression of additional inhibitory receptors such as Tim-3, LAG-3 or TIGIT,Citation9Citation-12 leading to impaired T cell functions and to tumor escape, upon ligation to its ligand PD-L1 expressed by tumor cells or immune infiltrating cells within tumor microenvironment. Constitutive PD-1 expression constitutes a form of immune adaptation to chronic stimulation, leading to a physiologic limitation of immune responses limiting auto-immune phenomena. This system is thus hijacked by tumor cells to favor peripheral tolerance and in this context PD-1 can be considered as a marker of dysfunctional T lymphocytes.
However, results from different groups highlighted the ambiguous role of PD-1 in defining efficient or ineffective immune T cell responses. Indeed, although leading to an inhibition signal upon ligation with its ligands, it is now clear that PD-1 expression is first a marker of T cell activation, allowing the identification of the tumor-reactive CD8+ T cell fraction in melanoma tumors,Citation13,14 and of high avidity CD8 T-cells specific for Melan-ACitation15 or neoantigens.Citation16 Indeed, the level of PD-1 expression is related to the strength of TCR signaling, and thus to the functional avidity of specific T cells, underlining the complex significance of PD-1 expression on tumor-specific T cells, also finely regulated by genetic and epigenetic dynamic mechanisms.Citation17 In this line of thoughts, the inability to identify exhausted or activated T cells based on the sole expression of inhibitory/co-stimulatory-receptors has been recently demonstrated. Transcriptomic analyses raised the possibility to uncouple activation and exhaustion gene programs in CD8+ T cells and provided new insight in the understanding of molecular mechanisms of CD8+ T cell dysfunction.Citation18,19 Thus PD-1 expression can also be considered as a marker of activated tumor-reactive T cells.
Nonetheless, immune tumor escape is a dynamic process involving the induction of an immunosuppressive microenvironment in which PD-1/PD-L1 signaling pathway plays definitively a multilayered role. In a growing numbers of solid tumors, targeting this pathway with PD-1 or PD-L1 specific antibodies progressively transformed patient's management and led to unprecedented clinical responses in a large spectrum of advanced human cancers.Citation20 In US and Europe, anti-PD-1 immunotherapy is used as a first line of treatment of metastatic melanoma patients. Despite its undisputed superior clinical efficacy compared with chemo- or radiotherapy, anti-PD-1 monotherapy remains inefficient on more than 60% of cancer patients. Acute toxicities are less common (around 14% of treated patients) than those reported for anti-CTLA-4 therapy but remain an appreciable risk for patients. For these reasons, defining early and robust predictive markers of clinical response is crucial both to improve patient's management and to reduce treatment costs. Furthermore, the comprehensive analysis of PD-1 regulation and signaling will also have a considerable impact on the optimization of other immunotherapies such as T-cell-based immunotherapies. Indeed, it could help define the best T cell subset to be used in adoptive cell transfer treatment in combination with check-point blockade.
This review will focus on this dual role of PD-1 as a reflect of efficient T cell activation and also of T cell dysfunction, and how these properties could be further exploited to improved the clinical efficacy of T-cell-based therapies.
Regulation of PD-1 expression
PD-1 expression on T cells is intricately regulated by different genetic and epigenetic programs adapted to either transient or chronic antigenic stimulations. PD-1 is rapidly induced on T cells following TCR-mediated activationCitation5 and this expression decreases with antigen clearance. In contrast, PD-1 expression is maintained on antigen-specific T cells in chronic disease settings and has been associated with a progressive loss of T-cell functions.
Oestreich and coll. described, in mice, 2 regulatory regions in Pdcd1 promoter, CR-C and CR-B, important crucial for Pdcd1 gene transcription. These regions, highly conserved in humans, contained numerous potential binding sites for transcription factors and CR-C, a CpG island, was mandatory for PD-1 expression.Citation21 In naïve T cells, CR-B and CR-C are highly methylated whereas following first antigen encounter, both regions are demethylated and concomitant coinciding with PD-1 expression. After antigen clearance, Pdcd1 promoter is progressively remethylated in effector or memory cells while Pdcd1 locus remained largely demethylated in hyporesponsive T cells during chronic antigen exposure.Citation6,22,23 This Pdcd1 gene demethylation was suggested as an active process, sensitive to TCR-mediated T-cell activation.Citation24
NFAT transcription factors were recently proposed as key modulators of this effector versus hyporesponsiveness T-cell states. Martinez and coll. described NFATs as an early transcriptional checkpoint progressively driving exhaustion.Citation25 NFATs are quickly activated in T cells following TCR stimulation. In effector T cells, NFATs form a protein complex with AP-1 (c-Fos and Jun proteins) induced by appropriate co-stimulation signaling and therefore regulate effector genes and T-cell functions.Citation26 In exhausted T cells, NFATs are predominantly “partnerless” thus binding to monomeric NFAT1 binding elements and promoted the activation of a transcriptional program associated with T cell dysfunction ().
Figure 1. Mechanisms leading to transitory or sustained PD-1 expression in activated and exhausted T cells. Left panel: Upon TCR-mediated stimulation, NFAT is dephosphorylated and translocated into the nucleus, where, upon association with AP-1 complex activated upon CD28 signaling, it drives effector gene and PD-1 expression. Right panel: In the context of chronic antigen stimulation, sustained TCR signaling leads to a continuous PD-1 expression. Upon PD-L1 ligation, induced by IFN-γ in the microenvironment, PD-1 pathway inhibits TCR and CD28 signaling, that decreases AP-1 activation. Once translocated into the nucleus, NFAT is mainly “partnerless” and drives exhaustion genes and a constant PD-1 expression, facilitated by a constitutively demethylated PDCD1 promoter.
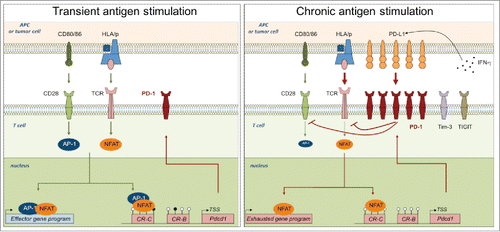
NFAT1 and NFAT2 were also described previously to directly bind Pdcd1 promoter and activate its transcription.Citation21 The mutation of NFAT1 binding site on the CR-C region completely abrogated PD-1 expression in a mouse model. NFAT1 rapidly activates PD-1 expression following TCR stimulation and this is via NFAT activation and nuclear translocation that PD-1 expression reflected the strength of TCR stimulation integrated by T cells. In absence of further activation signals, Blimp-1 actively repressed NFAT1 expression and modified the chromatin structure at PD-1 locus therefore down-regulating PD-1 expression.Citation27
Recent studies described, exclusively in exhausted T cells, a transcription enhancer in Pdcd1 gene implicated in PD-1 sustained expression. This activation region is equally accessible in cells genetically modified to express a NFAT1 protein unable to interact with AP-1 suggesting a role for the partnerless NFAT1 in the maintenance of PD-1 expression in hyporesponsive T cells.Citation28Citation-30 Fox01 transcription factor was also accumulated in turn of PD-1/PD-L1 signalisation and Fox01 directly sustained PD-1 expression.Citation31 Furthermore, PD-1 inhibitory signaling was shown to upregulate BATF expression and to inhibit CD28 positive co-stimulation.Citation8,32 These 2 mechanisms notably reduce AP-1 availability within the nucleus and favor T cell loss of function illustrating the feed-forward loop regulated by PD-1 upon chronic antigen stimulation.
This complex regulation of PD-1 expression clearly shows that PD-1 expression status alone cannot discriminate between exhausted and activated T cells, that are the result of distinct genetic and epigenetic programs, dictated by TCR signaling strength and microenvironment.
PD-1 expression and anti-tumor response
Although inhibiting T cell responses upon ligation to its ligands, PD-1 expression is the reflect of T cell activation. In HPV-positive head and neck cancers, it has been documented that a favorable clinical outcome was associated with a strong infiltration of activated PD-1+ T cells, able to get reinvigorated upon PD-1 blockade.Citation33 Same results have also been reported from non virus-induced solid tumors. In melanoma, PD-1 expression identified tumor reactive CD8+ T cells, within tumor infiltrating lymphocytes (TIL) derived from melanoma patients.Citation14 Furthermore, only this PD-1 positive fraction contains T lymphocytes specific for neoantigens, potentially expressing a high affinity TCR. These T lymphocytes are able to eliminate autologous tumor cells, despite their PD-1 expression.Citation14,16 PD-1 expression is proportional to the strength of TCR signaling to compensate T cell activation and to control immune response.Citation21 Furthermore, PD-1+ T-cell fraction was largely pauciclonal (TCRβ repertoire) suggesting an antigen-driven amplification of those PD-1+ T-cell clonotypes within the tumor. In addition, expression of PD-1 on circulating T cells also identifies patient-specific antitumor T cell response, similar to that detected within TIL.Citation16
In this line, we demonstrated the correlation between the expression of PD-1 by human CD8+ T cell clones specific for the shared melanoma antigens Melan-A,Citation34,35 and MELOE-136 and their high functional avidity.Citation15 We described the existence of melanoma-specific T cell clones (isolated from the blood of patients and healthy donors or from the TILs) unable to induce PD-1 after T cell stimulation. This absence of PD-1 expression was due to the persistent methylation of Pdcd1 promoter. These PD-1neg T cell clones were not susceptible to PD-L1 negative signaling but they exhibited lower anti-tumor reactivity than their PD-1pos counterparts in the absence of PD-L1 signaling. Logically, PD-1pos T cell clones exhibited greater functional avidity than PD-1neg T cell clones suggesting their interest for adoptive cell transfer (ACT) protocols. In this study, we also modulated PD-1 signaling during the melanoma-specific T cells production procedures with the addition of anti-PD-1 blocking antibody. Recovered specific-T cell Vβ repertoire, was different from that obtained in the control condition with the preferential expansion of clonotypes exhibiting high functional avidities that could potentially lead to a better anti-tumor activity in vivo, in combination with PD-1 blockade or gene inactivation in these T cells. Another recent study, deciphering the kinetics of TCR binding to peptide-major histocompatibility molecules (pMHC) with NTAmers resonates with these results. Indeed, this work documents that TCR-pMHC off-rate, associated with CD8+ T cell potency, closely follows costimulatory/coinhibitory receptor expression in activated CD8+ T cells, among them PD-1 expression.Citation37
The relevance of PD-1 expression to identify highly reactive specific T cells has also been underlined in vaccination studies. In mice engrafted with epithelial tumors expressing HPV-associated E7 protein, vaccination with E7 protein induced the proliferation of PD-1+ specific CD8+ T cells, partly inhibiting tumor growth. In this setting, PD-1 blockade synergized with vaccine in eliciting antitumor efficacy.Citation33 Another recent study demonstrated that vaccination with altered peptide ligands with optimized CMH or TCR affinity led to a strong but inefficient specific CD8 T cell response, impaired by a sustained expression of PD-1 (and other inhibitory receptors) by stimulated specific T cells.Citation38 The anti-tumor efficiency of such vaccines could thus be improved through a combination therapy targeting PD-1/PD-L1 signaling pathway.
All these studies concur to demonstrate the complex significance of PD-1 expression on tumor-specific T cells,Citation17 particularly because of its dynamic and fine regulation by genetic and epigenetic mechanisms. Globally, the expression of inhibitory receptors (among them PD-1) is a common feature of activated and exhausted T cells and recent single cell transcriptomic studies demonstrated that the sole expression of these inhibitory receptors does not allow discriminating these 2 functional status. The exhausted status of T lymphocytes can be documented on the basis of specific gene expression profile, notably involved in zinc metabolism.Citation18,39
Thus, setting up combination strategies associating PD-1 blockade and ACT with PD-1+ high avidity specific T-cells or vaccination strategies inducing such a CTL response would be a relevant approach to improve anti-tumor responses.
PD-1 expression by CD8 T lymphocytes in patients treated by anti-PD-1 therapy
The vast majority of studies about anti-PD-1 therapy, mainly documented immune markers related to the mode of action of PD-1 in the regulation of anti-tumor T cell response.Citation40 PD-1 regulates tumor-specific T cell response presumably on the tumor site, through the inhibition of specific T cell activation (Chen 2015). Therefore, the presence of a pre-existing CD8+ T cell infiltrate,Citation41 along with an increased infiltration by CD8+ T cells upon anti-PD-1 therapy have been associated with therapeutic responses. Indeed, several studies demonstrated that regressive lesions were densely infiltrated by cytotoxic CD8+ T cells.Citation42,43 More precisely, it has been documented that therapeutic responses were associated with the presence of PD-1 expressing CD8+ T cells at the tumor margin, before therapy, co-localized with PD-L1 expressing tumor cells.Citation41 Following anti-PD-1 treatment, in responding patients, cytotoxic CD8+ T cells accumulated within the tumor. This CD8+ T cell infiltrate (pre-existing or accumulating in tumors upon treatment) exhibited a low TCRß diversity, suggesting a clonal expansion of tumor-specific T cells. In addition, IFN-γ associated gene expression was also documented in patients responding to anti-PD-1 therapy.Citation44
These different studies concur to define an “active” tumor microenvironment, associating the accumulation of PD-1+ CD8+ activated effector T cells, the presence of IFN-γ, the activation of IFN-γ responsive genes and thus the expression of PD-L1 by tumor cells. Anti-PD-1 therapy could reinvigorate pre-existing tumor-specific T cell by removing the inhibition induced by the activation of PD-1/PD-L1 axis, and finally induce tumor rejection. This active microenvironment (present in about 38% of cancer patients) defines a sub-group able to respond to anti-PD-1 therapy.Citation45 A recent study confirmed the importance of this active microenvironment in melanoma tumors, identified by the expression of class II HLA on melanoma cells, and correlating with the expression of genes associated with T cell activation, notably PD-1 gene.Citation46 Thus, class II HLA expression, detected by immunohistochemistry on melanoma tumors could be a relevant immune marker for therapeutic decision support, associated with PD-1 expression on effector T cells.
In addition to the significance of PD-1+ tumor infiltrating CD8+ T cells in predicting anti-PD-1 therapeutic response, the presence of activated PD-1+ CD8+ T cells in patient blood was also associated with therapeutic efficacy. Indeed, most non-small cell lung cancer (NSCLC) patients receiving PD-1 therapy exhibited an early increase in circulating Ki67+ PD-1+ CD8+ T cells.Citation47 These effector-memory T cells are most likely recirculating tumor specific T cells, reinvigorated upon PD-1 blockade. Interestingly, this T cell subset was preferentially detected in responding patients, after the first cycle of anti-PD-1 administration, and could represent an immune biomarker of anti-PD-1 therapeutic efficacy.
PD-1 expression as a marker identifying efficient CD8 T cells for T-cell-based immunotherapy
The adoptive transfer of tumor-specific T lymphocytes is a therapeutic strategy that demonstrated a relative efficacy, notably for metastatic melanoma. This strategy aims at transferring high amounts of tumor-reactive lymphocytes, amplified and selected ex-vivo, to circumvent local tolerance mechanisms and eradicate tumor cells. The therapeutic efficacy of ACT relies both on antigenic specificity of infused T cells and on their functional properties (tumor reactivity, persistence, migration…). Several selection strategies of tumor-reactive T cells have been tested based on the expression of activation receptors (CD25, PD-1, 4–1BB…), the anti-tumor reactivity (cytokine secretion), or their specificity (HLA-peptide multimer sortingCitation48). In the periphery the initial frequency of circulating tumor-specific T cells could represent a limitation for the production of high amounts of specific T cells, with preserved properties of expansion and in vivo persistence. The genetic transfer of specificity in primary T cells or other T cell subsets through the use of TCR or CAR transduction allows bypassing this limitation. Whatever the approach for the production of tumor-specific T cells for T-cell-based therapy, a crucial issue is to identify the most relevant T-cell subset to select for ex-vivo amplification. In this line of thoughts, 2 recent studies investigated the therapeutic potential of T-cell-based therapy using PD-1-selected TIL, and came to the same conclusion. They documented, in myeloma and melanoma mouse models, that only T cells from the PD-1 positive fraction exhibited tumor reactivity and that their adoptive transfer to tumor-bearing mice resulted in tumor control, in contrast to the adoptive transfer of PD-1 negative T lymphocytes from the same TIL population.Citation49,50 Furthermore, PD-1+ transferred T cells were able to persist in vivo and to mount an adaptive memory immune response against the tumor.Citation50 In both studies, anti-tumor efficacy was further enhanced upon combination with PD-1 blockade. In the same line, and starting from the vast T cell repertoire specific for Melan-A antigen, we recently showed that PD-1 expression identified a peculiar specific T cell repertoire, exhibiting higher functional avidity and functional properties of melanoma specific effector T cells. We further documented in vitro that PD-1 blockade during a GMP-compliant procedure to produce Melan-A specific T cells for ACT, favored the amplification of peculiar TRCß subfamilies, with better functional avidity against their cognate antigen.Citation15 All these studies concur to strengthen the rationale for the use of PD-1 selected T lymphocytes for T-cell-based therapy. However, a major drawback for the use of PD-1 positive T cells for ACT purposes could be their impaired functions in vivo. To preserve the functions of these T cells after transfer, therapeutic combinations with PD-1 or PD-L1 blockade are explored, together with the possibility to inactivate PD-1 gene in selected T cells, before transfer. This latter perspective would offer twin benefits: first, the autoimmune adverse effects of the systemic infusion of anti-PD-1 would be avoided and the cost of the treatment would be significantly reduced. On this topic, TALEN or CRISPR-Cas9 genome editing tools provided an unprecedented and promising technological breakthrough modifying selected human T cell subsets to improve the anti-tumor efficiency of ACT treatments.Citation51 The successful use of this technology has been first reported for the silencing of CCR5 gene in HIV-1-susceptible human CD4+ T cells.Citation52 The feasibility of PD-1 inactivation following CRISPR/CAS9 edition has been initially reported in human primary T cells,Citation53 and resulted in a significant decrease of PD-1 expression on edited T cells, associated with enhanced IFN-γ production and cytotoxicity, without affecting the viability of these T cells. The edition of PD-1 gene with the TALEN technology has also be tested in polyclonal tumor-reactive T cells, from mice engrafted with fibrosarcoma cells.Citation54 The adoptive transfer of these PD-1 edited T cells in tumor-bearing mice resulted in tumor rejection and long-term protective memory. Thus, the edition of genes coding PD-1 and other inhibitory receptors could be done in high avidity tumor-specific T cells, selected on the basis of PD-1 expression, but also in the highly reactive CAR- or TCR transduced-T cells (). Indeed, a recent study illustrated the feasibility to inactivate PD-1 gene in human CD19-CAR-T cells. This inactivation led to an enhanced cytotoxicity of CAR-T cells against tumor cells in vitro, and an improved tumor reactivity in vivo against tumor xenografts.Citation55
Figure 2. PD-1 based selection and inactivation for optimal T-cell based therapies. 1/ High avidity tumor specific T cells can be expanded from the CD8+ PD-1+ TIL fraction or after antigen stimulation of PBMC upon PD-1 blockade that favors the amplification of highly reactive CD8+ T cells. 2/ PD-1+ recovered high avidity T cells, CAR-T cells or TCR-transduced T cells can be further inactivated for PD-1 expression (and other inhibitory receptors) by genome editing, to bypass immunosuppression mechanisms. 3/ High avidity tumor specific T cells inactivated for PD-1 expression can be infused to cancer patients, alone or in combination with other therapies such as radioimmunotherapy.
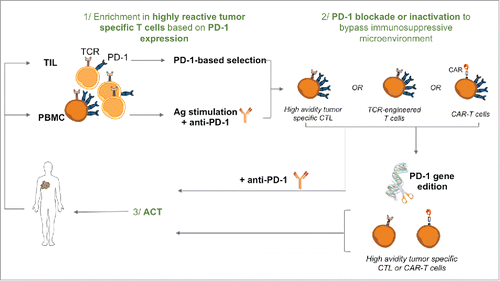
Furthermore, the transfer of genome-edited T cells to humans recently passed a key safety review in the USA, by the Recombinant DNA Advisory Committee (RAC) at the US. National Institutes of Health. This decision will accelerate the clinical transfer of new immunotherapy approaches, combining the selection of highly tumor-reactive T cell subset (based on the expression of PD-1 and potentially of other co-stimulation molecules) and genome engineering to counteract the tumor microenvironment driven immunosuppression.
Conclusion
In conclusion the fine regulation of PD-1 expression on tumor-specific T cells makes this molecule a valuable marker to select high avidity-specific T lymphocytes, for immunotherapy purposes. Furthermore, to prevent autoimmune adverse events related to anti-PD-1 systemic therapy, genome edition of specific T lymphocytes targeting immune checkpoint inhibitors appears a promising option, while leaving the possibility to associate another combination therapy.
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was performed in the context of the LabEX IGO program supported by the National Research Agency via the investment of the future program ANR-11-LABX-0016–01. Sylvain Simon is supported by a specific funding from the LabEx IGO program. This work was also supported by grants from the «Ligue contre le Cancer, Comite 44», the canceropole Grand-Ouest and the DHU Onco-greffe.
References
- Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11:3887-95. PMID:1396582
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999;11:141-51. doi:10.1016/S1074-7613(00)80089-8. PMID:10485649
- Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281-92. doi:10.1084/jem.20061496. PMID:16954372
- Liu Y, Yu Y, Yang S, Zeng B, Zhang Z, Jiao G, Zhang Y, Cai L, Yang R. Regulation of arginase I activity and expression by both PD-1 and CTLA-4 on the myeloid-derived suppressor cells. Cancer Immunol Immunother. 2009;58:687-97. doi:10.1007/s00262-008-0591-5. PMID:18828017
- Chikuma S, Terawaki S, Hayashi T, Nabeshima R, Yoshida T, Shibayama S, Okazaki T, Honjo T. PD-1-mediated suppression of IL-2 production induces CD8+ T cell anergy in vivo. J Immunol. 2009;182:6682-9. doi:10.4049/jimmunol.0900080. PMID:19454662
- Youngblood B, Oestreich KJ, Ha S-J, Duraiswamy J, Akondy RS, West EE, Wei Z, Lu P, Austin JW, Riley JL, et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity. 2011;35:400-12. doi:10.1016/j.immuni.2011.06.015. PMID:21943489
- Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209:1201-17. doi:10.1084/jem.20112741. PMID:22641383
- Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355:1428-33. doi:10.1126/science.aaf1292. PMID:28280247
- Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537-44. doi:10.1182/blood-2008-12-195792. PMID:19423728
- Fourcade J, Kudela P, Sun Z, Shen H, Land SR, Lenzner D, Guillaume P, Luescher IF, Sander C, Ferrone S, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol. 2009;182:5240-9. doi:10.4049/jimmunol.0803245. PMID:19380770
- Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175-86. doi:10.1084/jem.20100637. PMID:20819923
- Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ, et al. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J Clin Invest. 2015;125:2046-58. doi:10.1172/JCI80445. PMID:25866972
- Inozume T, Hanada K-I, Wang QJ, Ahmadzadeh M, Wunderlich JR, Rosenberg SA, Yang JC. Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J Immunother. 2010;33:956-64. doi:10.1097/CJI.0b013e3181fad2b0. PMID:20948441
- Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124:2246-59. doi:10.1172/JCI73639. PMID:24667641
- Simon S, Vignard V, Florenceau L, Dreno B, Khammari A, Lang F, Labarriere N. PD-1 expression conditions T cell avidity within an antigen-specific repertoire. Oncoimmunology. 2016;5:e1104448. doi:10.1080/2162402X.2015.1104448. PMID:26942093.
- Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016;22:433-8. doi:10.1038/nm.4051. PMID:26901407
- Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265-76. doi:10.1016/j.it.2015.02.008. PMID:25797516.
- Singer M, Wang C, Cong L, Marjanovic ND, Kowalczyk MS, Zhang H, Nyman J, Sakuishi K, Kurtulus S, Gennert D, et al. A Distinct Gene Module for Dysfunction Uncoupled from Activation in Tumor-Infiltrating T Cells. Cell. 2016;166:1500-9. doi:10.1016/j.cell.2016.08.052. PMID:27610572
- Wang C, Singer M, Anderson AC. Molecular Dissection of CD8(+) T-Cell Dysfunction. Trends Immunol. 2017;. doi:10.1016/j.it.2017.05.008
- Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125:3384-91. doi:10.1172/JCI80011. PMID:26325035
- Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 2008;181:4832-9. doi:10.4049/jimmunol.181.7.4832. PMID:18802087
- Youngblood B, Noto A, Porichis F, Akondy RS, Ndhlovu ZM, Austin JW, Bordi R, Procopio FA, Miura T, Allen TM, et al. Cutting edge: Prolonged exposure to HIV reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J Immunol. 2013;191:540-4. doi:10.4049/jimmunol.1203161. PMID:23772031
- Ahn E, Youngblood B, Lee J, Lee J, Sarkar S, Ahmed R. Demethylation of the PD-1 Promoter Is Imprinted during the Effector Phase of CD8 T Cell Exhaustion. J Virol. 2016;90:8934-46. doi:10.1128/JVI.00798-16. PMID:27466420
- McPherson RC, Konkel JE, Prendergast CT, Thomson JP, Ottaviano R, Leech MD, Kay O, Zandee SEJ, Sweenie CH, Wraith DC, et al. Epigenetic modification of the PD-1 (Pdcd1) promoter in effector CD4(+) T cells tolerized by peptide immunotherapy. Elife. 2014;3:765. doi:10.7554/eLife.03416
- Martinez GJ, Pereira RM, Äijö T, Kim EY, Marangoni F, Pipkin ME, Togher S, Heissmeyer V, Zhang YC, Crotty S, et al. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity. 2015;42:265-78. doi:10.1016/j.immuni.2015.01.006. PMID:25680272
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227-42. doi:10.1038/nri3405. PMID:23470321
- Lu P, Youngblood BA, Austin JW, Mohammed AUR, Butler R, Ahmed R, Boss JM. Blimp-1 represses CD8 T cell expression of PD-1 using a feed-forward transcriptional circuit during acute viral infection. J Exp Med. 2014;211:515-27. doi:10.1084/jem.20130208. PMID:24590765
- Scott-Browne JP, López-Moyado IF, Trifari S, Wong V, Chavez L, Rao A, Pereira RM. Dynamic Changes in Chromatin Accessibility Occur in CD8(+) T Cells Responding to Viral Infection. Immunity. 2016;45:1327-40. doi:10.1016/j.immuni.2016.10.028. PMID:27939672
- Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354:1160-5. doi:10.1126/science.aaf2807. PMID:27789795
- Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, Tsao H-W, Godec J, LaFleur MW, Brown FD, et al. The epigenetic landscape of T cell exhaustion. Science. 2016;354:1165-9. doi:10.1126/science.aae0491. PMID:27789799
- Staron MM, Gray SM, Marshall HD, Parish IA, Chen JH, Perry CJ, Cui G, Li MO, Kaech SM. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8(+) T cells during chronic infection. Immunity. 2014;41:802-14. doi:10.1016/j.immuni.2014.10.013. PMID:25464856
- Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med. 2010;16:1147-51. doi:10.1038/nm.2232. PMID:20890291
- Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128-38. doi:10.1158/0008-5472.CAN-12-2606. PMID:23135914
- Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, Appella E, Rosenberg SA. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med 1994;180:347-52. doi:10.1084/jem.180.1.347. PMID:7516411
- Coulie PG, Brichard V, Van Pel A, Wolfel T, Schneider J, Traversari C, Mattei S, De Plaen E, Lurquin C, Szikora JP, et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med 1994;180:35-42. doi:10.1084/jem.180.1.35. PMID:8006593
- Godet Y, Moreau-Aubry A, Guilloux Y, Vignard V, Khammari A, Dreno B, Jotereau F, Labarriere N. MELOE-1 is a new antigen overexpressed in melanomas and involved in adoptive T cell transfer efficiency. J Exp Med. 2008;205:2673-82. doi:10.1084/jem.20081356. PMID:18936238
- Allard M, Couturaud B, Carretero-Iglesia L, Duong MN, Schmidt J, Monnot GC, Romero P, Speiser DE, Hebeisen M, Rufer N. TCR-ligand dissociation rate is a robust and stable biomarker of CD8+ T cell potency. JCI Insight. 2017;2. doi:10.1172/jci.insight.92570. PMID:28724801
- Zahm CD, Colluru V, McNeel DG. Vaccination with High-Affinity Epitopes Impairs Antitumor Efficacy by Increasing PD-1Expression on CD8+ T Cells. Cancer Immunol Res. 2017;: canimm.0374.2016. doi:10.1158/2326-6066.CIR-16-0374. PMID:28634215
- Tirosh I, Izar B, Prakadan SM, Wadsworth MH, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189-96. doi:10.1126/science.aad0501. PMID:27124452
- Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275-87. doi:10.1038/nrc.2016.36. PMID:27079802
- Tumeh, P. C, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568-71. doi:10.1038/nature13954. PMID:25428505
- Hamid O, Robert C, Daud A, Hodi FS, Hwu W-J, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-44. doi:10.1056/NEJMoa1305133. PMID:23724846
- Ribas A, Shin DS, Zaretsky J, Frederiksen J, Cornish A, Avramis E, Seja E, Kivork C, Siebert J, Kaplan-Lefko P, et al. PD-1 Blockade Expands Intratumoral Memory T Cells. Cancer Immunol Res. 2016;4:194-203. doi:10.1158/2326-6066.CIR-15-0210. PMID:26787823
- Ribas A, Robert C, Hodi FS, Wolchok JD, Joshua AM, Hwu W-J, Weber JS, Zarour HM, Kefford R, Loboda A, et al. Association of response to programmed death receptor 1 (PD-1) blockade with pembrolizumab (MK-3475) with an interferon-inflammatory immune gene signature. JCO. 2015;33:3001-1
- Teng MWL, Ngiow SF, Ribas A, Smyth MJ. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75:2139-45. doi:10.1158/0008-5472.CAN-15-0255. PMID:25977340
- Johnson DB, Estrada MV, Salgado R, Sanchez V, Doxie DB, Opalenik SR, Vilgelm AE, Feld E, Johnson AS, Greenplate AR, et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun. 2016;7:10582. doi:10.1038/ncomms10582. PMID:26822383
- Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, Sica GL, Yu K, Koenig L, Patel NT, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci USA. 2017;114:4993-8. doi:10.1073/pnas.1705327114. PMID:28446615.
- Labarriere N, Fortun A, Bellec A, Khammari A, Dreno B, Saiagh S, Lang F. A full GMP process to select and amplify epitope-specific T lymphocytes for adoptive immunotherapy of metastatic melanoma. Clin Dev Immunol. 2013;2013:932318. doi:10.1155/2013/932318. PMID:24194775
- Fernandez-Poma SM, Salas-Benito D, Lozano T, Casares N, Riezu-Boj J-I, Mancheño U, Elizalde E, Alignani D, Zubeldia N, Otano I, et al. Expansion of Tumor-Infiltrating CD8(+) T cells Expressing PD-1 Improves the Efficacy of Adoptive T-cell Therapy. Cancer Res. 2017;77:3672-84. doi:10.1158/0008-5472.CAN-17-0236. PMID:28522749
- Jing W, Gershan JA, Blitzer GC, Palen K, Weber J, McOlash L, Riese M, Johnson BD. Adoptive cell therapy using PD-1(+) myeloma-reactive T cells eliminates established myeloma in mice. J Immunother Cancer. 2017;5:51. doi:10.1186/s40425-017-0256-z. PMID:28642819
- Lloyd A, Vickery ON, Laugel B. Beyond the antigen receptor: editing the genome of T-cells for cancer adoptive cellular therapies. Front Immunol. 2013;4:221. doi:10.3389/fimmu.2013.00221. PMID:23935598
- Wang W, Ye C, Liu J, Zhang D, Kimata JT, Zhou P. CCR5 gene disruption via lentiviral vectors expressing Cas9 and single guided RNA renders cells resistant to HIV-1 infection. PLoS ONE. 2014;9:e115987. doi:10.1371/journal.pone.0115987. PMID:25541967
- Su S, Hu B, Shao J, Shen B, Du J, Du Y, Zhou J, Yu L, Zhang L, Chen F, et al. CRISPR-Cas9 mediated efficient PD-1 disruption on human primary T cells from cancer patients. Sci Rep. 2016;6:20070. doi:10.1038/srep20070. PMID:26818188
- Menger L, Sledzinska A, Bergerhoff K, Vargas FA, Smith J, Poirot L, Pule M, Hererro J, Peggs KS, Quezada SA. TALEN-Mediated Inactivation of PD-1 in Tumor-Reactive Lymphocytes Promotes Intratumoral T-cell Persistence and Rejection of Established Tumors. Cancer Res. 2016;76:2087-93. doi:10.1158/0008-5472.CAN-15-3352. PMID:27197251
- Rupp LJ, Schumann K, Roybal KT, Gate RE, Ye CJ, Lim WA, Marson A. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017;7:737. doi:10.1038/s41598-017-00462-8. PMID:28389661
