ABSTRACT
Extracellular vesicles (EVs) have been discussed as a diagnostic tool for minimal residual disease (MRD) evaluation in breast cancer (BC) in addition to the analysis of circulating tumor cells (CTCs). Therefore, we investigated circulating EV levels as surrogate markers for disease monitoring and prediction of prognosis in primary, non-metastatic, locally advanced BC patients.
EVs were enriched from blood samples of BC patients before and after neoadjuvant chemotherapy (NACT) and from healthy females. EV marker expression analysis was performed and EV sizes and concentrations were determined by nanoparticle tracking analysis. The results were associated with disease status, outcome and CTC presence, evaluated by gene expression analysis after enrichment.
We demonstrated that i) the EV concentration was 40-fold higher in BC patients compared to healthy females, ii) the EV concentration increased during therapy, iii) an increased EV concentration pre-NACT was associated with therapy failure and iv) an elevated EV concentration post-NACT was associated with a reduced three-year progression-free and overall survival. Of note, residual stem cell-like and/or resistant CTCs after therapy were associated with a lower EV concentration post-NACT.
Our study highlights that the concentration of EVs within BC blood samples may serve as a complementary parameter reflecting the status of MRD as well as therapy and disease outcome in parallel with CTC investigation.
Introduction
Primary, non-metastatic breast cancer (BC) is considered as a systemic disease rather than a local disease, which implicates the requirement of early systemic, neoadjuvant therapies for treatment of systemic disease spread.Citation1 Originally, neoadjuvant chemotherapy (NACT) was used for the treatment of locally advanced and non-operable tumors, but presently, NACT has become a standard treatment in primary BC.Citation1,Citation2 Depending on the intrinsic BC subtype, a pathological complete response (pCR) can be achieved in a range from 7.5 – 36.4% being associated with an improved progression-free (PFS) and long-term survival.Citation3,Citation4 Despite high overall survival (OS) rates, a metastatic relapse occurs in 20% of all BC patients.Citation3
Routine BC diagnostics do not elucidate the complex intra-tumor heterogeneity and explain BC tumor biology. Therefore, potent non-invasive biomarkers are needed to assess the risk of recurrence. Extracellular vesicles (EVs) and circulating tumor cells (CTCs) have been discussed to be promising candidates.Citation5 For CTCs, the prognostic relevance has even already been demonstrated in early BC.Citation6,Citation7 EVs, such as exosomes and microvesicles, could be of further interest and relevance. These vesicles are released by all cell types including tumor cells and can be isolated from all body fluids.Citation8 EVs are able to fuse with local or distant recipient cells thereby altering their cellular phenotypes and biological properties.Citation9,Citation10
In vitro studies demonstrated that EVs derived from tumor cell lines contribute to tumor invasion,Citation11 chemoresistance,Citation12,Citation13 angiogenesis,Citation14 metastasis or immune escape.Citation15-17 BC-derived EVs are able to induce a neoplastic phenotype in fibroblastsCitation18 and mesenchymal stem cells.Citation19 EVs shed by BC cells are able to interact with normal breast cells, enhancing their proliferation, migration and invasiveness.Citation20 Further, BC-derived EVs can mediate drug resistance transfer from chemotherapy resistant BC cells to non-resistant BC cells via P-Glycoproteins or microRNAs.Citation21-23 Additionally, BC specific targeted drugs are reported to be neutralized by EVs expressing the drugs’ target protein.Citation24
Clinical studies analyzing tumor-derived EVs associated biomarkers have been conducted for quite a few tumor entities.Citation25 Up to now, we are not aware of any study analyzing the circulating EV concentration and size from BC patient samples. In our study, we enriched and analyzed for the first time the circulating EVs from 105 paired, locally advanced, NACT treated BC patients before and after therapy. We analyzed the recorded EV parameters in association with i) routinely determined clinical BC parameters, ii) their prognostic significance regarding PFS and OS and iii) CTC presence.
Results
Characterization of EVs in BC patients and healthy females
To verify the presence of EVs in the samples, SDS-Page and western blot () were performed for the detection of typical EV markers that included the tetraspanins CD9, CD63, CD81, ESCRT-associated proteins TSG101 and Syntenin as well as HSP70. Cytochrome C served as a negative control for cellular protein contamination.Citation26,Citation27 Characteristic EV markers were present in all sample preparations, but in different expression levels (Suppl. Table 1). The absence of Cytochrome C detection from EV samples of BC patients and healthy controls demonstrate that the samples were free of cellular protein contamination. Nanoparticle tracking analysis (NTA) revealed that EV particles size did not differ between pre- or post-NACT BC patient samples (), whereas the EV particle concentration differed substantially (). Healthy females displayed 40-fold lower EV particle concentrations in comparison to BC patient samples procured before and after therapy (p < 0.0001). Overall, the EV particle concentrations were significantly (p = 0.0082) increased in BC samples obtained post-NACT compared to pre-NACT. With the exception of patients who underwent hormonal therapy or chemotherapy combined with Avastin the clinical subgroup analyses revealed elevated EV levels after NACT without always reaching statistical significance (Suppl. Table 2).
Figure 1. EV characterization by western blot and nanoparticle tracking analysis. (A) EV marker expression analysis for HSP70, CD63, TSG101, Syntenin, CD9, CD81, Cytochrome C (Cyt C) in EV fractions from two healthy persons (NP1, NP2), three BC patients pre-NACT (1-3) and post-NACT (4-6). In all analyses, HEK cell culture supernatant derived EVs served as positive control, for Cyt C detection MCF-7 cell lysate (*) was used as control. (B) The EV size [median (interquartile range) nm] did not differ pre- and post-NACT (Mann-Whitney test). (C) Healthy females presented lower EV levels [median (interquartile range) 109/ml] compared to BC patients pre- and post-NACT. EV levels increased post-NACT in BC patients (Mann-Whitney test).
![Figure 1. EV characterization by western blot and nanoparticle tracking analysis. (A) EV marker expression analysis for HSP70, CD63, TSG101, Syntenin, CD9, CD81, Cytochrome C (Cyt C) in EV fractions from two healthy persons (NP1, NP2), three BC patients pre-NACT (1-3) and post-NACT (4-6). In all analyses, HEK cell culture supernatant derived EVs served as positive control, for Cyt C detection MCF-7 cell lysate (*) was used as control. (B) The EV size [median (interquartile range) nm] did not differ pre- and post-NACT (Mann-Whitney test). (C) Healthy females presented lower EV levels [median (interquartile range) 109/ml] compared to BC patients pre- and post-NACT. EV levels increased post-NACT in BC patients (Mann-Whitney test).](/cms/asset/21d20d68-e16a-4209-9d8f-d5898636b1a4/koni_a_1376153_f0001_b.gif)
Table 1. Association of pre- and post-NACT EV levels with clinical parameters.
Table 2. Post-NACT EV level cut-off discriminates between CTC subtypes. BC patients having post-NACT EV levels below the cut-off had a higher incidence of SL-CTCs, ALDH-1 positivity, ERCC1 positivity and ALDH-1/ERCC1 positivity. Data was evaluated by Fisher's exact test.
Increased EV concentrations pre-NACT are associated with lymph node infiltration and therapy failure
The association of EV particle concentrations obtained pre- and post-NACT with clinical parameters () showed that pre-NACT EVs levels increased with the number of infiltrated axillary lymph nodes pre- and post-NACT (p < 0.05). BC patients who did not respond to NACT, showed significantly (p < 0.05) higher pre-NACT EV concentrations compared to patients responding to the therapy. All other clinical parameters were not related to the EV concentration pre-NACT.
Increased EV concentrations post-NACT are associated with ER expression as well as reduced three-year progression-free and overall survival
Samples from post-NACT BC patients whose tumors were estrogen-receptor (ER) positive showed significantly (p < 0.05) elevated circulating EV concentrations when compared to samples from patients with ER negative tumors (). Further, the post-NACT EV concentration was associated with a reduced 3y-PFS and OS. A lower EV concentration post-NACT was related to survival of BC patients (p < 0.05) and the same was observed regarding 3y-PFS (p < 0.01). No association between EV particle concentrations determined post-NACT and other clinical parameters was observed.
Determination of EV concentrations as a prognostic marker for disease progression and outcome
Due to specific associations between EV particle concentrations post-NACT and 3y-PFS, ROC analysis was performed to define an optimal cut-off value (AUC = 0.800, sensitivity: 100.0%, specificity: 55.4%, p = 0.005) for risk assessment regarding progression prediction of BC patients post-NACT (). Kaplan-Meier Curve analyses combined with the log-rank test revealed that EV particle concentrations > 2484 ×109 EVs/ml post-NACT were significantly [p = 0.003; Hazard Ratio (HR): 8.27, 95% confidence interval (CI): 2.05 – 33.32] associated with a reduced 3y-PFS ().
Figure 2. ROC analyses for cut-off determination for EV levels pre- and post-NACT and Kaplan-Meier survival analysis regarding 3y-PFS and OS. (A)/(C) By ROC analysis a consistent cut-off value of 2484 ×109/ml for EV levels post-NACT was obtained for 3y-PFS and OS. (B/D) In Kaplan Meier analysis combined with the log rank test, BC patients having EV levels post-NACT above the determined cut-off value had a significantly reduced 3y-PFS and OS.
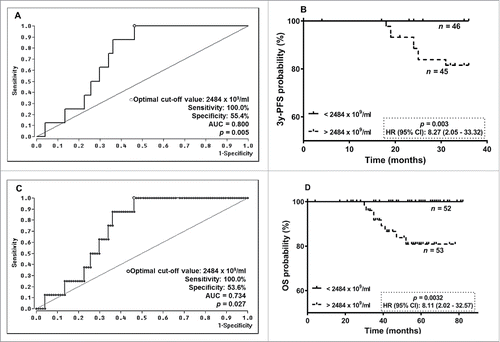
Regarding OS, an identical cut-off value of EV particle concentration post-NACT was calculated by ROC analysis (AUC = 0.734, sensitivity: 100.0%, specificity: 53.6%, p = 0.027, ). Patients with EV concentrations above 2484 ×109 EVs/ml after NACT (p = 0.0032; HR: 8.11, 95% CI: 2.02 – 32.57) had a reduced OS ().
Decreased EV concentrations post-NACT are associated with residual stem cell-like or resistant like CTCs after therapy
The EV concentration pre-NACT was not associated with the presence of a certain CTC subpopulation. Reduced concentrations of EVs obtained post-NACT were associated with the presence of certain CTC subpopulations. The presence of stem cell-like CTCs (SL-CTCs) was related to lower EV concentrations (p = 0.0815) post-NACT (). BC patients with ALDH-1 positive CTCs had significantly decreased EV concentrations post-NACT compared to patients with ALDH-1 negative CTCs (p = 0.0173, ). ERCC1 positive CTCs () as well as all other determined CTC marker post-NACT (e.g. PI3K, Akt2, TWIST) were not associated with EV concentrations after therapy (data not shown). Simultaneous ALDH-1/ERCC1 positivity post-NACT was associated (p = 0.0132) in the same manner (). Accordingly, using the cut-off value of OS and PFS, it became evident that the incidence of SL-CTC [73%, p = 0.0463], ALDH-1 positivity [86%, p = 0.0367] was higher in patients with EV concentrations below the cut-off value than in the ones above the cut-off. The combination of ALDH-1/ERCC1 positivity (65%, p = 0.0085) was associated in the same manner ().
Figure 3. Association between CTC subtypes and EV levels post-NACT. EV concentrations post-NACT were reduced if the following CTC subpopulations were detectable: (A) SL-CTCs, (B) ALDH-1 positive, (C) ERCC1 positive and (D) ALDH-1/ERCC1 positive. Data is shown as EV levels [median (interquartile range) 109/ml] and was evaluated by Mann-Whitney test. The dotted line indicates the cut-off value of 2484 ×109 EVs/ml.
![Figure 3. Association between CTC subtypes and EV levels post-NACT. EV concentrations post-NACT were reduced if the following CTC subpopulations were detectable: (A) SL-CTCs, (B) ALDH-1 positive, (C) ERCC1 positive and (D) ALDH-1/ERCC1 positive. Data is shown as EV levels [median (interquartile range) 109/ml] and was evaluated by Mann-Whitney test. The dotted line indicates the cut-off value of 2484 ×109 EVs/ml.](/cms/asset/7daa29da-1451-47b3-87d1-6b5e1ee17ec1/koni_a_1376153_f0003_b.gif)
Discussion
EVs have been reported as important mediators in BC development, maintenance and progression. Therefore, we hypothesize that the number of in vivo circulating EVs reflects the status of MRD and its outcome in BC patients. To the best of our knowledge, we are the first group to demonstrate a clinical relevance of EV concentrations before and after therapy in neoadjuvant treated BC patients. Indeed, the results of our study reveal that i) BC patients have a 40-fold higher EV concentration than healthy females, ii) therapy failure is associated with high EV concentrations pre-NACT, iii) elevated post-NACT EV concentrations are associated with 3y-PFS and OS in patients and iv) circulating EVs are related to post-NACT stem cell-like and/or resistant CTCs. These results have been carved out from a uniform patient cohort in whom the restrictive patient eligibility criteria focused solely on a primary, non-metastatic, locally advanced BC patient cohort with available samples pre- and post-NACT including a follow-up.
The characterization of EVs enriched from BC patient samples was performed according to the current recommendations.Citation27,Citation28 We detected different tetraspanins (CD9, CD63, CD81) in varying amounts which might be due to the different compositions of EV sources. All EV samples comprised Syntenin and TSG101, which are ESCRT complex associated proteins or linked to the release of EVs.Citation29,Citation30 In contrast, the intracellular protein Cytochrome C was not detectable. Thus, this marker profile showed that EVs are present in our preparations.
The circulating EV concentration was 40-fold elevated in NACT treated BC patients compared to healthy individuals, which was also described by others in BC.Citation31,Citation32 Pre-NACT EV concentrations were 1.5 fold increased in BC patients with advanced axillary lymph node spread at both time points compared to patients without lymph node involvement. This observation may indicate that a substantial amount of circulating EVs is released by tumor cells resident in lymph nodes, where an enhanced delivery into the periphery might be conceivable. Similar to pre-NACT, EV concentrations post-NACT were increasing with advanced axillary lymph node infiltration. An association of CTCs and SL-CTCs with lymph node spread was not observed in this cohort.Citation4 Consequently we hypothesize a different quality of EVs as a complementary marker in BC.
EV concentrations determined pre- and post-NACT have different aspects in patient risk management. BC patients not responding to NACT had 1.6-fold elevated EV concentrations pre-NACT compared to responders, which supports the hypothesis that EVs may be related to therapy outcome. In vitro studies suggested a similar tendency by demonstrating a chemotherapy resistance transfer and neutralizing anti-HER2 treatment by EVs.Citation21-24,Citation33
Post-NACT EV concentrations were associated with BC outcome of a reduced 3y-PFS and OS. Of note, the same cut-off value for EV concentrations post-NACT was obtained regarding 3y-PFS and OS for patient stratification. This also emphasizes the specificity of EV particle concentrations post-NACT as a clinically relevant predictor. In vitro studies demonstrated the induction of a neoplastic phenotype in fibroblasts and mesenchymal stem cells,Citation19 to enhance proliferation, migration and invasiveness of normal breast cells.Citation20 High numbers of circulating EVs in BC patients may use these mechanisms for tumor maintenance and spread, which may explain the worse BC prognosis.
An inverse association between CTC subgroups and EV concentrations post-NACT was observed. It is conceivable that remaining stem cell-like or resistant CTCs after NACT may be internalizing EVs for maintaining their BC phenotype. Additionally, the lowering EV concentrations might be due to TME alterations induced by the residual CTCs. The reason for this remains unclear and needs to be further investigated.
Both MRD markers – EVs and CTCs – were isolated from one sample clearly comprising different, but complementary information on BC disease status and prognosis.Citation4 Therefore, the simultaneous analysis of CTCs and EVs depicts an easy tool for completing the information of the MRD status. To this point, only quantitative EV analyses were performed, however providing clinical and prognostic importance. In CTC research, it has been demonstrated, that besides the enumeration of CTCs, their molecular characterization is essential.Citation4,Citation34,Citation35 In future studies, a detailed qualitative analysis of EVs by gene expression analyses via RNA-Seq or on the protein level by mass spectrometry will provide a comprehensive characterization of MRD in BC. The disease monitoring via EV analyses is a meaningful approach as the EV composition appears to reflect the disease status.
Conclusion
Our study highlights that the number of circulating EVs is of clinical and prognostic impact in BC patients. The association of circulating EVs with CTC presence underlines the requirement of analyzing both, EVs and CTCs, from one liquid biopsy for determination of EVs/CTC number and EV size and their molecular characterization. The simultaneous analysis from one sample will provide deeper insights into BC MRD and heterogeneity and facilitate individual patient risk management.
Material and methods
Human subjects and study design
In this study, 105 primary, non-metastatic, locally advanced BC patients treated with NACT at the Department of Gynecology and Obstetrics, University Hospital Essen, from January 2007 until June 2012, were enrolled. All samples were obtained after written informed consent and collected using protocols approved by the institutional review board (05/2856). The patient characteristics are documented in . The median follow-up time for 3y-PFS was 34 months (range: 4 to 36 months) and 52 months (range: 4 to 82 months) for OS. Eight patients experienced a disease relapse or died of BC. The patients’ eligibility criteria, NACT treatment regimens and definition of NACT response was described in detail elsewhere.Citation4
Table 3. Patient characteristics.
Plasma sampling
Ethylenediaminetetraacetic acid blood samples were collected pre- and post-NACT. Blood samples from age-matched, healthy females (n = 16; 47 [35-62] years) represented the control panel. The blood samples were processed immediately or not later than 4 hours after withdrawal, centrifuged at 1500 g for 10 min and the plasma supernatant was stored at −80°C until further usage.
Enrichment of circulating extracellular vesicles
The enrichment and precipitation of circulating EVs was performed using ExoQuick™ precipitation reagent (SBI Systems Bioscience Inc., #EXOQ20A-1) according to the manufacturer's instructions and described elsewhere.Citation26 Enriched EVs samples were stored at −20°C until further processing.
Nanoparticle tracking analysis for EV enumeration and size determination
NTA was performed for determination of size and particle concentration using the ZetaView (Particle Metrix GmbH, Microtrac) with corresponding software (Version 8.02.28) and defined settings: 85% sensitivity; 20% brightness; measuring size range: 5–200 nm. To obtain constant particle concentrations of approximately 1 × 106/ml in NTA, samples were regularly diluted by a factor of 1:50000 in phosphate buffered saline. In duplicate analysis a mean coefficient of variation for the EV particle concentration of 16.6% and for EV size of 1.8% was achieved. For serial plasma samples of healthy donors (n = 6) procured at least three different time points within one year a mean coefficient of variation for the EV particle concentration of 25.7% and for EV size of 4.4% was achieved
EVs characterization by SDS Page and western blot
EV marker expression was characterized by SDS-Page and western blot analysis under reducing conditions as described elsewhere for expression of CD9, CD63, CD81 and TSG101 (all SBI Systems Bioscience Inc., #EXOAB-KIT-1, #EXOAB-TSG101-1).Citation26 Additionally, the western blot detection of HSP70 (R&D Systems, #AF1663), Syntenin (clone EPR8102; Abcam, #ab133267) and Cytochrome C (clone 6H2.B4, Biolegend, # 612301) was performed. The primary antibodies were targeted either by the 1:200000 diluted secondary antibody goat anti-mouse HRP (ThermoFisher, #31430) or goat anti-rabbit HRP (ThermoFisher, #31460). Visualization was done using the ultra-sensitive ECL substrate (ThermoFisher, #34095) and signal detection was performed with the chemiluminescence detection system Fusion Fx7 and its corresponding software Bio ID (both Vilber Lourmat) for documentation as TIFF files. Images were cropped using Adobe Photoshop CC, but not further modified. A protein concentration of 35 µg was loaded per lane of the gel. For the densiometric evaluation either the positive control or a healthy person was defined as 100% reference (Suppl. Table 1).
Selection, detection and evaluation of CTCs and SL-CTCs
Two 5 ml blood samples obtained pre- and post-NACT were analyzed for CTC presence using the AdnaTest BreastCancer Select/Detect (Qiagen, # T-1-508-R/T-1-509-R) detecting EpCAM, MUC-1, HER2 and β-actin transcripts. The detection of the excision repair cross-complementing rodent repair deficiency complementation group 1 (ERCC1) nuclease was done in a singleplex PCR. For assessment of SL-CTCs, CTCs enriched using the AdnaTest BreastCancer Select were evaluated using the AdnaTest EMT-1/StemCell Detection kit (Qiagen, # T-1-533-R. A CTC expressing the stem cell marker ALDH-1 or at least one EMT marker was defined as a SL-CTC. All tests and results have been described in detail elsewhere.Citation4,Citation36
Statistical and graphical analysis
Statistical analysis was performed using SPSS 23.0 (SPSS Inc.), GraphPad Prism 7.0 (GraphPad Software) and BIAS 10.12 (Epsilon Verlag). Particle concentrations (109/ml) and sizes (nm) were given as median (interquartile range) or median (range). After testing for Gaussian distribution, the differences between two or more groups were assessed by Mann-Whitney test or Kruskal-Wallis test. In paired analysis Wilcoxon rank sum test was applied. ROC analysis was performed to obtain cut-off values for categorization of continuous patients’ characteristics into dichotomous variables representing the optimal separation of survival curve. Kaplan-Meier Curve analysis in combination with the log-rank test was executed for the analysis of 3y-PFS and OS probabilities. The 3y-PFS and OS were measured from time point of diagnosis until 36 months later or death/last contact. For categorical data analysis Fisher's exact test was used. Statistical significance was defined as p ≤ 0.05.
Disclosure of potential conflicts of interest
S. Kasimir-Bauer is a consultant for QIAGEN. All other authors declare no potential conflicts of interest.
Financial support
This work was supported by Deutsche Krebshilfe (number 109816) and IFORES (number D/107-81080), University of Duisburg-Essen.
Abbreviations
| ALDH-1 | = | aldehyde dehydrogenase 1 |
| A | = | Avastin |
| AUC | = | area under the curve |
| BC | = | breast cancer |
| CD | = | cluster of differentiation |
| CI | = | confidence interval |
| CTC | = | circulating tumor cell |
| CTX | = | chemotherapy |
| Cyt C | = | Cytochrome C |
| ECL | = | enhanced chemiluminescence |
| EMT | = | epithelial mesenchymal transition |
| EpCAM | = | epithelial cell adhesion molecule |
| ER | = | estrogen receptor |
| ERCC1 | = | excision repair cross-complementing rodent repair deficiency, complementation group 1 |
| EV | = | extracellular vesicle |
| HER2 | = | human epidermal growth factor receptor 2 |
| HR | = | hazard ratio |
| HSP70 | = | heat shock protein 70 |
| HTX | = | Hormonal therapy |
| L | = | Lapatinib |
| MRD | = | minimal residual disease |
| MUC-1 | = | mucin 1 |
| NACT | = | neoadjuvant chemotherapy |
| neg | = | negative |
| NP | = | Normal person |
| NTA | = | nanoparticle tracking analysis |
| OS | = | overall survival |
| pCR | = | pathological complete response |
| PCR | = | polymerase chain reaction |
| PFS | = | progression-free survival |
| pos | = | positive |
| PR | = | progesterone receptor |
| ROC | = | receiver operating curve |
| SL-CTC | = | stem cell-like circulating tumor cell |
| T | = | Trastuzumab |
| TME | = | tumor microenvironment |
| TSG101 | = | tumor susceptibility gene 101 |
supp_data.zip
Download Zip (29.2 KB)Acknowledgments
We gratefully thank the patients for study participation and kindly providing their samples. We highly value the technical support by the medical and laboratory team of the Department of Gynecology and Obstetrics and the colleagues from the Institute for Transfusion Medicine, both from the University Hospital Essen, especially Sabine Schramm and Monika Collenburg. Special thanks go to Bernd Giebel for the critical revision of the manuscript as well as for providing the NTA facility.
References
- Buchholz TA, Hunt KK, Whitman GJ, Sahin AA, Hortobagyi GN. Neoadjuvant chemotherapy for breast carcinoma: Multidisciplinary considerations of benefits and risks. Cancer. 2003;98:1150-60. https://doi.org/10.1002/cncr.11603
- Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta-analysis. J Natl Cancer Inst. 2005;97:188-94. https://doi.org/10.1093/jnci/dji021
- Bonnefoi H, Litiere S, Piccart M, MacGrogan G, Fumoleau P, Brain E, Petit T, Rouanet P, Jassem J, Moldovan C, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: A landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial. Ann Oncol. 2014;25:1128-36. https://doi.org/10.1093/annonc/mdu118
- Kasimir-Bauer S, Bittner AK, Konig L, Reiter K, Keller T, Kimmig R, Hoffmann O. Does primary neoadjuvant systemic therapy eradicate minimal residual disease? Analysis of disseminated and circulating tumor cells before and after therapy. Breast Cancer Res. 2016;18:20. https://doi.org/10.1186/s13058-016-0679-3
- Pantel K, Alix-Panabieres C. Liquid biopsy: Potential and challenges. Mol Oncol. 2016;10:371-3. https://doi.org/10.1016/j.molonc.2016.01.009
- Kasimir-Bauer S, Reiter K, Aktas B, Bittner AK, Weber S, Keller T, Kimmig R, Hoffmann O. Different prognostic value of circulating and disseminated tumor cells in primary breast cancer: Influence of bisphosphonate intake? Sci Rep. 2016;6:26355. https://doi.org/10.1038/srep26355
- Janni WJ, Rack B, Terstappen LW, Pierga JY, Taran FA, Fehm T, Hall C, de Groot MR, Bidard FC, Friedl TW, et al. Pooled Analysis of the Prognostic Relevance of Circulating Tumor Cells in Primary Breast Cancer. Clin Cancer Res. 2016;22:2583-93. https://doi.org/10.1158/1078-0432.CCR-15-1603
- Ludwig AK, Giebel B. Exosomes: Small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44:11-5. https://doi.org/10.1016/j.biocel.2011.10.005
- Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. https://doi.org/10.3402/jev.v4.27066
- Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373-83. https://doi.org/10.1083/jcb.201211138
- Graves LE, Ariztia EV, Navari JR, Matzel HJ, Stack MS, Fishman DA. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res. 2004;64:7045-9. https://doi.org/10.1158/0008-5472.CAN-04-1800
- Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, Howell SB. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4:1595-604. https://doi.org/10.1158/1535-7163.MCT-05-0102
- Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: Association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331-7
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470-6. https://doi.org/10.1038/ncb1800
- Ogorevc E, Kralj-Iglic V, Veranic P. The role of extracellular vesicles in phenotypic cancer transformation. Radiol Oncol. 2013;47:197-205. https://doi.org/10.2478/raon-2013-0037
- Yu S, Wei Y, Xu Y, Zhang Y, Li J, Zhang J. Extracellular vesicles in breast cancer drug resistance and their clinical application. Tumour biology: the journal of the International Society for Oncodevelopmental Biol. and Medicine. 2016;37:2849-61. https://doi.org/10.1007/s13277-015-4683-5
- Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66:9290-8. https://doi.org/10.1158/0008-5472.CAN-06-1819
- Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, Holowka DA, Cerione RA. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci U S A. 2011;108:4852-7. https://doi.org/10.1073/pnas.1017667108
- Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol. 2012;40:130-8.
- O'Brien K, Rani S, Corcoran C, Wallace R, Hughes L, Friel AM, McDonnell S, Crown J, Radomski MW, O'Driscoll L. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Eur J Cancer. 2013;49:1845-59. https://doi.org/10.1016/j.ejca.2013.01.017
- Chen WX, Cai YQ, Lv MM, Chen L, Zhong SL, Ma TF, Zhao JH, Tang JH. Exosomes from docetaxel-resistant breast cancer cells alter chemosensitivity by delivering microRNAs. Tumour Biol. 2014;35:9649-59. https://doi.org/10.1007/s13277-014-2242-0
- Lv MM, Zhu XY, Chen WX, Zhong SL, Hu Q, Ma TF, Zhang J, Chen L, Tang JH, Zhao JH. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumour Biol. 2014;35:10773-9. https://doi.org/10.1007/s13277-014-2377-z
- Wang T, Ning K, Lu TX, Sun X, Jin L, Qi X, et al. Increasing circulating exosomes-carrying TRPC5 predicts chemoresistance in metastatic breast cancer patients. Cancer Sci. 2017;108:448-54. https://doi.org/10.1111/cas.13150
- Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, Morelli D, Villa A, Della Mina P, Menard S, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012;227:658-67. https://doi.org/10.1002/jcp.22773
- Zocco D, Ferruzzi P, Cappello F, Kuo WP, Fais S. Extracellular vesicles as shuttles of tumor biomarkers and anti-tumor drugs. Front Oncol. 2014;4:267. https://doi.org/10.3389/fonc.2014.00267
- Konig L, Kasimir-Bauer S, Hoffmann O, Bittner AK, Wagner B, Manvailer LF, Schramm S, Bankfalvi A, Giebel B, Kimmig R, et al. The prognostic impact of soluble and vesicular HLA-G and its relationship to circulating tumor cells in neoadjuvant treated breast cancer patients. Hum Immunol. 2016;77(9):791-9. https://doi.org/10.1016/j.humimm.2016.01.002
- Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. https://doi.org/10.3402/jev.v3.26913
- Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J of Extracell Vesicles. 2013;2. https://doi.org/10.3402/jev.v2i0.20360
- Tauro BJ, Greening DW, Mathias RA, Mathivanan S, Ji H, Simpson RJ. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics. 2013;12:587-98. https://doi.org/10.1074/mcp.M112.021303
- Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J of Extracell Vesicles. 2012;1. https://doi.org/10.3402/jev.v1i0.18397
- Galindo-Hernandez O, Villegas-Comonfort S, Candanedo F, Gonzalez-Vazquez MC, Chavez-Ocana S, Jimenez-Villanueva X, Sierra-Martinez M, Salazar EP. Elevated concentration of microvesicles isolated from peripheral blood in breast cancer patients. Arch Med Res. 2013;44:208-14. https://doi.org/10.1016/j.arcmed.2013.03.002
- Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177-82. https://doi.org/10.1038/nature14581
- Conley A, Minciacchi VR, Lee DH, Knudsen BS, Karlan BY, Citrigno L, et al. High-throughput sequencing of two populations of extracellular vesicles provides an mRNA signature that can be detected in the circulation of breast cancer patients. RNA biol. 2017;14:305-16. https://doi.org/10.1002/ijc.26111
- Andreopoulou E, Yang LY, Rangel KM, Reuben JM, Hsu L, Krishnamurthy S, Valero V, Fritsche HA, Cristofanilli M. Comparison of assay methods for detection of circulating tumor cells in metastatic breast cancer: AdnaGen AdnaTest BreastCancer Select/Detect versus Veridex CellSearch system. Int J Cancer. 2012;130:1590-7. https://doi.org/10.1002/ijc.26111
- Lianidou ES, Markou A, Strati A. The role of CTCs as tumor biomarkers. Adv Exp Med Biol. 2015;867:341-67. https://doi.org/10.1007/978-94-017-7215-0_21
- Kasimir-Bauer S, Hoffmann O, Wallwiener D, Kimmig R, Fehm T. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res. 2012;14:R15. https://doi.org/10.1186/bcr3099
