ABSTRACT
Accumulating evidence support an important role for endogenous bystander dendritic cells (DCs) in the efficiency of autologous patient-derived DC-vaccines, as bystander DCs take up material from vaccine-DCs, migrate to draining lymph node and initiate antitumor T-cell responses. We examined the possibility of using allogeneic DCs as vaccine-DCs to activate bystander immune cells and promote antigen-specific T-cell responses. We demonstrate that human DCs matured with polyI:C, R848 and IFN-γ (denoted COMBIG) in combination with an infection-enhanced adenovirus vector (denoted Ad5M) exhibit a pro-inflammatory state. COMBIG/Ad5M-matured allogeneic DCs (alloDCs) efficiently activated T-cells and NK-cells in allogeneic co-culture experiments. The secretion of immunostimulatory factors during the co-culture promoted the maturation of bystander-DCs, which efficiently cross-presented a model-antigen to activate antigen-specific CD8+ T-cells in vitro. We propose that alloDCs, in combination with Ad5M as loading vehicle, may be a cost-effective and logistically simplified DC vaccination strategy to induce anti-tumor immune responses in cancer patients.
Introduction
As cancer vaccines, patient-derived, ex vivo-modified dendritic cells (DCs) aim to induce tumor-specific T-cell immunity by direct education of naïve T-cells against tumor-associated antigens (TAAs).Citation1 Although proof-of-principle was observed for DC vaccines in several clinical studies, clinical responses are yet suboptimal.Citation2 Variable clinical outcomes seem to correlate with the activation status of the vaccine DCs, it is important to optimize and standardize the production of DC-vaccines capable to induce T-helper type-1 (Th1)-polarized immune responses.Citation2,3 Interestingly though, recent findings indicate that ex vivo-modified vaccine DCs require help from endogenous bystander-DCs to prime CD8+ T-cell specific immunity.Citation4,5 This indirect priming of bystander-DCs has been found from many to be highly dependent on the active secretion of immune cell-recruiting pro-inflammatory factors.Citation5,6
Immature monocyte-derived DCs could secrete substantial amounts of Th1 cytokines and chemokines when stimulated by a cocktail of stimuli, such as Toll-like receptor (TLR) ligands and IFN-γ.Citation7,8 Additionally, allogeneic DCs (alloDCs) though not able to function as direct presenters, can create through the alloreaction an enriched milieu of Th1 inflammatory cytokines and sensitize immature bystander-DCs.Citation9 Thus, we hypothesized and examined the use of pro-inflammatory alloDCs as immune enhancers in order to create a strong, bystander-DC stimulating milieu with concomitant activation of NK-cells and antigen-specific CD8+ T-cells.
Materials and methods
Cell lines
The 911 cells (Crucell) were cultured in DMEM supplemented with 10% heat-inactivated FBS, 1% penicillin/streptomycin (PeSt) and 1% sodium pyruvate. K562(Luc), A549(pp65) and T2 cells (ATCC) were cultured in RPMI-1640 supplemented with 10% heat-inactivated FBS, 1% PeSt and 1% HEPES. All components and culture media were from Thermo Fisher Scientific. K562(Luc) cells were engineered with a lentivirus vector to express firefly luciferase and A549(pp65) cells to express the cytomegalovirus (CMV)-pp65 antigen. All cells were cultured in a humidified incubator with a 5% CO2 atmosphere at 37°C.
Production of the recombinant virus
The Ad5M vector was constructed and produced as previously described.Citation10 Ad5M is an E1-deleted human adenovirus serotype-5 (Ad5) vector with fiber shaft and knob from serotype-35 and a hexon modification to enhance transduction efficacy.Citation11 Ad5M does not encode any transgene. Titers were determined by quantitative PCR as encapsidated virus genomes (evg) per ml.Citation11
Isolation of human PBMCs and generation of DCs and NK-cells
Buffy coats from healthy donors were obtained from the blood bank at the Uppsala University Hospital, Uppsala, Sweden. PBMCs were isolated by Ficoll-Paque Premium separation (GE Healthcare Life Science) and cultured in RPMI-1640 supplemented with 10% heat-inactivated FBS, 1%PeSt, 0.5%L-glutamine, 1%HEPES and 20 mM β-mercaptoethanol (DC medium).
Generation and treatment of DCs. Monocytes isolated from PBMCs by CD14+ positive magnetic selection (Miltenyi Biotec) were differentiated to immature DCs (imDCs) using 20 ng/mL human IL-4 and 100 ng/mL GM-CSF (Gentaur) for 5 days. Medium was replaced every 2 days. On day 5, cells were either left untreated (imDCs) or matured for 18 h with a cocktail of maturation stimuli.Citation7,8 consisting of 2.5 μg/mL R848 (InvivoGen), 20 μg/mL polyinosinic:polycytidylic acid (polyI:C) (Sigma-Aldrich) and 1000IU/mL IFN-γ (Shenandoah Biotechnology). This maturation cocktail was named “Combined Toll-like receptor ligands with IFN-γ” (COMBIG). DCs matured with COMBIG used as allogeneic stimuli are referred to as allo-COMBIG-DCs. Immature DCs treated with Ad5M (2000 evg/cell) for 18 h are referred to as allo-Ad5M-DCs while allo-COMBIG/Ad5M-DCs were treated with both COMBIG and Ad5M for 18 h. After washing, cells were further cultured in fresh DC medium.
Generation of NK-cells. NK-cells were isolated from PBMCs by negative magnetic selection (Miltenyi Biotec) and cultured in DC medium.
Human Cytokine array and Cytokine release assay
Supernatants from DC cultures (2.5–5 × 105 cells/well in 48-well plates) were collected. The Proteome Profiler™ Human Cytokine Array Kit, Panel A (R&D Systems, Inc.) was used to screen for different cytokines and ELISA kits were used to validate the expression of IL-12p70 (Mabtech), IL-6 (BioLegend) and CXCL10 (BioLegend).
Flow cytometry
Human DC phenotyping. Anti-CD1a-BV510 (BD Biosciences), anti-CD14-APC/Cy7, anti-HLA-DR-perCP (MHC class II), anti-CD40-FITC, anti-CD80-PE, anti-CD83-APC and anti-CD86-BV421 were used to evaluate the DCs.
Activation of NK-cells and T-cells in allogeneic mixed leukocyte reaction. AlloDCs were co-cultured with PBMCs from unrelated donors at ratio 1:5 (alloDCs:PBMCs) or NK-cells at ratio 1:1 (alloDCs:NK-cells). Activation was assessed 24 h later by flow cytometry with anti-CD3-FITC, anti-CD56-APC and anti-CD69-BV510. ELISA was used for the detection of IFN-γ (Mabtech) in the allogeneic co-culture supernatant (allo-SN).
Inflammasome activation. The activation of inflammasome was assessed by a novel flow cytometric method described by Sester et al.Citation12 Briefly, alloDCs were prepared as described above and stained with the anti-ASC (TMS-1)-PE antibody. The transit of the ASC from a widespread localization into a single speck is detected as a decrease in the signal width parameter.
Activation of bystander immature DCs. AlloDCs were co-cultured with PBMCs at ratio 1:5 (alloDCs:PBMCs) for 24 h and the allo-SN was used as a maturation stimuli for immature bystander-DCs. Bystander-DC maturation was assessed 48 h later by flow cytometry as before.
All antibodies were purchased from BioLegend, unless specified elsewhere. Data acquisition was performed using a FACSCanto II (BD Biosciences) flow cytometer, and the analysis was performed using FlowJo software (version 7.6.5; Tree Star).
Cytotoxic assay for NK-cells
AlloDCs were co-cultured with NK-cells for 24 h at ratio 1:1. K562(Luc) (NK targets) cells were added in the co-culture at ratio 1:10 (K562(Luc):NK-cells) for a further 24 h. The luciferase activity was assessed by Bright-Glo (Promega) and measured with a luminometer (Wallac VICTOR2, PerkinElmer). Relative cell viability was calculated as the % of the luciferase activity from K562(Luc) cells alone.
Cultures of good manufacturing practice (GMP)-quality, FBS-free alloDCs
GMP-alloDCs tested in this study were manufactured in the GMP facilities of Eufets GmbH, Germany. The batch used for all experiments was a test batch manufactured in September 2015 according to the validated manufacturing process used for the manufacturing of clinical trial material. In brief, GMP-alloDCs were manufactured in a continuous 6-day process, starting with a fresh leukapheresis from a healthy donor and ending with cryopreserved GMP-alloDCs. At day 0, monocytes were isolated from the leukapheresis product and cultivated in CellGro media (CellGenix), supplemented with IL-4 and GM-CSF. Medium was replaced every 2 days. On day 4 cells were stimulated with COMBIG. On day 5, GMP-alloDCs were harvested, formulated in AB plasma and DMSO, and transferred to vials for cryopreservation (11.7 × 106 cells in 1 ml per vial). As part of the manufacturing process, GMP-alloDCs were tested according to the established release assays comprising testing of contents, viability, identity, potency and safety. GMP-alloDCs were shipped from Eufets to Uppsala University in liquid nitrogen (−150°C). Prior to use for in vitro experiments, GMP-alloDCs were thawed, washed and cultured in AIM-V media (Gibco) until use. PBMCs and NKs in the respective co-cultures were performed in identical settings as already described and cultured in AIM-V medium.
DC antigen cross-presentation and T-cell stimulation assays
Cross-presentation of CMV-pp65 by DCs for specific stimulation of autologous CMV-pp65495–503 TCR-modified T-cells. Immature DCs (HLA-A2+) were cultured for 2 h at 37°C with freeze/thawed cell-lysate from A549(pp65) tumor cells, as a mean of providing CMV-pp65 protein to DCs exogenously. The DCs were subsequently matured in supernatant (allo-SN) from alloDC and PBMCs co-cultures for 38 h. Autologous T-cells engineered to express an HLA-A*0201-restricted T-cell receptor (TCR) for the CMV-pp65495–503 epitopeCitation13 were then mixed with CMV-pp65 cross-presenting DCs at ratio 5:1 (T-cells:DCs) and cultured in fresh medium for 18 h. TCR-specific T-cell activation was assessed by the secretion of IFN-γ.
Expansion and re-stimulation of T-cells by cross-presenting DCs. T-cells (non-modified) from CMV-seropositive, HLA-A2+ donors were mixed with autologous CMV-pp65 cross-presenting DCs prepared as above at ratio of 20:1 (T-cells:DCs) and cultured in fresh medium for a total of 12 days. Medium with 30IU/ml IL-2 (Proleukin, Novartis) and 20 ng/ml IL-7 (Nordic Biosite) was replaced after 7 days. CMV-pp65-specific T-cell expansion was detected with an HLA-A*0201/pp65495–503 tetramer (Beckman Coulter).
T2 cells (HLA-A2+) were pulsed with 5 μg/ml CMV-pp65495–503 peptide or the HLA-A2 binding irrelevant TARP(P5L)4–13 peptide. The pulsed T2 cells were used to re-stimulate the expanded CMV-pp65-specific T-cells for 18 h. IFN-γ release was measured as an indicator of T-cell activation.
Statistics
The data are reported as mean±SEM. Statistical analysis was performed by GraphPad prism software version 6.01 (La Jolla). Statistical analyses were performed using parametric One-way ANOVA with Holm-Sidak test for multiple comparison correction. Student t-test was used when only two groups were evaluated. Values with P<0.05 were considered to be statistically significant.
Results and discussion
COMBIG-matured and COMBIG/Ad5M-matured DCs exhibit a phenotype and cytokine secretion profile associated with Th1 polarization
The maturation status of DCs is very crucial for the induction of optimal effector responses in autologous DC cancer vaccination.Citation3 Interestingly, it has also been found important for the activation of allogeneic T-cells.Citation14 In our experiments, immature DCs (imDCs) were left untreated or matured for 18 h by COMBIG, Ad5M or their combination. The use of the infection-enhanced adenoviral vector Ad5M can facilitate loading of DCs with immunomodulating agents or TAAs, a standard ex vivo practice to modify patient-derived DCs.Citation15 For the sake of having a simple system to assess the maturation effect of Ad5M on DCs in this study Ad5M was used without a transgene. Cells were washed and cultured for another 24 h in fresh DC medium without addition of any cytokines. DC phenotypic changes were assessed by flow cytometry and the supernatants (SN) were collected to map the secretion profiles ().
Figure 1. COMBIG/Ad5M-matured DCs express a mature phenotype and Th1-polarized cytokine secretion profile. (A) CD14+ monocytes were isolated from healthy donor PBMCs, differentiated into imDCs by GM-CSF/IL-4 for 5 days, matured under different conditions for 18 h, washed and further cultured for 24 h and analyzed. (B) DCs were characterized for HLA-DR, CD40, CD80, CD86 and CD83 expression by flow cytometry. Mean fluorescence intensity (MFI) for each marker on DCs (CD14−CD1a+) produced from eight donors are shown. (C) Secreted cytokines were assessed in supernatants of each treatment by proteome profiler where supernatants from six donors were pooled. (D) IL-12, IL-6 and CXCL10 secretion were also verified by ELISA for each donor. (E, F) Inflammasome activation was evaluated by the re-localization of the protein ASC, an inflammasome component, from a diffuse state to a single speck exhibited in representative FACS plots of ASC width (ASC-W) and ASC area (ASC-A) and % of speck+ DCs produced from six donors. Data are shown as mean±SEM (n.s. p ≥ 0.05; * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001).
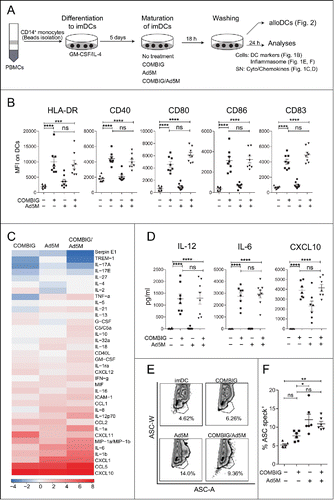
COMBIG maturation, alone or combined with Ad5M, induced upregulation of HLA-DR, CD40, CD80, CD86 and CD83, implying a mature and activated phenotype (). COMBIG-matured and COMBIG/Ad5M-matured DCs demonstrated also elevated secretion of pro-inflammatory cytokines and chemokines, among of which IL-12, IL-6, CXCL10, CCL5 and IL-1β had the highest fold-increases compared to imDCs (). High release of IL-12, IL-6 and CXCL10 was further verified by ELISA (), with the differences that IL-12 and IL-6 seemed rather low for Ad5M-matured DCs. Of notice, cytokines were measured after washing of cells indicating a sustained cytokine secretion ability of COMBIG-matured DCs, which will be important for vaccination efficiency and activation of bystander immune cells. IL-12 is associated with Th1 responses and antitumor effects as it supports the functions of NK-cells, CD4+ and CD8+ T-cells, and further enhances the release of other Th1 immune-modulating molecules.Citation16,17 In addition, CXCL-10 secreted by DCs in response to IFN-γ is a chemoattractant for several cell types, such as monocytes, T-cells and NK-cells, and it promotes T-cell adhesion to endothelial cells.Citation18,19 IL-1β signaling is important for the induction of strong effector immune responses and it has been reported to efficiently replace conventional receptor-dependent activation of DCs during anti-viral immune responses.Citation6 The ascending secretion of IL-1β found is in line with increased formation of inflammasome, the multiprotein assembly complex responsible for the maturation of IL-1β.Citation12 (, ). Interestingly, the presence of Ad5M during DC maturation provided an advantage over the use of COMBIG alone in IL-1β secretion and inflammasome formation (, ). This is in accordance with previous findings on the role of adenoviral infections in the activation of inflammasome.Citation20 Taken together, our data indicate that Ad5M/COMBIG-maturation is well tolerated by human monocyte-derived DCs and resulted in the generation of a fully matured and pro-inflammatory DC phenotype, a quality highly desirable in DC vaccination approaches.
Allogeneic DCs, matured by COMBIG/Ad5M, activate T-cells and NK-cells, mature bystander-DCs and promote NK-cell mediated killing in vitro
Desirable DC vaccination strategies involve the activation and polarization of several effector immune cells. We observed phenotypic and functional changes in the status of PBMCs in 24 h co-cultures with matured alloDCs (). T-cell and NK-cell activation in the PBMC pool was assessed by the upregulation of the early activation protein CD69. Higher CD69 expression was observed for both T-cells () and NK-cells () when cultured with allo-COMBIG/Ad5M-DCs than with allo-imDCs. Interestingly, T-cell and NK-cell activation was higher in co-cultures with allo-COMBIG/Ad5M-DCs compared to those of allo-COMBIC-DCs. In addition, co-culturing PBMCs with allo-Ad5M-DCs had a positive effect on the upregulation of CD69 in both T- and NK-cells (, ). Analogous to the observed phenotypic changes were functional changes monitored by the increased levels of secreted IFN-γ in the allogeneic-culture supernatant (allo-SN) (). Allo-SN from co-cultures of PBMCs with allo-COMBIG-DCs or allo-COMBIG/Ad5M-DCs was found to significantly induce upregulation of CD40 on immature bystander-DCs. Moreover, allo-SN from PBMC and allo-COMBIG/Ad5M-DC co-cultures led to significant upregulation of CD80 and CD86 on the bystander-DCs (). This is in line with previous findings where supernatants of mixed leukocyte reactions have been shown to activate and license bystander-DCs for Th1 priming.Citation9 In order to exclude any effects from the FBS present in the culturing medium, FBS-free co-cultures of GMP-alloDCs and PBMCs were evaluated for their ability to mature bystander-DCs (Supplementary Fig. S1A). Results were virtually identical to the ones obtained by FBS-cultured alloDCs (Supplementary Fig. S1B).
Figure 2. Allo-COMBIG/Ad5M-DCs activate innate and adaptive immune cells in vitro. (A) After maturation for 18 h and washing (samples as described in ) the DCs, here called alloDCs, were co-cultured with allogeneic PBMCs (meaning from a different donor). After 24 h of co-culture, activation of immune cells in the PBMC pool was characterized by the expression of CD69 on T-cells and NK-cells. Mean fluorescence intensities for CD69 on (B) T-cells (CD3+CD56−) and (C) NK-cells (CD3−CD56+) from sixteen individual combinations of eight unrelated donors are shown. (D) ELISA was used to measure the concentration of IFN-γ in the allogeneic co-culture supernatants, here called allo-SN. (E) The allo-SN from the alloDC-PBMC co-cultures was used as maturation stimuli for imDCs (DCs from the same donor as the PBMCs) to mimic a scenario for host “bystander” imDCs. Bystander-DC maturation was assessed by the upregulation of HLA-DR, CD40, CD80, CD86 and CD83 as shown in MFI scatter plots for each marker on bystander-DCs (CD14−CD1a+). Six individual combinations from three unrelated donors were evaluated. (F) NK-cells isolated from PBMCs were co-cultured with alloDCs for 24 h. NK-cell activation was then verified by (G) CD69 expression on NK-cell surface (MFI) and (H) IFN-γ secretion in the generated allo-SN. (I) The killing ability of the activated NK-cells were further investigated by adding NK-target cells (K562(Luc)). The viability of K562(Luc) cells was assessed 24 h later. (J) Relative cell viability was calculated as the percentage of the luciferase activity from K562(Luc) cells alone (without addition of NK-cells). Experimental duplicates were used for each condition and results of eight individual combinations from four unrelated donors are shown. Data are shown as mean±SEM (n.s. p ≥ 0.05; * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001).
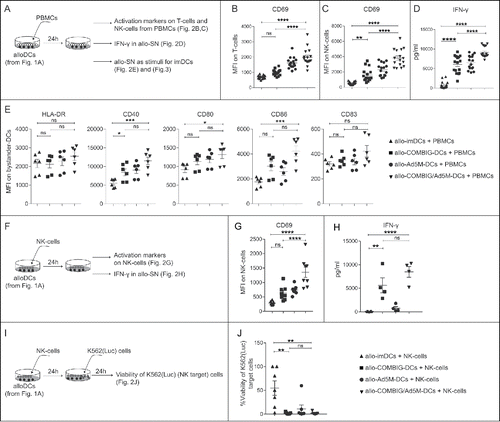
NK-cells are particularly important contributors in the effectiveness of DC vaccination because they cross-talk with bystander-DCs and T-cells and mediate tumor cell killing.Citation5,21 When NK-cells were added to allo-COMBIG-DCs or allo-COMBIG/Ad5M-DCs cultures () increased CD69 expression () and IFN-γ secretion () were observed. Furthermore, the activated NK-cells efficiently lysed target cells (K562(Luc)) in contrast to NK-cells activated in co-cultures with allo-imDCs (, ). Similar results were obtained from co-cultures of GMP-alloDCs and NK-cells under FBS-free conditions (Supplementary Fig. S1C-G). Interestingly, in a similar experimental setting autologous IL-4-differentiated DCs were found inefficient to activate and promote tumoricidal activity of NK-cells.Citation22
Bystander-DCs, matured by the allo-SN from PMBC allo-COMBIG/Ad5M-DC co-cultures, efficiently cross-present antigen to T-cells
Beside DC maturation, antigen-uptake and antigen-presentation play important roles in inducing adaptive tumor immunity.Citation23 At the tumor site, injected alloDCs are expected to create a milieu favoring effector activation and cytolytic functions, as shown in vitro (). This can result in the release of TAAs and neoantigens, which can be taken up by bystander-DCs and be presented to effector T-cells.Citation5 To test this hypothesis, we used an in vitro model system where DCs from HLA-A2+ donors (bystander-DCs) were incubated with cell lysate from tumor cells (A549(pp65)) expressing the full length CMV-pp65 protein, mimicking the condition of lysed tumor cells (, ). These antigen-loaded immature bystander-DCs were then matured with allo-SN from PBMC-alloDC co-cultures. The exogenous pp65 antigen needs to be processed by the bystander-DCs into the pp65495–503 peptide and cross-presented on HLA-A2 (MHC class I) in order to stimulate autologous T-cells, engineered with a TCR specific for CMV-pp65495–503. We found that antigen-loaded bystander-DCs matured in allo-SN could efficiently cross-present the antigen and stimulate engineered CMV-pp65495–503 TCR-specific T-cells () to secrete high amounts of IFN-γ, with allo-SN from PBMC and allo-COMBIG/Ad5M-DC co-cultures yielding significantly better activation compared to the other allo-SNs ().
Figure 3. Activation and expansion of antigen-directed T-cells induced by cross-presentation of the antigen by autologous bystander-DCs matured by the allogeneic supernatant from PMBC and allo-COMBIG/Ad5M-DC co-cultures. (A) DCs from an HLA-A2+ donors (bystander-DCs) were pulsed for 2 h with lysate from tumor cells expressing the CMV-pp65 antigen (A549(pp65)) and matured for 38 h by allo-SN from the various co-cultures (). The cross-presenting bystander-DCs were then mixed for 18 h with autologous T-cells, engineered with a TCR against the HLA-A2-restricted CMV-pp65495–503 peptide. (B) ELISA was used to measure the concentration of IFN-γ released by the T-cells into the supernatants. Six individual combinations from three unrelated donors were examined. (C) Bystander-DCs from CMV-seropositive, HLA-A2+ donor were prepared as mentioned above and used to stimulate autologous T-cells (non-engineered) for 12 days in the presence of low dose IL-2 (20IU/ml). (D) CMV-pp65-specific T-cell expansion was quantified by flow cytometry using PE-conjugated HLA-A*0201/pp65495–503 tetramer. FACS plots from one representative individual combination out of four using the allo-SN from PBMC and allo-COMBIG/Ad5M-DC co-cultures as maturation stimuli are shown. (E) The expanded T-cells were then re-stimulated by exposure to T2 cells loaded either with CMV-pp65495–503 peptide (relevant target) or TARP4––13 peptide (irrelevant target). ELISA was used to measure the concentration of IFN-γ in supernatants harvested 18 h after re-stimulation. The dotted line indicates the background IFN-γ release from T-cells stimulated by immature bystander-DCs. Data are shown as mean±SEM (* P < 0.05; ** P < 0.01; **** P < 0.0001).
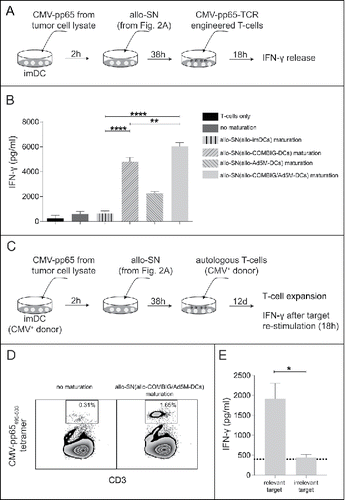
We next wanted to test whether endogenous CMV-pp65-specific T-cells (non-engineered) could be activated and expanded. In this case we started with bystander-DCs from HLA-A2+ CMV-seropositive donors, loaded with pp65-containing tumor cell lysate and matured with allo-SN from PBMC and allo-COMBIG/Ad5M-DC co-cultures (). These bystander-DCs yielded a 5-fold expansion of autologous CMV-pp65-specific T-cells from CMV-seropositive donors (). The ability of those T-cells to recognize their target and exert effector functions was tested in a re-stimulation assay. The expanded T-cells were exposed to T2 cells pulsed with either the HLA-A2-restricted pp65495–503 peptide or an irrelevant HLA-A2-restricted peptide and IFN-γ release was measured. Upon re-stimulation with the pp65-positive target cells, high IFN-γ release could be detected from the T-cells expanded by pp65-presenting bystander-DCs matured with allo-SN from PBMC and allo-COMBIG/Ad5M-DCs co-cultures (). The expanded T-cells were also confirmed as antigen-specific since exposure to T2 cells pulsed with an irrelevant peptide led only to background amounts of IFN-γ release ().
In summary, we postulate a rationale for the concept of intratumoral injections of alloDCs as cellular adjuvant cancer immunotherapy. The engagement of NK-cells and bystander-DCs has recently been proven to be of major importance for the successful induction of effector T-cell immunity by intratumoral vaccine DCs.Citation5 Additional findings support that the maturation of bystander-DCs, which is crucial in several cases of viral infections, is mediated through the secretion of pro-inflammatory factors.Citation6,24 In this sense, pro-inflammatory alloDCs were found not only to efficiently activate effector immune cells and support cytolytic functions of NK-cells, but to create a milieu in favor of bystander-DC maturation. When a model antigen was provided exogenously, mimicking tumor cell lysate, allo-SN-matured bystander-DCs could efficiently digest and cross-present the model antigen and provoke antigen-specific cytotoxic T-cell expansion and activation. The current findings are pertinent to the clinical setting, as the alloDCs approach using intratumorally injected non-transduced pro-inflammatory alloDCs has been tested successfully in a phase I/II clinical trial in patients with metastatic renal cell carcinoma (mRCC)Citation25 and is ongoing in a randomized multicenter phase II study in mRCC diagnosed patients (NCT02432846). We speculate that modification of alloDCs with Ad5M-encoded TAAs or neoantigens might be sufficient in promoting antigen-specific adaptive immune responses. However, this is an interesting concept which requires further studies.
Conflict of interest
AKP is an employee of Immunicum and owns stocks in Immunicum. The other authors have no conflicting financial interests.
Financial support
The Swedish Cancer Society (CAN 2013/373; CAN 2016/318), The Swedish Children Cancer Foundation (PR2015-0049), the Swedish Research Council (2015-03688) and Immunicum AB supported this work.
Supplementary_figure_S1.tif
Download TIFF Image (704.1 KB)Acknowledgments
The authors would like to thank Jing Ma and Berith Nilsson for technical assistance. The Swedish Cancer Society (CAN 2013/373; CAN 2016/318), The Swedish Children Cancer Foundation (PR2015-0049), the Swedish Research Council (2015-03688) and Immunicum AB supported this work. Conceived and designed the experiments: GF, CJ, IK, MR, AKP, DY, ME. Performed the experiments and analyzed the data: GF, CJ, IK. Wrote the paper: GF, CJ, DY, ME. All authors read and approved the final version of the manuscript.
References
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–77. doi:10.1038/nrc3258. PMID:22437871.
- Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15:e257–67. doi:10.1016/S1470-2045(13)70585-0. PMID:24872109.
- Sabado RL, Bhardwaj N. Directing dendritic cell immunotherapy towards successful cancer treatment. Immunotherapy. 2010;2:37–56. doi:10.2217/imt.09.43. PMID:20473346.
- Yewdall AW, Drutman SB, Jinwala F, Bahjat KS, Bhardwaj N. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen presenting cells. PLoS One. 2010;5:e11144. doi:10.1371/journal.pone.0011144. PMID:20585396.
- Liu C, Lou Y, Lizee G, Qin H, Liu S, Rabinovich B, Kim GJ, Wang YH, Ye Y, Sikora AG, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118:1165–75. PMID:18259609.
- Pang IK, Ichinohe T, Iwasaki A. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8(+) T cell responses to influenza A virus. Nat Immunol. 2013;14:246–53. doi:10.1038/ni.2514. PMID:23314004.
- Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–76. doi:10.1038/ni1223. PMID:15995707.
- Lovgren T, Sarhan D, Truxova I, Choudhary B, Maas R, Melief J, et al. Enhanced stimulation of human tumor-specific T cells by dendritic cells matured in the presence of interferon-gamma and multiple toll-like receptor agonists. Cancer Immunol Immunother. 2017;66:1133–44. doi:10.1007/s00262-017-2029-4. PMID:28601925.
- Wallgren AC, Andersson B, Backer A, Karlsson-Parra A. Direct allorecognition promotes activation of bystander dendritic cells and licenses them for Th1 priming: A functional link between direct and indirect allosensitization. Scand J Immunol. 2005;62:234–42. doi:10.1111/j.1365-3083.2005.01663.x. PMID:16179010.
- Yu D, Jin C, Leja J, Majdalani N, Nilsson B, Eriksson F, Essand M. Adenovirus with hexon Tat-protein transduction domain modification exhibits increased therapeutic effect in experimental neuroblastoma and neuroendocrine tumors. J Virol. 2011;85:13114–23. doi:10.1128/JVI.05759-11. PMID:21957304.
- Yu D, Jin C, Ramachandran M, Xu J, Nilsson B, Korsgren O, Le Blanc K, Uhrbom L, Forsberg-Nilsson K, Westermark B, et al. Adenovirus serotype 5 vectors with Tat-PTD modified hexon and serotype 35 fiber show greatly enhanced transduction capacity of primary cell cultures. PLoS One. 2013;8:e54952. doi:10.1371/journal.pone.0054952. PMID:23372800.
- Sester DP, Thygesen SJ, Sagulenko V, Vajjhala PR, Cridland JA, Vitak N, Chen KW, Osborne GW, Schroder K, Stacey KJ, et al. A novel flow cytometric method to assess inflammasome formation. J Immunol. 2015;194:455–62. doi:10.4049/jimmunol.1401110. PMID:25404358.
- Hillerdal V, Boura VF, Bjorkelund H, Andersson K, Essand M. Avidity characterization of genetically engineered T-cells with novel and established approaches. BMC Immunol. 2016;17:23. doi:10.1186/s12865-016-0162-z. PMID:27411667.
- Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi:10.1084/jem.192.9.1213. PMID:11067871.
- Tacken PJ, Figdor CG. Targeted antigen delivery and activation of dendritic cells in vivo: steps towards cost effective vaccines. Semin Immunol. 2011;23:12–20. doi:10.1016/j.smim.2011.01.001. PMID:21269839.
- Kerkar SP, Leonardi AJ, van Panhuys N, Zhang L, Yu Z, Crompton JG, Pan JH, Palmer DC, Morgan RA, Rosenberg SA, et al. Collapse of the tumor stroma is triggered by IL-12 induction of Fas. Mol Ther. 2013;21:1369–77. doi:10.1038/mt.2013.58. PMID:23568260.
- Tugues S, Burkhard SH, Ohs I, Vrohlings M, Nussbaum K, Vom Berg J, Kulig P, Becher B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015;22:237–46. doi:10.1038/cdd.2014.134. PMID:25190142.
- Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–62. doi:10.1084/jem.182.1.155. PMID:7540647.
- Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–204. doi:10.4049/jimmunol.168.7.3195. PMID:11907072.
- Barlan AU, Griffin TM, McGuire KA, Wiethoff CM. Adenovirus membrane penetration activates the NLRP3 inflammasome. J Virol. 2011;85:146–55. doi:10.1128/JVI.01265-10. PMID:20980503.
- Bouwer AL, Saunderson SC, Caldwell FJ, Damani TT, Pelham SJ, Dunn AC, Jack RW, Stoitzner P, McLellan AD. NK cells are required for dendritic cell-based immunotherapy at the time of tumor challenge. J Immunol. 2014;192:2514–21. doi:10.4049/jimmunol.1202797. PMID:24477907.
- Anguille S, Van Acker HH, Van den Bergh J, Willemen Y, Goossens H, Van Tendeloo VF, Smits EL, Berneman ZN, Lion E. Interleukin-15 Dendritic Cells Harness NK Cell Cytotoxic Effector Function in a Contact- and IL-15-Dependent Manner. PLoS One. 2015;10:e0123340. doi:10.1371/journal.pone.0123340. PMID:25951230.
- Vandenberk L, Belmans J, Van Woensel M, Riva M, Van Gool SW. Exploiting the Immunogenic Potential of Cancer Cells for Improved Dendritic Cell Vaccines. Front Immunol 2015;6:663. PMID:26834740.
- Pascutti MF, Rodriguez AM, Falivene J, Giavedoni L, Drexler I, Gherardi MM. Interplay between modified vaccinia virus Ankara and dendritic cells: Phenotypic and functional maturation of bystander dendritic cells. J Virol. 2011;85:5532–45. doi:10.1128/JVI.02267-10. PMID:21411535.
- Laurell A, Lönnemark M, Brekkan E, Magnusson A, Tolf A, Wallgren AC, Andersson B, Adamson L, Kiessling R, Karlsson-Parra A, et al. Intratumorally injected pro-inflammatory allogeneic dendritic cells as immune enhancers: a first-in-human study in unfavourable risk patients with metastatic renal cell carcinoma. J Immunother Cancer. 2017;5:52. doi:10.1186/s40425-017-0255-0. PMID:28642820.
